Pyridoxamine Alleviates Cardiac Fibrosis and Oxidative Stress in Western Diet-Induced Prediabetic Rats
Abstract
:1. Introduction
2. Results
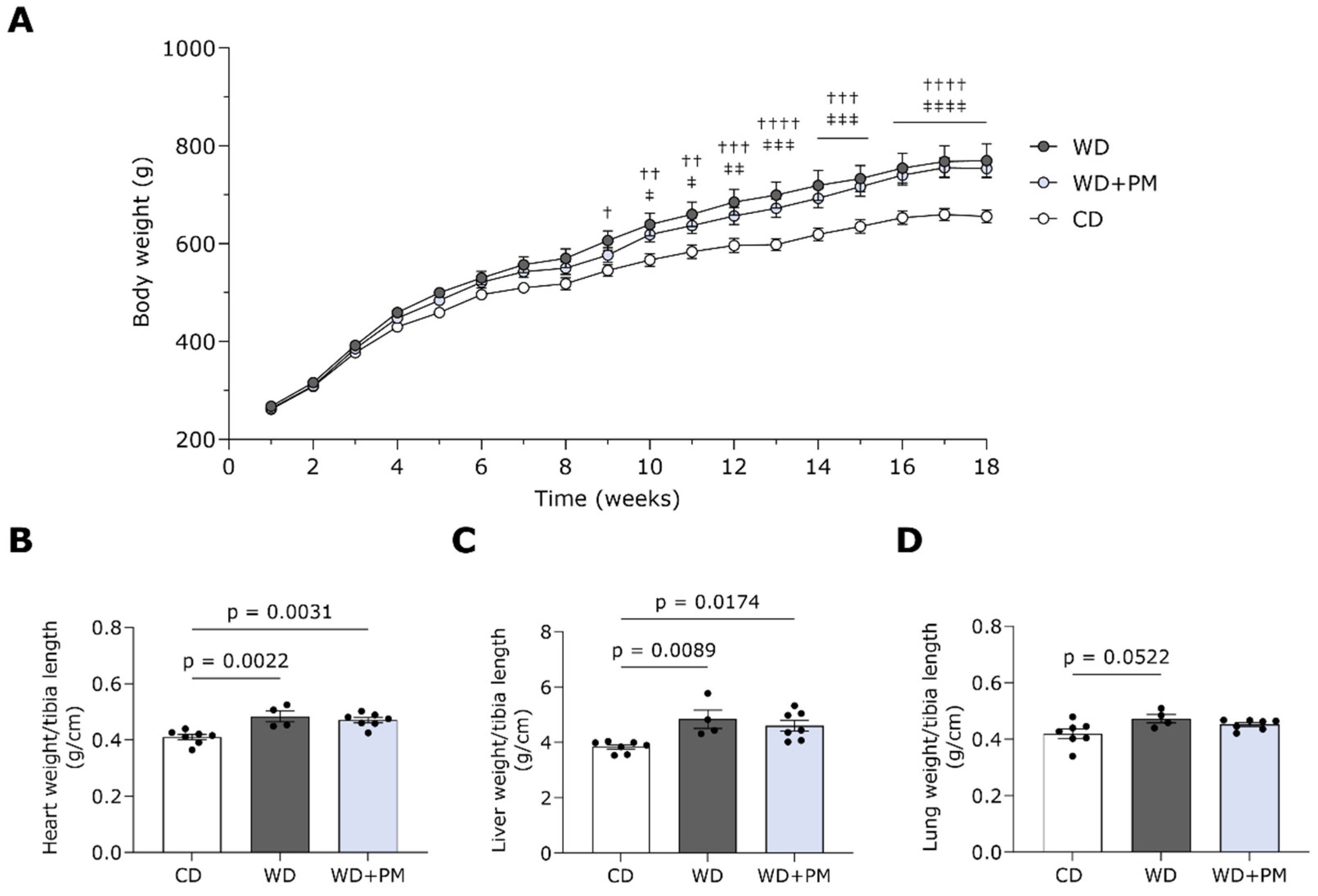
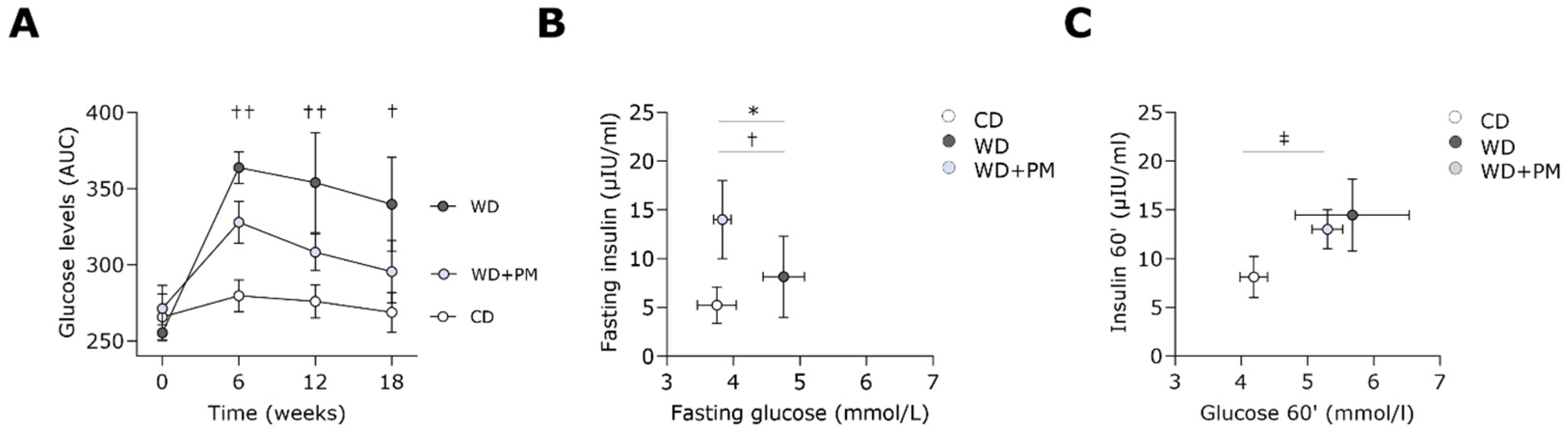
2.1. Pyridoxamine Improves Fasting Blood Glucose Levels in Western Diet-Fed Rats
2.2. Pyridoxamine Tends to Prevent Western Diet-Induced LV Dilation
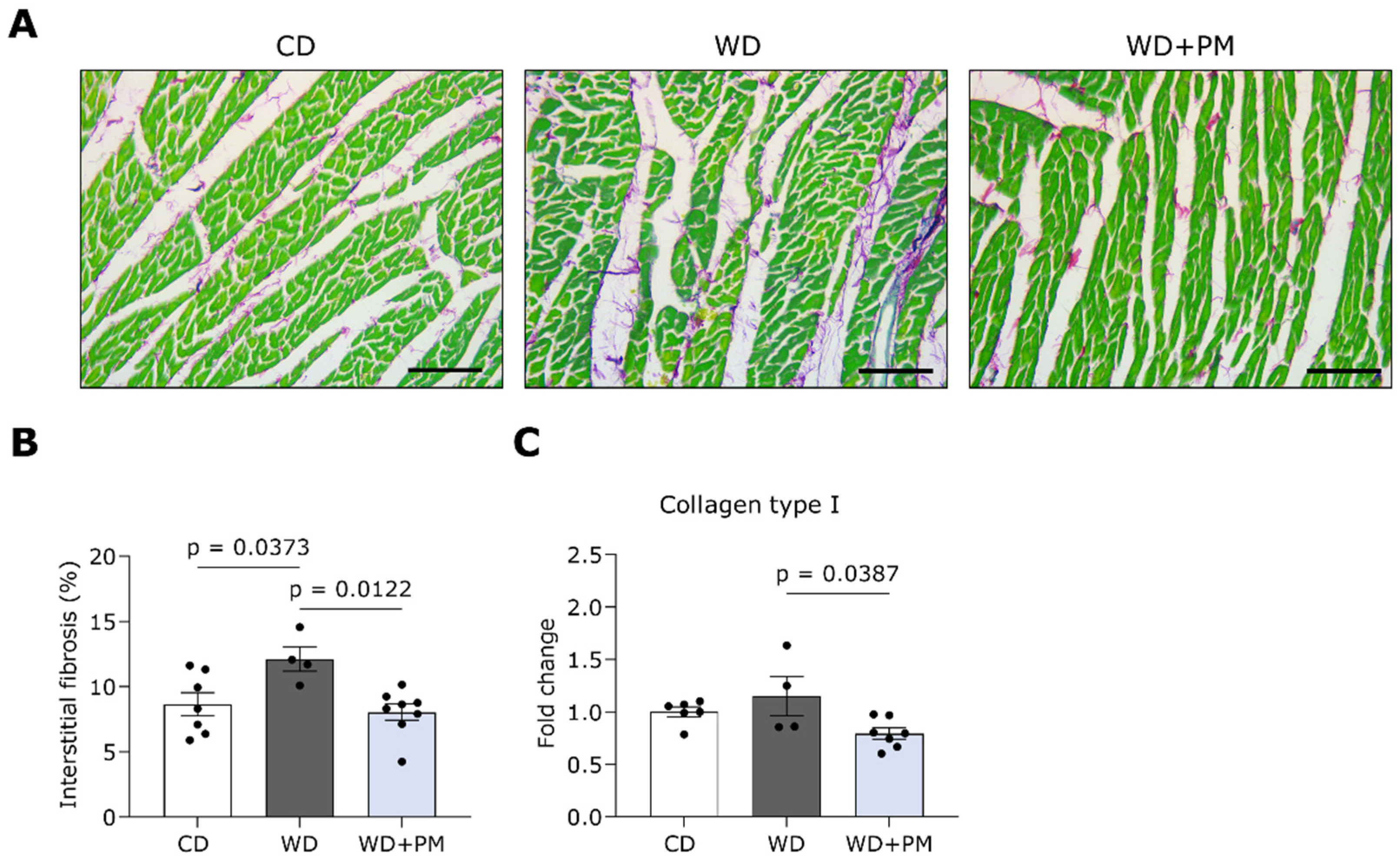
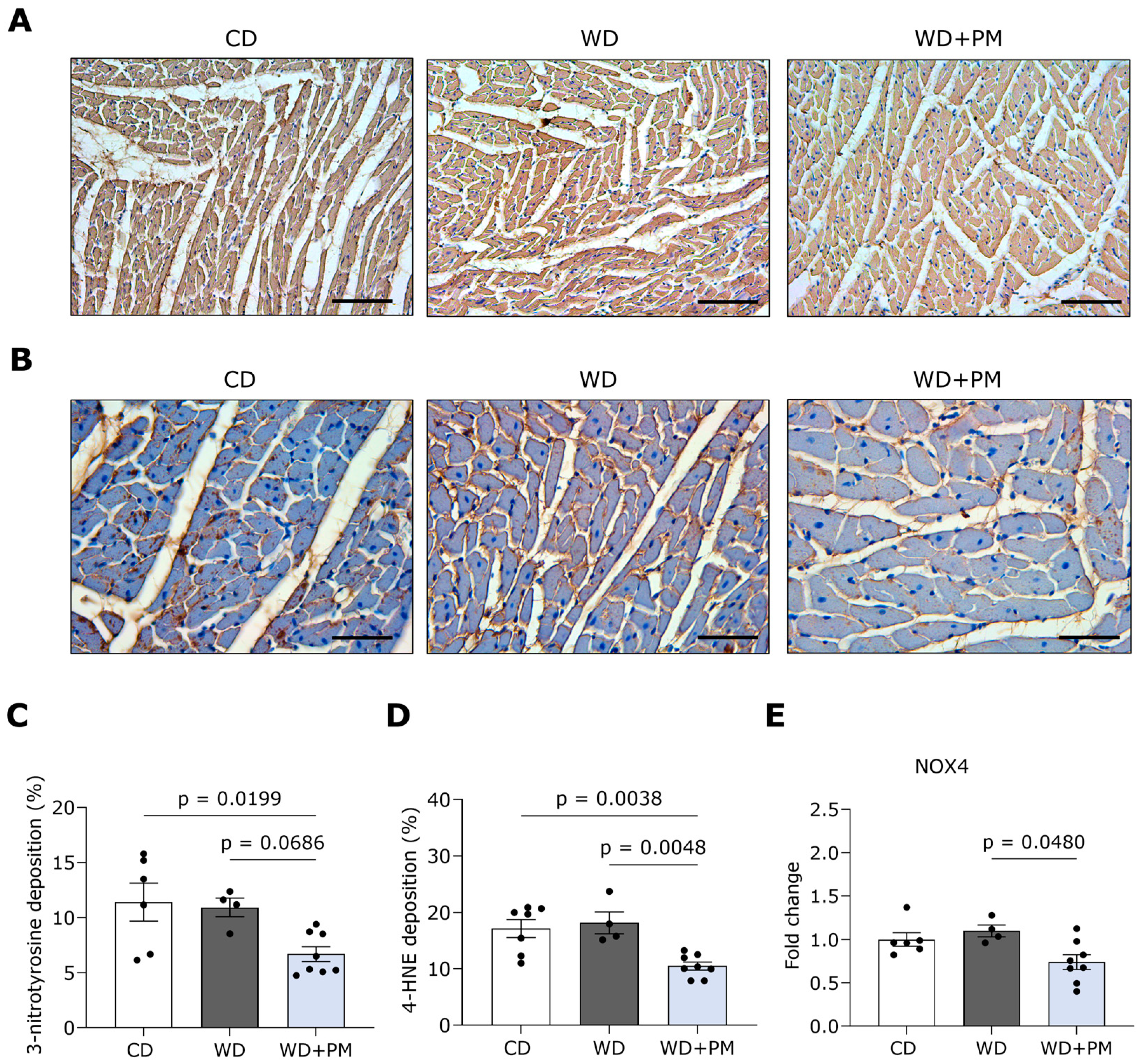
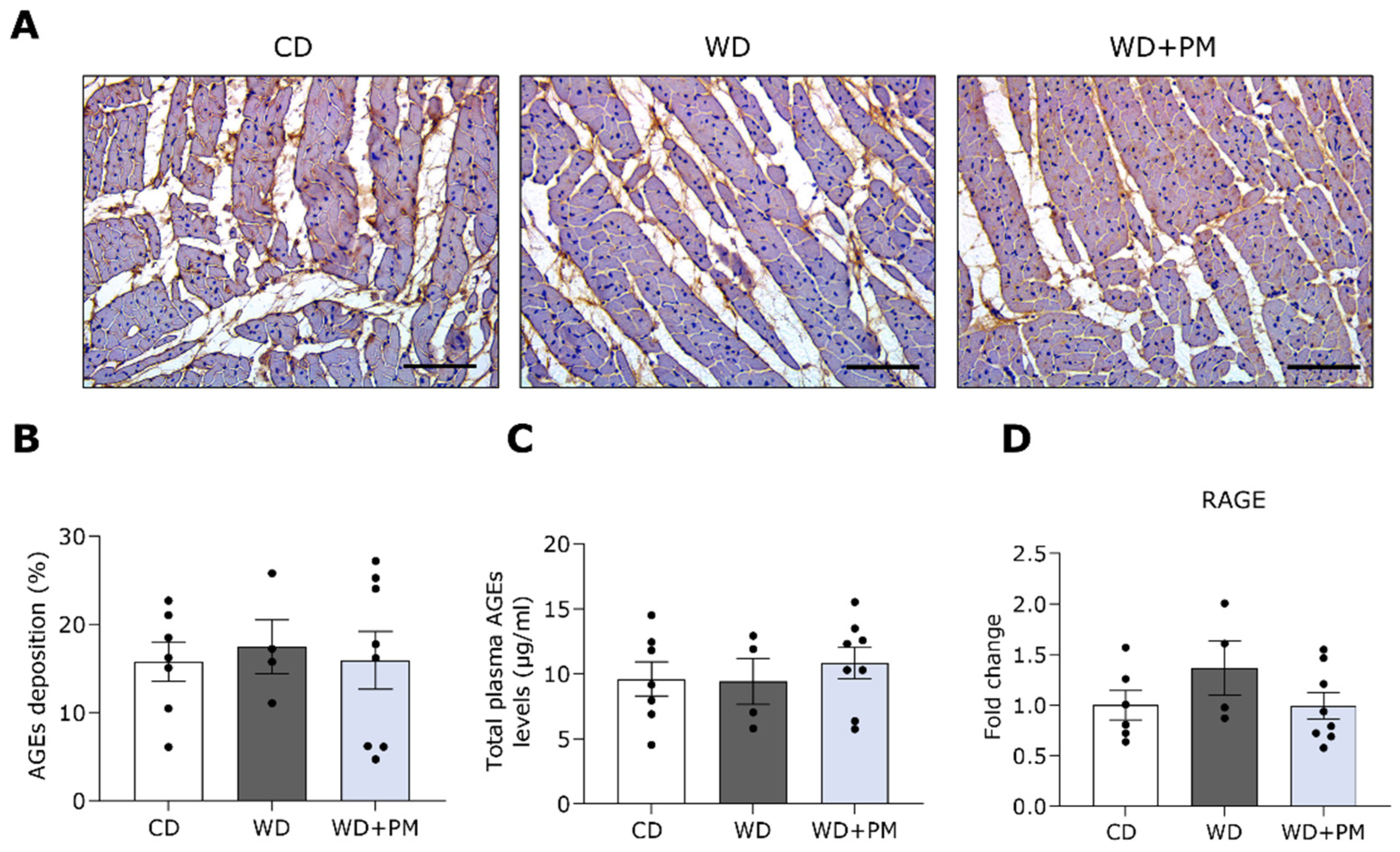
2.3. Pyridoxamine Limits LV Fibrosis and Oxidative Stress in Western Diet-Fed Rats
3. Discussion
3.1. A Translatable Rat Model of Diet-Induced Diabetic Cardiomyopathy
3.2. PM as a Potential Cardioprotective Strategy in T2DM Development
3.3. Safety and Efficacy of PM
3.4. Limitations
4. Materials and Methods
4.1. Animal Model and Study Design
4.2. Oral Glucose Tolerance Test and Insulin Measurements
4.3. Conventional Echocardiographic Measurements
4.4. Hemodynamic Measurements
4.5. Histology
4.6. Immunohistochemistry
4.7. RT-qPCR
4.8. Plasma Advanced Glycation End Products Determination
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-HNE | 4-hydroxynenonal |
| Ad | Diastolic area |
| AGEs | Advanced glycation end products |
| As | Systolic area |
| AUC | Area under the curve |
| AWTd | Anterior wall thickness in diastole |
| AWTs | Anterior wall thickness in systole |
| BSA | Body surface area |
| CO | Cardiac output |
| CSA | Cross-sectional cardiomyocyte area |
| CVD | Cardiovascular diseases |
| DCM | Diabetic cardiomyopathy |
| E/A | Ratio of peak mitral flow velocity in early versus late filling |
| E’ | Peak septal mitral annulus velocity in the early filling phase |
| E/E’ | Ratio of peak mitral flow velocity versus peak mitral annular velocity |
| EDP | End-diastolic pressure |
| EDV | End-diastolic volume |
| EF | Ejection fraction |
| ESP | End-systolic pressure |
| ESV | End-systolic volume |
| FS | Fractional shortening |
| GPx1 | Glutathione peroxidase 1 |
| H&E | Hematoxylin and eosin |
| HFD | High-fat diet |
| HFpEF | Heart failure with a preserved ejection fraction |
| HFrEF | Heart failure with reduced ejection fraction |
| HOMA-IR | Homeostatic model assessment for insulin resistance |
| HPRT | Hypoxanthine-guanine phosphoribosyl transferase |
| HR | Heart rate |
| i.p. | Intraperitoneally |
| IDd | Internal diameter in diastole |
| IDs | Internal diameter in systole |
| IVSd | Interventricular septum thickness in diastole |
| IVSs | Interventricular septum thickness in systole |
| LV | Left ventricular |
| NOX4 | Nicotinamide adenine dinucleotide phosphate oxidase 4 |
| OGTT | Oral glucose tolerance test |
| PGK1 | Phosphoglycerate kinase 1 |
| PM | Pyridoxamine |
| PWTd | Posterior wall thickness in diastole |
| PWTs | Posterior wall thickness in systole |
| RAGE | Receptor for advanced glycation end products |
| RCS | Reactive carbonyl species |
| ROS | Reactive oxygen species |
| RT | Room temperature |
| RT-qPCR | Real-Time quantitative polymerase chain reaction |
| SEM | Standard error of the mean |
| SOD2 | Superoxide dismutase |
| SV | Stroke volume |
| T2DM | Type 2 diabetes mellitus |
| Tau | Time constant for isovolumetric relaxation |
| WD | Western diet |
References
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; Hjortland, M.; Castelli, W.P. Role of diabetes in congestive heart failure: The Framingham study. Am. J. Cardiol. 1974, 34, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Zheng, C.; Wintergerst, K.A.; Keller, B.B.; Cai, L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: Preclinical and clinical evidence. Nat. Rev. Cardiol. 2020, 17, 585–607. [Google Scholar] [CrossRef] [PubMed]
- Seferović, P.M.; Paulus, W.J. Clinical diabetic cardiomyopathy: A two-faced disease with restrictive and dilated phenotypes. Eur. Heart J. 2015, 36, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Bodiga, V.L.; Eda, S.R.; Bodiga, S. Advanced glycation end products: Role in pathology of diabetic cardiomyopathy. Heart Fail. Rev. 2014, 19, 49–63. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Micali, L.R.; Wouters, K. Advanced glycation endproducts in diabetes-related macrovascular complications: Focus on methylglyoxal. Trends Endocrinol. Metab. 2023, 34, 49–60. [Google Scholar] [CrossRef]
- Marx, N.; Federici, M.; Schütt, K.; Müller-Wieland, D.; Ajjan, R.A.; Antunes, M.J.; Christodorescu, R.M.; Crawford, C.; Di Angelantonio, E.; Eliasson, B.; et al. 2023 ESC Guidelines for the management of cardiovascular disease in patients with diabetes: Developed by the task force on the management of cardiovascular disease in patients with diabetes of the European Society of Cardiology (ESC). Eur. Heart J. 2023, 44, 4043–4140. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef]
- Alderson, N.L.; Chachich, M.E.; Youssef, N.N.; Beattie, R.J.; Nachtigal, M.; Thorpe, S.R.; Baynes, J.W. The AGE inhibitor pyridoxamine inhibits lipemia and development of renal and vascular disease in Zucker obese rats. Kidney Int. 2003, 63, 2123–2133. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.C.; Liang, J.T.; Tsai, P.S.; Wu, M.S.; Hsu, K.L. Prevention of arterial stiffening by pyridoxamine in diabetes is associated with inhibition of the pathogenic glycation on aortic collagen. Br. J. Pharmacol. 2009, 157, 1419–1426. [Google Scholar] [CrossRef]
- Degenhardt, T.P.; Alderson, N.L.; Arrington, D.D.; Beattie, R.J.; Basgen, J.M.; Steffes, M.W.; Thorpe, S.R.; Baynes, J.W. Pyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic rat. Kidney Int. 2002, 61, 939–950. [Google Scholar] [CrossRef]
- Haesen, S.; Jager, M.M.; Brillouet, A.; de Laat, I.; Vastmans, L.; Verghote, E.; Delaet, A.; D’Haese, S.; Hamad, I.; Kleinewietfeld, M.; et al. Pyridoxamine Limits Cardiac Dysfunction in a Rat Model of Doxorubicin-Induced Cardiotoxicity. Antioxidants 2024, 13, 112. [Google Scholar] [CrossRef] [PubMed]
- Reeve, E.H.; Kronquist, E.K.; Wolf, J.R.; Lee, B.; Khurana, A.; Pham, H.; Cullen, A.E.; Peterson, J.A.; Meza, A.; Bramwell, R.C.; et al. Pyridoxamine treatment ameliorates large artery stiffening and cerebral artery endothelial dysfunction in old mice. J. Cereb. Blood Flow Metab. 2023, 43, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.M.; Soro-Paavonen, A.; Sheehy, K.; Li, J.; Calkin, A.C.; Koitka, A.; Rajan, S.N.; Brasacchio, D.; Allen, T.J.; Cooper, M.E.; et al. Delayed intervention with AGE inhibitors attenuates the progression of diabetes-accelerated atherosclerosis in diabetic apolipoprotein E knockout mice. Diabetologia 2011, 54, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Voziyan, P.A.; Hudson, B.G. Pyridoxamine as a multifunctional pharmaceutical: Targeting pathogenic glycation and oxidative damage. Cell Mol. Life Sci. 2005, 62, 1671–1681. [Google Scholar] [CrossRef]
- Metz, T.O.; Alderson, N.L.; Thorpe, S.R.; Baynes, J.W. Pyridoxamine, an inhibitor of advanced glycation and lipoxidation reactions: A novel therapy for treatment of diabetic complications. Arch. Biochem. Biophys. 2003, 419, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.; Hinkel, R. Research(’s) Sweet Hearts: Experimental Biomedical Models of Diabetic Cardiomyopathy. Front. Cardiovasc. Med. 2021, 8, 703355. [Google Scholar] [CrossRef]
- Heather, L.C.; Hafstad, A.D.; Halade, G.V.; Harmancey, R.; Mellor, K.M.; Mishra, P.K.; Mulvihill, E.E.; Nabben, M.; Nakamura, M.; Rider, O.J.; et al. Guidelines on models of diabetic heart disease. Am. J. Physiol.-Heart Circ. Physiol. 2022, 323, H176–H200. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Popkin, B.M.; Bray, G.A.; Després, J.P.; Willett, W.C.; Hu, F.B. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: A meta-analysis. Diabetes Care 2010, 33, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- Malik, V.S.; Li, Y.; Pan, A.; De Koning, L.; Schernhammer, E.; Willett, W.C.; Hu, F.B. Long-Term Consumption of Sugar-Sweetened and Artificially Sweetened Beverages and Risk of Mortality in US Adults. Circulation 2019, 139, 2113–2125. [Google Scholar] [CrossRef] [PubMed]
- Velagic, A.; Li, M.Y.; Deo, M.; Li, J.C.; Kiriazis, H.; Donner, D.G.; Anderson, D.; Blasio, M.J.D.; Woodman, O.L.; Kemp-Harper, B.K.; et al. A high-sucrose diet exacerbates the left ventricular phenotype in a high fat-fed streptozotocin rat model of diabetic cardiomyopathy. Am. J. Physiol.-Heart Circ. Physiol. 2023, 324, H241–H257. [Google Scholar] [CrossRef] [PubMed]
- Verboven, M.; Deluyker, D.; Ferferieva, V.; Lambrichts, I.; Hansen, D.; Eijnde, B.O.; Bito, V. Western diet given to healthy rats mimics the human phenotype of diabetic cardiomyopathy. J. Nutr. Biochem. 2018, 61, 140–146. [Google Scholar] [CrossRef] [PubMed]
- D’Haese, S.; Verboven, M.; Evens, L.; Deluyker, D.; Lambrichts, I.; Eijnde, B.O.; Hansen, D.; Bito, V. Moderate- and High-Intensity Endurance Training Alleviate Diabetes-Induced Cardiac Dysfunction in Rats. Nutrients 2023, 15, 3950. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, S.; Gohda, T.; Tanimoto, M.; Ito, T.; Murakoshi, M.; Ohara, I.; Yamazaki, T.; Matsumoto, M.; Horikoshi, S.; Funabiki, K.; et al. Effects of pyridoxamine (K-163) on glucose intolerance and obesity in high-fat diet C57BL/6J mice. Metabolism 2009, 58, 934–945. [Google Scholar] [CrossRef] [PubMed]
- Maessen, D.E.; Brouwers, O.; Gaens, K.H.; Wouters, K.; Cleutjens, J.P.; Janssen, B.J.; Miyata, T.; Stehouwer, C.D.; Schalkwijk, C.G. Delayed Intervention with Pyridoxamine Improves Metabolic Function and Prevents Adipose Tissue Inflammation and Insulin Resistance in High-Fat Diet-Induced Obese Mice. Diabetes 2016, 65, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Ahn, H.; Park, H.; Lee, J.-I.; Park, K.Y.; Hwang, D.; Lee, S.; Son, K.H.; Byun, K. The attenuating effects of pyridoxamine on adipocyte hypertrophy and inflammation differ by adipocyte location. J. Nutr. Biochem. 2019, 72, 108173. [Google Scholar] [CrossRef]
- Chiazza, F.; Cento, A.S.; Collotta, D.; Nigro, D.; Rosa, G.; Baratta, F.; Bitonto, V.; Cutrin, J.C.; Aragno, M.; Mastrocola, R.; et al. Protective Effects of Pyridoxamine Supplementation in the Early Stages of Diet-Induced Kidney Dysfunction. Biomed. Res. Int. 2017, 2017, 2682861. [Google Scholar] [CrossRef]
- Rangel Silvares, R.; Nunes Goulart da Silva Pereira, E.; Eduardo Ilaquita Flores, E.; Lino Rodrigues, K.; Ribeiro Silva, A.; Gonçalves-de-Albuquerque, C.F.; Daliry, A. High-fat diet-induced kidney alterations in rats with metabolic syndrome: Endothelial dysfunction and decreased antioxidant defense. Diabetes Metab. Syndr. Obes. 2019, 12, 1773–1781. [Google Scholar] [CrossRef]
- Ren, X.; Sun, H.; Zhang, C.; Li, C.; Wang, J.; Shen, J.; Yu, D.; Kong, L. Protective function of pyridoxamine on retinal photoreceptor cells via activation of the p-Erk1/2/Nrf2/Trx/ASK1 signalling pathway in diabetic mice. Mol. Med. Rep. 2016, 14, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, M.R.; Bouvet, C.; Bouchard, S.; Moreau, S.; Leblond, J.; Deblois, D.; Moreau, P. Reduction of advanced-glycation end products levels and inhibition of RAGE signaling decreases rat vascular calcification induced by diabetes. PLoS ONE 2014, 9, e85922. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Deluyker, D.; Ferferieva, V.; Driesen, R.B.; Verboven, M.; Lambrichts, I.; Bito, V. Pyridoxamine improves survival and limits cardiac dysfunction after MI. Sci. Rep. 2017, 7, 16010. [Google Scholar] [CrossRef] [PubMed]
- Alshanwani, A.R.; Hagar, H.; Shaheen, S.; Alhusaini, A.M.; Arafah, M.M.; Faddah, L.M.; Alharbi, F.M.; Sharma, A.K.; Fayed, A.; Badr, A.M. A promising antifibrotic drug, pyridoxamine attenuates thioacetamide-induced liver fibrosis by combating oxidative stress, advanced glycation end products, and balancing matrix metalloproteinases. Eur. J. Pharmacol. 2022, 923, 174910. [Google Scholar] [CrossRef]
- Shao, C.H.; Capek, H.L.; Patel, K.P.; Wang, M.; Tang, K.; DeSouza, C.; Nagai, R.; Mayhan, W.; Periasamy, M.; Bidasee, K.R. Carbonylation contributes to SERCA2a activity loss and diastolic dysfunction in a rat model of type 1 diabetes. Diabetes 2011, 60, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Moore, C.J.; Shao, C.H.; Nagai, R.; Kutty, S.; Singh, J.; Bidasee, K.R. Malondialdehyde and 4-hydroxynonenal adducts are not formed on cardiac ryanodine receptor (RyR2) and sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA2) in diabetes. Mol. Cell Biochem. 2013, 376, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Obrosova, I.G.; Mabley, J.G.; Szabó, C. Role of nitrosative stress and peroxynitrite in the pathogenesis of diabetic complications.: Emerging new therapeutical strategies. Curr. Med. Chem. 2005, 12, 267–275. [Google Scholar] [CrossRef]
- Dham, D.; Roy, B.; Gowda, A.; Pan, G.; Sridhar, A.; Zeng, X.; Thandavarayan, R.A.; Palaniyandi, S.S. 4-Hydroxy-2-nonenal, a lipid peroxidation product, as a biomarker in diabetes and its complications: Challenges and opportunities. Free Radic. Res. 2021, 55, 547–561. [Google Scholar] [CrossRef]
- Gawargi, F.I.; Mishra, P.K. Regulation of cardiac ferroptosis in diabetic human heart failure: Uncovering molecular pathways and key targets. Cell Death Discov. 2024, 10, 268. [Google Scholar] [CrossRef] [PubMed]
- Han, H.J.; Lee, Y.J.; Park, S.H.; Lee, J.H.; Taub, M. High glucose-induced oxidative stress inhibits Na+/glucose cotransporter activity in renal proximal tubule cells. Am. J. Physiol. Ren. Physiol. 2005, 288, F988–F996. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Thornalley, P.J. Measurement of methylglyoxal by stable isotopic dilution analysis LC-MS/MS with corroborative prediction in physiological samples. Nat. Protoc. 2014, 9, 1969–1979. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tao, H.; Yancey, P.G.; Leuthner, Z.; May-Zhang, L.S.; Jung, J.-Y.; Zhang, Y.; Ding, L.; Amarnath, V.; Liu, D.; et al. Scavenging dicarbonyls with 5′-O-pentyl-pyridoxamine increases HDL net cholesterol efflux capacity and attenuates atherosclerosis and insulin resistance. Mol. Metab. 2023, 67, 101651. [Google Scholar] [CrossRef] [PubMed]
- Wu, E.-T.; Liang, J.-T.; Wu, M.-S.; Chang, K.-C. Pyridoxamine prevents age-related aortic stiffening and vascular resistance in association with reduced collagen glycation. Exp. Gerontol. 2011, 46, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.E.; Bolton, W.K.; Khalifah, R.G.; Degenhardt, T.P.; Schotzinger, R.J.; McGill, J.B. Effects of pyridoxamine in combined phase 2 studies of patients with type 1 and type 2 diabetes and overt nephropathy. Am. J. Nephrol. 2007, 27, 605–614. [Google Scholar] [CrossRef]
- Lewis, E.J.; Greene, T.; Spitalewiz, S.; Blumenthal, S.; Berl, T.; Hunsicker, L.G.; Pohl, M.A.; Rohde, R.D.; Raz, I.; Yerushalmy, Y.; et al. Pyridorin in type 2 diabetic nephropathy. J. Am. Soc. Nephrol. 2012, 23, 131–136. [Google Scholar] [CrossRef]
- Garg, S.; Syngle, A.; Vohra, K. Efficacy and Tolerability of Advanced Glycation End-Products Inhibitor in Osteoarthritis: A Randomized, Double-Blind, Placebo-controlled Study. Clin. J. Pain 2013, 29, 717–724. [Google Scholar] [CrossRef]
- Van den Eynde, M.D.G.; Houben, A.; Scheijen, J.; Linkens, A.M.A.; Niessen, P.M.; Simons, N.; Hanssen, N.M.J.; Kusters, Y.; Eussen, S.; Miyata, T.; et al. Pyridoxamine reduces methylglyoxal and markers of glycation and endothelial dysfunction, but does not improve insulin sensitivity or vascular function in abdominally obese individuals: A randomized double-blind placebo-controlled trial. Diabetes Obes. Metab. 2023, 25, 1280–1291. [Google Scholar] [CrossRef]
- Levin, B.E.; Dunn-Meynell, A.A.; Balkan, B.; Keesey, R.E. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1997, 273, R725–R730. [Google Scholar] [CrossRef]
- D’Haese, S.; Claes, L.; de Laat, I.; Van Campenhout, S.; Deluyker, D.; Heeren, E.; Haesen, S.; Lambrichts, I.; Wouters, K.; Schalkwijk, C.G.; et al. Moderate-Intensity and High-Intensity Interval Exercise Training Offer Equal Cardioprotection, with Different Mechanisms, during the Development of Type 2 Diabetes in Rats. Nutrients 2024, 16, 431. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]

| CD | WD | WD + PM | |
|---|---|---|---|
| EDV/BSA (µL/cm2) | 0.841 ± 0.046 | 1.037 ± 0.104 | 0.866 ± 0.043 |
| ESV/BSA (µL/cm2) | 0.196 ± 0.027 | 0.327 ± 0.028 * | 0.232 ± 0.028 |
| Ad/BSA (mm2/cm2) | 0.149 ± 0.007 | 0.157 ± 0.009 | 0.144 ± 0.005 |
| As/BSA (mm2/cm2) | 0.062 ± 0.005 | 0.081 ± 0.002 * | 0.063 ± 0.005 |
| EF (%) | 78 ± 2 | 68 ± 3 | 73 ± 3 |
| HR (bpm) | 326 ± 15 | 340 ± 10 | 341 ± 10 |
| BSA (cm2) | 739 ± 10 | 822 ± 24 * | 811 ± 14 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Haese, S.; Claes, L.; Jaeken, E.; Deluyker, D.; Evens, L.; Heeren, E.; Haesen, S.; Vastmans, L.; Lambrichts, I.; Wouters, K.; et al. Pyridoxamine Alleviates Cardiac Fibrosis and Oxidative Stress in Western Diet-Induced Prediabetic Rats. Int. J. Mol. Sci. 2024, 25, 8508. https://doi.org/10.3390/ijms25158508
D’Haese S, Claes L, Jaeken E, Deluyker D, Evens L, Heeren E, Haesen S, Vastmans L, Lambrichts I, Wouters K, et al. Pyridoxamine Alleviates Cardiac Fibrosis and Oxidative Stress in Western Diet-Induced Prediabetic Rats. International Journal of Molecular Sciences. 2024; 25(15):8508. https://doi.org/10.3390/ijms25158508
Chicago/Turabian StyleD’Haese, Sarah, Lisa Claes, Eva Jaeken, Dorien Deluyker, Lize Evens, Ellen Heeren, Sibren Haesen, Lotte Vastmans, Ivo Lambrichts, Kristiaan Wouters, and et al. 2024. "Pyridoxamine Alleviates Cardiac Fibrosis and Oxidative Stress in Western Diet-Induced Prediabetic Rats" International Journal of Molecular Sciences 25, no. 15: 8508. https://doi.org/10.3390/ijms25158508






