Biological Properties of Oleanolic Acid Derivatives Bearing Functionalized Side Chains at C-3
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation of Oleanolic Acid Derivatives
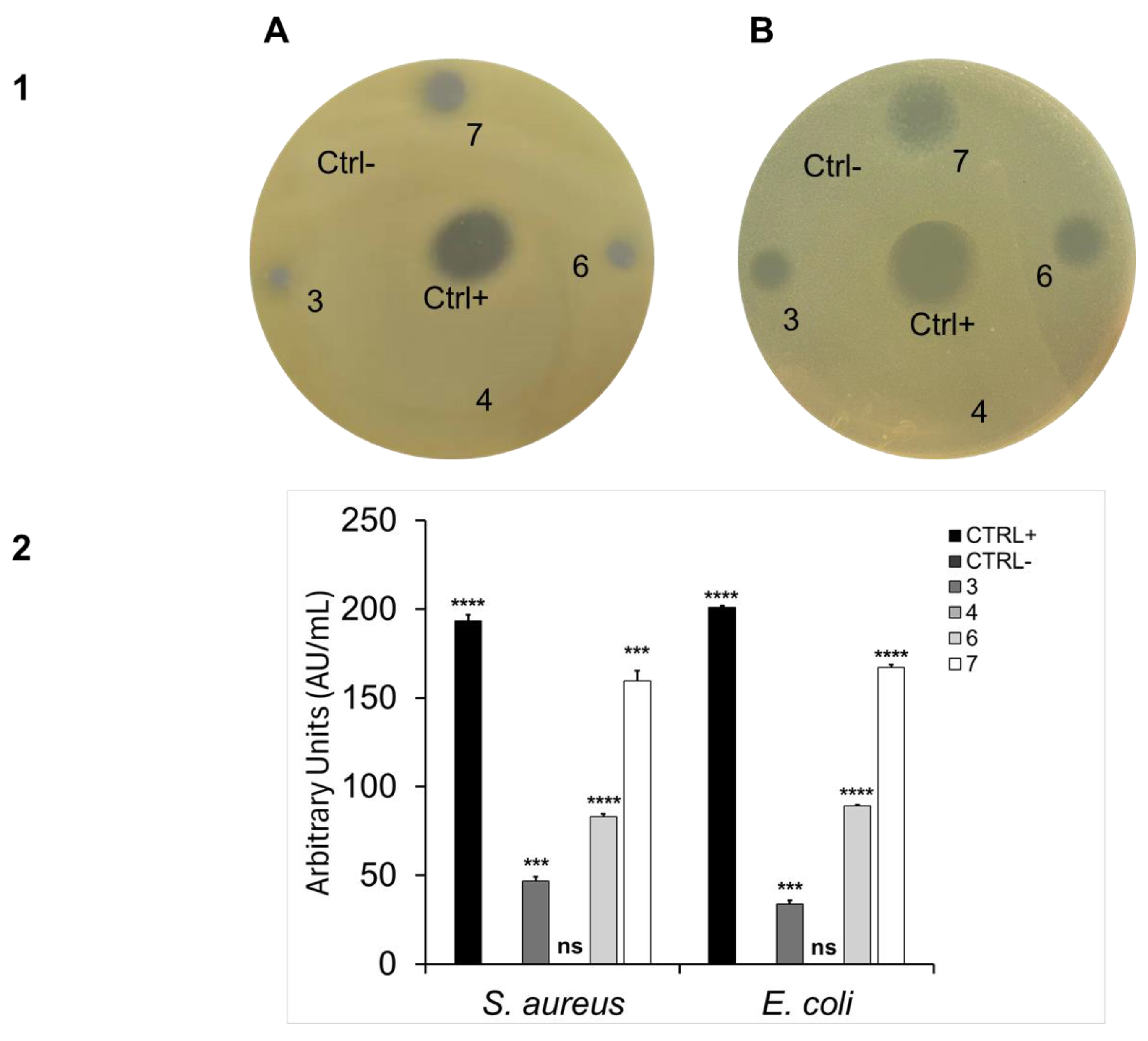
2.2. Antibacterial Activity
2.3. Cytotoxic Activity
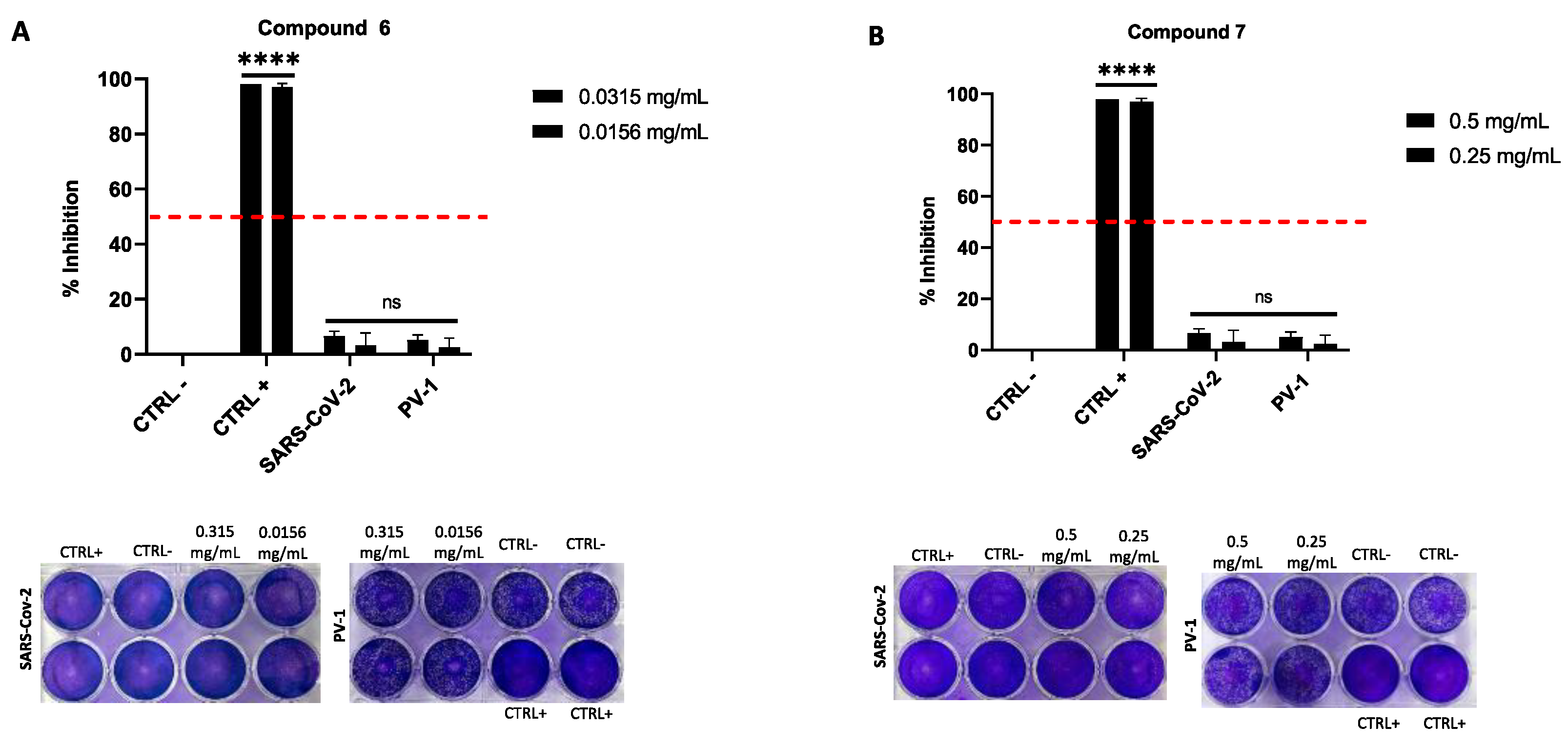
2.4. Antiviral Activity
3. Materials and Methods
3.1. General Procedures
3.2. Preparation of Oleanolic Derivatives
3.2.1. Synthesis of Compounds 2, 5, 8, and 9
3.2.2. Synthesis of Compound 3
3.2.3. Synthesis of Compound 4
3.2.4. Synthesis of Compounds 6 and 7
3.2.5. Synthesis of Compounds 10–13
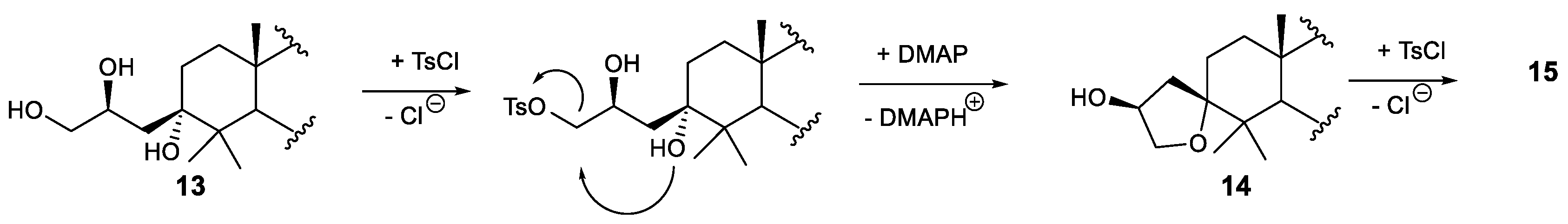
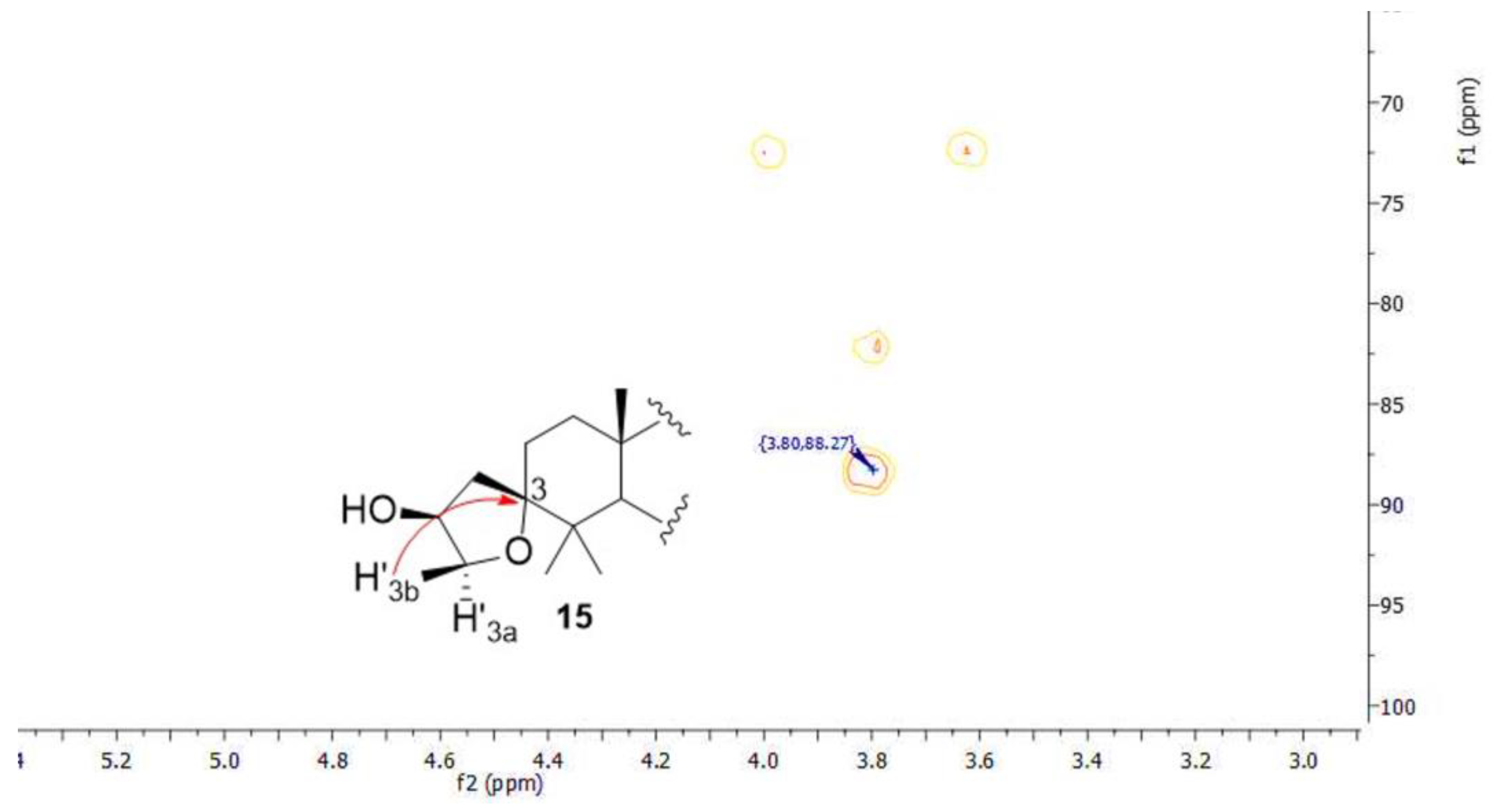
3.2.6. Synthesis of Compounds 14 and 15
3.3. Bacterial Strains and Antimicrobial Activity
3.4. Determination of Minimal Inhibitory Concentration
3.5. Cell Culture Condition
3.6. Viruses
3.7. Cell Cytotoxicity Assay
3.8. Antiviral Activity Assays
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fontana, G.; Badalamenti, N.; Bruno, M.; Castiglione, D.; Notarbartolo, M.; Poma, P.; Spinella, A.; Tutone, M.; Labbozzetta, M. Synthesis, In Vitro and In Silico Analysis of New Oleanolic Acid and Lupeol Derivatives against Leukemia Cell Lines: Involvement of the NF-κB Pathway. Int. J. Mol. Sci. 2022, 23, 6594. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef] [PubMed]
- Ovesná, Z.; Vachálková, A.; Horváthová, K.; Tóthová, D. Pentacyclic triterpenoic acids: New chemoprotective compounds. Minirev. Neoplasma 2004, 51, 327–333. [Google Scholar]
- Gill, B.S.; Kumar, S. Triterpenes in cancer: Significance and their influence. Mol. Biol. Rep. 2016, 43, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Liby, K.T.; Yore, M.M.; Sporn, M.B. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat. Rev. Cancer 2007, 7, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Setzer, W.N.; Setzer, M.C. Plant-derived triterpenoids as potential antineoplastic agents. Mini Rev. Med. Chem. 2003, 3, 540–556. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P.G.; Awad, A.B. Phytosterols as anticancer compounds. Mol. Nutr. Food Res. 2007, 51, 161–170. [Google Scholar] [CrossRef]
- Hisham Shady, N.; Youssif, K.A.; Sayed, A.M.; Belbahri, L.; Oszako, T.; Hassan, H.M.; Abdelmohsen, U.R. Sterols and Triterpenes: Antiviral Potential Supported by In-Silico Analysis. Plants 2021, 10, 41. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, L.; Wang, H.; Xiong, Y. Recent Advances in Antiviral Activities of Triterpenoids. Pharmaceuticals 2022, 15, 1169. [Google Scholar] [CrossRef]
- Wolska, K.; Grudniak, A.; Fiecek, B.; Kraczkiewicz-Dowjat, A.; Kurek, A. Antibacterial Activity of Oleanolic and Ursolic Acids and Their Derivatives. Open Life Sci. 2010, 5, 543–553. [Google Scholar] [CrossRef]
- Wang, L.; Geng, J.; Wang, H. Delivery of Oleanolic Acid with Improved Antifibrosis Efficacy by a Cell Penetrating Peptide P10. ACS Pharmacol. Transl. Sci. 2023, 6, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Rebamang, A.M.; Mandlakayise, L.N.; Thandeka, V.D.; Andy, R.O. Antibacterial Activity of Two Triterpenes from Stem Bark of Protorhus longifolia. J. Med. Plants Res. 2014, 8, 686–702. [Google Scholar] [CrossRef]
- Wang, C.-M.; Chen, H.-T.; Wu, Z.-Y.; Jhan, Y.-L.; Shyu, C.-L.; Chou, C.-H. Antibacterial and Synergistic Activity of Pentacyclic Triterpenoids Isolated from Alstonia scholaris. Molecules 2016, 21, 139. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.; Albarella, L.; Scognamiglio, G.; Uriz, M.; Cimino, G. Structural and Stereochemical Studies of C-21 Terpenoids from Mediterranean Spongiidae Sponges. J. Nat. Prod. 1996, 59, 869–872. [Google Scholar] [CrossRef]
- Medina-O’Donnell, M.; Rivas, F.; Reyes-Zurita, F.J.; Cano-Muñoz, M.; Martinez, A.; Lupiañez, J.A.; Parra, A. Oleanolic Acid Derivatives as Potential Inhibitors of HIV-1 Protease. J. Nat. Prod. 2019, 82, 2886–2896. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Cabra, N.; Vega-Granados, K.; Moya-Andérico, L.; Vukomanović, M.; Parra, A.; Álvarez de Cienfuegos, L.; Torrents, E. Novel Oleanolic and Maslinic Acids derivatives as a promising treatment against bacterial biofilm in nosocomial infections: An in Vitro and in Vivo study. ACS Infect. Dis. 2019, 5, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Kazakova, O.; Rubanik, L.; Smirnova, I.; Poleschuk, N.; Petrova, A.; Kapustsina, Y.; Baikova, I.; Tret’yakova, E.; Khusnutdinova, E. Synthesis and in vitro activity of oleanolic acid derivatives against Chlamydia trachomatis and Staphylococcus aureus. Med. Chem. Res. 2021, 30, 1408–1418. [Google Scholar] [CrossRef]
- Fontana, G.; Bruno, M.; Notarbartolo, M.; Labbozzetta, M.; Poma, P.; Spinella, A.; Rosselli, S. Cytotoxicity of oleanolic and ursolic acid derivatives toward hepatocellular carcinoma and evaluation of NF-κB involvement. Bioorg. Chem. 2019, 90, 103054. [Google Scholar] [CrossRef]
- Talybov, G.M.; Mamedbeyli, E.G.; Yusubov, F.V. Synthesis of Propyne(Ene)Oxy-Substituted Spirotetrahydrofurans. Russ. J. Gen. Chem. 2018, 88, 2684–2688. [Google Scholar] [CrossRef]
- Baltina, L.A.; Tasi, Y.T.; Huang, S.H.; Lai, H.C.; Baltina, L.A.; Petrova, S.F.; Yunusov, M.S.; Lin, C.W. Glycyrrhizic acid derivatives as Dengue virus inhibitors. Bioorg. Med. Chem. Lett. 2019, 29, 126645. [Google Scholar] [CrossRef]
- Hattori, T.; Ikematsu, S.; Koito, A.; Matsushita, S.; Maeda, Y.; Hada, M.; Fujimaki, M.; Takatsuki, K. Preliminary evidence for inhibitory effect of glycyrrhizin on HIV replication in patients with AIDS. Antivir. Res. 1989, 11, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Takei, M.; Kobayashi, M.; Pollard, R.B.; Suzuki, F. Effect of glycyrrhizin, an active component of licorice roots, on HIV replication in cultures of peripheral blood mononuclear cells from HIV-seropositive patients. Pathobiology 2003, 70, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Lahmadi, G.; Horchani, M.; Dbeibia, A.; Mahdhi, A.; Romdhane, A.; Lawson, A.M.; Daïch, A.; Harrath, A.H.; Ben Jannet, H.; Othman, M. Novel Oleanolic Acid-Phtalimidines Tethered 1,2,3 Triazole Hybrids as Promising Antibacterial Agents: Design, Synthesis, In Vitro Experiments and In Silico Docking Studies. Molecules 2023, 28, 4655. [Google Scholar] [CrossRef] [PubMed]
- Gamedze, M.P.; Nkambule, C.M. Dibutyltin Oxide Mediated Diastereoselective Cyclodehydration/Sulfonylation of 1,2,4-Triols. Tetrahedron Lett. 2015, 56, 1825–1829. [Google Scholar] [CrossRef]
- Gowravaram Sabitha, V.; Rama Subba Rao, K.; Sudhakar, M.; Raj Kumar, E.; Venkata Reddy, J.S. Study of conventional versus microwave-assisted reactions of 3,4-epoxyalcohols by CeCl3·7H2O: Synthesis of tetrahydrofurans and 1-chloro-3-substituted-2-propanols. J. Mol. Catal. A Chem. 2008, 280, 16–19. [Google Scholar] [CrossRef]
- Chirskaya, M.V.; Vasil’ev, A.A.; Shorshnev, S.V.; Sviridov, S.I. Transformation of homoallylic alcohol oxides into 3-hydroxytetrahydrofurans in aqueous HClO4. Russ. Chem. Bull. 2006, 55, 1300–1303. [Google Scholar] [CrossRef]
- Bubonja-Šonje, M.; Knežević, S.; Abram, M. Challenges to Antimicrobial Susceptibility Testing of Plant-Derived Polyphenolic Compounds. Arh. Hig. Rada. Toksikol. 2020, 71, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Magréault, S.; Jauréguy, F.; Carbonnelle, E.; Zahar, J.-R. When and How to Use MIC in Clinical Practice? Antibiotics 2022, 11, 1748. [Google Scholar] [CrossRef] [PubMed]
- Sultana, N.; Ata, A. Oleanolic acid and related derivatives as medicinally important compounds. J. Enzyme Inhib. Med. Chem. 2008, 23, 739–756. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Zhang, W.; Hong, D.; Shi, L.; Shen, Q.; Li, J.-Y.; Li, J.; Hu, L.-H. Oleanolic acid and its derivatives: New inhibitor of protein tyrosine phosphatase 1B with cellular activities. Bioorg. Med. Chem. 2008, 16, 8697–8705. [Google Scholar] [CrossRef]
- Kuete, V.; Wabo, G.F.; Ngameni, B.; Mbaveng, A.T.; Metuno, R.; Etoa, F.X. Antimicrobial activity ofthe methanolic extract, fractions and compounds from the stem bark of Irvingia gabonensis (Ixonanthaceae). J. Ethnopharmacol. 2007, 114, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Woldemichael, G.M.; Franzblau, S.G.; Zhang, F.; Wang, Y.; Timmerman, B.N. Inhibitory effect of sterols from Ruprechtia triflora and diterpenes from Calceolaria pinnifolia on the growth of Mycobacterium tuberculosis. Planta Med. 2003, 69, 628–631. [Google Scholar]
- Farina, C.; Pinza, M.; Pifferi, G. Synthesis and antiulcer activity of new derivatives of glycyrrhetic, oleanolic and ursolic acids. Pharmacology 1998, 53, 22–32. [Google Scholar]
- Fontanay, S.; Grare, M.; Mayer, J.; Finance, C.; Duval, R.M. Ursolic, oleanolic and betulic acids: Antibacterial spectra and selectivity indexes. J. Ethnopharmacol. 2008, 120, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Gnoatto, S.C.B.; Vechia, L.D.; Lencina, C.L.; Dassonville-Klimpt, A.; Da Nascimento, S.; Mossalayi, D.; Guillon, J.; Gosmann, G.; Sonnet, P. Synthesis and preliminary evaluation of new ursolic and oleanolic acids derivatives as antileishmanial agents. J. Enzyme Inhib. Med. Chem. 2008, 23, 604–610. [Google Scholar] [CrossRef]
- Lou, H.; Li, H.; Zhang, S.; Lu, H.; Chen, Q. A Review on Preparation of Betulinic Acid and Its Biological Activities. Molecules 2021, 26, 5583. [Google Scholar] [CrossRef] [PubMed]
- Ogbole, O.; Adeniji, J.; Ajaiyeoba, E.; Kamdem, R.; Choudhary, M. Anthraquinones and triterpenoids from Senna siamea (Fabaceae) Lam inhibit poliovirus activity. Afr. J. Microbiol. Res. 2014, 8, 2955–2963. [Google Scholar]
- Badalamenti, N.; Rosselli, S.; Zito, P.; Bruno, M. Phytochemical profile and insecticidal activity of Drimia pancration (Asparagaceae) against adults of Stegobium paniceum (Anobiidae). Nat. Prod. Res. 2021, 35, 4468–4478. [Google Scholar] [CrossRef]
- Candela, R.G.; Lazzara, G.; Piacente, S.; Bruno, M.; Cavallaro, G.; Badalamenti, N. Conversion of Organic Dyes into Pigments: Extraction of Flavonoids from Blackberries (Rubus ulmifolius) and Stabilization. Molecules 2021, 26, 6278. [Google Scholar] [CrossRef]
- Chatow, L.; Nudel, A.; Eyal, N.; Lupo, T.; Ramirez, S.; Zelinger, E.; Nesher, I.; Boxer, R. Terpenes and cannabidiol against human corona and influenza viruses-anti-inflammatory and antiviral in vitro evaluation. Biotechnol. Rep. Amst. 2024, 41, e00829. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single-disk method. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef]
- Wen, X.; Sun, H.; Liu, J.; Cheng, K.; Zhang, P.; Zhang, L.; Hao, J.; Zhang, L.; Ni, P.; Zographos, S.E.; et al. Naturally Occurring Pentacyclic Triterpenes as Inhibitors of Glycogen Phosphorylase: Synthesis, Structure−Activity Relationships, and X-ray Crystallographic Studies. J. Med. Chem. 2008, 51, 3540–3554. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.; Tomita, Y.; Tori, K. Carbon-13 NMR spectra of urs-12-enes and application to structural assignments of components of Isodon japonicus hara tissue cultures. Tetrahedron Lett. 1975, 16, 7–10. [Google Scholar] [CrossRef]
- Castagliuolo, G.; Di Napoli, M.; Vaglica, A.; Badalamenti, N.; Antonini, D.; Varcamonti, M.; Bruno, M.; Zanfardino, A.; Bazan, G. Thymus richardii Subsp. nitidus (Guss.) Jalas Essential Oil: An Ally against Oral Pathogens and Mouth Health. Molecules 2023, 28, 4803. [Google Scholar] [CrossRef] [PubMed]
- Iyapparaj, P.; Maruthiah, T.; Ramasubburayan, R.; Prakash, S.; Kumar, C.; Immanuel, G.; Palavesam, A. Optimization of Bacteriocin Production by Lactobacillus Sp. MSU3IR against Shrimp Bacterial Pathogens. Aquat. Biosyst. 2013, 9, 12. [Google Scholar] [CrossRef]
- Di Napoli, M.; Castagliuolo, G.; Badalamenti, N.; Vaglica, A.; Ilardi, V.; Varcamonti, M.; Bruno, M.; Zanfardino, A. Chemical Composition, Antimicrobial and Antioxidant Activities of the Essential Oil of Italian Prangos trifida (Mill.) Herrnst. & Heyn. Nat. Prod. Res. 2023, 37, 3772–3786. [Google Scholar] [CrossRef] [PubMed]
- Di Napoli, M.; Castagliuolo, G.; Pio, S.; Di Nardo, I.; Russo, T.; Antonini, D.; Notomista, E.; Varcamonti, M.; Zanfardino, A. Study of the Antimicrobial Activity of the Human Peptide SQQ30 against Pathogenic Bacteria. Antibiotics 2024, 13, 145. [Google Scholar] [CrossRef] [PubMed]
- Dell’Annunziata, F.; Sellitto, C.; Franci, G.; Marcotullio, M.C.; Piovan, A.; Della Marca, R.; Folliero, V.; Galdiero, M.; Filippelli, A.; Conti, V.; et al. Antiviral Activity of Ficus rubiginosa Leaf Extracts against HSV-1, HCoV-229E and PV-1. Viruses 2022, 14, 2257. [Google Scholar] [CrossRef]
- Dell’Annunziata, F.; Morone, M.V.; Gioia, M.; Cione, F.; Galdiero, M.; Rosa, N.; Franci, G.; De Bernardo, M.; Folliero, V. Broad-Spectrum Antimicrobial Activity of Oftasecur and Visuprime Ophthalmic Solutions. Microorganisms 2023, 11, 503. [Google Scholar] [CrossRef]


| Comp. | 8 | 9 | 12 | 13 | 14 | 15 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C/H | C | H | C | H | C | H | C | H | C | H | C | H |
| 1 | 36.9 | 36.7 | 36.9 | 1.25 m 1.27 m | 34.4 | 34.8 | 1.29 m 1.31 m | 34.7 | ||||
| 2 | 32.6 | 28.4 | 32.4 | 1.49 m 1.65 m | 33.9 | 31.3 | 1.45 m 1.66 m | 32.4 | ||||
| 3 | 75.0 | 76.2 | 77.1 | 75.9 | 85.1 | 88.2 | ||||||
| 4 | 41.2 | 40.6 | 41.7 | 41.7 | 42.0 | 41.7 | ||||||
| 5 | 50.9 | 1.21 m | 51.1 | 1.19 m | 51.5 | 1.17 m | 51.0 | 1.18 m | 51.6 | 1.24 m | 51.5 | 1.22 m |
| 6 | 18.8 | 18.9 | 18.8 | 1.37 m 1.43 m | 20.8 | 18.7 | 1.40 m 1.42 m | 18.8 | ||||
| 7 | 33.8 | 32.9 | 32.7 | 1.20 m 1.29 m | 30.7 | 32.5 | 1.20 m 1.22 m | 32.5 | ||||
| 8 | 39.2 | 39.3 | 39.2 | 37.0 | 37.1 | 36.8 | ||||||
| 9 | 47.5 | 47.6 | 47.7 | 1.62 m | 47.6 | 46.5 | 1.62 m | 47.4 | ||||
| 10 | 30.7 | 37.1 | 36.7 | 36.8 | 31.2 | 30.9 | ||||||
| 11 | 23.5 | 23.4 | 23.0 | 1.88 m 1.90 m | 23.2 | 23.3 | 1.83 m 1.84 m | 23.2 | ||||
| 12 | 122.4 | 5.29 t (3.4) | 122.3 | 5.29 t (3.4) | 122.3 | 5.29 t br (3.5) | 122.2 | 5.28 t (3.5) | 122.4 | 5.28 m | 122.4 | 5.28 m |
| 13 | 143.8 | 143.8 | 143.8 | 143.7 | 143.4 | 143.7 | ||||||
| 14 | 41.7 | 41.6 | 40.8 | 39.2 | 40.7 | 40.4 | ||||||
| 15 | 27.6 | 27.7 | 27.9 | 1.04 m 1.60 m | 27.6 | 27.9 | 1.00 m 1.58 m | 27.6 | ||||
| 16 | 23.2 | 23.1 | 23.5 | 1.61 m 1.96 m | 23.0 | 23.2 | 1.60 m 1.87 m | 23.0 | ||||
| 17 | 46.7 | 46.0 | 45.8 | 45.8 | 41.5 | 45.8 | ||||||
| 18 | 40.9 | 2.86 dd (14.4, 4.4) | 41.3 | 2.87 dd (14.0, 4.6) | 41.2 | 2.85 dd (13.4, 4.3) | 41.3 | 2.85 dd (13.9, 4.1) | 41.3 | 2.85 m | 41.2 | 2.84 dd (14.1, 4.3) |
| 19 | 45.8 | 46.8 | 46.7 | 1.13 m 1.15 m | 46.7 | 46.5 | 1.10 m 1.12 m | 46.7 | ||||
| 20 | 30.7 | 30.7 | 30.7 | 30.2 | 30.8 | 30.7 | ||||||
| 21 | 34.3 | 33.9 | 33.8 | 1.17 m 1.32 m | 32.4 | 34.0 | 1.15 m 1.17 m | 33.8 | ||||
| 22 | 32.4 | 32.4 | 34.4 | 1.20 m 1.45 m | 32.7 | 29.2 | 1.65 m 1.73 m | 29.1 | ||||
| 23 | 20.7 | 0.91 s | 19.5 | 0.87 s | 16.8 | 0.73 s | 16.8 | 0.71 s | 17.2 | 0.71 s | 16.8 | 0.70 s |
| 24 | 23.0 | 0.82 s | 24.1 | 0.95 s | 23.6 | 0.93 s | 23.8 | 0.92 s | 23.7 | 0.91 s | 23.4 | 0.92 s |
| 25 | 14.9 | 0.91 s | 15.8 | 0.91 s | 14.9 | 0.90 s | 14.9 | 0.90 s | 15.1 | 0.93 s | 15.0 | 0.90 s |
| 26 | 16.8 | 0.73 s | 16.9 | 0.73 s | 20.7 | 0.78 s | 18.8 | 0.79 s | 20.4 | 0.76 s | 20.5 | 0.75 s |
| 27 | 26.1 | 1.15 s | 26.0 | 1.16 s | 26.0 | 1.14 s | 26.1 | 1.14 s | 26.2 | 1.12 s | 26.1 | 1.14 s |
| 28 | 178.3 | 178.3 | 178.3 | 178.4 | 178.4 | 178.3 | ||||||
| 29 | 33.1 | 0.93 s | 33.1 | 0.91 s | 33.1 | 0.90 s | 33.1 | 0.90 s | 33.2 | 0.91 s | 33.1 | 0.89 s |
| 30 | 23.6 | 0.94 s | 23.6 | 0.93 s | 23.7 | 0.94 s | 23.6 | 0.92 s | 23.8 | 0.76 s | 23.6 | 0.92 s |
| 1′ | 40.5 | 2.11 dd (13.8, 6.8) 2.41 dd (13.8, 8.2) | 41.1 | 2.23 dd (13.5, 7.2) 2.48 dd (14.0, 7.4) | 36.8 | 1.25 m 1.87 m | 40.9 | 1.85 m 1.90 m | 43.8 | 2.00 m 2.24 dd m (14.1, 6.6) | 39.2 | 1.67 m 1.70 m |
| 2′ | 134.7 | 5.93 ddt (17.1, 10.0, 7.3) | 135.0 | 5.91 ddt. (17.0, 10.2, 7.5) | 69.0 | 4.11 m | 69.6 | 4.04 m | 67.3 | 4.44 m | 82.1 | 5.02 m |
| 3′ | 118.9 | 5.12 m 5.16 m | 118.0 | 5.10 dd (17.0, 2.3) 5.16 dd (10.2, 2.3) | 67.4 | 3.44 dd (11.2, 6.7) 3.59 m | 67.6 | 3.48 dd (10.9, 6.5) 3.58 m | 78.2 | 3.81 m | 72.3 | 3.81 m 3.90 m |
| 1″ | 133.9 | |||||||||||
| 2″ = 6″ | 129.9 | 7.34 d (8.5) | ||||||||||
| 3″ = 5″ | 127.7 | 7.79 m | ||||||||||
| 4″ | 144.8 | |||||||||||
| Tosyl-CH3 | 21.6 | 2.45 s | ||||||||||
| -OMe | 51.5 | 3.63 s | 51.5 | 3.62 s | 50.7 | 3.63 s | 51.6 | 3.62 s | 51.5 | 3.62 s | 51.5 | 3.62 s |
| Strain | Compounds | MIC100 [mg/mL] |
|---|---|---|
| E. coli DH5α | 1 | >10 |
| 3 | >10 | |
| 4 | >10 | |
| 6 | >10 | |
| 7 | 10 | |
| 8 | >10 | |
| 9 | >10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fontana, G.; Badalamenti, N.; Bruno, M.; Maggi, F.; Dell’Annunziata, F.; Capuano, N.; Varcamonti, M.; Zanfardino, A. Biological Properties of Oleanolic Acid Derivatives Bearing Functionalized Side Chains at C-3. Int. J. Mol. Sci. 2024, 25, 8480. https://doi.org/10.3390/ijms25158480
Fontana G, Badalamenti N, Bruno M, Maggi F, Dell’Annunziata F, Capuano N, Varcamonti M, Zanfardino A. Biological Properties of Oleanolic Acid Derivatives Bearing Functionalized Side Chains at C-3. International Journal of Molecular Sciences. 2024; 25(15):8480. https://doi.org/10.3390/ijms25158480
Chicago/Turabian StyleFontana, Gianfranco, Natale Badalamenti, Maurizio Bruno, Filippo Maggi, Federica Dell’Annunziata, Nicoletta Capuano, Mario Varcamonti, and Anna Zanfardino. 2024. "Biological Properties of Oleanolic Acid Derivatives Bearing Functionalized Side Chains at C-3" International Journal of Molecular Sciences 25, no. 15: 8480. https://doi.org/10.3390/ijms25158480
APA StyleFontana, G., Badalamenti, N., Bruno, M., Maggi, F., Dell’Annunziata, F., Capuano, N., Varcamonti, M., & Zanfardino, A. (2024). Biological Properties of Oleanolic Acid Derivatives Bearing Functionalized Side Chains at C-3. International Journal of Molecular Sciences, 25(15), 8480. https://doi.org/10.3390/ijms25158480










