Nanostructured Transition Metal Oxides on Carbon Fibers for Supercapacitor and Li-Ion Battery Electrodes: An Overview
Abstract
1. Introduction
2. TMOs for Energy-Storage Applications
2.1. TMOs as Supercapacitor Electrodes

- Sulfurization or selenization. These elements, which are softer Pearson bases than oxygen, polarize the metal bond less, reducing the band gap [54].
- Creation of oxygen vacancies. Oxygen vacancies lead to the presence of metal cations with a higher oxidation state within the crystalline structure, resulting in the appearance of discrete energy levels allowed in the band gap, facilitating the passage of electrons from the valence band to the conduction band [55,56].
- Doping with donor dopants. Doping with elements such as P, which promotes electrons to the conduction band [57].
2.2. TMOs as Li-Ion Batteries Anodes
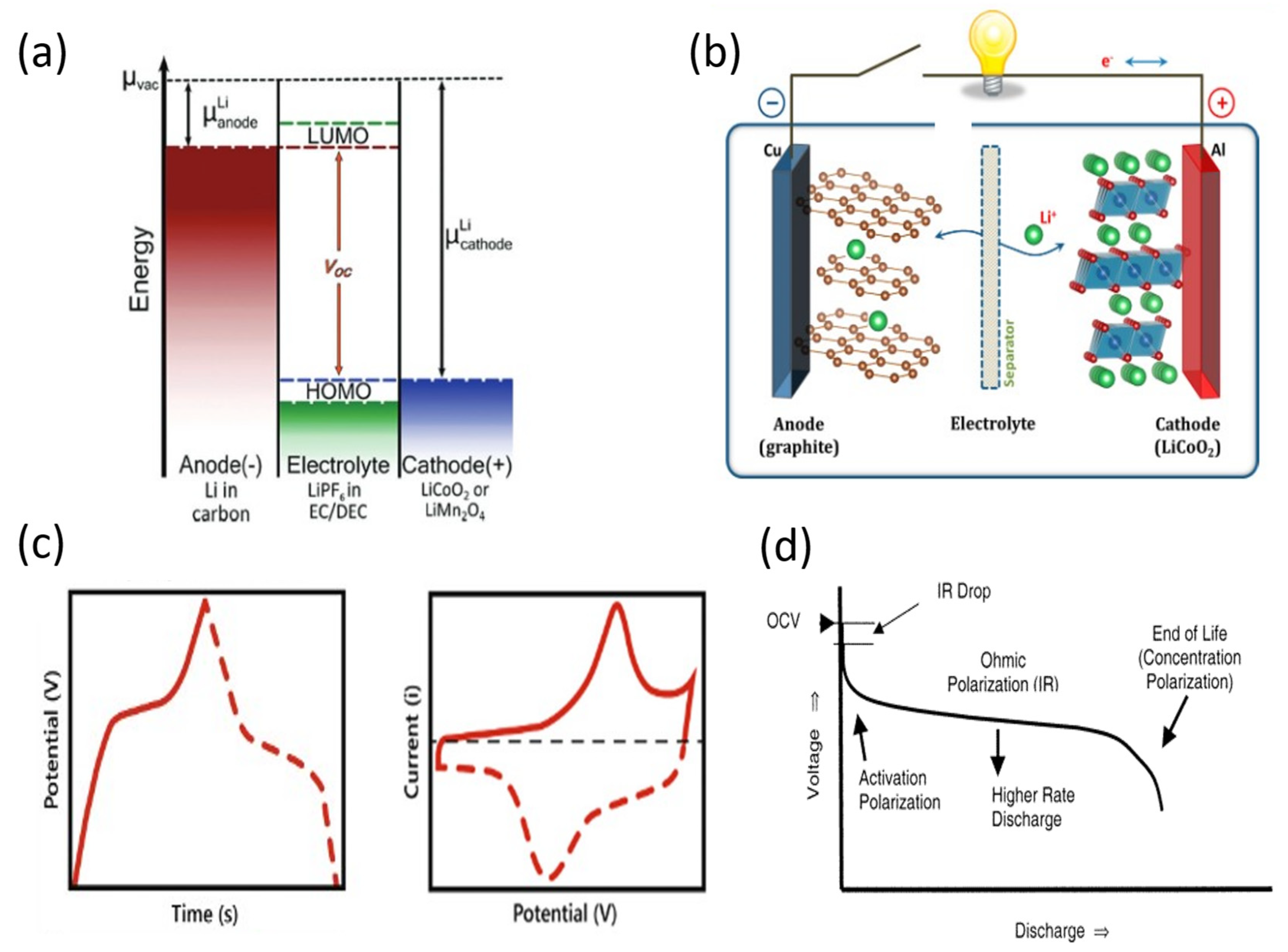
3. Nanostructured TMOs on Carbon Fiber for Energy-Storage Applications: Obtention Methods
3.1. Solvothermal Synthesis
3.2. MOF-Derived TMOs

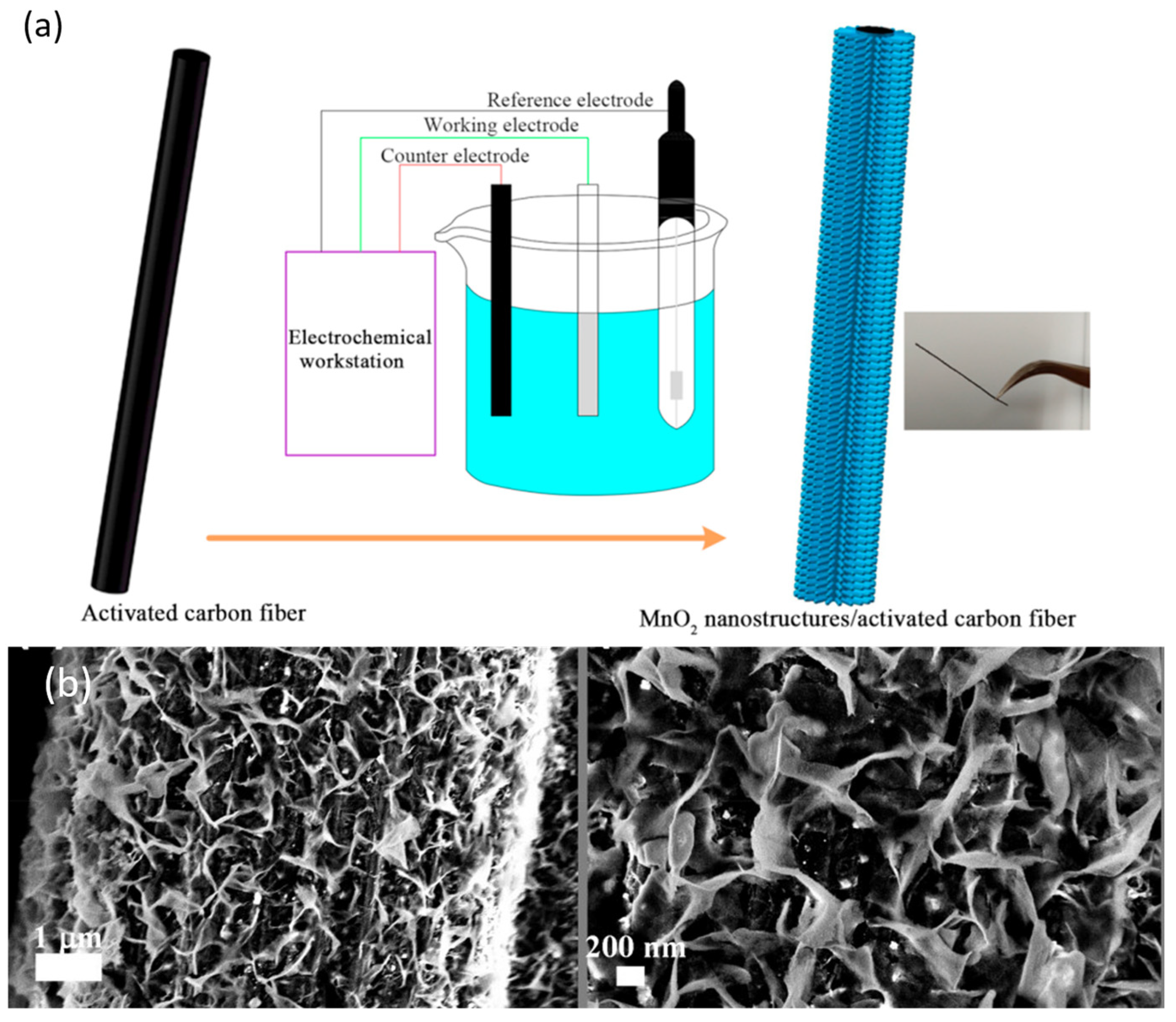
3.3. Electrochemical Deposition (ECD)
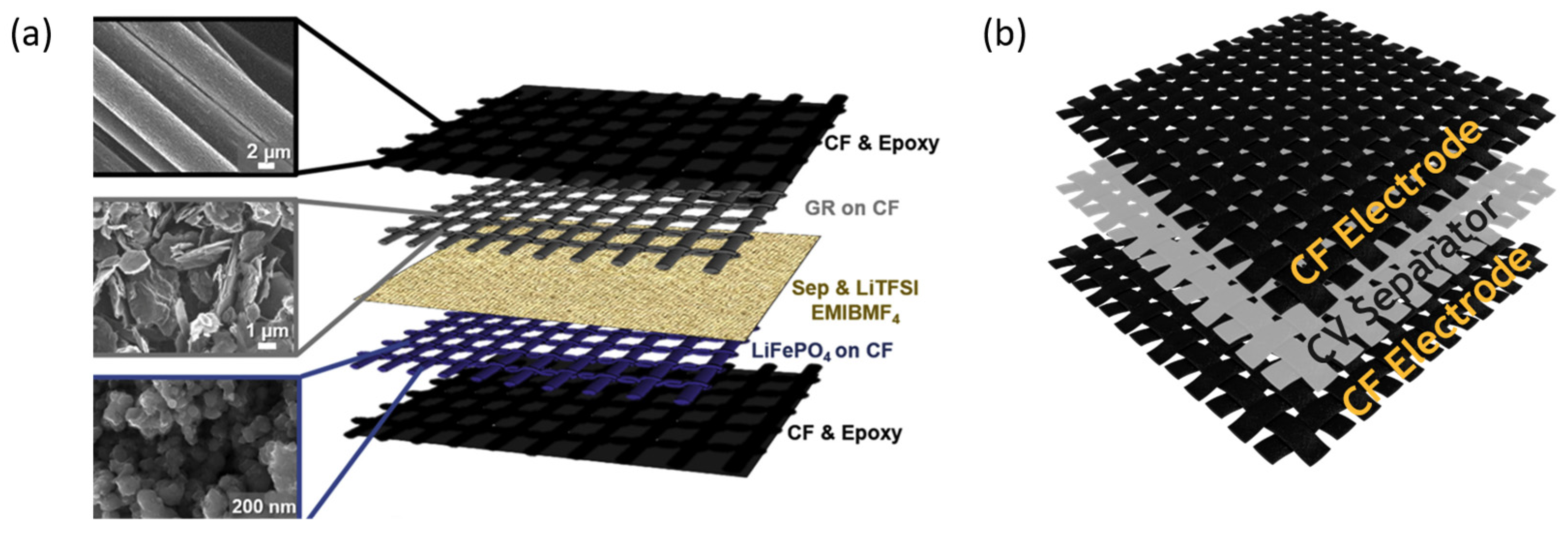
4. Conclusions and Outlook
- Solvothermal/hydrothermal synthesis: This technique offers the significant advantage of achieving a wide range of transition metal oxide compositions and nanostructure morphologies by varying only a few parameters. As Li-ion anodes, different transition metal oxides yield different theoretical capacities due to various conversion reactions. Additionally, different morphologies can affect cyclability. On the other hand, as supercapacitor electrodes, diverse transition metal oxides provide varying pseudocapacitive reactions and electrical conductivities, with different nanostructured morphologies offering different surface areas, a crucial factor for supercapacitor electrode capacitance. Consequently, the extensive possibilities offered by hydrothermal synthesis make it the most developed technique for these applications while also opening up a wide range of potential optimizations and new materials for future research.
- Metal–organic framework-derived synthesis: Using MOFs as precursors to obtain transition metal oxides results in more porous coatings. Increased porosity enhances the surface area of transition metal oxides, leading to higher supercapacitor electrode capacitance. On the other, for Li-ion battery anodes, increased porosity improves capacity and cyclability. However, a major drawback of this technique is that MOF-derived coatings are primarily limited to the zeolitic–imidazole frameworks series, resulting in a limited variety of available transition metal oxide structures. Therefore, the primary goal of this technique should be to develop new MOF coatings on the carbon fiber to expand the range of available materials.
- Electrochemical deposition: This is the least developed of the three techniques, particularly for Li-ion battery applications. Consequently, it presents significant potential for future research. One advantage of electrochemical deposition is the ability to deposit transition metal oxide alongside other compounds, such as conductive polymers, which can act synergistically with the oxides.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pan, Z.; Liu, X.; Yang, J.; Li, X.; Liu, Z.; Loh, X.J.; Wang, J. Aqueous Rechargeable Multivalent Metal-Ion Batteries: Advances and Challenges. Adv. Energy Mater. 2021, 11, 2100608. [Google Scholar] [CrossRef]
- Abdillah, O.B.; Bin Rus, Y.; Ulfa, M.; Dedi, F.; Iskandar, F. Recent progress on reduced graphene oxide and polypyrrole composites for high performance supercapacitors: A review. J. Energy Storage 2023, 74, 109300. [Google Scholar] [CrossRef]
- Battery Market Size, Share & Trends Analysis Report by Product (Lead Acid, Li-Ion, Nickle Metal Hydride, Ni-Cd), by Application, by End-Use, by Region, and Segment Forecasts, 2023–2030; Grand View Research: San Francisco, CA, USA, 2020.
- Supercapacitors Market Size, Share, and Trends 2024 to 2034. Available online: https://www.precedenceresearch.com/supercapacitors-market (accessed on 23 July 2024).
- Aydin, A.; Zajonz, F.; Günther, T.; Dermenci, K.B.; Berecibar, M.; Urrutia, L. Lithium-Ion Battery Manufacturing: Industrial View on Processing Challenges, Possible Solutions and Recent Advances. Batteries 2023, 9, 555. [Google Scholar] [CrossRef]
- Hong, H.; Tu, H.; Jiang, L.; Du, Y.; Wong, C.-P. Advances in fabric-based supercapacitors and batteries: Harnessing textiles for next-generation energy storage. J. Energy Storage 2024, 75, 109561. [Google Scholar] [CrossRef]
- Hesar, M.E.; Seyedsadrkhani, N.S.; Khan, D.; Naghashian, A.; Piekarski, M.; Gall, H.; Schermuly, R.; Ghofrani, H.A.; Ingebrandt, S. AI-enabled epidermal electronic system to automatically monitor a prognostic parameter for hypertension with a smartphone. Biosens. Bioelectron. 2023, 241, 115693. [Google Scholar] [CrossRef] [PubMed]
- del Bosque, A.; Sánchez–Romate, X.F.; Patrizi, D.; Sáez, J.S.d.R.; Wang, D.-Y.; Sánchez, M.; Ureña, A. Ultrasensitive flexible strain sensors based on graphene nanoplatelets doped poly(ethylene glycol) diglycidyl ether: Mask breathing monitoring for the Internet of Things. Sensors Actuators A Phys. 2023, 358, 114448. [Google Scholar] [CrossRef]
- Pasta, M.; Armstrong, D.; Brown, Z.L.; Bu, J.; Castell, M.R.; Chen, P.; Cocks, A.; Corr, S.A.; Cussen, E.J.; Darnbrough, E.; et al. 2020 roadmap on solid-state batteries. J. Phys. Energy 2020, 2, 032008. [Google Scholar] [CrossRef]
- Electric Vehicle Market Size, Share & Trends Analysis Report by Product (BEV, PHEV, FCEV), by Application (Passenger Cars, Commercial Vehicles), by Region, and Segment Forecasts, 2023–2030, Grand View Research, n.d. Available online: https://www.grandviewresearch.com/industry-analysis/electric-vehicles-ev-market (accessed on 23 July 2024).
- Niri, A.J.; Poelzer, G.A.; Zhang, S.E.; Rosenkranz, J.; Pettersson, M.; Ghorbani, Y. Sustainability challenges throughout the electric vehicle battery value chain. Renew. Sustain. Energy Rev. 2024, 191, 114176. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Belenkov, E.; Greshnyakov, V.; Yalovega, G.; Bashkin, I. New aspects in the study of carbon-hydrogen interaction in hydrogenated carbon nanotubes for energy storage applications. J. Alloys Compd. 2019, 792, 713–720. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Shmatko, V.; Yalovega, G.; Krestinin, A.; Bashkin, I.; Bogoslavskaja, E. Electronic structure of hydrogenated carbon nanotubes studied by core level spectroscopy. J. Electron Spectrosc. Relat. Phenom. 2014, 196, 99–103. [Google Scholar] [CrossRef]
- Ouramdane, O.; Elbouchikhi, E.; Amirat, Y.; Gooya, E.S. Optimal sizing and energy management of microgrids with Vehicle-to-Grid technology: A critical review and future trends. Energies 2021, 14, 4166. [Google Scholar] [CrossRef]
- González, C.; Vilatela, J.; Molina-Aldareguía, J.; Lopes, C.; Llorca, J. Structural composites for multifunctional applications: Current challenges and future trends. Prog. Mater. Sci. 2017, 89, 194–251. [Google Scholar] [CrossRef]
- Petrushenko, D.; Rahmati, Z.; Barazanchy, D.; De Backer, W.; Mustain, W.E.; White, R.E.; Ziehl, P.; Coman, P.T. Dip-Coating of Carbon Fibers for the Development of Lithium Iron Phosphate Electrodes for Structural Lithium-Ion Batteries. Energy Fuels 2023, 37, 711–723. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, S.; Li, D.; Liao, J.; Ji, F.; Liu, H.; Ci, L. Commercial carbon cloth: An emerging substrate for practical lithium metal batteries. Energy Storage Mater. 2022, 48, 172–190. [Google Scholar] [CrossRef]
- Seo, M.-K.; Pandey, P.; Sohn, J.I. Metal-free flexible triboelectric nanogenerator based on bifunctional carbon fiber for mechanical energy harvesting and human activity monitoring. Sens. Actuators A Phys. 2024, 370, 115247. [Google Scholar] [CrossRef]
- Xu, Y.; Lu, W.; Xu, G.; Chou, T.-W. Structural supercapacitor composites: A review. Compos. Sci. Technol. 2021, 204, 108636. [Google Scholar] [CrossRef]
- Asp, L.E.; Bouton, K.; Carlstedt, D.; Duan, S.; Harnden, R.; Johannisson, W.; Johansen, M.; Johansson, M.K.G.; Lindbergh, G.; Liu, F.; et al. A Structural Battery and its Multifunctional Performance. Adv. Energy Sustain. Res. 2021, 2, 2000093. [Google Scholar] [CrossRef]
- Moyer, K.; Meng, C.; Marshall, B.; Assal, O.; Eaves, J.; Perez, D.; Karkkainen, R.; Roberson, L.; Pint, C.L. Carbon fiber reinforced structural lithium-ion battery composite: Multifunctional power integration for CubeSats. Energy Storage Mater. 2020, 24, 676–681. [Google Scholar] [CrossRef]
- Danzi, F.; Salgado, R.M.; Oliveira, J.E.; Arteiro, A.; Camanho, P.P.; Braga, M.H. Structural Batteries: A Review. Molecules 2021, 26, 2203. [Google Scholar] [CrossRef]
- Kalnaus, S.; Asp, L.E.; Li, J.; Veith, G.M.; Nanda, J.; Daniel, C.; Chen, X.C.; Westover, A.; Dudney, N.J. Multifunctional approaches for safe structural batteries. J. Energy Storage 2021, 40, 102747. [Google Scholar] [CrossRef]
- Deng, R.; He, T. Flexible Solid-State Lithium-Ion Batteries: Materials and Structures. Energies 2023, 16, 4549. [Google Scholar] [CrossRef]
- Sanchez, J.S.; Xu, J.; Xia, Z.; Sun, J.; Asp, L.E.; Palermo, V. Electrophoretic coating of LiFePO4/Graphene oxide on carbon fibers as cathode electrodes for structural lithium ion batteries. Compos. Sci. Technol. 2021, 208, 108768. [Google Scholar] [CrossRef]
- Moyer, K.; Carter, R.; Hanken, T.; Douglas, A.; Oakes, L.; Pint, C.L. Electrophoretic deposition of LiFePO4 onto 3-D current collectors for high areal loading battery cathodes. Mater. Sci. Eng. B 2019, 241, 42–47. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, H.; Lu, Y.-C.; Hou, Y.; Li, Q. Electrophoretic lithium iron phosphate/reduced graphene oxide composite for lithium ion battery cathode application. J. Power Sources 2015, 284, 236–244. [Google Scholar] [CrossRef]
- Hagberg, J.; Maples, H.A.; Alvim, K.S.; Xu, J.; Johannisson, W.; Bismarck, A.; Zenkert, D.; Lindbergh, G. Lithium iron phosphate coated carbon fiber electrodes for structural lithium ion batteries. Compos. Sci. Technol. 2018, 162, 235–243. [Google Scholar] [CrossRef]
- Mishra, A.; Shetti, N.P.; Basu, S.; Reddy, K.R.; Aminabhavi, T.M. Carbon Cloth-based Hybrid Materials as Flexible Electrochemical Supercapacitors. ChemElectroChem 2019, 6, 5771–5786. [Google Scholar] [CrossRef]
- Artigas-Arnaudas, J.; Sánchez-Romate, X.F.; Sánchez, M.; Ureña, A. Effect of electrode surface treatment on carbon fiber based structural supercapacitors: Electrochemical analysis, mechanical performance and proof-of-concept. J. Energy Storage 2023, 59, 106599. [Google Scholar] [CrossRef]
- Jin, T.; Singer, G.; Liang, K.; Yang, Y. Structural batteries: Advances, challenges and perspectives. Mater. Today 2023, 62, 151–167. [Google Scholar] [CrossRef]
- O’Brien, D.J.; Baechle, D.M.; Wetzel, E.D. Design and performance of multifunctional structural composite capacitors. J. Compos. Mater. 2011, 45, 2797–2809. [Google Scholar] [CrossRef]
- Snyder, J.; Gienger, E.; Wetzel, E. Performance metrics for structural composites with electrochemical multifunctionality. J. Compos. Mater. 2015, 49, 1835–1848. [Google Scholar] [CrossRef]
- Meena, D.; Kumar, R.; Gupta, S.; Khan, O.; Gupta, D.; Singh, M. Energy storage in the 21st century: A comprehensive review on factors enhancing the next-generation supercapacitor mechanisms. J. Energy Storage 2023, 72, 109323. [Google Scholar] [CrossRef]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Zhang, S.; Pan, N. Supercapacitors performance evaluation. Adv. Energy Mater. 2015, 5, 1401401. [Google Scholar] [CrossRef]
- Show, Y. Electric double-layer capacitor fabricated with addition of carbon nanotube to polarizable electrode. J. Nanomater. 2012, 2012, 929343. [Google Scholar] [CrossRef]
- Rajkumar, M.; Hsu, C.-T.; Wu, T.-H.; Chen, M.-G.; Hu, C.-C. Advanced materials for aqueous supercapacitors in the asymmetric design. Prog. Nat. Sci. Mater. Int. 2015, 25, 527–544. [Google Scholar] [CrossRef]
- Reveles-Miranda, M.; Ramirez-Rivera, V.; Pacheco-Catalán, D. Hybrid energy storage: Features, applications, and ancillary benefits. Renew. Sustain. Energy Rev. 2024, 192, 114196. [Google Scholar] [CrossRef]
- Biswas, S.; Chowdhury, A. Organic Supercapacitors as the Next Generation Energy Storage Device: Emergence, Opportunity, and Challenges. Chemphyschem 2023, 24, e202200567. [Google Scholar] [CrossRef]
- George, J.; Balachandran, M. Extrinsic pseudocapacitance: Tapering the borderline between pseudocapacitive and battery type electrode materials for energy storage applications. J. Energy Storage 2023, 74, 109292. [Google Scholar] [CrossRef]
- Lichchhavi; Kanwade, A.; Shirage, P.M. A review on synergy of transition metal oxide nanostructured materials: Effective and coherent choice for supercapacitor electrodes. J. Energy Storage 2022, 55, 105692. [Google Scholar] [CrossRef]
- Chen, T.; Dai, L. Carbon nanomaterials for high-performance supercapacitors. Mater. Today 2013, 16, 272–280. [Google Scholar] [CrossRef]
- Arumugam, B.; Mayakrishnan, G.; Manickavasagam, S.K.S.; Kim, S.C.; Vanaraj, R. An Overview of Active Electrode Materials for the Efficient High-Performance Supercapacitor Application. Crystals 2023, 13, 1118. [Google Scholar] [CrossRef]
- Mathis, T.S.; Kurra, N.; Wang, X.; Pinto, D.; Simon, P.; Gogotsi, Y. Energy Storage Data Reporting in Perspective—Guidelines for Interpreting the Performance of Electrochemical Energy Storage Systems. Adv. Energy Mater. 2019, 9, 1902007. [Google Scholar] [CrossRef]
- Eftekhari, A. Lithium-Ion Batteries with High Rate Capabilities. ACS Sustain. Chem. Eng. 2017, 5, 2799–2816. [Google Scholar] [CrossRef]
- Parveen, N. Resent Development of Binder-Free Electrodes of Transition Metal Oxides and Nanohybrids for High Performance Supercapacitors—A Review. Chem. Rec. 2023, 24, e202300065. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Shahzad, A.; Danish, M.; Fatima, M.; Adnan, M.; Atiq, S.; Asim, M.; Khan, M.A.; Ain, Q.U.; Perveen, R. Recent developments in transition metal oxide-based electrode composites for supercapacitor applications. J. Energy Storage 2024, 81, 110430. [Google Scholar] [CrossRef]
- Zhu, X. Recent advances of transition metal oxides and chalcogenides in pseudo-capacitors and hybrid capacitors: A review of structures, synthetic strategies, and mechanism studies. J. Energy Storage 2022, 49, 104148. [Google Scholar] [CrossRef]
- Yalovega, G.E.; Brzhezinskaya, M.; Dmitriev, V.O.; Shmatko, V.A.; Ershov, I.V.; Ulyankina, A.A.; Chernysheva, D.V.; Smirnova, N.V. Interfacial Interaction in MeOx/MWNTs (Me–Cu, Ni) Nanostructures as Efficient Electrode Materials for High-Performance Supercapacitors. Nanomaterials 2024, 14, 947. [Google Scholar] [CrossRef] [PubMed]
- Shmatko, V.; Leontyeva, D.; Nevzorova, N.; Smirnova, N.; Brzhezinskaya, M.; Yalovega, G. Interaction between NiOx and MWCNT in NiOx/MWCNTs composite: XANES and XPS study. J. Electron Spectrosc. Relat. Phenom. 2017, 220, 76–80. [Google Scholar] [CrossRef]
- Sankar, B.D.; Sekar, S.; Sathish, S.; Dhanasekaran, S.; Nirmala, R.; Kim, D.Y.; Lee, Y.; Lee, S.; Navamathavan, R. Recent advancements in MXene with two-dimensional transition metal chalcogenides/oxides nanocomposites for supercapacitor application—A topical review. J. Alloys Compd. 2024, 978, 173481. [Google Scholar] [CrossRef]
- Rahat, S.S.M.; Hasan, K.M.Z.; Mondol, M.H.; Mallik, A.K. A comprehensive review of carbon nanotube-based metal oxide nanocomposites for supercapacitors. J. Energy Storage 2023, 73, 108847. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Q.; Xie, Q.; Ou, H.; Lin, X.; Zeb, A.; Hu, L.; Wu, Y.; Ma, G. Recent progress in Co–based metal–organic framework derivatives for advanced batteries. J. Mater. Sci. Technol. 2022, 96, 262–284. [Google Scholar] [CrossRef]
- Portillo-Vélez, N.; Olvera-Neria, O.; Hernández-Pérez, I.; Rubio-Ponce, A. Localized electronic states induced by oxygen vacancies on anatase TiO2 (101) surface. Surf. Sci. 2013, 616, 115–119. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, X.; Li, Y.; Liu, Y. Exploring transition metal oxide-based oxygen vacancy supercapacitors: A review. J. Energy Storage 2024, 80, 110350. [Google Scholar] [CrossRef]
- Chen, J.; Wang, K.; Sun, M.; Ni, W.; Wang, M.; Yu, M.; Yu, D.; Ling, M.; Liang, C. Superior lithium storage in Fe2O3 nanoporous arrays endowed by surface phosphorylation and bulk phosphorous doping. Appl. Surf. Sci. 2022, 604, 154668. [Google Scholar] [CrossRef]
- Li, M.; Meng, Z.; Feng, R.; Zhu, K.; Zhao, F.; Wang, C.; Wang, J.; Wang, L.; Chu, P.K. Fabrication of bimetallic oxides (MCo2O4: M=Cu, Mn) on ordered microchannel electro-conductive plate for high-performance hybrid supercapacitors. Sustainability 2021, 13, 9896. [Google Scholar] [CrossRef]
- Liu, J.; Dong, L.; Chen, D.; Han, Y.; Liang, Y.; Yang, M.; Han, J.; Yang, C.; He, W. Metal Oxides with Distinctive Valence States in an Electron-Rich Matrix Enable Stable High-Capacity Anodes for Li Ion Batteries. Small Methods 2020, 4, 1900753. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices System Power Ratings, Module Size, n.d. Available online: www.sciencemag.org (accessed on 23 July 2024).
- Palacín, M.R. Understanding ageing in Li-ion batteries: A chemical issue. Chem. Soc. Rev. 2018, 47, 4924–4933. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.-S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Meng, J.; Wu, J.; Deng, Z.; Lin, M.; Mao, S.; Stroe, D.-I. A comprehensive overview and comparison of parameter benchmark methods for lithium-ion battery application. J. Energy Storage 2023, 71, 108197. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef]
- Gittins, J.W.; Chen, Y.; Arnold, S.; Augustyn, V.; Balducci, A.; Brousse, T.; Frackowiak, E.; Gómez-Romero, P.; Kanwade, A.; Köps, L.; et al. Interlaboratory study assessing the analysis of supercapacitor electrochemistry data. J. Power Sources 2023, 585, 233637. [Google Scholar] [CrossRef]
- Melot, B.C.; Tarascon, J.-M. Design and Preparation of Materials for Advanced Electrochemical Storage. Acc. Chem. Res. 2013, 46, 1226–1238. [Google Scholar] [CrossRef]
- Zhu, J.; Ding, Y.; Ma, Z.; Tang, W.; Chen, X.; Lu, Y. Recent Progress on Nanostructured Transition Metal Oxides As Anode Materials for Lithium-Ion Batteries. J. Electron. Mater. 2022, 51, 3391–3417. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, A. Importance and challenges of hydrothermal technique for synthesis of transition metal oxides and composites as supercapacitor electrode materials. J. Energy Storage 2021, 44, 103295. [Google Scholar] [CrossRef]
- Li, S.; Nechache, R.; Davalos, I.A.V.; Goupil, G.; Nikolova, L.; Nicklaus, M.; Laverdiere, J.; Ruediger, A.; Rosei, F. Ultrafast Microwave Hydrothermal Synthesis of BiFeO3 Nanoplates. J. Am. Ceram. Soc. 2013, 96, 3155–3162. [Google Scholar] [CrossRef]
- Chen, T.; Li, Z.; Chen, J.; Ge, W.; Liu, M.; Lu, Y. Hydrothermal synthesis and formation mechanism of Aurivillius Bi5Fe0.9Co0.1Ti3O15nanosheets. CrystEngComm 2016, 18, 7449–7456. [Google Scholar] [CrossRef]
- Wu, L.; Sun, L.; Li, X.; Zhang, Q.; Si, H.; Zhang, Y.; Wang, K.; Zhang, Y. Mesoporous ZnCo2O4-CNT microflowers as bifunctional material for supercapacitive and lithium energy storage. Appl. Surf. Sci. 2020, 506, 144964. [Google Scholar] [CrossRef]
- Liu, Y.; Bai, J.; Ma, X.; Li, J.; Xiong, S. Formation of quasi-mesocrystal ZnMn2O4 twin microspheres via an oriented attachment for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 14236–14244. [Google Scholar] [CrossRef]
- Li, Y.; Hou, X.; Zhang, Z.; Hai, Z.; Xu, H.; Cui, D.; Zhuiykov, S.; Xue, C. NiCo2O4 particles with diamond-shaped hexahedron structure for high-performance supercapacitors. Appl. Surf. Sci. 2018, 436, 242–251. [Google Scholar] [CrossRef]
- Liao, W.; Chen, H.; Zeng, Y.; Liu, L. Recent progress in the fabrication of nanostructured zinc-based ternary metal oxides for high-performance lithium-ion batteries. J. Appl. Electrochem. 2023, 53, 1077–1107. [Google Scholar] [CrossRef]
- Redekar, R.; Avatare, A.; Chouhan, J.; Patil, K.; Pawar, O.; Patil, S.; Bhoite, A.; Patil, V.; Patil, P.; Tarwal, N. Review on recent advancements in chemically synthesized manganese cobalt oxide (MnCo2O4) and its composites for energy storage application. Chem. Eng. J. 2022, 450, 137425. [Google Scholar] [CrossRef]
- Hu, L.; Qu, B.; Li, C.; Chen, Y.; Mei, L.; Lei, D.; Chen, L.; Li, Q.; Wang, T. Facile synthesis of uniform mesoporous ZnCo2O4 microspheres as a high-performance anode material for Li-ion batteries. J. Mater. Chem. A 2013, 1, 5596–5602. [Google Scholar] [CrossRef]
- Dang, W.; Wang, F.; Ding, Y.; Feng, C.; Guo, Z. Synthesis and electrochemical properties of ZnMn2O4 microspheres for lithium-ion battery application. J. Alloys Compd. 2017, 690, 72–79. [Google Scholar] [CrossRef]
- Qin, X.; Zhou, M.; Zong, B.; Guo, J.; Gong, J.; Wang, L.; Liang, G. Urea-assisted hydrothermal synthesis of a hollow hierarchical LiNi0.5Mn1.5O4 cathode material with tunable morphology characteristics. RSC Adv. 2018, 8, 30087–30097. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Elizondo, L.; Rendón-Ángeles, J.; Matamoros-Veloza, Z.; López-Cuevas, J.; Yanagisawa, K. Urea decomposition enhancing the hydrothermal synthesis of lithium iron phosphate powders: Effect of the lithium precursor. Adv. Powder Technol. 2017, 28, 1593–1602. [Google Scholar] [CrossRef]
- Nataraj, N.; Kubendhiran, S.; Gan, Z.-W.; Chen, S.-M.; Sakthivel, R. HMTA-assisted synthesis of praseodymium oxide nanostructures integrated multiwalled carbon nanotubes for efficient levofloxacin electrochemical sensing. Mater. Today Chem. 2022, 26, 101136. [Google Scholar] [CrossRef]
- Kong, K.; Deka, B.K.; Seo, J.W.; Park, Y.-B.; Park, H.W. Effect of CuO nanostructure morphology on the mechanical properties of CuO/woven carbon fiber/vinyl ester composites. Compos. Part A Appl. Sci. Manuf. 2015, 78, 48–59. [Google Scholar] [CrossRef]
- Kong, K.; Deka, B.K.; Kwak, S.K.; Oh, A.; Kim, H.; Park, Y.-B.; Park, H.W. Processing and mechanical characterization of ZnO/polyester woven carbon–fiber composites with different ZnO concentrations. Compos. Part A Appl. Sci. Manuf. 2013, 55, 152–160. [Google Scholar] [CrossRef]
- Zou, J.; Liu, B.; Liu, H.; Ding, Y.; Xin, T.; Wang, Y. Facile synthesis of interconnected mesoporous ZnMn2O4 nano-peanuts for Li-storage via distinct structure design. Mater. Res. Bull. 2018, 107, 468–476. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Liu, B.; Wang, Q.; Tan, D.; Song, W.; Hou, X.; Chen, D.; Shen, G. Advanced rechargeable lithium-ion batteries based on bendable ZnCo2O4-urchins-on-carbon-fibers electrodes. Nano Res. 2013, 6, 525–534. [Google Scholar] [CrossRef]
- Chen, H.; Deng, L.; Luo, S.; Ren, X.; Li, Y.; Sun, L.; Zhang, P.; Chen, G.; Gao, Y. Flexible Three-Dimensional Heterostructured ZnO-Co3O4 on Carbon Cloth as Free-Standing Anode with Outstanding Li/Na Storage Performance. J. Electrochem. Soc. 2018, 165, A3932–A3942. [Google Scholar] [CrossRef]
- Mo, Y.; Ru, Q.; Chen, J.; Song, X.; Guo, L.; Hu, S.; Peng, S. Three-dimensional NiCo2O4 nanowire arrays: Preparation and storage behavior for flexible lithium-ion and sodium-ion batteries with improved electrochemical performance. J. Mater. Chem. A 2015, 3, 19765–19773. [Google Scholar] [CrossRef]
- Huang, R.; Zhang, J.; Dong, Z.; Lin, H.; Han, S. Flexible carbon fiber/reduced-TiO2 composites for constructing remarkable performance supercapacitors. J. Power Sources 2022, 550, 232169. [Google Scholar] [CrossRef]
- Javed, M.S.; Lei, H.; Shah, H.U.; Asim, S.; Raza, R.; Mai, W. Achieving high rate and high energy density in an all-solid-state flexible asymmetric pseudocapacitor through the synergistic design of binder-free 3D ZnCo2O4 nano polyhedra and 2D layered Ti3C2Tx-MXenes. J. Mater. Chem. A 2019, 7, 24543–24556. [Google Scholar] [CrossRef]
- Ye, S.; Liu, F.; Xu, R.; Yao, Y.; Zhou, X.; Feng, Y.; Cheng, X.; Yu, Y. RuO2 Particles Anchored on Brush-Like 3D Carbon Cloth Guide Homogenous Li/Na Nucleation Framework for Stable Li/Na Anode. Small 2019, 15, e1903725. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zhang, W.; Han, Z.; Wang, F.; Geng, D.; Li, X.; Li, Y.; Zhang, X. Preparation of PAN-based carbon fiber@MnO2 composite as an anode material for structural lithium-ion batteries. J. Mater. Sci. 2019, 54, 11972–11982. [Google Scholar] [CrossRef]
- Hong, C.; Wang, X.; Yu, H.; Wu, H.; Wang, J.; Liu, A. MnO2 nanowires-decorated carbon fiber cloth as electrodes for aqueous asymmetric supercapacitor. Funct. Mater. Lett. 2018, 11, 1850034. [Google Scholar] [CrossRef]
- Li, F.; Liu, Y.-L.; Wang, G.-G.; Zhang, H.-Y.; Zhang, B.; Li, G.-Z.; Wu, Z.-P.; Dang, L.-Y.; Han, J.-C. Few-layered Ti3C2Tx MXenes coupled with Fe2O3 nanorod arrays grown on carbon cloth as anodes for flexible asymmetric supercapacitors. J. Mater. Chem. A 2019, 7, 22631–22641. [Google Scholar] [CrossRef]
- Peng, S.; Yu, L.; Lan, B.; Sun, M.; Cheng, G.; Liao, S.; Cao, H.; Deng, Y. Low-cost superior solid-state symmetric supercapacitors based on hematite nanocrystals. Nanotechnology 2016, 27, 505404. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Shi, M.; Han, Z.; Zhang, W.; Li, Y.; Zhang, X.; Sheng, Y. Synthesis of one-dimensional PAN-based carbon fiber/NiO composite as an anode material for structural lithium-ion batteries. Ionics 2020, 26, 5935–5940. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, R.; Dong, Z.; Lin, H.; Han, S. An illumination-assisted supercapacitor of rice-like CuO nanosheet coated flexible carbon fiber. Electrochim. Acta 2022, 430, 140789. [Google Scholar] [CrossRef]
- Xu, W.; Dai, S.; Liu, G.; Xi, Y.; Hu, C.; Wang, X. CuO Nanoflowers growing on Carbon Fiber Fabric for Flexible High-Performance Supercapacitors. Electrochim. Acta 2016, 203, 1–8. [Google Scholar] [CrossRef]
- Sui, Y.; Zhang, M.; Hu, H.; Zhang, Y.; Qi, J.; Wei, F.; Meng, Q.; He, Y.; Ren, Y.; Sun, Z. ZnO@Ni-Co-S Core-Shell Nanorods-Decorated Carbon Fibers as Advanced Electrodes for High-Performance Supercapacitors. Nano 2018, 13, 1850148. [Google Scholar] [CrossRef]
- Deka, B.K.; Hazarika, A.; Kwon, O.; Kim, D.; Park, Y.-B.; Park, H.W. Multifunctional enhancement of woven carbon fiber/ZnO nanotube-based structural supercapacitor and polyester resin-domain solid-polymer electrolytes. Chem. Eng. J. 2017, 325, 672–680. [Google Scholar] [CrossRef]
- Wang, G.; Sun, Z.; Huang, F.; Gong, C.; Liu, H.; Zheng, G.; Wen, S. Carbon cloth supported anatase TiO2 aligned arrays as a high-performance anode material for Li-ion batteries. Mater. Lett. 2016, 171, 150–153. [Google Scholar] [CrossRef]
- Padmanathan, N.; Selladurai, S.; Razeeb, K.M. Ultra-fast rate capability of a symmetric supercapacitor with a hierarchical Co3O4 nanowire/nanoflower hybrid structure in non-aqueous electrolyte. RSC Adv. 2015, 5, 12700–12709. [Google Scholar] [CrossRef]
- Song, K.; Wang, X.; Wang, J.; Zhang, B.; Yang, R. Hierarchical structure of CoFe2O4 core-shell microsphere coating on carbon fiber cloth for high-performance asymmetric flexible supercapacitor applications. Ionics 2019, 25, 4905–4914. [Google Scholar] [CrossRef]
- Song, K.; Wang, X.; Wang, J.; Zhang, B.; Yang, R. Bifunctional Conducting Polymer Coated CoFe2O4 Core-Shell Nanolayer on Carbon Fiber Cloth for 2.0 V Wearable Aqueous Supercapacitors. ChemistrySelect 2019, 4, 1685–1695. [Google Scholar] [CrossRef]
- Luo, G.; Diao, G.; He, Q.; Han, S.; Dang, D.; Su, X. Morphology-controlled synthesis of MnO2 grown on carbon fiber paper for high-rate supercapacitor electrode. J. Mater. Sci. Mater. Electron. 2022, 33, 18284–18293. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, H.; Tang, J.; Jiang, X.; Bao, Y.; Chen, Y. Preparation of ternary composite CF@γ-MnO2/PANI material in electrochemical supercapacitors. J. Mater. Sci. Mater. Electron. 2021, 32, 25300–25317. [Google Scholar] [CrossRef]
- Huang, Q.; Hu, J.; Zhang, M.; Li, M.; Li, T.; Yuan, G.; Liu, Y.; Zhang, X.; Cheng, X. Li-ion charge storage performance of wood-derived carbon fibers@MnO as a battery anode. Chin. Chem. Lett. 2021, 33, 1091–1094. [Google Scholar] [CrossRef]
- Sha, Z.; Zhou, Y.; Huang, F.; Yang, W.; Yu, Y.; Zhang, J.; Wu, S.; Brown, S.A.; Peng, S.; Han, Z.; et al. Carbon fibre electrodes for ultra long cycle life pseudocapacitors by engineering the nano-structure of vertical graphene and manganese dioxides. Carbon 2021, 177, 260–270. [Google Scholar] [CrossRef]
- Subhani, K.; Hameed, N.; Al-Qatatsheh, A.; Ince, J.; Mahon, P.J.; Lau, A.; Salim, N.V. Multifunctional structural composite supercapacitors based on MnO2-nanowhiskers decorated carbon fibers. J. Energy Storage 2022, 56, 105936. [Google Scholar] [CrossRef]
- Ling, X.; Zhang, G.; Long, Z.; Lu, X.; He, Z.; Li, J.; Wang, Y.; Zhang, D. Core–shell structure γ-MnO2-PANI carbon fiber paper-based flexible electrode material for high-performance supercapacitors. J. Ind. Eng. Chem. 2021, 99, 317–325. [Google Scholar] [CrossRef]
- Yao, S.; Zhang, G.; Zhang, X.; Shi, Z. Mace-like carbon fibers@Fe3O4@carbon composites as anode materials for lithium-ion batteries. Ionics 2020, 26, 5923–5934. [Google Scholar] [CrossRef]
- Zhang, K.; Cen, Z.; Yang, F.; Xu, K. Rational construction of NiCo2O4@Fe2O3 core-shell nanowire arrays for high-performance supercapacitors. Prog. Nat. Sci. Mater. Int. 2021, 31, 19–24. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, Z.; Zhang, H.; Shen, A.; Zhao, Y.; Zhou, Y.; Weng, Y. Binder-Free Charantia-Like Metal-Oxide Core/Shell Nanotube Arrays for High-Performance Lithium-Ion Anodes. Front. Chem. 2020, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Wang, S.; Yu, Y.; Feng, Q.; Yang, J.; Zhang, B. Carboxyl functionalized carbon fibers with preserved tensile strength and electrochemical performance used as anodes of structural lithium-ion batteries. Appl. Surf. Sci. 2017, 392, 27–35. [Google Scholar] [CrossRef]
- Xie, Q.; Zhang, Y.; Zhu, Y.; Fu, W.; Zhang, X.; Zhao, P.; Wu, S. Graphene enhanced anchoring of nanosized Co3O4 particles on carbon fiber cloth as free-standing anode for lithium-ion batteries with superior cycling stability. Electrochim. Acta 2017, 247, 125–131. [Google Scholar] [CrossRef]
- Deka, B.K.; Kong, K.; Seo, J.; Kim, D.; Park, Y.-B.; Park, H.W. Controlled growth of CuO nanowires on woven carbon fibers and effects on the mechanical properties of woven carbon fiber/polyester composites. Compos. Part A Appl. Sci. Manuf. 2015, 69, 56–63. [Google Scholar] [CrossRef]
- Li, M.; Zhu, K.; Zhao, H.; Meng, Z.; Wang, C.; Chu, P.K. Construction of α-MnO2 on Carbon Fibers Modified with Carbon Nanotubes for Ultrafast Flexible Supercapacitors in Ionic Liquid Electrolytes with Wide Voltage Windows. Nanomaterials 2022, 12, 2020. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, K.; Ye, P.; Huang, Q.; Wang, L.; Li, S. Optimized NiCo2O4/rGO hybrid nanostructures on carbon fiber as an electrode for asymmetric supercapacitors. RSC Adv. 2018, 8, 37550–37556. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.M.; Kitiphatpiboon, N.; An, X.; Hao, X.; Li, S.; Hao, X.; Abudula, A.; Guan, G. Fabrication of High Energy Flexible All-Solid-State Supercapacitor Using Pseudocapacitive 2D-Ti3C2Tx-MXene and Battery-Type Reduced Graphene Oxi-de/Nickel-Cobalt Bimetal Oxide Electrode Materials. ACS Appl. Mater. Interfaces 2020, 12, 52749–52762. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, H.; Xiong, T.; Adekoya, D.; Qiu, W.; Wang, Z.; Zhang, S.; Balogun, M.-S. Adsorption energy engineering of nickel oxide hybrid nanosheets for high areal capacity flexible lithium-ion batteries. Energy Storage Mater. 2020, 25, 41–51. [Google Scholar] [CrossRef]
- Razali, S.; Majid, S. Electrochemical performance of binder-free NiO-PANI on etched carbon cloth as active electrode material for supercapacitor. Mater. Des. 2018, 153, 24–35. [Google Scholar] [CrossRef]
- Ma, L.; Fan, H.; Wei, X.; Chen, S.; Hu, Q.; Liu, Y.; Zhi, C.; Lu, W.; Zapien, J.A.; Huang, H. Towards high areal capacitance, rate capability, and tailorable supercapacitors: Co3O4@polypyrrole core–shell nanorod bundle array electrodes. J. Mater. Chem. A 2018, 6, 19058–19065. [Google Scholar] [CrossRef]
- Chen, T.; Fan, Y.; Wang, G.; Zhang, J.; Chuo, H.; Yang, R. Rationally Designed Carbon Fiber@NiCo2O4@Polypyrrole Core–Shell Nanowire Array for High-Performance Supercapacitor Electrodes. Nano 2016, 11, 1650015. [Google Scholar] [CrossRef]
- Wang, X.-X.; Yu, G.-F.; Zhang, J.; Yu, M.; Ramakrishna, S.; Long, Y.-Z. Conductive polymer ultrafine fibers via electrospinning: Preparation, physical properties and applications. Prog. Mater. Sci. 2021, 115, 100704. [Google Scholar] [CrossRef]
- Chi, H.Z.; Zhu, H.; Gao, L. Boron-doped MnO2/carbon fiber composite electrode for supercapacitor. J. Alloys Compd. 2015, 645, 199–205. [Google Scholar] [CrossRef]
- Sari, F.N.I.; Ting, J. MoS2/MoOx-Nanostructure-Decorated Activated Carbon Cloth for Enhanced Supercapacitor Performance. ChemSusChem 2018, 11, 897–906. [Google Scholar] [CrossRef]
- Govindasamy, M.; Shanthi, S.; Elaiyappillai, E.; Wang, S.-F.; Johnson, P.M.; Ikeda, H.; Hayakawa, Y.; Ponnusamy, S.; Muthamizhchelvan, C. Fabrication of hierarchical NiCo2S4@CoS2 nanostructures on highly conductive flexible carbon cloth substrate as a hybrid electrode material for supercapacitors with enhanced electrochemical performance. Electrochim. Acta 2019, 293, 328–337. [Google Scholar] [CrossRef]
- Tian, Z.; Yin, J.; Wang, X.; Wang, Y. Construction of Ni3S2 wrapped by rGO on carbon cloth for flexible supercapacitor application. J. Alloys Compd. 2019, 777, 806–811. [Google Scholar] [CrossRef]
- Huang, F.; Sui, Y.; Wei, F.; Qi, J.; Meng, Q.; He, Y. Ni3S4 supported on carbon cloth for high-performance flexible all-solid-state asymmetric supercapacitors. J. Mater. Sci. Mater. Electron. 2018, 29, 2525–2536. [Google Scholar] [CrossRef]
- Xu, X.; Tian, X.; Li, X.; Yang, T.; He, Y.; Wang, K.; Song, Y.; Liu, Z. Structural and chemical synergistic effect of NiCo2S4 nanoparticles and carbon cloth for high performance binder-free asymmetric supercapacitors. Appl. Surf. Sci. 2019, 465, 635–642. [Google Scholar] [CrossRef]
- Su, C.; Xu, S.; Zhang, L.; Chen, X.; Guan, G.; Hu, N.; Su, Y.; Zhou, Z.; Wei, H.; Yang, Z.; et al. Hierarchical CoNi2S4 nanosheet/nanotube array structure on carbon fiber cloth for high-performance hybrid supercapacitors. Electrochim. Acta 2019, 305, 81–89. [Google Scholar] [CrossRef]
- Wan, L.; Jiang, T.; Zhang, Y.; Chen, J.; Xie, M.; Du, C. 1D-on-1D core–shell cobalt iron selenide @ cobalt nickel carbonate hydroxide hybrid nanowire arrays as advanced battery-type supercapacitor electrode. J. Colloid Interface Sci. 2022, 621, 149–159. [Google Scholar] [CrossRef]
- Zhan, J.; Wu, K.; Yu, X.; Yang, M.; Cao, X.; Lei, B.; Pan, D.; Jiang, H.; Wu, M. α-Fe2O3 Nanoparticles Decorated C@MoS2 Nanosheet Arrays with Expanded Spacing of (002) Plane for Ultrafast and High Li/Na-Ion Storage. Small 2019, 15, e1901083. [Google Scholar] [CrossRef] [PubMed]
- Thakur, S.; Maiti, S.; Paul, T.; Besra, N.; Sarkar, S.; Chattopadhyay, K.K. Geometrically intricate sheet-on-pillar/flake hierarchy embracing cobaltosic and manganese oxides over flexible carbon scaffold for binder-free high-energy-density supercapacitor. CrystEngComm 2018, 20, 6183–6196. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Hu, Z.; Miao, Y.; Sui, Y.; Qi, J.; Wei, F.; Ren, Y.; Zhan, Z.; Liu, J.; et al. In-situ growth of core-shell NiCo2O4@Ni-Co layered double hydroxides for all-solid-state flexible hybrid supercapacitor. Colloids Surf. A Physicochem. Eng. Asp. 2020, 607, 125417. [Google Scholar] [CrossRef]
- Qian, Y.; Liu, R.; Wang, Q.; Xu, J.; Chen, D.; Shen, G. Efficient synthesis of hierarchical NiO nanosheets for high-performance flexible all-solid-state supercapacitors. J. Mater. Chem. A 2014, 2, 10917–10922. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Wang, M.; Ren, G.; Wu, S.; Shen, J. Facilely prepared oxidized carbon Fiber@Co3O4@RGO as negative electrode for a novel asymmetric supercapacitor with high areal energy and power density. Appl. Surf. Sci. 2018, 450, 66–76. [Google Scholar] [CrossRef]
- Abbas, Q.; Javed, M.S.; Ahmad, A.; Siyal, S.H.; Asim, I.; Luque, R.; Albaqami, M.D.; Tighezza, A.M. Zno nano-flowers assembled on carbon fiber textile for high-performance supercapacitor’s electrode. Coatings 2021, 11, 1337. [Google Scholar] [CrossRef]
- Sun, X.; Wang, J.; Chen, B.; Dai, G.; Situ, Y.; Huang, H. High-performance adjustable manganese oxides hybrid nanostructure for supercapacitors. Electrochim. Acta 2021, 381, 138213. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.; Xu, J.; Pan, Y.; Huang, Y.; Han, S.; Li, Y. Preparation of high-performance asymmetric supercapacitors based on NiCo2S4 nanospheres and CuO-MOF nanosheets on carbon fibers. Fuel 2024, 356, 129542. [Google Scholar] [CrossRef]
- Song, Z.; Shu, K.; Hu, H.; Wu, X.; Tang, X.; Zhou, X.; Li, Y.; Zhang, Y. Facile synthesis and modification of Fe2O3 nanorod arrays on carbon paper as efficient negative electrodes for supercapacitors. J. Alloys Compd. 2024, 979, 173578. [Google Scholar] [CrossRef]
- Simonenko, T.L.; Simonenko, N.P.; Gorobtsov, P.Y.; Simonenko, E.P.; Kuznetsov, N.T. Hydrothermal Synthesis of a Cellular NiO Film on Carbon Paper as a Promising Way to Obtain a Hierarchically Organized Electrode for a Flexible Supercapacitor. Materials 2023, 16, 5208. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-M.; Yang, W.-D. Boosting the Capacitive Performance of Supercapacitors by Hybridizing N, P-Codoped Carbon Polycrystalline with Mn3O4-Based Flexible Electrodes. Nanomaterials 2023, 13, 2060. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, B.K.; González-Banciella, A.; Ureña, D.; Sánchez, M.; Ureña, A. Electrochemical Comparison of 2D-Flexible Solid-State Supercapacitors Based on a Matrix of PVA/H3PO4. Polymers 2023, 15, 4036. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, Y.-H.; Liu, X. SnO2 nanospheres and V2O5/SnO2 nanoparticles with mesoporous structures for flexible asymmetric supercapacitors. J. Mater. Sci. Mater. Electron. 2023, 34, 1–19. [Google Scholar] [CrossRef]
- Radhi, A.A.; Al-Rubaiey, S.I.J.; Al-Rubaye, S. NH4F-assisted and morphology-controlled fabrication of iron cobaltite FeCo2O4 nanoparticles on carbon fiber cloth for supercapacitor applications. Chem. Pap. 2023, 78, 733–746. [Google Scholar] [CrossRef]
- Li, S.; Liu, G.; Liu, J.; Lu, Y.; Yang, Q.; Yang, L.-Y.; Yang, H.-R.; Liu, S.; Lei, M.; Han, M. Carbon fiber cloth@VO2(B): Excellent binder-free flexible electrodes with ultrahigh mass-loading. J. Mater. Chem. A 2016, 4, 6426–6432. [Google Scholar] [CrossRef]
- Pan, G.; Xia, X.; Cao, F.; Chen, J.; Zhang, Y. Carbon cloth supported vanadium pentaoxide nanoflake arrays as high-performance cathodes for lithium ion batteries. Electrochim. Acta 2014, 149, 349–354. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, X.; Zhang, W.; Li, Y.; Zhang, Z. Preparation of multifunctional structural P-CF@ZnCo2O4 composites used as structural anode materials. J. Alloys Compd. 2020, 842, 155743. [Google Scholar] [CrossRef]
- Han, Q.; Sheng, Y.; Zhang, X. Preparation of a multifunctional P-CF@Mn3O4 composite as a structural anode material. New J. Chem. 2021, 45, 15808–15817. [Google Scholar] [CrossRef]
- Purvika, A.; Yadav, S.; Jijoe, S.P.; Tenzin, T.; Divya, V.; Shahmoradi, B.; Wantala, K.; Jenkins, D.; McKay, G.; Shivaraju, H.P. Improved metal-organic frameworks (MOFs) and their application in catalytic CO2 reduction: A review. Mater. Today Sustain. 2024, 26, 100745. [Google Scholar] [CrossRef]
- Li, L.; Han, J.; Huang, X.; Qiu, S.; Liu, X.; Liu, L.; Zhao, M.; Qu, J.; Zou, J.; Zhang, J. Organic pollutants removal from aqueous solutions using metal-organic frameworks (MOFs) as adsorbents: A review. J. Environ. Chem. Eng. 2023, 11, 111217. [Google Scholar] [CrossRef]
- Meskher, H.; Belhaouari, S.B.; Sharifianjazi, F. Mini review about metal organic framework (MOF)-based wearable sensors: Challenges and prospects. Heliyon 2023, 9, e21621. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xu, X.; Shao, Z.; Jiang, S.P. Metal-organic frameworks derived porous carbon, metal oxides and metal sulfides-based compounds for supercapacitors application. Energy Storage Mater. 2020, 26, 1–22. [Google Scholar] [CrossRef]
- Wang, K.B.; Xun, Q.; Zhang, Q. Recent progress in metal-organic frameworks as active materials for supercapacitors. Energy Chem. 2020, 2, 100025. [Google Scholar] [CrossRef]
- Salunkhe, R.R.; Young, C.; Tang, J.; Takei, T.; Ide, Y.; Kobayashi, N.; Yamauchi, Y. A high-performance supercapacitor cell based on ZIF-8-derived nanoporous carbon using an organic electrolyte. Chem. Commun. 2016, 52, 4764–4767. [Google Scholar] [CrossRef]
- Deng, X.; Zhu, S.; Li, J.; Ma, L.; He, F.; Liu, E.; He, C.; Shi, C.; Li, Q.; Zhao, N. Ball-in-cage nanocomposites of metal–organic frameworks and three-dimensional carbon networks: Synthesis and capacitive performance. Nanoscale 2017, 9, 6478–6485. [Google Scholar] [CrossRef]
- Huang, J.; Hao, F.; Zhang, X.; Chen, J. N-doped porous carbon sheets derived from ZIF-8: Preparation and their electrochemical capacitive properties. J. Electroanal. Chem. 2018, 810, 86–94. [Google Scholar] [CrossRef]
- Liu, B.; Shioyama, H.; Jiang, H.; Zhang, X.; Xu, Q. Metal–organic framework (MOF) as a template for syntheses of nanoporous carbons as electrode materials for supercapacitor. Carbon 2010, 48, 456–463. [Google Scholar] [CrossRef]
- Hu, J.; Wang, H.; Gao, Q.; Guo, H. Porous carbons prepared by using metal–organic framework as the precursor for supercapacitors. Carbon 2010, 48, 3599–3606. [Google Scholar] [CrossRef]
- Tan, X.; Wu, Y.; Lin, X.; Zeb, A.; Xu, X.; Luo, Y.; Liu, J. Application of MOF-derived transition metal oxides and composites as anodes for lithium-ion batteries. Inorg. Chem. Front. 2020, 7, 4939–4955. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Zhang, B.; Tian, S.; Yang, X.; Ye, H.; Xia, Z.; Zheng, G. Application of MOFs-derived mixed metal oxides in energy storage. J. Electroanal. Chem. 2020, 878, 114576. [Google Scholar] [CrossRef]
- Bajwa, R.A.; Farooq, U.; Ullah, S.; Salman, M.; Haider, S.; Hussain, R. Metal-organic framework (MOF) attached and their derived metal oxides (Co, Cu, Zn and Fe) as anode for lithium ion battery: A review. J. Energy Storage 2023, 72, 108708. [Google Scholar] [CrossRef]
- Wu, X.; Meng, L.; Wang, Q.; Zhang, W.; Wang, Y. Highly flexible and large areal/volumetric capacitances for asymmetric supercapacitor based on ZnCo2O4 nanorods arrays and polypyrrole on carbon cloth as binder-free electrodes. Mater. Lett. 2019, 234, 1–4. [Google Scholar] [CrossRef]
- Javed, M.S.; Aslam, M.K.; Asim, S.; Batool, S.; Idrees, M.; Hussain, S.; Shah, S.S.A.; Saleem, M.; Mai, W.; Hu, C. High-performance flexible hybrid-supercapacitor enabled by pairing binder-free ultrathin Ni–Co–O nanosheets and metal-organic framework derived N-doped carbon nanosheets. Electrochim. Acta 2020, 349, 136384. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.; Feng, M.; Yang, J.; Zhang, B. MOF-derived ZnCo2O4/C wrapped on carbon fiber as anode materials for structural lithium-ion batteries. Chin. Chem. Lett. 2019, 30, 529–532. [Google Scholar] [CrossRef]
- Wang, F.; Han, Q.; Yi, Z.; Geng, D.; Li, X.; Wang, Z.; Wang, L. Synthesis and performances of carbon fiber@Co3O4 based on metal organic frameworks as anode materials for structural lithium-ion battery. J. Electroanal. Chem. 2017, 807, 196–202. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Zhang, J.; Jung, E.; Lee, S.; Jun, S.C. Structural engineering and surface modification of MOF-derived cobalt-based hybrid nanosheets for flexible solid-state supercapacitors. Energy Storage Mater. 2020, 32, 167–177. [Google Scholar] [CrossRef]
- Dai, S.; Han, F.; Tang, J.; Tang, W. MOF-derived Co3O4 nanosheets rich in oxygen vacancies for efficient all-solid-state symmetric supercapacitors. Electrochim. Acta 2019, 328, 135103. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, H.; Yao, J. Synthesis of 2D nanoporous zeolitic imidazolate framework nanosheets for diverse applications. Co-ord. Chem. Rev. 2021, 431, 213677. [Google Scholar] [CrossRef]
- Fu, Y.; Zhou, H.; Hu, Z.; Yin, S.; Zhou, L. Temperature-induced microstructure optimization of Co3O4 for the achievement of a high-areal-capacity carbon cloth-based lithium ion battery anode. Compos. Commun. 2020, 22, 100446. [Google Scholar] [CrossRef]
- Wu, F.; Bai, J.; Feng, J.; Xiong, S. Porous mixed metal oxides: Design, formation mechanism, and application in lithium-ion batteries. Nanoscale 2015, 7, 17211–17230. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Bin Wu, H.; Xie, Y.; Lou, X.W. Mixed Transition-Metal Oxides: Design, Synthesis, and Energy-Related Applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef] [PubMed]
- Kavinkumar, T.; Vinodgopal, K.; Neppolian, B. Development of nanohybrids based on porous spinel MCo2O4 (M = Zn, Cu, Ni and Mn)/reduced graphene oxide/carbon nanotube as promising electrodes for high performance energy storage devices. Appl. Surf. Sci. 2020, 513, 145781. [Google Scholar] [CrossRef]
- Qi, J.; Wang, P.; Yan, Y.; Zheng, X.; Cao, J.; Feng, J. MCo2O4 (M = Co, Mn, Ni, Zn) nanosheet arrays constructed by two-dimension metal-organic frameworks as binder-free electrodes for lithium-ion batteries. Vacuum 2019, 169, 108959. [Google Scholar] [CrossRef]
- Liu, X.; Zang, W.; Guan, C.; Zhang, L.; Qian, Y.; Elshahawy, A.M.; Zhao, D.; Pennycook, S.J.; Wang, J. Ni-Doped Cobalt–Cobalt Nitride Heterostructure Arrays for High-Power Supercapacitors. ACS Energy Lett. 2018, 3, 2462–2469. [Google Scholar] [CrossRef]
- Lim, G.J.; Liu, X.; Guan, C.; Wang, J. Co/Zn bimetallic oxides derived from metal organic frameworks for high performance electrochemical energy storage. Electrochim. Acta 2018, 291, 177–187. [Google Scholar] [CrossRef]
- Huang, T.; Lou, Z.; Lu, Y.; Li, R.; Jiang, Y.; Shen, G.; Chen, D. Metal-Organic-Framework-Derived MCo2O4 (M = Mn and Zn) Nanosheet Arrays on Carbon Cloth as Integrated Anodes for Energy Storage Applications. ChemElectroChem 2019, 6, 5836–5843. [Google Scholar] [CrossRef]
- Guan, C.; Liu, X.; Ren, W.; Li, X.; Cheng, C.; Wang, J. Rational Design of Metal-Organic Framework Derived Hollow NiCo2O4 Arrays for Flexible Supercapacitor and Electrocatalysis. Adv. Energy Mater. 2017, 7, 1602391. [Google Scholar] [CrossRef]
- Guan, C.; Zhao, W.; Hu, Y.; Lai, Z.; Li, X.; Sun, S.; Zhang, H.; Cheetham, A.K.; Wang, J. Cobalt oxide and N-doped carbon nanosheets derived from a single two-dimensional metal–organic framework precursor and their application in flexible asymmetric supercapacitors. Nanoscale Horiz. 2017, 2, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Tian, R.; Zhou, Y.; Duan, H.; Guo, Y.; Li, H.; Chen, K.; Xue, D.; Liu, H. MOF-Derived Hollow Co3S4 Quasi-polyhedron/MWCNT Nanocomposites as Electrodes for Advanced Lithium Ion Batteries and Supercapacitors. ACS Appl. Energy Mater. 2018, 1, 402–410. [Google Scholar] [CrossRef]
- Han, X.; Tao, K.; Wang, D.; Han, L. Design of a porous cobalt sulfide nanosheet array on Ni foam from zeolitic imidazolate frameworks as an advanced electrode for supercapacitors. Nanoscale 2018, 10, 2735–2741. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, C.; Xiao, D.; Kopold, P.; Gu, L.; van Aken, P.A.; Maier, J.; Yu, Y. MOF-Derived Hollow Co9S8 Nanoparticles Embedded in Graphitic Carbon Nanocages with Superior Li-Ion Storage. Small 2016, 12, 2354–2364. [Google Scholar] [CrossRef]
- Li, H.; Wu, P.; Xiao, Y.; Shao, M.; Shen, Y.; Fan, Y.; Chen, H.; Xie, R.; Zhang, W.; Li, S.; et al. Metal–Organic Frameworks as Metal Ion Precursors for the Synthesis of Nanocomposites for Lithium-Ion Batteries. Angew. Chem. 2020, 132, 4793–4799. [Google Scholar] [CrossRef]
- Aslam, M.K.; Shah, S.S.A.; Li, S.; Chen, C. Kinetically controlled synthesis of MOF nanostructures: Single-holed hollow core–shell ZnCoS@Co9S8/NC for ultra-high performance lithium-ion batteries. J. Mater. Chem. A 2018, 6, 14083–14090. [Google Scholar] [CrossRef]
- Li, H.; Su, Y.; Sun, W.; Wang, Y. Carbon Nanotubes Rooted in Porous Ternary Metal Sulfide@N/S-Doped Carbon Dodecahedron: Bimetal-Organic-Frameworks Derivation and Electrochemical Application for High-Capacity and Long-Life Lithium-Ion Batteries. Adv. Funct. Mater. 2016, 26, 8345–8353. [Google Scholar] [CrossRef]
- Jia, H.; Wang, Z.; Zheng, X.; Cai, Y.; Lin, J.; Liang, H.; Qi, J.; Cao, J.; Feng, J.; Fei, W. Controlled synthesis of MOF-derived quadruple-shelled CoS2 hollow dodecahedrons as enhanced electrodes for supercapacitors. Electrochim. Acta 2019, 312, 54–61. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, J.; Guan, B.; Lou, X.W. Unusual Formation of CoSe@carbon Nanoboxes, which have an Inhomogeneous Shell, for Efficient Lithium Storage. Angew. Chem. 2016, 128, 9666–9670. [Google Scholar] [CrossRef]
- Yang, J.; Gao, H.; Men, S.; Shi, Z.; Lin, Z.; Kang, X.; Chen, S. CoSe2 Nanoparticles Encapsulated by N-Doped Carbon Framework Intertwined with Carbon Nanotubes: High-Performance Dual-Role Anode Materials for Both Li- and Na-Ion Batteries. Adv. Sci. 2018, 5, 1800763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Dai, P.; Liu, H.; Yan, L.; Song, H.; Liu, D.; Zhao, X. Metal-organic framework derived porous flakes of cobalt chalcogenides (CoX, X = O, S, Se and Te) rooted in carbon fibers as flexible electrode materials for pseudocapacitive energy storage. Electrochim. Acta 2021, 369, 137681. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, Y.; Yan, M.; Lei, Y.; Shi, W. MOF-derived hierarchical nanosheet arrays constructed by interconnected NiCo-alloy@NiCo-sulfide core-shell nanoparticles for high-performance asymmetric supercapacitors. Chem. Eng. J. 2019, 370, 666–676. [Google Scholar] [CrossRef]
- Dai, S.; Yuan, Y.; Yu, J.; Tang, J.; Zhou, J.; Tang, W. Metal–organic framework-templated synthesis of sulfur-doped core–sheath nanoarrays and nanoporous carbon for flexible all-solid-state asymmetric supercapacitors. Nanoscale 2018, 10, 15454–15461. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Pu, W.; Zheng, Y.; Wu, H.; Li, L.; Wei, X. Excellent rate capability supercapacitor electrodes with highly hydroxyl ion adsorption capacity enabled by P-doped MnCo2O4 nanotube arrays. Appl. Surf. Sci. 2022, 599, 153908. [Google Scholar] [CrossRef]
- Gong, H.; Bie, S.; Zhang, J.; Ke, X.; Wang, X.; Liang, J.; Wu, N.; Zhang, Q.; Luo, C.; Jia, Y. In Situ Construction of ZIF-67-Derived Hybrid Tricobalt Tetraoxide@Carbon for Supercapacitor. Nanomaterials 2022, 12, 1571. [Google Scholar] [CrossRef] [PubMed]
- Mateen, A.; Javed, M.S.; Khan, S.; Saleem, A.; Majeed, M.K.; Khan, A.J.; Tahir, M.F.; Ahmad, M.A.; Assiri, M.A.; Peng, K.-Q. Metal-organic framework-derived walnut-like hierarchical Co-O-nanosheets as an advanced binder-free electrode material for flexible supercapacitor. J. Energy Storage 2022, 49, 104150. [Google Scholar] [CrossRef]
- Liu, T.; Wang, W.; Yi, M.; Chen, Q.; Xu, C.; Cai, D.; Zhan, H. Metal-organic framework derived porous ternary ZnCo2O4 nanoplate arrays grown on carbon cloth as binder-free electrodes for lithium-ion batteries. Chem. Eng. J. 2018, 354, 454–462. [Google Scholar] [CrossRef]
- Fang, G.; Zhou, J.; Liang, C.; Pan, A.; Zhang, C.; Tang, Y.; Tan, X.; Liu, J.; Liang, S. MOFs nanosheets derived porous metal oxide-coated three-dimensional substrates for lithium-ion battery applications. Nano Energy 2016, 26, 57–65. [Google Scholar] [CrossRef]
- Han, Q.; Li, X.; Wang, F.; Han, Z.; Geng, D.; Zhang, W.; Li, Y.; Deng, Y.; Zhang, J.; Niu, S.; et al. Carbon fiber@ pore-ZnO composite as anode materials for structural lithium-ion batteries. J. Electroanal. Chem. 2018, 833, 39–46. [Google Scholar] [CrossRef]
- Zhu, F.; Liu, W.; Liu, Y.; Shi, W. Self-supported hierarchical core–shell Co9S8@NiCo2O4 hollow nanoneedle arrays for asymmetric supercapacitors. Inorg. Chem. Front. 2019, 6, 982–987. [Google Scholar] [CrossRef]
- Chen, Y.; Guan, J.-H.; Gan, H.; Chen, B.-Z.; Shi, X.-C. Electrochemical growth of α-MnO2 on carbon fibers for high-performance binder-free electrodes of supercapacitors. J. Appl. Electrochem. 2018, 48, 105–113. [Google Scholar] [CrossRef]
- Ko, W.-Y.; Liu, Y.-C.; Lai, J.-Y.; Chung, C.-C.; Lin, K.-J. Vertically Standing MnO2 Nanowalls Grown on AgCNT-Modified Carbon Fibers for High-Performance Supercapacitors. ACS Sustain. Chem. Eng. 2019, 7, 669–678. [Google Scholar] [CrossRef]
- Sha, Z.; Huang, F.; Zhou, Y.; Zhang, J.; Wu, S.; Chen, J.; Brown, S.A.; Peng, S.; Han, Z.; Wang, C.-H. Synergies of vertical graphene and manganese dioxide in enhancing the energy density of carbon fibre-based structural supercapacitors. Compos. Sci. Technol. 2021, 201, 108568. [Google Scholar] [CrossRef]
- Wang, Z.; Du, J.; Zhang, M.; Yu, J.; Liu, H.; Chai, X.; Yang, B.; Zhu, C.; Xu, J. Continuous preparation of high performance flexible asymmetric supercapacitor with a very fast, low-cost, simple and scalable electrochemical co-deposition method. J. Power Sources 2019, 437, 226827. [Google Scholar] [CrossRef]
- Jiang, S.; Cheng, S.; Huang, Y.; Shi, T.; Tang, Z. High performance wire-shaped supercapacitive electrodes based onactivated carbon fibers core/manganese dioxide shell structures. Ceram. Int. 2017, 43, 7916–7921. [Google Scholar] [CrossRef]
- Velayutham, R.; Manikandan, R.; Raj, C.J.; Kale, A.M.; Kaya, C.; Palanisamy, K.; Kim, B.C. Electrodeposition of vanadium pentoxide on carbon fiber cloth as a binder-free electrode for high-performance asymmetric supercapacitor. J. Alloys Compd. 2021, 863, 158332. [Google Scholar] [CrossRef]
- Kazemi, S.; Asghari, A.; Kiani, M. High Performance Supercapacitors Based on the Electrodeposited Co3O4 Nanoflakes on Electro-etched Carbon Fibers. Electrochim. Acta 2014, 138, 9–14. [Google Scholar] [CrossRef]
- Ko, T.H.; Kwak, C.-S.; Seong, J.-G.; Cho, Y.-H.; Lei, D.; Choi, W.-K.; Kuk, Y.-S.; Seo, M.-K.; Kim, B.-S. Influence of electrochemically deposited polypyrrole layers on NiCo2O4-decorated carbon fiber paper electrodes for high-performance hybrid supercapacitor applications. Funct. Compos. Struct. 2019, 1, 045003. [Google Scholar] [CrossRef]
- Zhang, J.; Yi, X.-B.; Wang, X.-C.; Ma, J.; Liu, S.; Wang, X.-J. Nickel oxide grown on carbon nanotubes/carbon fiber paper by electrodeposition as flexible electrode for high-performance supercapacitors. J. Mater. Sci. Mater. Electron. 2015, 26, 7901–7908. [Google Scholar] [CrossRef]
- Li, H.; Feng, Z.; Che, H.; Liu, Y.; Guo, Z.; Zhang, X.; Zhang, Z.; Wang, Y.; Mu, J. Direct growth of NiCo2O4nanosheet arrays on 3D-Ni-modified CFs for enhanced electrochemical storage in flexible supercapacitors. J. Mater. Sci. Mater. Electron. 2020, 31, 17879–17891. [Google Scholar] [CrossRef]
- Li, W.; Xin, L.; Xu, X.; Liu, Q.; Zhang, M.; Ding, S.; Zhao, M.; Lou, X. Facile synthesis of three-dimensional structured carbon fiber-NiCo2O4-Ni(OH)2 high-performance electrode for pseudocapacitors. Sci. Rep. 2015, 5, srep09277. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Hu, Y.; Zhou, Y.; Li, N.; Ding, Y.; Guo, J.; Zhao, C.; Yang, Y. Aerobic Recovered Carbon Fiber Support-Based MoO2//MnO2 Asymmetric Supercapacitor with a Widened Voltage Window. Energy Fuels 2021, 35, 6909–6920. [Google Scholar] [CrossRef]
- Xie, H.; Liu, X.; Wu, R.; Liu, J.; Wu, J.; Li, L. High-Performance Supercapacitor with Faster Energy Storage and Long Cyclic Life Based on CuO@MnO2 Nano-Core–Shell Array on Carbon Fiber Surface. ACS Appl. Energy Mater. 2020, 3, 7325–7334. [Google Scholar] [CrossRef]
- Huang, F.; Zhou, Y.; Sha, Z.; Peng, S.; Chang, W.; Cheng, X.; Zhang, J.; Brown, S.A.; Han, Z.; Wang, C.-H. Surface Functionalization of Electrodes and Synthesis of Dual-Phase Solid Electrolytes for Structural Supercapacitors. ACS Appl. Mater. Interfaces 2022, 14, 30857–30871. [Google Scholar] [CrossRef]
- Qiu, W.; Balogun, M.-S.; Luo, Y.; Chen, K.; Zhu, Y.; Xiao, X.; Lu, X.; Liu, P.; Tong, Y. Three-dimensional Fe3O4 Nanotube Array on Carbon Cloth Prepared from A Facile Route for Lithium ion Batteries. Electrochim. Acta 2016, 193, 32–38. [Google Scholar] [CrossRef]
| Material | Other Reactants/ Solvents | Solvothermal Treatment | Post-Treatment | Capacitance | Current Density | Capacitance Retention | Cycles | Electrolyte | Reference |
|---|---|---|---|---|---|---|---|---|---|
| MnO2 nanosheets + CNTs | -/H2O | 180 °C 2 h | - | 367.44 F/g | 2 mA/cm2 | 97.43% | 10,000 | Na2SO4 1 M | [115] |
| Fe2O3 nanodots + CNTs | Urea/H2O | 130 °C 8 h | - | 361.8 F/g | 3 mA/cm2 | 98.07% | 10,000 | Na2SO4 1 M | [115] |
| MnO2 nanowhiskers | -/H2O | 160 °C 3 h | - | 490 F/g | 1 A/g | 87.5% | 10,000 | KOH 6 M | [107] |
| B-doped MnO2 worm-like films | H3BO3/H2O | 80 °C 8 h | - | 210 F/g | 5 A/g | 80.4% | 900 | Na2SO4 0.5 M | [123] |
| MnO2 nanowires | -/H2O | 140 °C 3 h | - | 3.88 F/cm2 | 5 mA/cm2 | 91.5% | 3000 | Na2SO4 1 M | [91] |
| MnO2 + PCC | -/H2O | 90 °C 6 h | - | 1065 mF/cm2 | 1 mA/cm2 | - | - | LiCl 5 M | [92] |
| Fe2O3 nanorod arrays + MXenes | Na2SO4/H2O | 120 °C 8 h | 450 °C 2 h in N2 + dipping in Mxene solution | 725 mF/cm2 | 1 mA/cm2 | 88.6% | 10,000 | LiCl 5 M | [92] |
| MnO2 nanocrystals | NaNO3/H2O | 100 °C 24 h | - | 1.66 F/cm2 | 2 mA/cm2 | 88.6% | 5000 | Na2SO4 1 M | [93] |
| NiO nanosheets | -/H2O | 80 °C 6 h | Soaking in KOH 2 M | 842 mF/cm2 | 1 mA/cm2 | - | - | KOH 3 M | [134] |
| NiO + PANI | Urea/H2O | 120 °C 6 h | 300 °C 3 h + PANI ECD | 192.3 F/g | 0.5 A/g | 72% | 4500 | Na2SO4 0.5 M | [119] |
| Co3O4 nanorod bundle arrays + PPy | Urea + NH4F/H2O | 120 °C 8 h | Annealing 350 °C 3 h + immersing in PPy solution | 6.67 mF/cm2 | 2 mA/cm2 | ~100% | 2000 | KOH 1 M | [120] |
| Co3O4 flakes + MnO2 sheets | I (Urea) /H2O | I (180 °C 2 h) + II (160 °C 2 h) | - | 1396 F/g | 0.3 A/g | 98% | 5000 | KOH 1 M | [132] |
| Co3O4 nanowires/nanoflowers | Urea/EtOH | 180 °C 12 h | 300 °C 3 h | 7.8 mF/cm2 | 0.2 mA/cm2 | 54% | 5000 | KOH 3 M | [100] |
| Co3O4 + RGO | -/EtOH | 140 °C 12 h | Soaking in GO solution + 500 °C 2 h in Ar | 5.91 F/cm2 | 4 mA/cm2 | 58.64% | 5000 | KOH 1 M | [135] |
| NiCo2O4 nanowire arrays + PPy | Urea/H2O | 80 °C 6 h | 300 °C 2 h in air + PPy ECD | 1.44 F/cm2 | 2 mA/cm2 | 85% | 5000 | KOH 3 M | [121] |
| NiCo2O4 nanowires + rGO | GO + urea /H2O + EtOH | 120 °C 8 h | 350 °C 2 h | 931.7 F/g | 1 A/g | - | - | KOH 3 M | [116] |
| NiCo2O4 nanospikes + rGO | Urea + NH4F/H2O | 150 °C 5 h | Annealing 300 °C 3 h | 1338 mF/cm2 | 3 mA/cm2 | 88.2% | 10,000 | KOH 3 M | [117] |
| NiCo2O4 rods + Ni-Co LDH | Urea + NH4F/H2O | 120 °C 6 h | Ni-Co LDH ECD | 4901.8 mF/cm2 | 2 mA/cm2 | 86.7% | 5000 | KOH 1 M | [133] |
| CuO nanosheets | NaOH /H2O + EG | 120 °C 12 h | - | 1.64 F/g | 40 mA/g | 99.45% | 10,000 | Na2SO4 1 M | [95] |
| CuO nanoflowers | NaOH + urea/EtOH | 373 K 12 h | - | 839.9 F/g | 1 mV/s | 91% | 2000 | KOH 6 M | [96] |
| ZnO + Ni-Co-S core–shell nanorods | HMTA + ammonia/H2O | 90 °C 24 h | Ni-Co-S ECD | 1302.5 F/g | 1 A/g | 65% | 5000 | KOH 1 M | [97] |
| ZnO nanoflowers | NaOH + CTAB/H2O | 100 °C 10 h | 350 °C 2 h | 201.25 F/g | 1 A/g | 90.31% | 3000 | KOH 1 M | [136] |
| ZnO nanotubes | HMTA/H2O | 100 °C 8 h | Dipping in KOH solution | 14.25 F/g | 1 mV/s | 93.2% | 2500 | IL (15%)/LiS (5%)/PES (78%)/PANI (2%) | [98] |
| TiO2 | -/C3H8O3 + C2HhO | 150 °C 15 h | 320 °C 4 h in air + reduction in NaBH4 solution | 115.3 F/g | 1200 mA/g | 98.7% | 10,000 | Na2SO4 1 M + Fe(CN)63−/4− | [87] |
| ZnCo2O4 nano polyhedra | -/H2O + EG | 180 °C 20 h | 350 °C 2 h in air | 2643.66 F/g* | 2 A/g | 96.54% | 5000 | KOH | [88] |
| CoFe2O4 microspheres | -/H2O + glycol | 200 °C 8 h + 200 °C 4 h | 300 °C 3 h | 393 mF/cm2 | 1 A/cm2 | 95.8% | 2000 | KOH 3 M | [101] |
| CoFe2O4 | -/H2O + EG | 180 °C 24 h | 350 °C 3 h + spraying PEDOT:PSS | 472.5 F/g | 1 A/g | ~90% | 5000 | Na2SO4 1 M | [102] |
| MnO2-Mn3O4 | -/H2O // -/EtOH + H2O + EG | 160 °C 6 h + 180 °C 5 h | - | 1708.8 F/g | 1 A/g | 91.9% | 6000 | Saturated KCl | [137] |
| CuO-MOF nanosheets | BTC/H2O + EtOH | 150 °C 15 h | - | 953 F/g | 400 mA/g | 97.5% | 10,000 | Na2SO4 1 M + Fe(CN)63−/4− 0.05 M | [138] |
| Fe2O3 nanorod arrays | Urea/H2O | 100 °C 12 h | 500 °C 3 h in air | 976.4 mF/cm2 | 4 mA/cm2 | 104% | 10,000 | KOH 1 M | [139] |
| NiO film | C6H15NO3/ H2O | 200 °C 1 h | 300 °C 2 h in air | 207 F/g | 0.5 A/g | 95% | 2000 | KOH 3 M | [140] |
| Mn3O4@ NPC | Chitosan/ H2O | 180 °C 10 h | 600 °C 1 h in N2 | 256.8 F/g | 1 A/g | 97.3% | 5000 | Na2SO4 1 M | [141] |
| CuO nanoparticles | -/ H2O | 80 °C 2 h | - | 5.42 F/g | 10 mV/s | - | - | KCl 3 M | [142] |
| V2O5/SnO2 | CTAB/EtOH + H2O | 180 °C 12 h | - | 151.3 F/g | 1 A/g | 92.1% | 10,000 | KOH 4 M | [143] |
| FeCo2O4 nanoparticles | Urea + NH4F/EtOH + H2O | 140 °C 7 h | 400 °C 2 h | 912 F/g | 2 A/g | 90% | 10,000 | KOH 1 M | [144] |
| Material | Other Reactants/ Solvent | Solvothermal Treatment | Post-Treatment | Reversible Capacity | Current Density | Cycles | Reference |
|---|---|---|---|---|---|---|---|
| NiO nanosheets + CD | HMTA/H2O | 120 °C 10 h | 900 °C 90 min in N2 +500 °C 3 h in air | 2.91 mAh/cm2 | 3 mA/cm2 | 250 | [118] |
| NiO | NH4F + urea/H2O + isopropanol | 180 °C 8 h | 3 h in Ar | 171 mAh/g | 100 mA/g | 60 | [94] |
| MnO2 | -/H2O + EG | 3 h stirring + 30 min ultrasonication | 350 °C 40 min | 648 mAh/g | 100 mA/g | 150 | [90] |
| RuO2 nanoparticles | - | Soaking | 500 °C 15 min in air | 1 mAh/g | 1 mA/cm2 | 3500 h | [89] |
| TiO2 + Fe2O3 nanotube arrays | I (glycerinum/EtOH) + II (ammonia/H2O) | I (175 °C 20 h) + II (95 °C 4 h) | I (350 °C 1 h in N2) + II (450 °C 2 h) | 896 mAh/g | 200 mA/g | 200 | [111] |
| TiO2 arrays | Glycerinum/EtOH | 180 °C 4 h | - | 188 mAh/g | 0.2 C | 500 | [99] |
| MoS2 + Fe 3 nanosheets | I (l-cysteine + glucose/H2O + DMF) + II (HMTA; ammonia/H2O) | I (200 °C 12 h) + II (85 °C 24 h) | I (700 °C 2 h in Ar) + II (self-sacrificing template + 550 °C 5 h in air) | 1162.3 mAh/g | 0.5 A/g | 200 | [131] |
| VO2 nanobelt arrays | H2O2 (30%) + oxalic acid/H2O + EtOH | 180 °C 3 h | - | 130 mAh/g | 1000 mA/g | 200 | [145] |
| V2O5 nanoflake arrays | H2O2 (30%) + oxalic acid/H2O + EtOH | 180 °C 3 h | - | 230 mAh/g | 2000 mA/g | 300 | [146] |
| Co3O4 nanoparticles + graphene | -/H2O + EtOH | 180 °C 2 h | 700 °C 2 h in Ar + 250 °C 2 h in air | 391 mAh/g | 100 mA/g | 300 | [113] |
| Co3O4 + ZnO nanosheets | Urea + CTAB/H2O + EtOH + EG | 120 °C 10 h | 450 °C 2 h in Ar + 200 °C 8 h in air | 1102.5 mAh/g | 0.5 A/g | 400 | [85] |
| ZnCo2O4 urchins | Urea/H2O | 200 °C 12 h | 400 °C 2 h | 1180 mAh/g | 0.2 C | 100 | [84] |
| NiCo2O4 nanowire arrays | Urea + NH4F/H2O | 100 °C 10 h | 400 °C 2 h in N2 | 1085.5 mAh/g | 500 mA/g | 100 | [86] |
| ZnCo2O4 | Urea + NH4F/H2O | 120 °C 5 h | 400 °C 2 h in air | 787.2 mAh/g | 100 mA/g | 150 | [147] |
| Mn3O4 | Oxalic acid/H2O | 200 °C 10 h | 400 °C 3 h in air | 610.5 mAh/g | 100 mA/g | 150 | [148] |
| MOF Precursor | TMO | MOF Obtention | Heat Treatment | Capacitance/ Capacity | Current Density | Capacity Retention | Cycles | Electrolyte | Reference |
|---|---|---|---|---|---|---|---|---|---|
| ZIF-67 | Co3O4 nanosheets with O vacancies | Coprecipitation + reduction with NaBH4 | 500 °C 30 min in N2 + 2 h 350 °C 2 h in air | 414 C/g | 1 A/g | 73.9% | 15,000 | LiOH 2 M | [167] |
| ZIF-67 | S-Co3O4 polyhedrons | Coprecipitation | 2.5 h in autoclave with TAA solution | 970 mF/cm2 | 5 mA/cm2 | 67.7% | 5000 | KOH 2 M | [190] |
| Co(2-mIM) MOF | Co3O4 nanosheets | Coprecipitation | 500 °C 1 h in N2 + 350 °C 2 h in air | 12.731 mAh/g | 2 mA/cm2 | 89.6% | 2000 | KOH 3 M | [175] |
| CoZn(2-mIM) MOF nanosheets | ZnCo2O4 nanosheets | Coprecipitation | 500 °C 1 h in N2 + 350 °C 2 h in air | 40.741 mAh/g | 2 mA/cm2 | 95.1% | 2000 | KOH 3 M | [175] |
| Co(2-mIM) MOF nanosheets | NiCo2O4 nanosheets | Coprecipitation + ion exchange (Ni) | 350 °C 2 h in air | 1055.3 F/g | 2.5 mA/cm2 | - | - | KOH 2 M | [177] |
| ZIF-L | Ni-doped Co2N nanosheets | Coprecipitation + ion exchange (Ni) | 350 °C 2 h in air + 350 °C 2 h in NH3 | 361.93 C/g (Ni-doped Co2N) | 2 mA/cm2 (Ni-doped Co2N) | 82.4% (Ni-doped Co2N) | 5000 (Ni-doped Co2N) | KOH 1 M | [174] |
| Co(2-mIM) MOF | P-Co3O4 nanosheets | Coprecipitation | 700 °C 2 h in Ar/H2 + 350 °C 2 h in air + 350 °C 2 h in air with NaH2PO2 | 337 mF/cm2 | 1 A/cm2 | 97.6% | 10,000 | KOH 2 M | [166] |
| CoZn(2-mIM) MOF nanosheets | ZnCo2O4 nanosheets | Coprecipitation + ion exchange (Zn) | 350 °C 2 h in air | 536.8 mF/cm2 | 2.5 mA/cm2 | - | - | KOH 2 M | [176] |
| CoMn(2-mIM) MOF | MnCo2O4 nanosheets | Coprecipitation + ion exchange (Mn) | 350 °C 2 h in air | 515 mF/cm2 | 2.5 mA/cm2 | 95.5% | 20,000 | KOH 2 M | [176] |
| Co(2-mIM) MOF | Co3O4 nanosheets | Coprecipitation | 500 °C 1 h in N2 + 350 °C 2 h in air | 225 mF/cm2 | 10 mA/cm2 | - | - | KOH 2 M | [178] |
| Co(2-mIM) MOF | P-MnCo2O4 nanotube arrays | Coprecipitation + ion exchange | 350 °C 2 h in air with NaH2PO2 | 996.7 F/g | 1 A/g | 85.7% | 3000 | KOH 6 M | [191] |
| ZIF-67 | Co3O4 nanosheets | Coprecipitation | 290 °C 1 h in air | 251 F/g | 1 A/g | 90% | 5000 | KOH 1 M | [192] |
| ZIF-67 | Walnut-like CoO nanosheets | Coprecipitation | 250 °C 2 h in air | 842 F/g | 1 A/g | 96.4 | 10,000 | KOH 6 M | [193] |
| MOF Precursor | TMO | MOF Obtention | Heat-Treatment | Reversible Capacity | Current Density | Cycles | Reference |
|---|---|---|---|---|---|---|---|
| Co(2-mIM) MOF | Co3O4 nanosheets | Coprecipitation | 500 °C 1 h in air | 3.1 mAh/cm2 | 0.5 mA/cm2 | 100 | [169] |
| CoZn(2-mIM) ZIF | ZnCo2O4 nanosheets | Coprecipitation | 450 °C 2 h in air | 3.01 mAh/cm2 | 0.24 mA/cm2 | 100 | [194] |
| Co(2-mIM) ZIF | Co3O4 nanosheets | Coprecipitation | 450 °C 2 h in air | 1.93 mAh/cm2 | 0.24 mA/cm2 | 100 | [194] |
| CoZn(2-mIM) MOF | ZnCo2O4 nanosheets | Coprecipitation + ion exchange | 350 °C 2 h in air | 1376 mAh/g | 1 A/g | 200 | [176] |
| CoMn(2-mIM) MOF | MnCo2O4 nanosheets | Coprecipitation + ion exchange | 350 °C 2 h in air | 1289 mAh/g | 1 A/g | 200 | [176] |
| Co(2-mIM) MOF | ZnCo2O4 polyhedrons | Coprecipitation | 400 °C 3 h in N2 | 463 mAh/g | 50 mA/g | 100 | [164] |
| Co(2-mIM) MOF | Co3O4 nanosheets | Coprecipitation | 300 °C 1 h in air | - | - | - | [195] |
| Zn(2-mIM) MOF | ZnO nanosheets | Coprecipitation | 300 °C 1 h in air | - | - | - | [195] |
| ZIF 67 | Co3O4 polyhedrons | Coprecipitation | 400 °C 1 h in air | 420 mAh/g | 100 mA/g | 300 | [165] |
| ZIF-8 | ZnO polyhedrons | Coprecipitation | 450 °C 1 h in air | 510 mA/g | 100 mA/g | 300 | [196] |
| Material | ECD Electrolyte | Current Density/ Potential/ CV Potential Window | Time/Cycles (Scan Rate) | Capacitance | Current Density | Capacitance Retention | Cycles | Electrolyte | Reference |
|---|---|---|---|---|---|---|---|---|---|
| VG/MnO2 nanoflowers | Na2SO4 + MnSO4 | 0.4 mA/cm2 | 40 min | 30.7 mF/cm2 | 0.5 mA/cm2 | 93.8 | 2000 | Na2SO4 1 M | [200] |
| Silane-treated MnO2 | Mn(CH3CO2)2 | 1 mA/cm2 | 10 min | 5.68 mF/cm2 | 2.5 µA/cm2 | 92 | 5000 | EP-IL | [211] |
| AgCNTs/MnO2 nanosheets | Na2SO4 + MnSO4 | −1.8 V | 10 min | 325 F/g | 1 A/g | 82.2 | 5000 | Na2SO4 0.5 M | [199] |
| MnO2/PEDOT | Mn(CH3CO2)2*4H2O +PEDOT:PSS | 7.0 V | - | 386 mF/cm2 | 1 mA/cm2 | 98.4 | 1800 | LiCl 1 M | [201] |
| Fe2O3 | Fe(NO3)2*9H2O + Na2SO4 | 7.0 V | - | 315 mF/cm2 | 1 mA/cm2 | 94.6 | 10,000 | LiCl 1 M | [201] |
| V2O5 | VOSO4*xH2O | 1.2 V | 30 min | 354 mF/g | 1 mA/cm2 | - | - | Na2SO4 1 M | [203] |
| Co9S8/NiCo2O4 core–shell nanoneedles | Co(NO3)2 + Ni(NO3)2 | −0.6 → −1.2 V | 5 (20 mV/s) | 1022.5 F/g | 1 A/g | 88.9 | 6000 | KOH 6 M | [197] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Banciella, A.; Martinez-Diaz, D.; Sánchez, M.; Ureña, A. Nanostructured Transition Metal Oxides on Carbon Fibers for Supercapacitor and Li-Ion Battery Electrodes: An Overview. Int. J. Mol. Sci. 2024, 25, 8514. https://doi.org/10.3390/ijms25158514
González-Banciella A, Martinez-Diaz D, Sánchez M, Ureña A. Nanostructured Transition Metal Oxides on Carbon Fibers for Supercapacitor and Li-Ion Battery Electrodes: An Overview. International Journal of Molecular Sciences. 2024; 25(15):8514. https://doi.org/10.3390/ijms25158514
Chicago/Turabian StyleGonzález-Banciella, Andrés, David Martinez-Diaz, María Sánchez, and Alejandro Ureña. 2024. "Nanostructured Transition Metal Oxides on Carbon Fibers for Supercapacitor and Li-Ion Battery Electrodes: An Overview" International Journal of Molecular Sciences 25, no. 15: 8514. https://doi.org/10.3390/ijms25158514
APA StyleGonzález-Banciella, A., Martinez-Diaz, D., Sánchez, M., & Ureña, A. (2024). Nanostructured Transition Metal Oxides on Carbon Fibers for Supercapacitor and Li-Ion Battery Electrodes: An Overview. International Journal of Molecular Sciences, 25(15), 8514. https://doi.org/10.3390/ijms25158514








