Size-Exclusion Chromatography: A Path to Higher Yield and Reproducibility Compared to Sucrose Cushion Ultracentrifugation for Extracellular Vesicle Isolation in Multiple Myeloma
Abstract
:1. Introduction
2. Results
2.1. Characterization of Isolated EVs
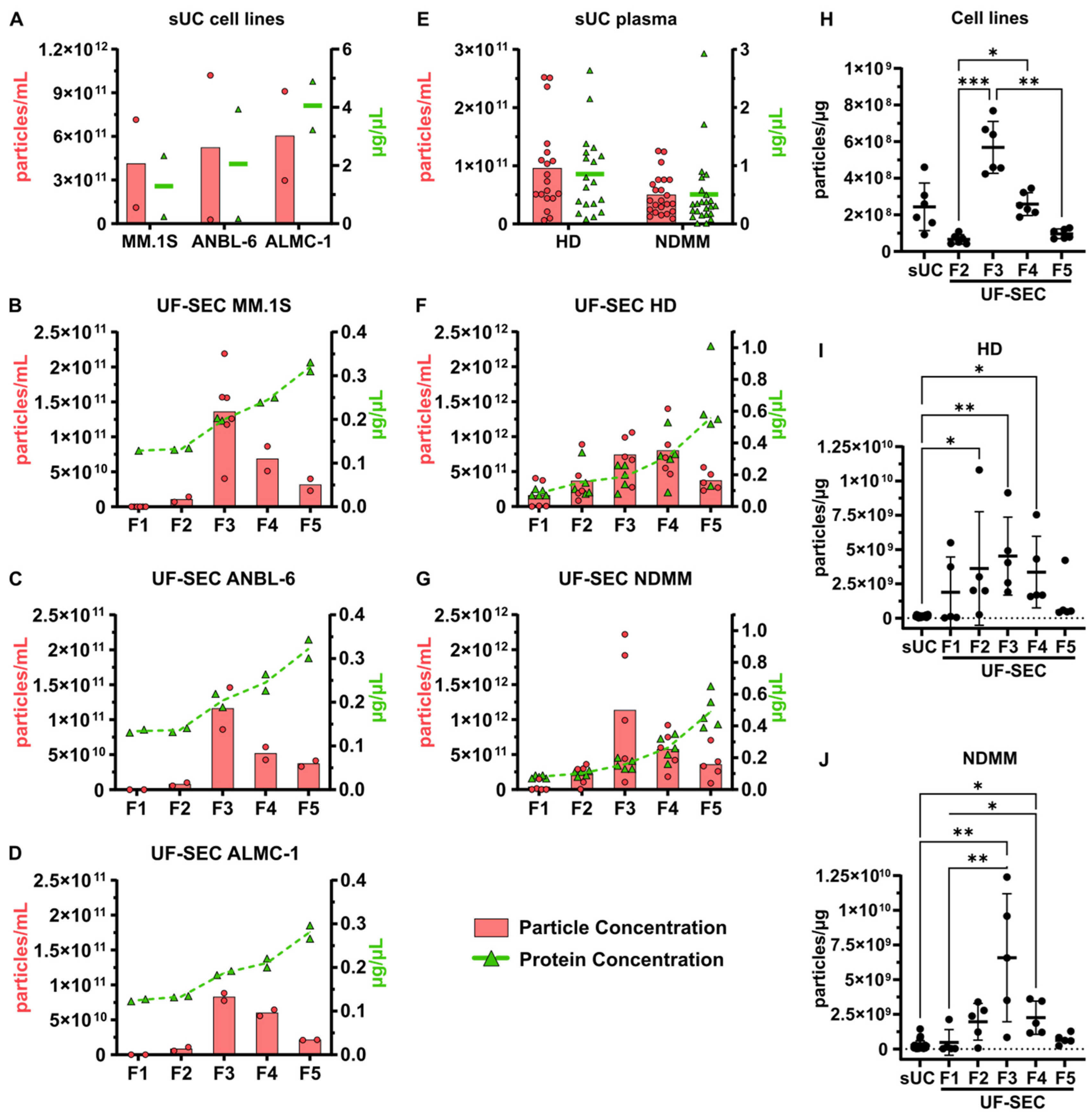
2.1.1. Particle and Protein Concentrations
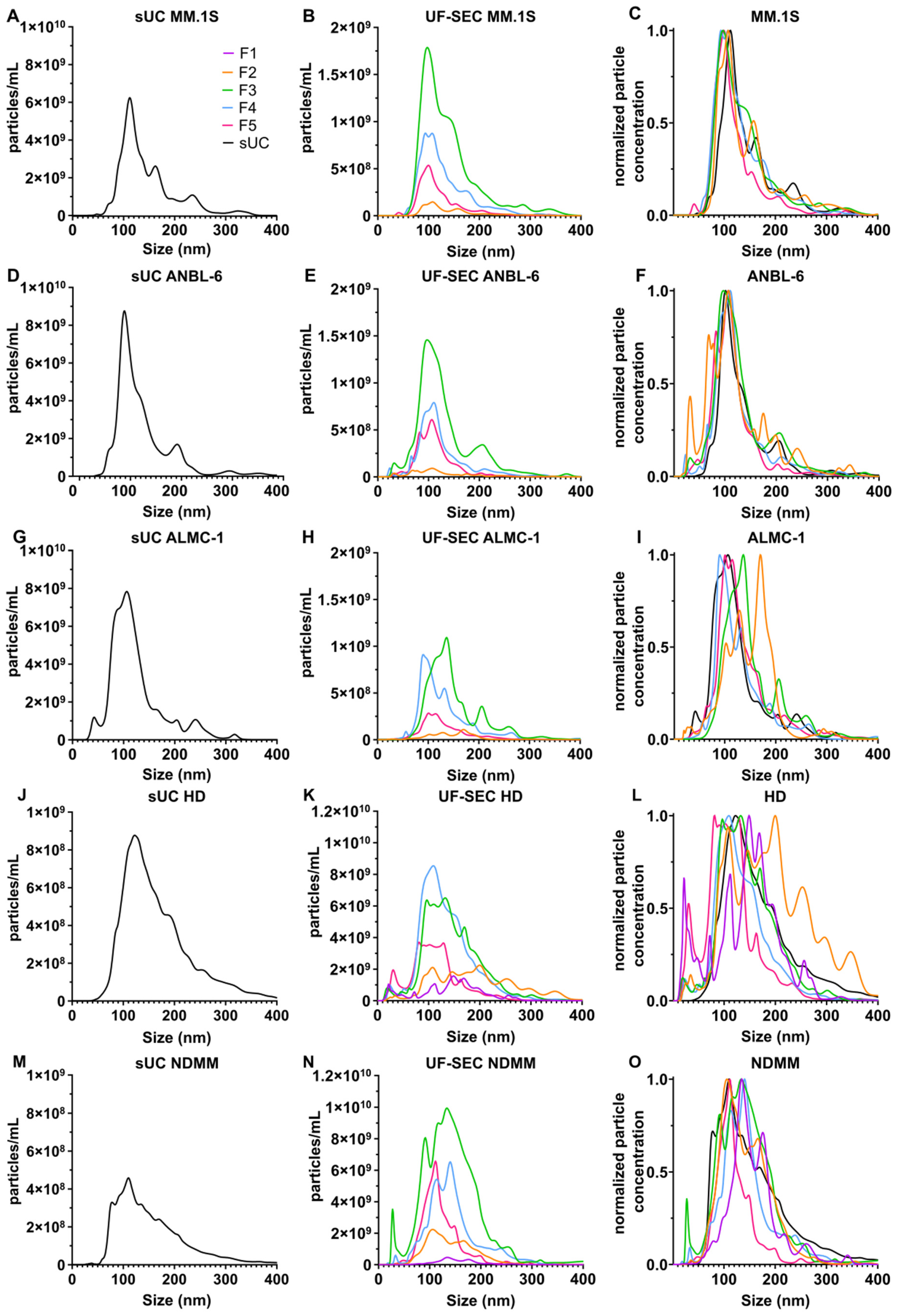
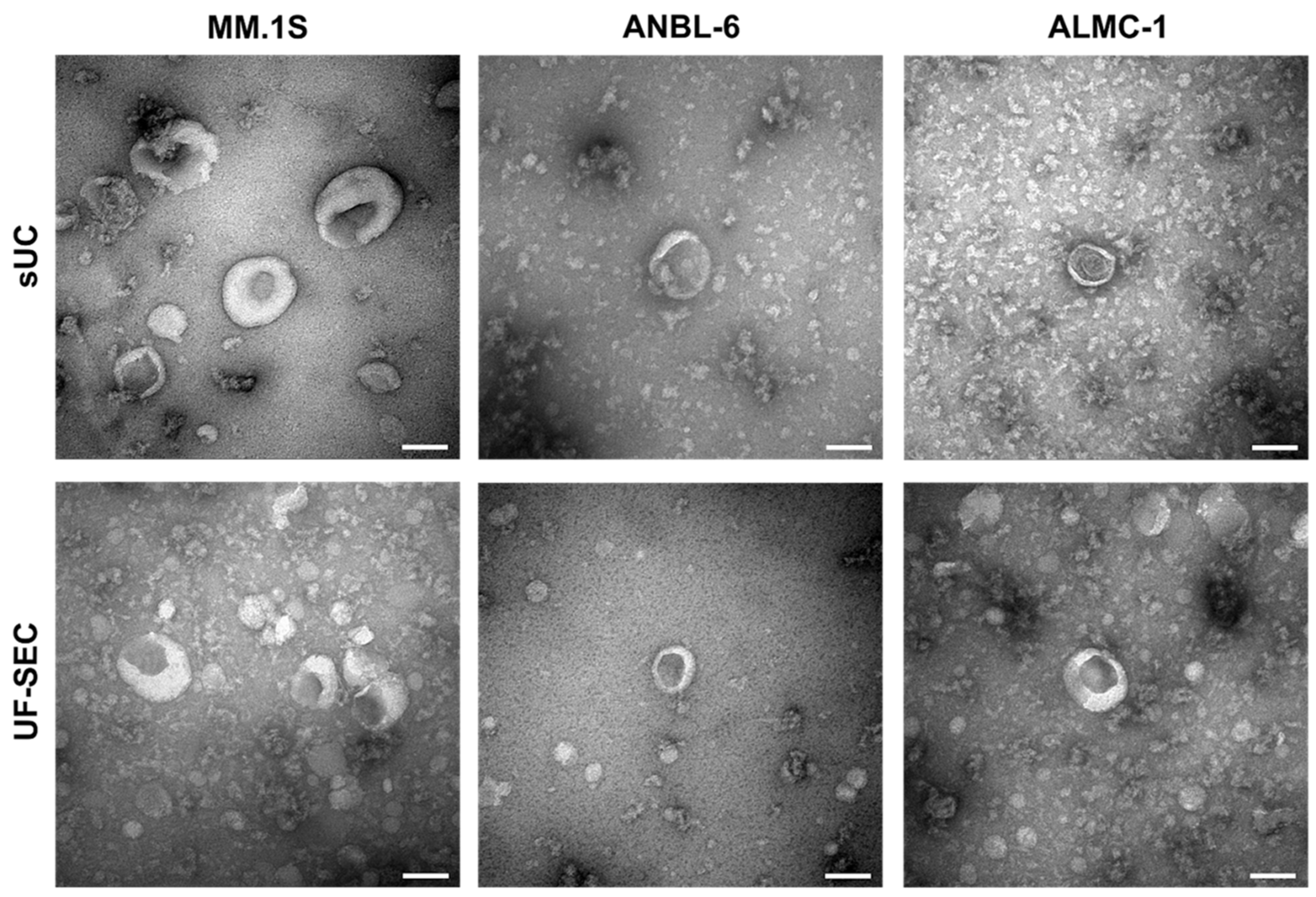
2.1.2. EV Size and Morphology
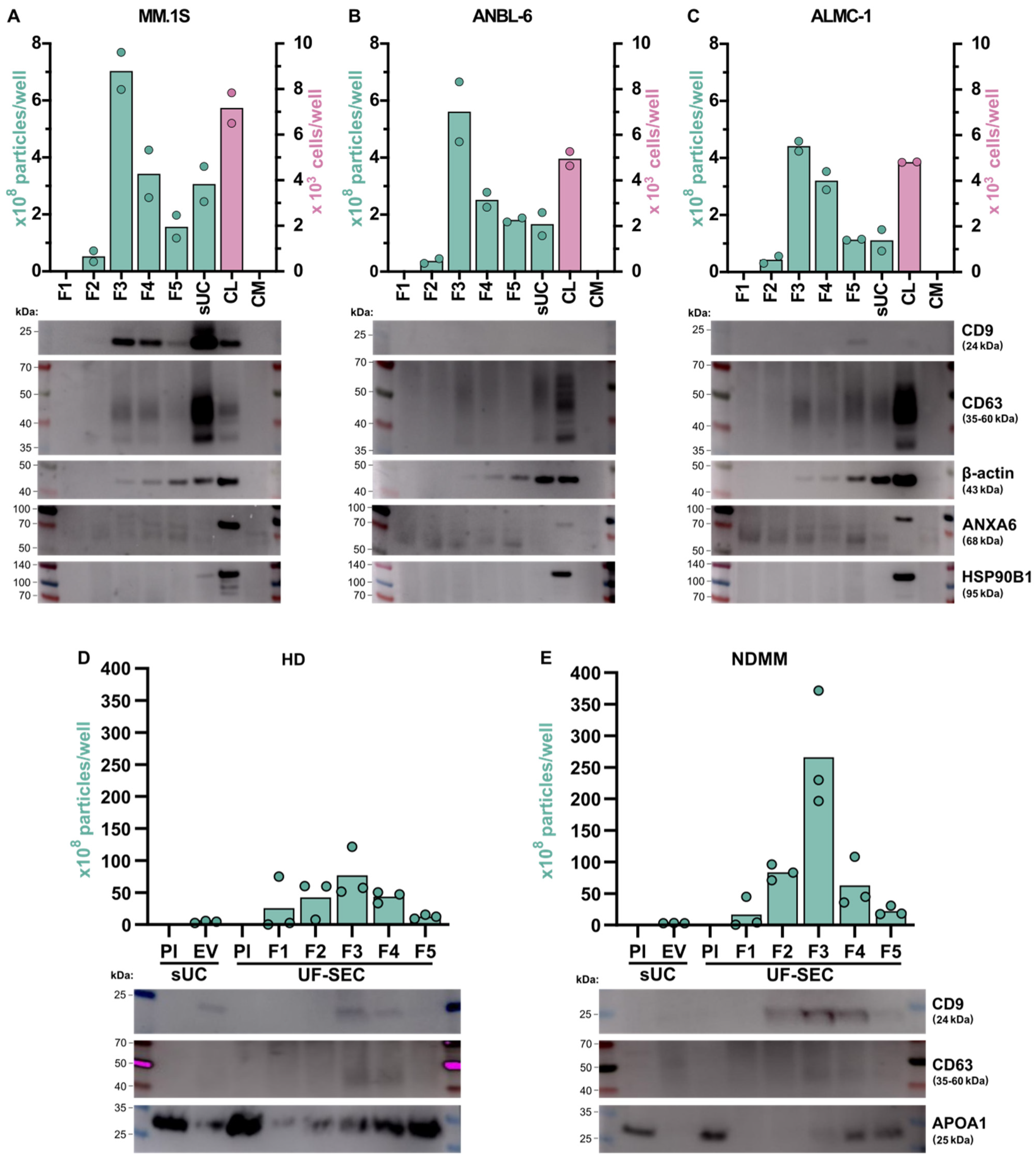
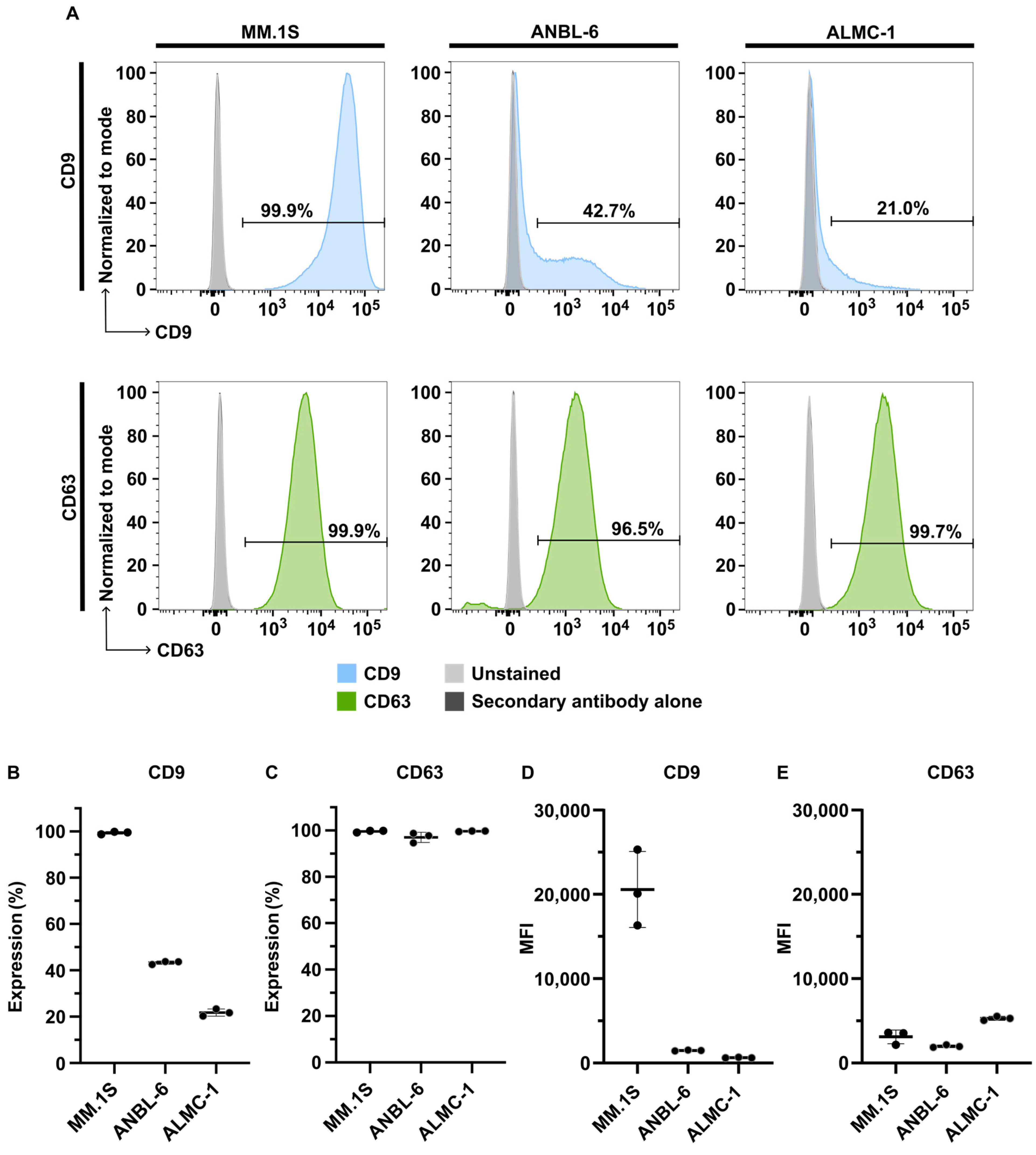
2.1.3. Detection of EV Markers
2.2. Yield and Reproducibility of EV Isolation by sUC vs. UF-SEC
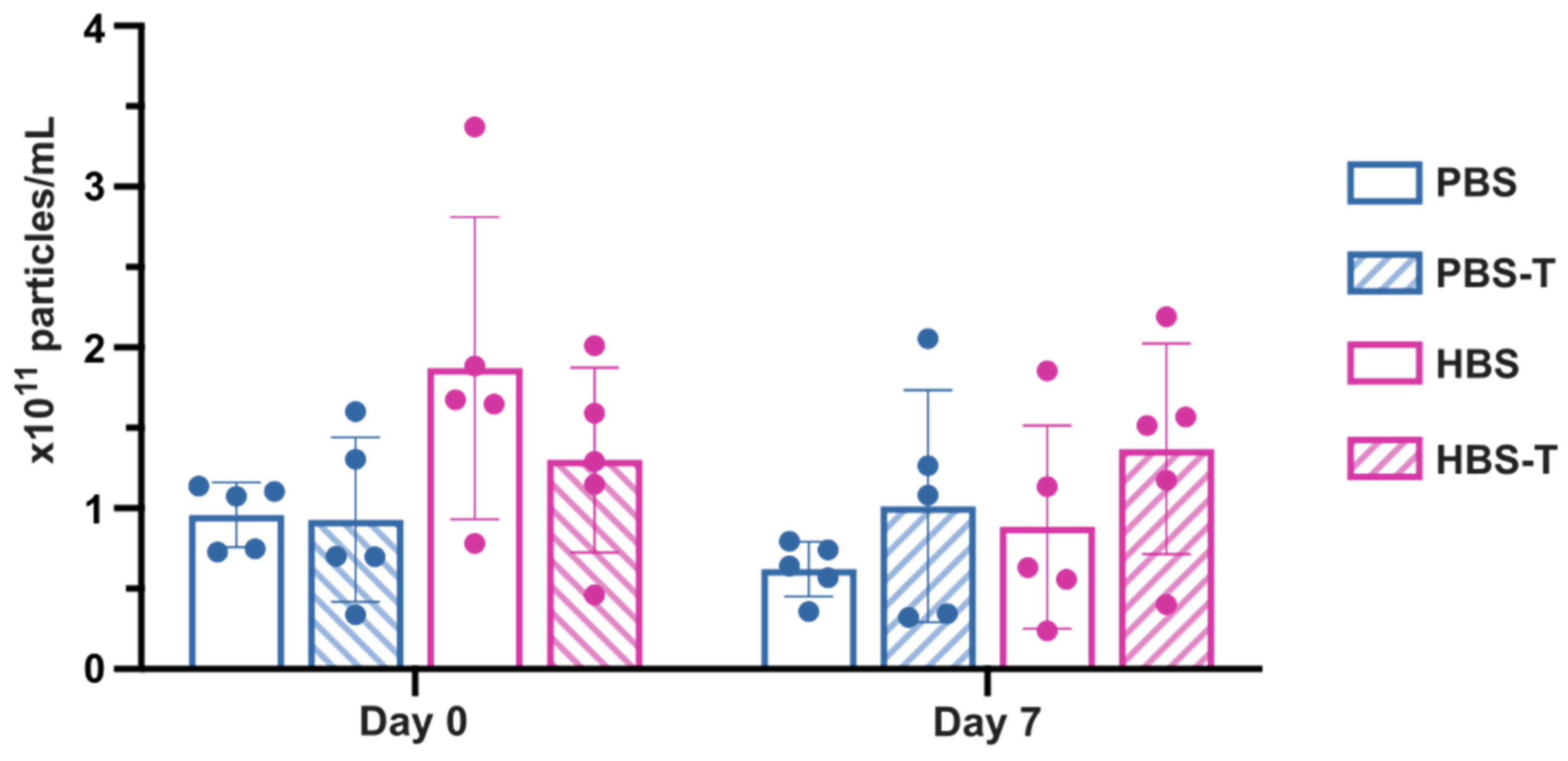
2.3. Comparison of Buffers for EV Recovery and Preservation
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Culture Medium Conditioning
4.3. Patients’ Inclusion and Plasma Samples Collection
4.4. EV Isolation by Ultrafiltration Followed by Size-Exclusion Chromatography
4.5. EV Isolation by Sucrose Cushion Ultracentrifugation
4.6. Nanoparticle Tracking Analysis
4.7. Bicinchoninic Acid Protein Assay
4.8. Western Blot
4.9. Flow Cytometry
4.10. Transmission Electron Microscopy
4.11. Statistical Analysis
4.12. EV-TRACK
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BCA | Bicinchoninic acid protein assay |
| CCM | Conditioned culture medium |
| CL | Cell lysates |
| CM | Culture medium |
| EDTA | Ethylenediaminetetraacetic acid |
| EV | Extracellular vesicle |
| F | fraction |
| FACS | Fluorescence-activated cell sorting |
| FBS | Fetal bovine serum |
| HBS | HEPES buffered saline |
| HBS-T | HBS + 0.005% Tween 20 |
| HD | Healthy donor |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| HRP | Horseradish peroxidase |
| IMDM | Iscove’s Modified Dulbecco’s Medium |
| LDS | Lithium dodecyl sulfate |
| MES | 2-(N-morpholino) ethane sulfonic acid |
| MISEV | Minimal information for studies of extracellular vesicles |
| MM | Multiple myeloma |
| NDMM | Newly diagnosed Multiple Myeloma |
| NTA | Nanoparticle tracking analysis |
| p | p-value |
| PBS | Phosphate-buffered saline |
| PBS-T | PBS + 0.005% Tween 20 |
| PVDF | Polyvinylidene difluoride |
| RIPA | Radioimmunoprecipitation assay |
| RT | Room temperature |
| SDS | Sodium dodecyl sulfate |
| SEC | Size-exclusion chromatography |
| sUC | Sucrose cushion ultracentrifugation |
| TBS | Tris-buffered saline |
| TBS-T | TBS + 0.1% Tween 20 |
| UC | Ultracentrifugation |
| UF | Ultrafiltration |
| UF-SEC | Ultrafiltration combined with size-exclusion chromatography |
References
- Kalluri, R.; LeBleu, V.S. The Biology, Function, and Biomedical Applications of Exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, Biologic Function and Clinical Potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Ghodasara, A.; Raza, A.; Wolfram, J.; Salomon, C.; Popat, A. Clinical Translation of Extracellular Vesicles. Adv. Healthc. Mater. 2023, 12, 2301010. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Sun, H.-T.; Wang, S.; Huang, S.-L.; Zheng, Y.; Wang, C.-Q.; Hu, B.-Y.; Qin, W.; Zou, T.-T.; Fu, Y.; et al. Isolation and Characterization of Exosomes for Cancer Research. J. Hematol. Oncol. 2020, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, C.; Vizio, D.D.; Sahoo, S.; Théry, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques Used for the Isolation and Characterization of Extracellular Vesicles: Results of a Worldwide Survey. J. Extracell. Vesicles 2016, 5, 32945. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Chen, Y.; Pei, F.; Zeng, C.; Yao, Y.; Liao, W.; Zhao, Z. Extracellular Vesicles in Liquid Biopsies: Potential for Disease Diagnosis. BioMed Res. Int. 2021, 2021, 6611244. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Rawat, S.; Arora, V.; Kottarath, S.K.; Dinda, A.K.; Vaishnav, P.K.; Nayak, B.; Mohanty, S. An Improvised One-Step Sucrose Cushion Ultracentrifugation Method for Exosome Isolation from Culture Supernatants of Mesenchymal Stem Cells. Stem Cell Res. Ther. 2018, 9, 180. [Google Scholar] [CrossRef]
- Taylor, D.D.; Shah, S. Methods of Isolating Extracellular Vesicles Impact Down-Stream Analyses of Their Cargoes. Methods 2015, 87, 3–10. [Google Scholar] [CrossRef]
- Monguió-Tortajada, M.; Gálvez-Montón, C.; Bayes-Genis, A.; Roura, S.; Borràs, F.E. Extracellular Vesicle Isolation Methods: Rising Impact of Size-Exclusion Chromatography. Cell. Mol. Life Sci. 2019, 76, 2369–2382. [Google Scholar] [CrossRef] [PubMed]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef] [PubMed]
- Liangsupree, T.; Multia, E.; Riekkola, M.-L. Modern Isolation and Separation Techniques for Extracellular Vesicles. J. Chromatogr. A 2021, 1636, 461773. [Google Scholar] [CrossRef]
- Benedikter, B.J.; Bouwman, F.G.; Vajen, T.; Heinzmann, A.C.A.; Grauls, G.; Mariman, E.C.; Wouters, E.F.M.; Savelkoul, P.H.; Lopez-Iglesias, C.; Koenen, R.R.; et al. Ultrafiltration Combined with Size Exclusion Chromatography Efficiently Isolates Extracellular Vesicles from Cell Culture Media for Compositional and Functional Studies. Sci. Rep. 2017, 7, 15297. [Google Scholar] [CrossRef] [PubMed]
- Mol, E.A.; Goumans, M.-J.; Doevendans, P.A.; Sluijter, J.P.G.; Vader, P. Higher Functionality of Extracellular Vesicles Isolated Using Size-Exclusion Chromatography Compared to Ultracentrifugation. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2061–2065. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.K.; Khan, M.A.; Zubair, H.; Srivastava, S.K.; Khushman, M.; Singh, S.; Singh, A.P. Comparative Analysis of Exosome Isolation Methods Using Culture Supernatant for Optimum Yield, Purity and Downstream Applications. Sci. Rep. 2019, 9, 5335. [Google Scholar] [CrossRef] [PubMed]
- Sarin, V.; Yu, K.; Ferguson, I.D.; Gugliemini, O.; Nix, M.A.; Hann, B.; Sirota, M.; Wiita, A.P. Evaluating the Efficacy of Multiple Myeloma Cell Lines as Models for Patient Tumors via Transcriptomic Correlation Analysis. Leukemia 2020, 34, 2754–2765. [Google Scholar] [CrossRef]
- Roccaro, A.M.; Sacco, A.; Maiso, P.; Azab, A.K.; Tai, Y.-T.; Reagan, M.; Azab, F.; Flores, L.M.; Campigotto, F.; Weller, E.; et al. BM Mesenchymal Stromal Cell–Derived Exosomes Facilitate Multiple Myeloma Progression. J. Clin. Invest. 2013, 123, 1542–1555. [Google Scholar] [CrossRef]
- Wang, J.; Hendrix, A.; Hernot, S.; Lemaire, M.; De Bruyne, E.; Van Valckenborgh, E.; Lahoutte, T.; De Wever, O.; Vanderkerken, K.; Menu, E. Bone Marrow Stromal Cell–Derived Exosomes as Communicators in Drug Resistance in Multiple Myeloma Cells. Blood 2014, 124, 555–566. [Google Scholar] [CrossRef]
- Ferreira, B.V.; Carneiro, E.A.; Pestana, C.; Barahona, F.; Caetano, J.; Lopes, R.; Lúcio, P.; Neves, M.; Beck, H.C.; Carvalho, A.S.; et al. Patient-Derived Extracellular Vesicles Proteins as New Biomarkers in Multiple Myeloma—A Real-World Study. Front. Oncol. 2022, 12, 860849. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.; Caetano, J.; Barahona, F.; Lopes, R.; Carneiro, E.; Costa-Silva, B.; João, C. Liquid Biopsies for Multiple Myeloma in a Time of Precision Medicine. J. Mol. Med. 2020, 98, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T. Realization of Osteolysis, Angiogenesis, Immunosuppression, and Drug Resistance by Extracellular Vesicles: Roles of RNAs and Proteins in Their Cargoes and of Ectonucleotidases of the Immunosuppressive Adenosinergic Noncanonical Pathway in the Bone Marrow Niche of Multiple Myeloma. Cancers 2021, 13, 2969. [Google Scholar] [CrossRef] [PubMed]
- Smith, Z.J.; Lee, C.; Rojalin, T.; Carney, R.P.; Hazari, S.; Knudson, A.; Lam, K.; Saari, H.; Ibañez, E.L.; Viitala, T.; et al. Single Exosome Study Reveals Subpopulations Distributed among Cell Lines with Variability Related to Membrane Content. J. Extracell. Vesicles 2015, 4, 28533. [Google Scholar] [CrossRef] [PubMed]
- Gelibter, S.; Marostica, G.; Mandelli, A.; Siciliani, S.; Podini, P.; Finardi, A.; Furlan, R. The Impact of Storage on Extracellular Vesicles: A Systematic Study. J. Extracell. Vesicles 2022, 11, e12162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A Review of Its Classification, Isolation Techniques, Storage, Diagnostic and Targeted Therapy Applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef] [PubMed]
- Van De Wakker, S.I.; Van Oudheusden, J.; Mol, E.A.; Roefs, M.T.; Zheng, W.; Görgens, A.; El Andaloussi, S.; Sluijter, J.P.G.; Vader, P. Influence of Short Term Storage Conditions, Concentration Methods and Excipients on Extracellular Vesicle Recovery and Function. Eur. J. Pharm. Biopharm. 2022, 170, 59–69. [Google Scholar] [CrossRef]
- Dixson, A.C.; Dawson, T.R.; Di Vizio, D.; Weaver, A.M. Context-Specific Regulation of Extracellular Vesicle Biogenesis and Cargo Selection. Nat. Rev. Mol. Cell Biol. 2023, 24, 454–476. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular Vesicles and Nanoparticles: Emerging Complexities. Trends Cell Biol. 2023, 33, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Jeyaram, A.; Jay, S.M. Preservation and Storage Stability of Extracellular Vesicles for Therapeutic Applications. AAPS J. 2018, 20, 1. [Google Scholar] [CrossRef]
- Kusuma, G.D.; Barabadi, M.; Tan, J.L.; Morton, D.A.V.; Frith, J.E.; Lim, R. To Protect and to Preserve: Novel Preservation Strategies for Extracellular Vesicles. Front. Pharmacol. 2018, 9, 1199. [Google Scholar] [CrossRef]
- Maia, J.; Batista, S.; Couto, N.; Gregório, A.C.; Bodo, C.; Elzanowska, J.; Strano Moraes, M.C.; Costa-Silva, B. Employing Flow Cytometry to Extracellular Vesicles Sample Microvolume Analysis and Quality Control. Front. Cell Dev. Biol. 2020, 8, 593750. [Google Scholar] [CrossRef] [PubMed]
- EV-TRACK Consortium; Van Deun, J.; Mestdagh, P.; Agostinis, P.; Akay, Ö.; Anand, S.; Anckaert, J.; Martinez, Z.A.; Baetens, T.; Beghein, E.; et al. EV-TRACK: Transparent Reporting and Centralizing Knowledge in Extracellular Vesicle Research. Nat. Methods 2017, 14, 228–232. [Google Scholar] [CrossRef] [PubMed]

| Antigen | Clone | Host | Supplier | Reference | Dilution used | Electrophoresis Condition |
|---|---|---|---|---|---|---|
| CD9 | Ts9 | Mouse | Invitrogen | 10626D | 1:1000 | Non-reducing |
| CD63 | Ts63 | Mouse | Invitrogen | 10628D | 1:250 | Non-reducing |
| β-actin | AC-15 | Mouse | Invitrogen | MA1-91399 | 1:8000 | Reducing |
| ANXA6 | ns | Rabbit | Novus Biologicals | NBP1-90149 | 1:1000 | Reducing |
| HSP90B1 | 4G7C7 | Mouse | Proteintech | 60012-2-Ig | 1:5000 | Reducing |
| ApoA1 | 532 | Mouse | Invitrogen | MA5-14732 | 1:500 | Reducing |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grenhas, M.; Lopes, R.; Ferreira, B.V.; Barahona, F.; João, C.; Carneiro, E.A. Size-Exclusion Chromatography: A Path to Higher Yield and Reproducibility Compared to Sucrose Cushion Ultracentrifugation for Extracellular Vesicle Isolation in Multiple Myeloma. Int. J. Mol. Sci. 2024, 25, 8496. https://doi.org/10.3390/ijms25158496
Grenhas M, Lopes R, Ferreira BV, Barahona F, João C, Carneiro EA. Size-Exclusion Chromatography: A Path to Higher Yield and Reproducibility Compared to Sucrose Cushion Ultracentrifugation for Extracellular Vesicle Isolation in Multiple Myeloma. International Journal of Molecular Sciences. 2024; 25(15):8496. https://doi.org/10.3390/ijms25158496
Chicago/Turabian StyleGrenhas, Madalena, Raquel Lopes, Bruna Velosa Ferreira, Filipa Barahona, Cristina João, and Emilie Arnault Carneiro. 2024. "Size-Exclusion Chromatography: A Path to Higher Yield and Reproducibility Compared to Sucrose Cushion Ultracentrifugation for Extracellular Vesicle Isolation in Multiple Myeloma" International Journal of Molecular Sciences 25, no. 15: 8496. https://doi.org/10.3390/ijms25158496





