Biochemical and Structural Characterization of a Novel Psychrophilic Laccase (Multicopper Oxidase) Discovered from Oenococcus oeni 229 (ENOLAB 4002)
Abstract
:1. Introduction
2. Results
2.1. Isolation and Sequence Analysis of the Laccase Gene of the O. oeni 229
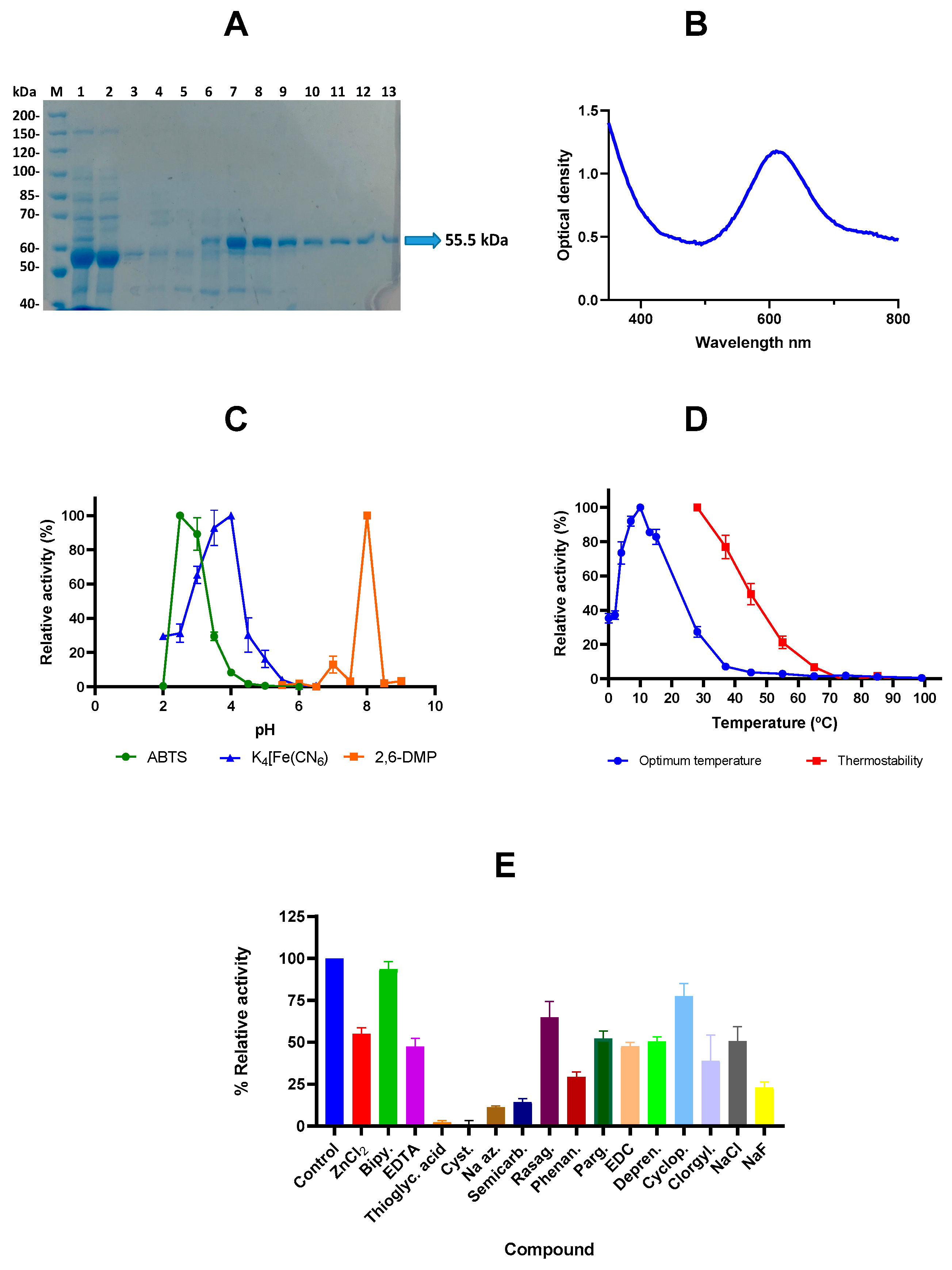
2.2. Cloning, Expression, and Purification and Characterization of the Laccase LcOe 229

2.3. Structure of LcOe 229
2.4. Organization around the Copper Binding Sites of LcOe
2.5. Comparison among LcOe 229 and Other Laccases
3. Discussion
4. Material and Methods
4.1. Materials: Chemicals and DNA and Protein Purification Systems
4.2. Strains, Enzymes, and Plasmids
4.3. Cloning of the Gene Encoding the LcOe 229 Laccase, and Construction of the Expression Plasmid
4.4. Recombinant Laccase Expression and Purification
4.5. Biochemical Characterization of LcOe 229 Laccase
4.6. Oligomeric State Determination by SEC
4.7. Protein Crystallography
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, J.; Wang, L.; Sun, J.; Ju, N.; Jin, G. Malolactic fermentation: New approaches to old problems. Microorganisms 2022, 10, 2363. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Pio, L.E.; Poveda, M.; Alberto, M.R.; Ferrer, S.; Pardo, I. Exploring the biodiversity of two groups of Oenococcus oeni isolated from grape musts and wines: Are they equally diverse? Syst. Appl. Microbiol. 2017, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Maicas, S.; Ferrer, S.; Pardo, I. NAD(P)H regeneration is the key for heterolactic fermentation of hexoses in Oenococcus oeni. Microbiol. 2002, 148, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Akram, F.; Ashraf, S.; Haq, I.U.; Shah, F.I.; Aqeel, A. Eminent industrial and biotechnological applications of laccases from bacterial source: A current overview. Appl. Biochem. Biotechnol. 2022, 194, 2336–2356. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.D.; Ibarra, D.; Eugenio, M.E.; Tomás-Pejó, E. Laccases as versatile enzymes: From industrial uses to novel applications. J. Chem. Technol. Biotechnol. 2019, 95, 481–494. [Google Scholar] [CrossRef]
- Brijwani, K.; Rigdon, A.; Vadlani, P.V. Fungal laccases: Production, function, and applications in food processing. Enzym. Res. 2010, 2010, 149748. [Google Scholar] [CrossRef] [PubMed]
- Khatami, S.H.; Vakili, O.; Movahedpour, A.; Ghesmati, Z.; Ghasemi, H.; Taheri-Anganeh, M. Laccase: Various types and applications. Biotechnol. Appl. Biochem. 2022, 69, 2658–2672. [Google Scholar] [CrossRef] [PubMed]
- Giovanelli, G.; Ravasini, G. Apple juice stabilization by combined enzyme—Membrane filtration process. LWT Food Sci. Technol. 1993, 26, 1–7. [Google Scholar] [CrossRef]
- Benucci, I.; Mazzocchi, C.; Lombardelli, C.; Esti, M. Phenolic-degrading enzymes: Effect on haze active phenols and chill haze in India pale ale beer. Foods 2023, 12, 77. [Google Scholar] [CrossRef]
- Callejón, S.; Sendra, R.; Ferrer, S.; Pardo, I. Recombinant laccase from Pediococcus acidilactici CECT 5930 with ability to degrade tyramine. PLoS ONE 2017, 12, e0186019. [Google Scholar] [CrossRef]
- Olmeda, I.; Casino, P.; Collins, R.E.; Sendra, R.; Callejón, S.; Huesa, J.; Soares, A.S.; Ferrer, S.; Pardo, I. Structural analysis and biochemical properties of laccase enzymes from two Pediococcus species. Microb. Biotechnol. 2021, 14, 1026–1043. [Google Scholar] [CrossRef] [PubMed]
- Feller, G.; Gerday, C. Psychrophilic enzymes: Hot topics in cold adaptation. Nat. Rev. Microbiol. 2003, 1, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Adrio, J.L.; Demain, A.L. Microbial enzymes: Tools for biotechnological processes. Biomolecules 2014, 4, 117–139. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Choudhary, G.; Thakur, I.S. Characterization of laccase activity produced by Cryptococcus albidus. Prep. Biochem. Biotechnol. 2012, 42, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Rovati, J.I.; Pajot, H.F.; Ruberto, L.; Mac Cormack, W.; Figueroa, L.I.C. Polyphenolic substrates and dyes degradation by yeasts from 25 de Mayo/King George Island (Antarctica). Yeast 2013, 30, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Roulling, F.; Godin, A.; Cipolla, A.; Collins, T.; Miyazaki, K.; Feller, G. Activity–stability relationships revisited in blue oxidases catalyzing electron transfer at extreme temperatures. Extremophiles 2016, 20, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, K.M.; Twarda-Clapa, A.; Białkowska, A.M. Screening of novel laccase producers—Isolation and characterization of cold-adapted laccase from Kabatiella bupleuri G3 capable of synthetic dye decolorization. Biomolecules 2021, 11, 828. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewska, K.M.; Twarda-Clapa, A.; Białkowska, A.M. Novel cold-adapted recombinant laccase Kblcc1 from Kabatiella bupleuri G3 IBMiP as a green catalyst in biotransformation. Int. J. Mol. Sci. 2021, 22, 9593. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, Y.; Zhang, S.; Lin, X.; Liang, H.; Chen, Y.; Ji, C. Heterologous expression of the Lactobacillus sakei multiple copper oxidase to degrade histamine and tyramine at different environmental conditions. Foods 2022, 11, 3306. [Google Scholar] [CrossRef]
- Bisaccia, M.; Binda, E.; Rosini, E.; Caruso, G.; Dell’Acqua, O.; Azzaro, M.; Laganà, P.; Tedeschi, G.; Maffioli, E.M.; Pollegioni, L.; et al. A novel promising laccase from the psychrotolerant and halotolerant Antarctic marine Halomonas sp. M68 strain. Front. Microbiol. 2023, 14, 1078382. [Google Scholar] [CrossRef]
- Su, J.; Fu, J.; Wang, Q.; Silva, C.; Cavaco-Paulo, A. Laccase: A green catalyst for the biosynthesis of poly-phenols. Crit. Rev. Biotechnol. 2018, 38, 294–307. [Google Scholar] [CrossRef] [PubMed]
- Roulling, F. Extremophilic Cuproxidases: New Pattern of Temperature Adaptation and Function of Their Methionine-Rich Regions. Ph.D. Thesis, University of Liège, Liège, Belgium, 2022. [Google Scholar]
- Pei, J.; Kim, B.-H.; Grishin, N.V. PROMALS3D: A tool for multiple protein sequence and structure alignments. Nucleic Acids Res. 2008, 36, 2295–2300. [Google Scholar] [CrossRef]
- Pei, J.; Tang, M.; Grishin, N.V. PROMALS3D web server for accurate multiple protein sequence and structure alignments. Nucleic Acids Res. 2008, 36, W30–W34. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Goel, R.; Capalash, N. Bacterial laccases. World J. Microbiol. Biotechnol. 2007, 23, 823–832. [Google Scholar] [CrossRef]
- Xie, T.; Liu, Z.; Liu, Q.; Wang, G. Structural insight into the oxidation of sinapic acid by CotA laccase. J. Struct. Biol. 2015, 190, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Kallio, J.P.; Auer, S.; Jänis, J.; Andberg, M.; Kruus, K.; Rouvinen, J.; Koivula, A.; Hakulinen, N. Structure–function studies of a Melanocarpus albomyces laccase suggest a pathway for oxidation of phenolic compounds. J. Mol. Biol. 2009, 392, 895–909. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Blancas, R.; Avelar, M.; Rodriguez-Arteaga, A.; Sinicropi, A.; Rudiño-Piñera, E. The β-hairpin from the Thermus thermophilus HB27 laccase works as a pH-dependent switch to regulate laccase activity. J. Struct. Biol. 2021, 213, 107740. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Roberts, S.A.; McDevitt, S.F.; Weichsel, A.; Wildner, G.F.; Grass, G.B.; Rensing, C.; Montfort, W.R. Crystal structures of multicopper oxidase CueO bound to copper(I) and silver(I): Functional role of a methionine-rich sequence. J. Biol. Chem. 2011, 286, 37849–37857. [Google Scholar] [CrossRef]
- Serrano-Posada, H.; Centeno-Leija, S.; Rojas-Trejo, S.P.; Rodríguez-Almazán, C.; Stojanoff, V.; Rudiño-Piñera, E. X-ray-induced catalytic active-site reduction of a multicopper oxidase: Structural insights into the proton-relay mechanism and O2-reduction states. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 2396–2411. [Google Scholar] [CrossRef]
- Kovaľ, T.; Švecová, L.; Østergaard, L.H.; Skalova, T.; Dušková, J.; Hašek, J.; Kolenko, P.; Fejfarová, K.; Stránský, J.; Trundová, M.; et al. Trp–His covalent adduct in bilirubin oxidase is crucial for effective bilirubin binding but has a minor role in electron transfer. Sci. Rep. 2019, 9, 13700. [Google Scholar] [CrossRef]
- Moser, C.C.; Anderson, J.L.R.; Dutton, P.L. Guidelines for tunneling in enzymes. Biochim. Biophys. Acta 2010, 1797, 1573–1586. [Google Scholar] [CrossRef] [PubMed]
- Kothe, C.I.; Monnet, C.; Irlinger, F.; Virsolvy, M.; Frühling, A.; Neumann-Schaal, M.; Wolf, J.; Renault, P. Halomonas citrativorans sp. nov., Halomonas casei sp. nov. and Halomonas colorata sp. nov., isolated from French cheese rinds. Int. J. Syst. Evol. Microbiol. 2024, 74, 006234. [Google Scholar] [CrossRef] [PubMed]
- Horikoshi, K. General physiology of alkaliphiles. In Extremophiles Handbook; Horikoshi, K., Ed.; Springer: Tokyo, Japan, 2011; pp. 99–118. [Google Scholar] [CrossRef]
- Wang, H.; You, S.; Wang, W.; Zeng, Y.; Su, R.; Qi, W.; Wang, K.; He, Z. Laccase-catalyzed soy protein and gallic acid complexation: Effects on conformational structures and antioxidant activity. Food Chem. 2022, 375, 131865. [Google Scholar] [CrossRef] [PubMed]
- Monroy, I.; Olmeda, I.; Ferrer, S.; Pardo, I. Biochemical characterization of three heterologous lactic acid bacteria laccases from Pediococcus, Lactobacillus, and Lactococcus genus and their potential to degrade biogenic amines using ABTS and epicatechin as mediators. Fermentation 2023, 10, 32. [Google Scholar] [CrossRef]
- Callejón, S.; Sendra, R.; Ferrer, S.; Pardo, I. Cloning and characterization of a new laccase from Lactobacillus plantarum J16 CECT 8944 catalyzing biogenic amines degradation. Appl. Microbiol. Biotechnol. 2016, 100, 3113–3124. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. The rapid generation of mutation data matrices from protein sequences. Bioinformatics 1992, 8, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Georlette, D.; Damien, B.; Blaise, V.; Depiereux, E.; Uversky, V.N.; Gerday, C.; Feller, G. Structural and functional adaptations to extreme temperatures in psychrophilic, mesophilic, and thermophilic DNA ligases. J. Biol. Chem. 2003, 278, 37015–37023. [Google Scholar] [CrossRef] [PubMed]
- Collins, T.; Meuwis, M.-A.; Gerday, C.; Feller, G. Activity, stability and flexibility in glycosidases adapted to extreme thermal environments. J. Mol. Biol. 2003, 328, 419–428. [Google Scholar] [CrossRef]
- D’Amico, S.; Marx, J.-C.; Gerday, C.; Feller, G. Activity-stability relationships in extremophilic enzymes. J. Biol. Chem. 2003, 278, 7891–7896. [Google Scholar] [CrossRef]
- Caspritz, G.; Radler, F. Malolactic enzyme of Lactobacillus plantarum. Purification, properties, and distribution among bacteria. J. Biol. Chem. 1983, 258, 4907–4910. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.G.; Ayling, A.; Baneyx, F. Molecular chaperones, folding catalysts, and the recovery of active recombinant proteins from E. coli. To fold or to refold. Appl. Biochem. Biotechnol. 1997, 66, 197–238. [Google Scholar] [CrossRef] [PubMed]
- Kabsch, W. XDS. Acta Crystallogr. Sect. D Struct. Biol. 2010, 66, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.W.; McCoy, A.; et al. Overview of the CCP4 suite and current developments. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011, 67, 235–242. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007, 40, 658–674. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef]
- Murshudov, G.N.; Skubák, P.; Lebedev, A.A.; Pannu, N.S.; Steiner, R.A.; Nicholls, R.A.; Winn, M.D.; Long, F.; Vagin, A.A. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011, 67, 355–367. [Google Scholar] [CrossRef]


| Substrate | Km (mM) | Vmax (U/mg) | Kcat (s−1) | Kcat/Km (mM−1 s−1) |
|---|---|---|---|---|
| ABTS | 0.07 (±0.00) a | 12.06 (±0.39) | 11.20 (±0.38) | 161.67 |
| 2,6-DMP | 0.73 (±0.17) | 0.01 (±0.00) | 0.01 (±0.00) | 0.01 |
| Ferrocyanide | 1.14 (±0.14) | 11.10 (±1.16) | 10.27 (±1.07) | 9.02 |
| Histag-LcOe 229 | LcOe 229 | |
|---|---|---|
| Data collection | ||
| Space group | C 1 2 1 | P 1 2 1 |
| Cell dimensions | ||
| a, b, c (Å) | 206.26, 56.69, 106.05 | 52.75, 97.96, 96.34 |
| α, β, γ (°) | 90.00, 118.03, 90.00 | 90.00, 91.61, 90.00 |
| Resolution (Å) | 93.61–3.50 (3.83–3.50) | 68.55–3.00 (3.18–3.00) |
| No. reflections | 93,984 (22,297) | 90,396 (14,384) |
| Rsym or Rmerge | 0.419 (1.135) | 0.273 (0.715) |
| Rpim | 0.262 (0.715) | 0.199 (0.532) |
| I/σI | 4.8 (2.0) | 4.4 (2.3) |
| Completeness (%) | 99.9 (100) | 97.8 (97.8) |
| Refinement | ||
| Rwork/Rfree | 0.21/0.26 | 0.21/0.26 |
| No. atoms | ||
| Protein | 7348 | 7405 |
| Ligand/Ion | 8 | 8 |
| Water | 11 | 74 |
| B-factors | ||
| Protein | 67.1 | 20.88 |
| Ligand/Ion | 94.29 | 24.6 |
| Water | 38.54 | 6.88 |
| R.m.s deviations | ||
| Bond lengths (Å) | 0.004 | 0.005 |
| Bond Angles (°) | 1.067 | 1.132 |
| PDB Code | 9F3Z | 9F1T |
| Organism Type | Laccase | pI | Mw (Da) | Enzymatic Optimal T (°C) | Activity at 20 °C (%) | Total Aas | M | G | P | R | C | D | E | N | Q | Aliphatic Index |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | % | % | % | % | ||||||||
| Antarctic Gram− | Hmer-R1t3 | 5.70 | 67373.47 | ?? | 607 | 5.9 | 10.0 | 5.6 | 7.2 | 0.2 | 5.8 | 6.9 | 3.1 | 3.5 | 75.1 | |
| Hpie-resA | 5.65 | 67402.35 | ?? | 607 | 6.1 | 10.2 | 5.4 | 7.2 | 0.2 | 5.4 | 7.2 | 3.3 | 3.6 | 72.9 | ||
| Hcol-FME66 | 5.51 | 67397.35 | ?? | 607 | 6.3 | 10.2 | 5.6 | 7.2 | 0.2 | 5.6 | 7.1 | 3.5 | 3.6 | 72.5 | ||
| PhaMOx | 5.47 | 64212.83 | 35 | 576 | 6.4 | 7.5 | 5.4 | 5.2 | 0.2 | 8.0 | 4.5 | 4.3 | 3.5 | 75.3 | ||
| Mesophylic Gram− | Ecol-6898 | 6.07 | 53420.43 | 55 | 488 | 5.7 | 10.2 | 6.8 | 7.8 | 0.6 | 6.1 | 4.3 | 3.5 | 3.6 | 83.1 | |
| Mesophilic Gram+ | Bsub-6077 | 5.91 | 58498.99 | 75 | 513 | 1.6 | 6.6 | 9.4 | 5.7 | 0.8 | 6.6 | 7.2 | 3.8 | 2.5 | 77.9 | |
| LAB Gram+ | Ppar-3909 | 5.11 | 58216.56 | 28 | 93 | 511 | 4.1 | 7.6 | 6.8 | 3.1 | 1.0 | 9.2 | 5.9 | 4.3 | 4.1 | 71.1 |
| Llac-5298 | 5.32 | 58613.52 | 28 | 86 | 520 | 4.2 | 7.9 | 7.9 | 3.7 | 0.8 | 8.1 | 5.8 | 3.1 | 3.5 | 74.2 | |
| Ppen-4816 | 5.37 | 57312.97 | 55 | 25 | 509 | 3.9 | 7.9 | 7.9 | 3.7 | 0.8 | 8.3 | 5.5 | 2.9 | 3.7 | 73.7 | |
| Lpar-4314 | 5.37 | 57298.94 | 28 | 57 | 509 | 3.9 | 7.9 | 7.9 | 3.7 | 0.8 | 8.4 | 5.3 | 2.9 | 3.7 | 73.7 | |
| Lpla-J16 | 4.93 | 56875.9 | 60 | 20 | 501 | 3.4 | 7.0 | 8.0 | 4.4 | 1.0 | 10.2 | 5.4 | 3.8 | 4.4 | 71.5 | |
| Paci-5930 | 5.42 | 54361.44 | 28 | 95 | 477 | 3.1 | 7.8 | 7.1 | 4.0 | 0.8 | 7.8 | 6.1 | 5.7 | 4.2 | 79.5 | |
| Lsak-LS | 5.37 | 57327.00 | 25 | 84 | 509 | 3.9 | 7.9 | 7.9 | 3.7 | 0.8 | 8.3 | 5.5 | 2.5 | 3.1 | 73.9 | |
| Ooen-2291 | 5.70 | 55544.12 | 10 | 70 | 488 | 4.1 | 7.4 | 6.6 | 4.3 | 0.8 | 8.4 | 5.3 | 4.8 | 2.6 | 80.5 | |
| Thermophylic Gram− | Tth-MCO | 7.09 | 48855.73 | 95 | 440 | 3.0 | 8.4 | 10.0 | 7.3 | 0.2 | 4.1 | 6.8 | 1.6 | 2.3 | 96.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olmeda, I.; Paredes-Martínez, F.; Sendra, R.; Casino, P.; Pardo, I.; Ferrer, S. Biochemical and Structural Characterization of a Novel Psychrophilic Laccase (Multicopper Oxidase) Discovered from Oenococcus oeni 229 (ENOLAB 4002). Int. J. Mol. Sci. 2024, 25, 8521. https://doi.org/10.3390/ijms25158521
Olmeda I, Paredes-Martínez F, Sendra R, Casino P, Pardo I, Ferrer S. Biochemical and Structural Characterization of a Novel Psychrophilic Laccase (Multicopper Oxidase) Discovered from Oenococcus oeni 229 (ENOLAB 4002). International Journal of Molecular Sciences. 2024; 25(15):8521. https://doi.org/10.3390/ijms25158521
Chicago/Turabian StyleOlmeda, Isidoro, Francisco Paredes-Martínez, Ramón Sendra, Patricia Casino, Isabel Pardo, and Sergi Ferrer. 2024. "Biochemical and Structural Characterization of a Novel Psychrophilic Laccase (Multicopper Oxidase) Discovered from Oenococcus oeni 229 (ENOLAB 4002)" International Journal of Molecular Sciences 25, no. 15: 8521. https://doi.org/10.3390/ijms25158521





