Nanocellulose-Based Materials for Water Pollutant Removal: A Review
Abstract
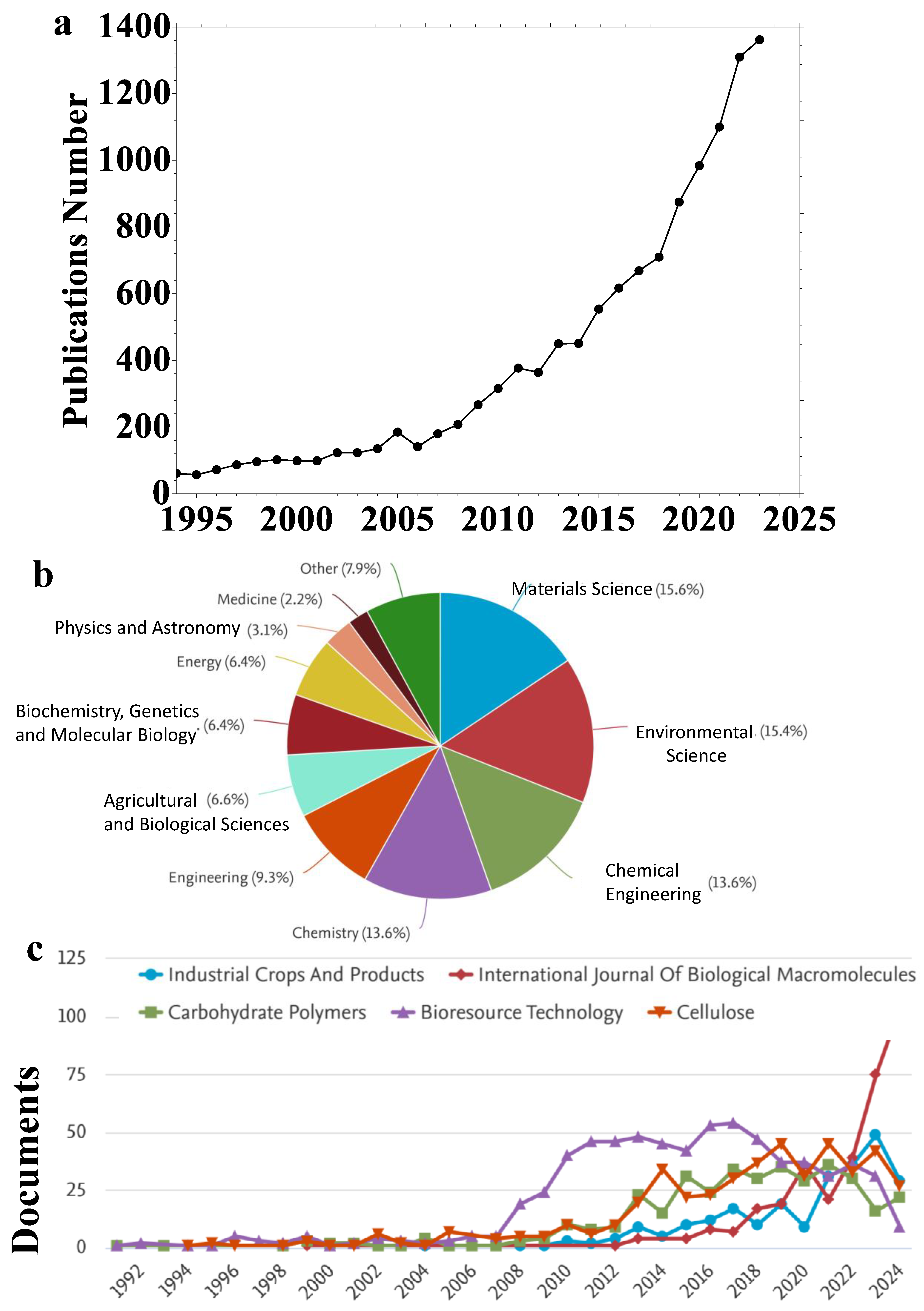
1. Introduction
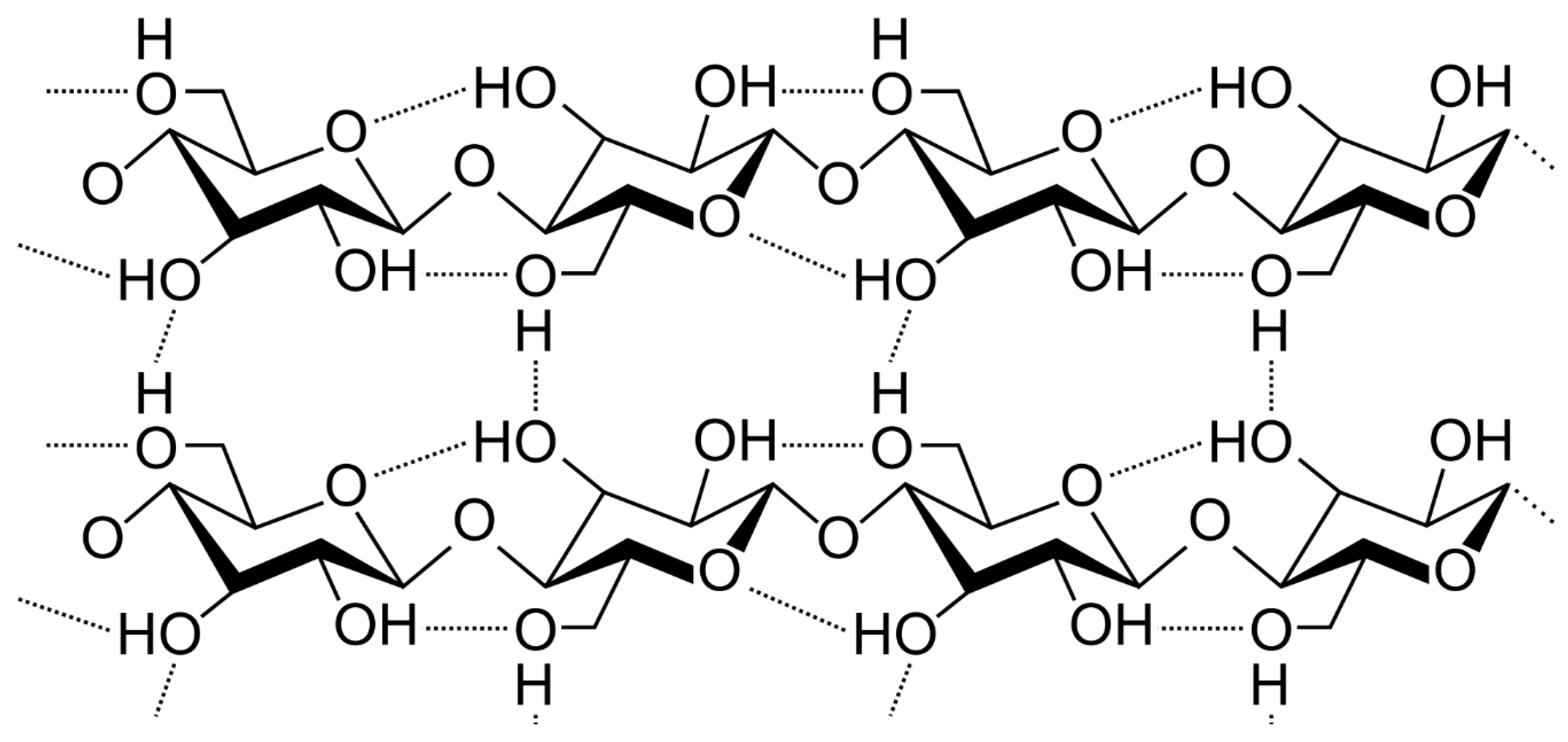
2. Chemical Structure of Cellulose
3. Nanotechnology and Nanocellulose
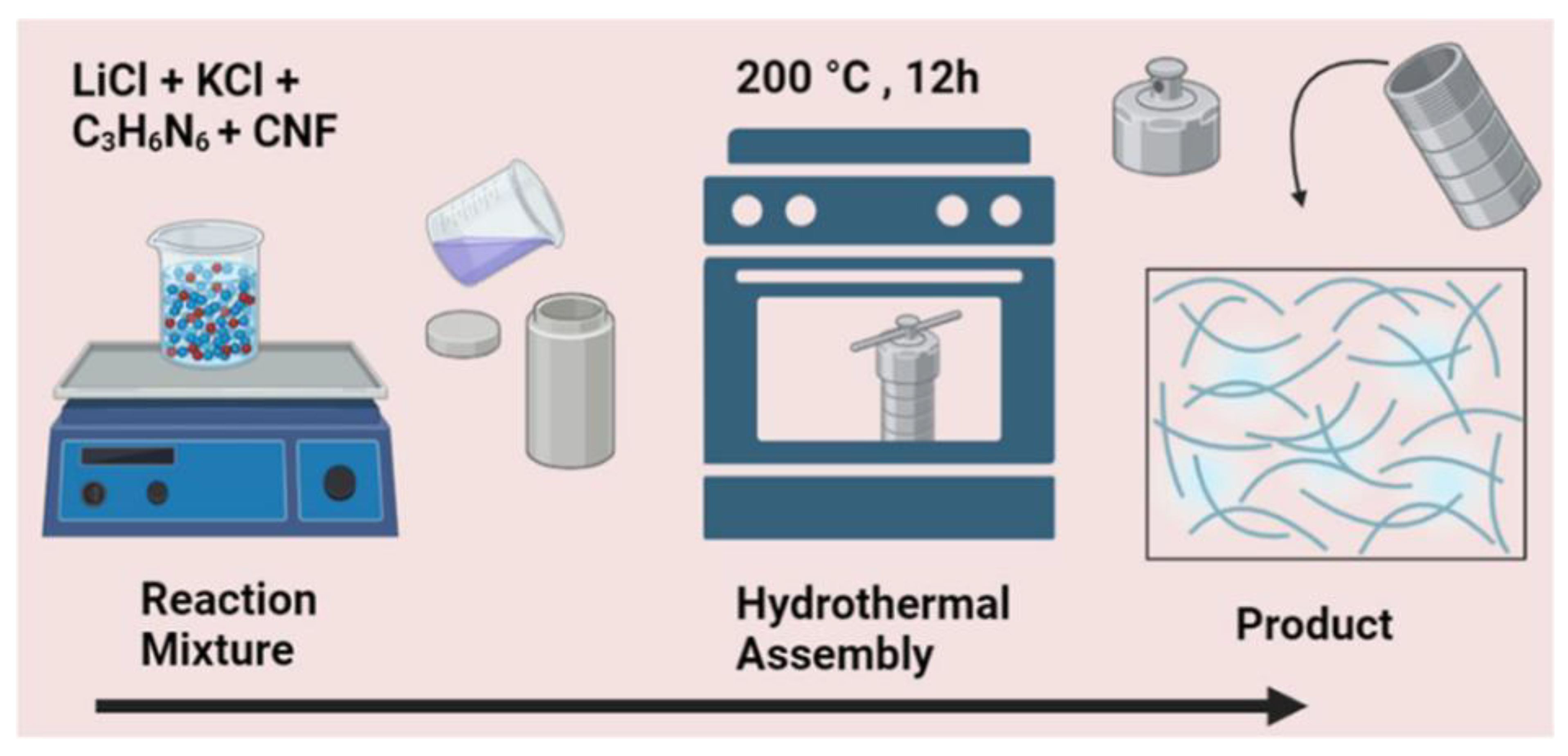
4. Production Methods for Nanocellulose
4.1. Acid Hydrolysis
4.2. Mechanical Method
4.3. Biological Methods
5. Nanocellulose-Based Composites
6. Applications of Nanocellulose for Pollutant Removal
6.1. Removal of Heavy Metal Ions
| Materials | Prepared Methods | Source | Pollutants | Techniques | Conditions | Capacity (mg/g) | Efficiency (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| NOCNF | Acid hydrolysis | Grass from Australia, named 104 spinifex | Cd2+ | Adsorption | Cd2+ concentration of 250 ppm, 25 °C | 2550 | 84 | [38] |
| CNF@C3N4 | Salt melt approach | Commercial | Ni2+ and Cu2+ | Separation | Adsorbent, 500 mg; pH of 6.0, 100 mL of the solution containing metal ions | 340 385 | 99.5 99.2 | [54] |
| MIP-NC | Acid hydrolysis; reversed-phase microemulsion method | Cotton wool | Pb2+ Hg2+ | Adsorption | pH 6, | 27.55 161.31 | [88] | |
| CE-SPES | Base and acid extraction; stirring | Rice waste | Zn2+ | Adsorption | Adsorbent, 0.02 g, pH = 5; 30 min, T = 30 °C for SPES; and Zn2+ concentration = 2 ppm | 5.96 | 80.4–96.08 | [89] |
| Cellulose methacrylate hydrogels | Extraction self-crosslinking thiol-ene click chemistry | Wood | Dyes: methyl blue and methylene blue | Adsorption | Hydrogel, 20 mg; dye, 1000 mg·L−1; 25 °C | 934.63 mg·g−1 706.64 mg·g−1 | 98.15–90.90 | [113] |
| Ag/lignin/cellulose | Freezing-thawing process | Commercial | dyes, pollutants, and antibiotics | Catalytic reduction | 2 mM NaBH4 (75 mg) or 100 mg of studied aerogels, 25 mg/L MB solution, 30 min | 99.8–99.9 | [114] | |
| MCNC | Hydrothermal | Commercial | DOX | Adsorption | 0.1 g of samples, 100 mL of DOX solution (20 mg/L) at pH 7, 3 h at 300 rpm shaking | 61.2 mg/m2 | 70 | [115] |
| Sr/Alg/CMC/GO/TiO2 | Hummers method freeze drying processes; cross-linking | Commercial | Dye: Congo red | Photocatalysis | 1.2 g/L of photocatalyst, 30 mL of aqueous solution of CR dye (30 mg/L), for 2 min and stirred for 30 min under dark conditions, 900 W/m2 | 98% | 240 | [116] |
| CNC | Cotton pulp | Acid hydrolysis | L = 90 ± 10 nm, diameter, D = 8 ± 1 nm and zeta potential of 51.5 ± 0.8 mV | Bacteria | Pseudomanas aeruginosa | Flocculation | P. aeruginosa suspension, 1.0 × 108 cells/mL in 10 mM NaCl solution, bacteria to CNC ratios of 1:100,000, 24 h | [117] |
| Imidazolyl CNCs | Cotton wool | Acid hydrolysis | DS, 0.06; pH adjusted to 10.8 using sodium carbonate (ζ = −16.1 ± 1.3 mV). pH = 5.7 and ζ = 9.9 ± 0.8 mV | microalgae | Chlorella Vulgaris | Flocculation | 200 mg L−1 dose, 2–3% CO2, biomass concentration of 0.35 g L−1 | [118] |
6.2. Removal Anionic Species
6.3. Desalination: Salts Rejection
6.4. Removal Organic-Based Pollutants: Adsorption and Catalysis
6.5. Bacteria Removal from Wastewater
6.6. Nanocellulose for Oil Removal
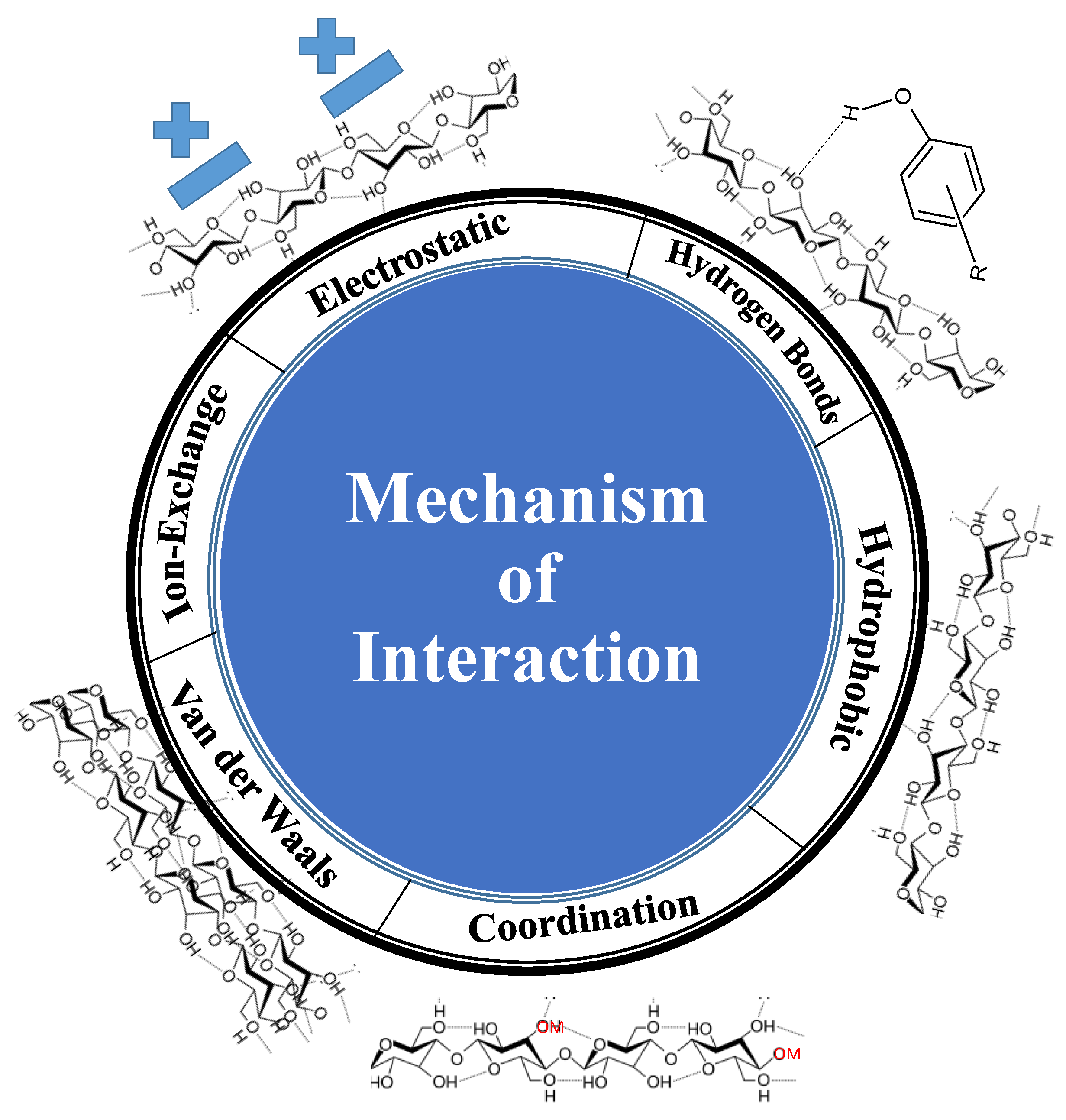
7. Key Parameters Affecting Adsorption and Mechanism of Pollutant Removal
7.1. Functional Groups
7.2. Chemical Compositions
7.3. Synthesis Procedure
7.4. The Solution’s pH Values
8. Advantages and Challenges
9. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, S.; Shi, W.; Li, K.; Cai, J.; Chen, L. Recent advances on sustainable bio-based materials for water treatment: Fabrication, modification and application. J. Environ. Chem. Eng. 2022, 10, 108921. [Google Scholar] [CrossRef]
- Zanaty, M.; Zaki, A.H.; El-Dek, S.I.; Abdelhamid, H.N. Zeolitic imidazolate framework@hydrogen titanate nanotubes for efficient adsorption and catalytic oxidation of organic dyes and microplastics. J. Environ. Chem. Eng. 2024, 12, 112547. [Google Scholar] [CrossRef]
- Pérez, J.; Muñoz-Dorado, J.; de la Rubia, T.; Martínez, J. Biodegradation and biological treatments of cellulose, hemicellulose and lignin: An overview. Int. Microbiol. 2002, 5, 53–63. [Google Scholar] [CrossRef]
- Saito, T.; Nishiyama, Y.; Putaux, J.-L.; Vignon, M.; Isogai, A. Homogeneous Suspensions of Individualized Microfibrils from TEMPO-Catalyzed Oxidation of Native Cellulose. Biomacromolecules 2006, 7, 1687–1691. [Google Scholar] [CrossRef]
- Pääkkö, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic Hydrolysis Combined with Mechanical Shearing and High-Pressure Homogenization for Nanoscale Cellulose Fibrils and Strong Gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose-Based Nanomaterials Advance Biomedicine: A Review. Int. J. Mol. Sci. 2022, 23, 5405. [Google Scholar] [CrossRef]
- Etale, A.; Onyianta, A.J.; Turner, S.R.; Eichhorn, S.J. Cellulose: A Review of Water Interactions, Applications in Composites, and Water Treatment. Chem. Rev. 2023, 123, 2016–2048. [Google Scholar] [CrossRef]
- Goswami, R.; Singh, S.; Narasimhappa, P.; Ramamurthy, P.C.; Mishra, A.; Mishra, P.K.; Joshi, H.C.; Pant, G.; Singh, J.; Kumar, G.; et al. Nanocellulose: A comprehensive review investigating its potential as an innovative material for water remediation. Int. J. Biol. Macromol. 2024, 254, 127465. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, N.; Grishkewich, N.; Tam, K.C. Cellulose nanomaterials: Promising sustainable nanomaterials for application in water/wastewater treatment processes. Environ. Sci. Nano 2018, 5, 623–658. [Google Scholar] [CrossRef]
- Jing, L.; Shi, T.; Chang, Y.; Meng, X.; He, S.; Xu, H.; Yang, S.; Liu, J. Cellulose-based materials in environmental protection: A scientometric and visual analysis review. Sci. Total Environ. 2024, 929, 172576. [Google Scholar] [CrossRef]
- Das, R.; Lindström, T.; Sharma, P.R.; Chi, K.; Hsiao, B.S. Nanocellulose for Sustainable Water Purification. Chem. Rev. 2022, 122, 8936–9031. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, Z.; Zheng, Z.; Liu, H.; Zhu, L.; Yang, M.; Chen, Y. Nanocellulose-based membranes for highly efficient molecular separation. Chem. Eng. J. 2023, 451, 138711. [Google Scholar] [CrossRef]
- Payen, A. Mémoire sur la composition du tissu propre des plantes et du ligneux. Comptes Rendus 1838, 1838, 1052–1056. [Google Scholar]
- Niemz, P.; Mai, C.; Schmitt, U. Introduction to Wood Science. In Springer Handbook of Wood Science and Technology; Springer International Publishing: Cham, Switzerland, 2023; pp. 25–40. [Google Scholar]
- Sponsler, O.L.; Dore, W.H. The structure of mercerized cellulose. I. The space lattice of mercerized ramie cellulose as developed from X-ray data 1. J. Am. Chem. Soc. 1928, 50, 1940–1950. [Google Scholar] [CrossRef]
- Sharma, R.; Nath, P.C.; Mohanta, Y.K.; Bhunia, B.; Mishra, B.; Sharma, M.; Suri, S.; Bhaswant, M.; Nayak, P.K.; Sridhar, K. Recent advances in cellulose-based sustainable materials for wastewater treatment: An overview. Int. J. Biol. Macromol. 2024, 256, 128517. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Huang, J.; Dufresne, A. Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: A review. Nanoscale 2012, 4, 3274. [Google Scholar] [CrossRef] [PubMed]
- Radoor, S.; Karayil, J.; Jayakumar, A.; Kandel, D.R.; Kim, J.T.; Siengchin, S.; Lee, J. Recent advances in cellulose- and alginate-based hydrogels for water and wastewater treatment: A review. Carbohydr. Polym. 2024, 323, 121339. [Google Scholar] [CrossRef]
- Marchessault, R.H.; Morehead, F.F.; Walter, N.M. Liquid Crystal Systems from Fibrillar Polysaccharides. Nature 1959, 184, 632–633. [Google Scholar] [CrossRef]
- Revol, J.-F.; Bradford, H.; Giasson, J.; Marchessault, R.H.; Gray, D.G. Helicoidal self-ordering of cellulose microfibrils in aqueous suspension. Int. J. Biol. Macromol. 1992, 14, 170–172. [Google Scholar] [CrossRef]
- Revol, J.-F.; Godbout, L.; Dong, X.-M.; Gray, D.G.; Chanzy, H.; Maret, G. Chiral nematic suspensions of cellulose crystallites; phase separation and magnetic field orientation. Liq. Cryst. 1994, 16, 127–134. [Google Scholar] [CrossRef]
- Kimura, F.; Kimura, T.; Tamura, M.; Hirai, A.; Ikuno, M.; Horii, F. Magnetic Alignment of the Chiral Nematic Phase of a Cellulose Microfibril Suspension. Langmuir 2005, 21, 2034–2037. [Google Scholar] [CrossRef] [PubMed]
- Westerhoff, P.; Alvarez, P.; Li, Q.; Gardea-Torresdey, J.; Zimmerman, J. Overcoming implementation barriers for nanotechnology in drinking water treatment. Environ. Sci. Nano 2016, 3, 1241–1253. [Google Scholar] [CrossRef]
- Tan, K.B.; Vakili, M.; Horri, B.A.; Poh, P.E.; Abdullah, A.Z.; Salamatinia, B. Adsorption of dyes by nanomaterials: Recent developments and adsorption mechanisms. Sep. Purif. Technol. 2015, 150, 229–242. [Google Scholar] [CrossRef]
- Santhosh, C.; Velmurugan, V.; Jacob, G.; Jeong, S.K.; Grace, A.N.; Bhatnagar, A. Role of nanomaterials in water treatment applications: A review. Chem. Eng. J. 2016, 306, 1116–1137. [Google Scholar] [CrossRef]
- Qu, X.; Alvarez, P.J.J.; Li, Q. Applications of nanotechnology in water and wastewater treatment. Water Res. 2013, 47, 3931–3946. [Google Scholar] [CrossRef] [PubMed]
- Wang, D. A critical review of cellulose-based nanomaterials for water purification in industrial processes. Cellulose 2019, 26, 687–701. [Google Scholar] [CrossRef]
- Olivera, S.; Muralidhara, H.B.; Venkatesh, K.; Guna, V.K.; Gopalakrishna, K.; Yogesh Kumar, K. Potential applications of cellulose and chitosan nanoparticles/composites in wastewater treatment: A review. Carbohydr. Polym. 2016, 153, 600–618. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.K.; Voicu, S.I. Recent advances in cellulose and chitosan based membranes for water purification: A concise review. Carbohydr. Polym. 2016, 146, 148–165. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, A.C.; Zhang, M.; Lin, Z. Water treatment via non-membrane inorganic nanoparticles/cellulose composites. Mater. Today 2021, 50, 329–357. [Google Scholar] [CrossRef]
- Qiu, K.; Netravali, A.N. A Review of Fabrication and Applications of Bacterial Cellulose Based Nanocomposites. Polym. Rev. 2014, 54, 598–626. [Google Scholar] [CrossRef]
- Oun, A.A.; Shankar, S.; Rhim, J.-W. Multifunctional nanocellulose/metal and metal oxide nanoparticle hybrid nanomaterials. Crit. Rev. Food Sci. Nutr. 2020, 60, 435–460. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Rodriguez, K.; Renneckar, S.; Vikesland, P.J. Environmental science and engineering applications of nanocellulose-based nanocomposites. Environ. Sci. Nano 2014, 1, 302–316. [Google Scholar] [CrossRef]
- Saud, A.; Saleem, H.; Khan, A.W.; Munira, N.; Khan, M.; Zaidi, S.J. Date Palm Tree Leaf-Derived Cellulose Nanocrystal Incorporated Thin-Film Composite forward Osmosis Membranes for Produced Water Treatment. Membranes 2023, 13, 513. [Google Scholar] [CrossRef] [PubMed]
- Rånby, B.G.; Banderet, A.; Sillén, L.G. Aqueous Colloidal Solutions of Cellulose Micelles. Acta Chem. Scand. 1949, 3, 649–650. [Google Scholar] [CrossRef]
- Turbak, A.K.; Snyder, F.W.; Sandberg, K.R. Microfibrillated Cellulose: Morphology and Accessibility (Conference)|OSTI.GOV. Available online: https://www.osti.gov/biblio/5039044-microfibrillated-cellulose-morphology-accessibility (accessed on 23 July 2020).
- Zimmermann, T.; Pöhler, E.; Geiger, T. Cellulose Fibrils for Polymer Reinforcement. Adv. Eng. Mater. 2004, 6, 754–761. [Google Scholar] [CrossRef]
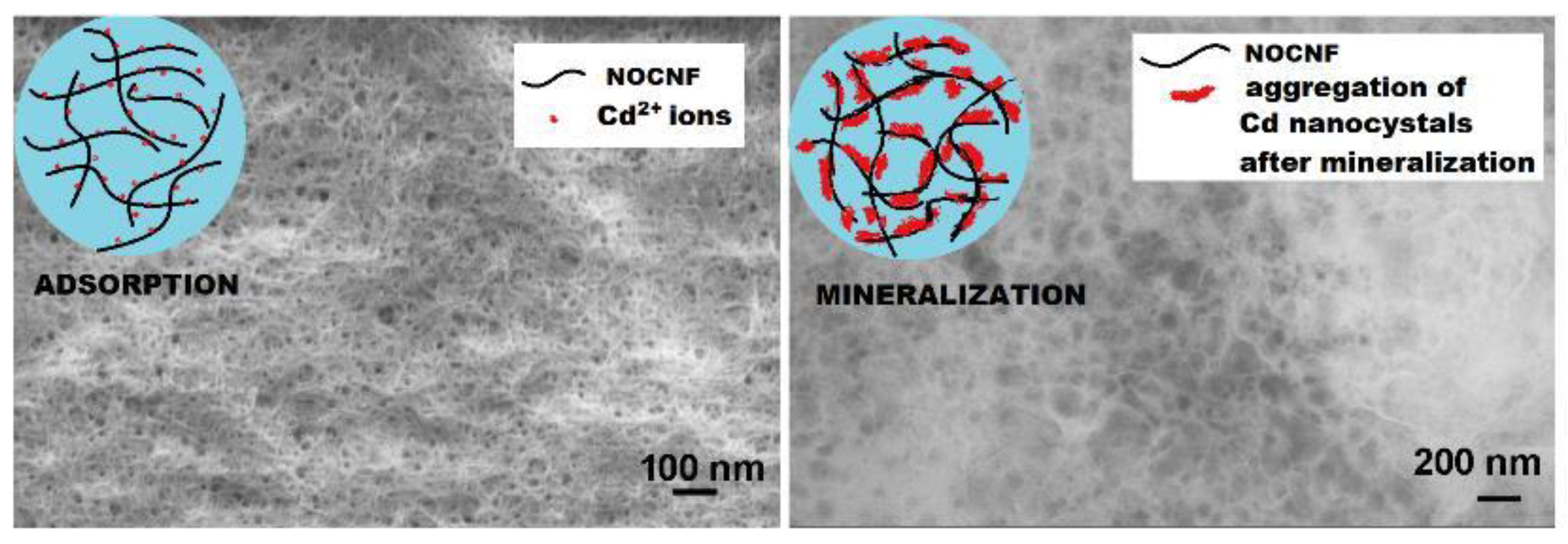
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Geng, L.; Amiralian, N.; Martin, D.; Hsiao, B.S. Nanocellulose from Spinifex as an Effective Adsorbent to Remove Cadmium(II) from Water. ACS Sustain. Chem. Eng. 2018, 6, 3279–3290. [Google Scholar] [CrossRef]
- Onyianta, A.J.; Dorris, M.; Williams, R.L. Aqueous morpholine pre-treatment in cellulose nanofibril (CNF) production: Comparison with carboxymethylation and TEMPO oxidisation pre-treatment methods. Cellulose 2018, 25, 1047–1064. [Google Scholar] [CrossRef]
- Rocha, I.; Ferraz, N.; Mihranyan, A.; Strømme, M.; Lindh, J. Sulfonated nanocellulose beads as potential immunosorbents. Cellulose 2018, 25, 1899–1910. [Google Scholar] [CrossRef]
- dos Santos, D.M.; Leite, I.S.; Bukzem, A.d.L.; de Oliveira Santos, R.P.; Frollini, E.; Inada, N.M.; Campana-Filho, S.P. Nanostructured electrospun nonwovens of poly(ε-caprolactone)/quaternized chitosan for potential biomedical applications. Carbohydr. Polym. 2018, 186, 110–121. [Google Scholar] [CrossRef]
- Laitinen, O.; Suopajärvi, T.; Österberg, M.; Liimatainen, H. Hydrophobic, Superabsorbing Aerogels from Choline Chloride-Based Deep Eutectic Solvent Pretreated and Silylated Cellulose Nanofibrils for Selective Oil Removal. ACS Appl. Mater. Interfaces 2017, 9, 25029–25037. [Google Scholar] [CrossRef]
- Ninomiya, K.; Abe, M.; Tsukegi, T.; Kuroda, K.; Tsuge, Y.; Ogino, C.; Taki, K.; Taima, T.; Saito, J.; Kimizu, M.; et al. Lignocellulose nanofibers prepared by ionic liquid pretreatment and subsequent mechanical nanofibrillation of bagasse powder: Application to esterified bagasse/polypropylene composites. Carbohydr. Polym. 2018, 182, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Nakagaito, A.N.; Yano, H. The effect of morphological changes from pulp fiber towards nano-scale fibrillated cellulose on the mechanical properties of high-strength plant fiber based composites. Appl. Phys. A Mater. Sci. Process. 2004, 78, 547–552. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Henriksson, M.; Henriksson, G.; Berglund, L.A.; Lindström, T. An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur. Polym. J. 2007, 43, 3434–3441. [Google Scholar] [CrossRef]
- Berghem, L.E.R.; Pettersson, L.G. The Mechanism of Enzymatic Cellulose Degradation. Eur. J. Biochem. 1973, 37, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Gatenholm, P.; Klemm, D. Bacterial Nanocellulose as a Renewable Material for Biomedical Applications. MRS Bull. 2010, 35, 208–213. [Google Scholar] [CrossRef]
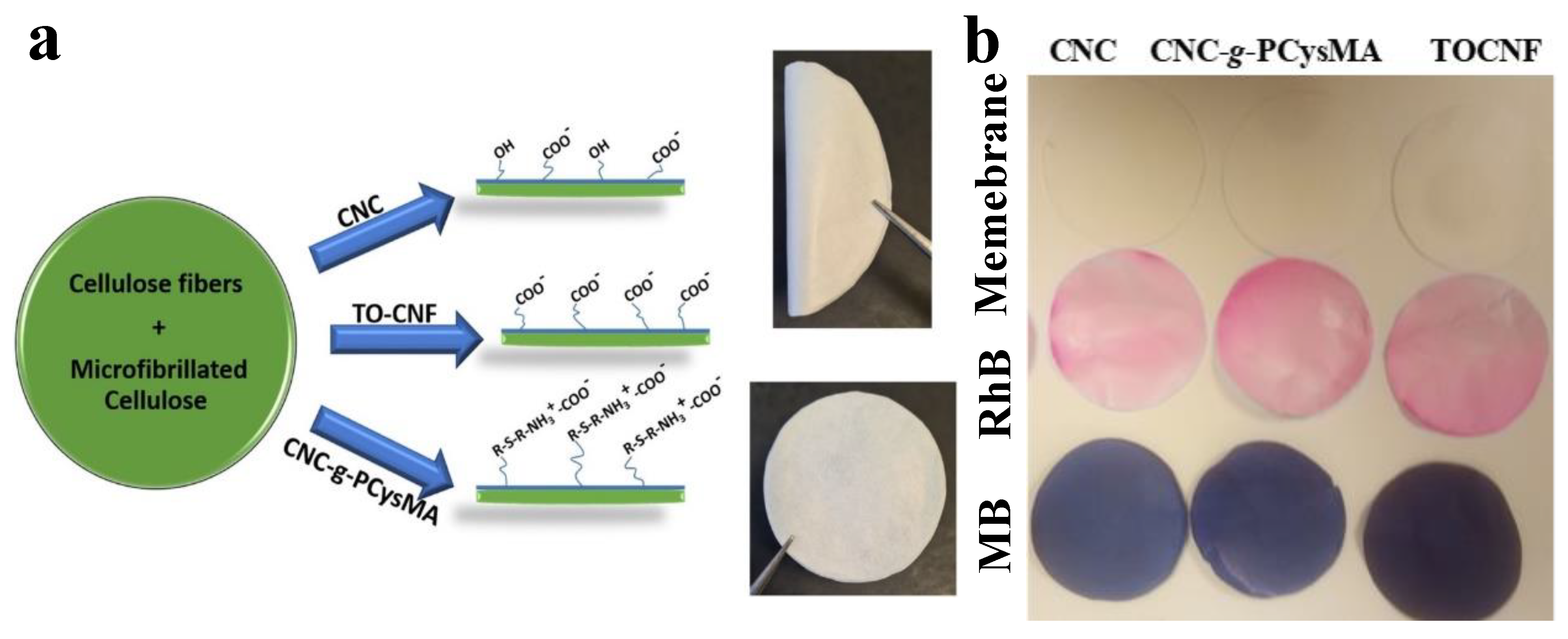
- Georgouvelas, D.; Abdelhamid, H.N.; Li, J.; Edlund, U.; Mathew, A.P. All-cellulose functional membranes for water treatment: Adsorption of metal ions and catalytic decolorization of dyes. Carbohydr. Polym. 2021, 264, 118044. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Sanchez, A.; Jalvo, B.; Mautner, A.; Rissanen, V.; Kontturi, K.S.; Abdelhamid, H.N.; Tammelin, T.; Mathew, A.P. Charged ultrafiltration membranes based on TEMPO-oxidized cellulose nanofibrils/poly(vinyl alcohol) antifouling coating. RSC Adv. 2021, 11, 6859–6868. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Aramesh, N.; Lee, H.K. Chitosan- and/or cellulose-based materials in analytical extraction processes: A review. TrAC Trends Anal. Chem. 2022, 157, 116770. [Google Scholar] [CrossRef]
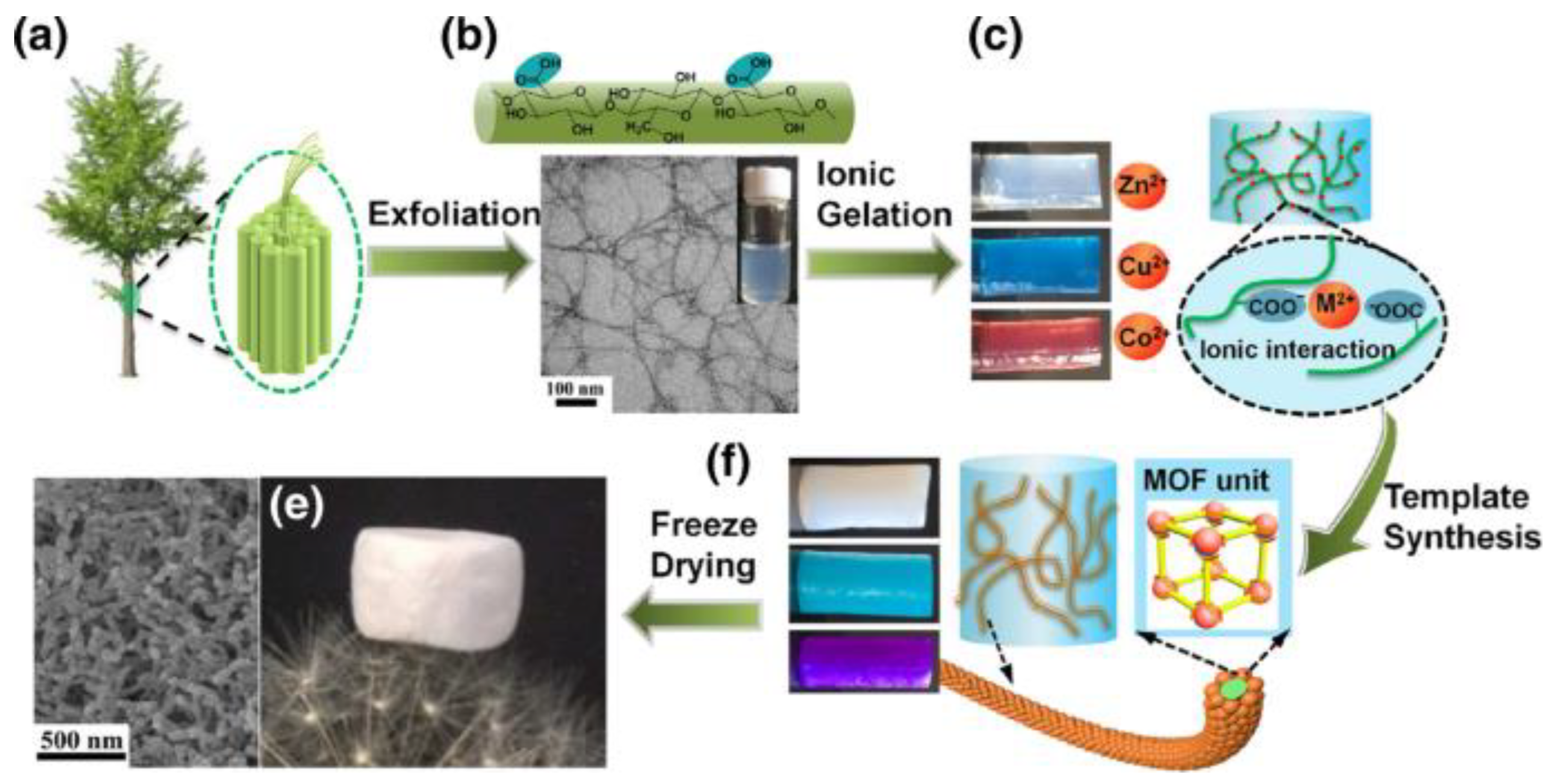
- Georgouvelas, D.; Abdelhamid, H.N.; Edlund, U.; Mathew, A.P. In situ modified nanocellulose/alginate hydrogel composite beads for purifying mining effluents. Nanoscale Adv. 2023, 5, 5892–5899. [Google Scholar] [CrossRef]
- Soliman, M.; Sadek, A.A.; Abdelhamid, H.N.; Hussein, K. Graphene oxide-cellulose nanocomposite accelerates skin wound healing. Res. Vet. Sci. 2021, 137, 262–273. [Google Scholar] [CrossRef] [PubMed]
- Haseen, U.; Kapoor, S.; Khan, R.A.; Ahmad, H.; Koo, B.H. In Situ Fabrication and Characterization of g-C3N4 onto Cellulose Nanofibers and Selective Separation of Heavy Metal Ions. ACS Omega 2024, 9, 1620–1626. [Google Scholar] [CrossRef] [PubMed]
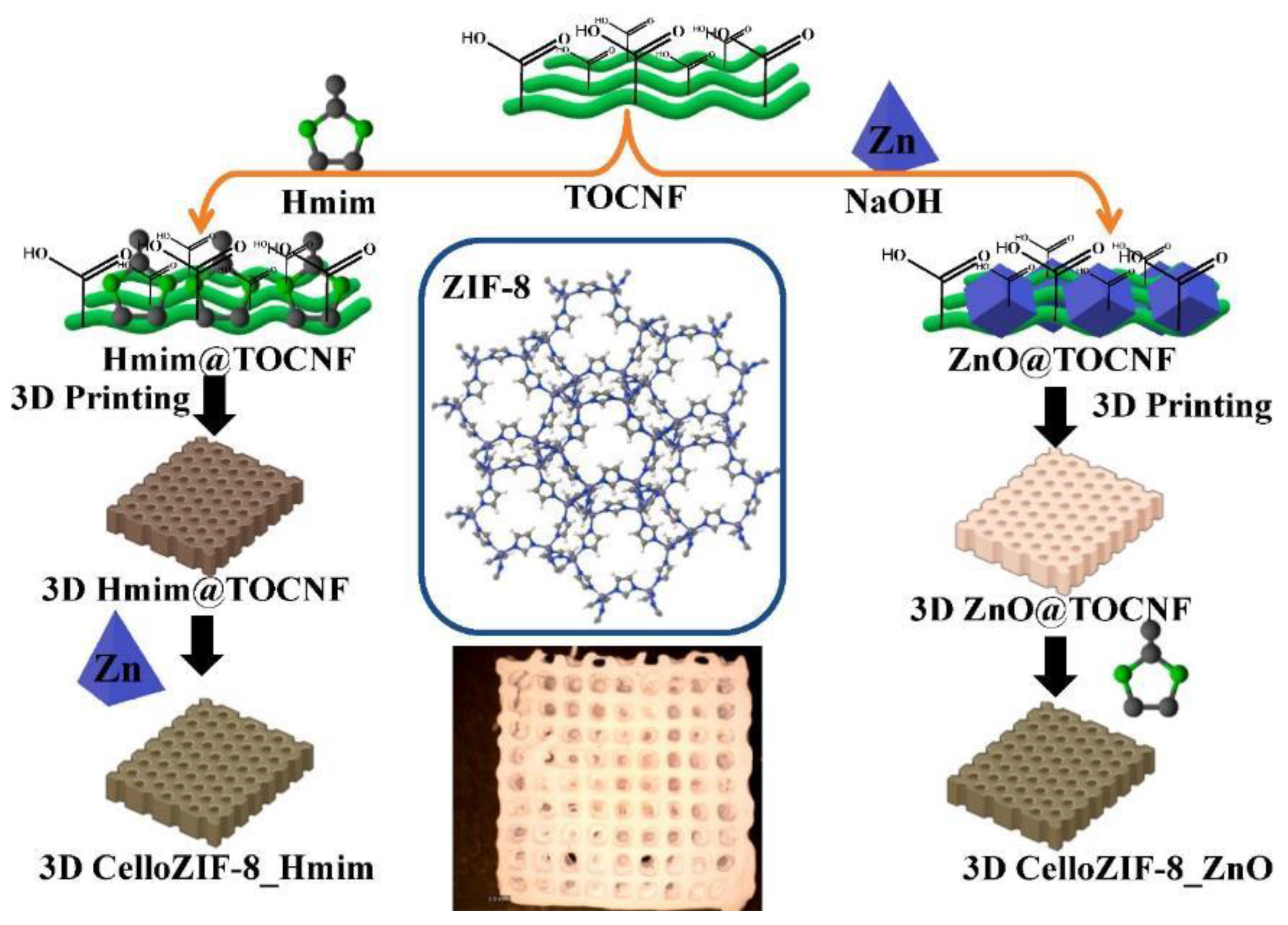
- Nasser Abdelhamid, H.; Sultan, S.; Mathew, A.P. Binder-free Three-dimensional (3D) printing of Cellulose-ZIF8 (CelloZIF-8) for water treatment and carbon dioxide (CO2) adsorption. Chem. Eng. J. 2023, 468, 143567. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. MOFTextile: Metal-Organic Frameworks Nanosheets Incorporated Cotton Textile for Selective Vapochromic Sensing and Capture of Pyridine. Appl. Organomet. Chem. 2023, 78, 547–552. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Sultan, S.; Mathew, A.P. 3D printing of cellulose/leaf-like zeolitic imidazolate frameworks (CelloZIF-L) for adsorption of carbon dioxide (CO2) and heavy metal ions. Dalt. Trans. 2023, 52, 2988–2998. [Google Scholar] [CrossRef] [PubMed]
- Nasser Abdelhamid, H.; Georgouvelas, D.; Edlund, U.; Mathew, A.P. CelloZIFPaper: Cellulose-ZIF Hybrid Paper for Heavy Metal Removal and Electrochemical Sensing. Chem. Eng. J. 2022, 446, 136614. [Google Scholar] [CrossRef]
- Nasser Abdelhamid, H.; Mathew, A.P. Cellulose-zeolitic imidazolate frameworks (CelloZIFs) for multifunctional environmental remediation: Adsorption and catalytic degradation. Chem. Eng. J. 2021, 426, 131733. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, P.; Mathew, A.P. Self-Assembled TEMPO Cellulose Nanofibers: Graphene Oxide-Based Biohybrids for Water Purification. ACS Appl. Mater. Interfaces 2017, 9, 21048–21058. [Google Scholar] [CrossRef]
- Ray, S.S.; Iroegbu, A.O.C. Nanocellulosics: Benign, Sustainable, and Ubiquitous Biomaterials for Water Remediation. ACS Omega 2021, 6, 4511–4526. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Z.; Hui, L.; Liu, H.; Liu, P.; Zhang, F.; An, X.; Wen, Y.; Wu, S. Cationic cellulose nanofibers as sustainable flocculant and retention aid for reconstituted tobacco sheet with high performance. Carbohydr. Polym. 2019, 210, 372–378. [Google Scholar] [CrossRef]
- Nkalane, A.; Oyewo, O.A.; Leswifi, T.; Onyango, M.S. Application of coagulant obtained through charge reversal of sawdust-derived cellulose nanocrystals in the enhancement of water turbidity removal. Mater. Res. Express 2019, 6, 105060. [Google Scholar] [CrossRef]
- Oyewo, O.A.; Mutesse, B.; Leswifi, T.Y.; Onyango, M.S. Highly efficient removal of nickel and cadmium from water using sawdust-derived cellulose nanocrystals. J. Environ. Chem. Eng. 2019, 7, 103251. [Google Scholar] [CrossRef]
- Dogan, H.; Hilmioglu, N.D. Zeolite-filled regenerated cellulose membranes for pervaporative dehydration of glycerol. Vacuum 2010, 84, 1123–1132. [Google Scholar] [CrossRef]
- Ali, I.; Gupta, V.K. Advances in water treatment by adsorption technology. Nat. Protoc. 2006, 1, 2661–2667. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.B.; Nagpal, G.; Agrawal, S. Rachna Water purification by using Adsorbents: A Review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Faiz Norrrahim, M.N.; Mohd Kasim, N.A.; Knight, V.F.; Mohamad Misenan, M.S.; Janudin, N.; Ahmad Shah, N.A.; Kasim, N.; Wan Yusoff, W.Y.; Mohd Noor, S.A.; Jamal, S.H.; et al. Nanocellulose: A bioadsorbent for chemical contaminant remediation. RSC Adv. 2021, 11, 7347–7368. [Google Scholar] [CrossRef] [PubMed]
- Doyo, A.N.; Kumar, R.; Barakat, M.A. Recent advances in cellulose, chitosan, and alginate based biopolymeric composites for adsorption of heavy metals from wastewater. J. Taiwan. Inst. Chem. Eng. 2023, 151, 105095. [Google Scholar] [CrossRef]
- Motloung, M.T.; Magagula, S.I.; Kaleni, A.; Sikhosana, T.S.; Lebelo, K.; Mochane, M.J. Recent Advances on Chemically Functionalized Cellulose-Based Materials for Arsenic Removal in Wastewater: A Review. Water 2023, 15, 793. [Google Scholar] [CrossRef]
- Rana, A.K.; Gupta, V.K.; Hart, P.; Thakur, V.K. Cellulose-alginate hydrogels and their nanocomposites for water remediation and biomedical applications. Environ. Res. 2024, 243, 117889. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Borges, W.; Chen, H.; Hsiao, B.S. Remediation of UO22+ from Water by Nitro-Oxidized Carboxycellulose Nanofibers: Performance and Mechanism. In Contaminants in Our Water: Identification and Remediation Methods; American Chemical Society: Washington, DC, USA, 2020; pp. 269–283. [Google Scholar]
- Sharma, S.K.; Sharma, P.R.; Chen, H.; Johnson, K.; Zhan, C.; Wang, R.; Hsiao, B. Cellulose-Supported Nanosized Zinc Oxide: Highly Efficient Bionanomaterial for Removal of Arsenic from Water. In Contaminants in Our Water: Identification and Remediation Methods; American Chemical Society: Washington, DC, USA, 2020; pp. 253–267. [Google Scholar]
- Chen, H.; Sharma, S.K.; Sharma, P.R.; Yeh, H.; Johnson, K.; Hsiao, B.S. Arsenic(III) Removal by Nanostructured Dialdehyde Cellulose–Cysteine Microscale and Nanoscale Fibers. ACS Omega 2019, 4, 22008–22020. [Google Scholar] [CrossRef]
- Qin, F.; Fang, Z.; Zhou, J.; Sun, C.; Chen, K.; Ding, Z.; Li, G.; Qiu, X. Efficient Removal of Cu2+ in Water by Carboxymethylated Cellulose Nanofibrils: Performance and Mechanism. Biomacromolecules 2019, 20, 4466–4475. [Google Scholar] [CrossRef] [PubMed]
- Movaghgharnezhad, S.; Mirabi, A.; Toosi, M.R.; Rad, A.S. Synthesis of cellulose nanofibers functionalized by dithiooxamide for preconcentration and determination of trace amounts of Cd(II) ions in water samples. Cellulose 2020, 27, 8885–8898. [Google Scholar] [CrossRef]
- Awang, N.A.; Wan Salleh, W.N.; Ismail, A.F.; Yusof, N.; Aziz, F.; Jaafar, J. Adsorption Behavior of Chromium(VI) onto Regenerated Cellulose Membrane. Ind. Eng. Chem. Res. 2019, 58, 720–728. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Nolan, M.; Li, W.; Kundal, L.; Hsiao, B.S. Sequential Oxidation on Wood and Its Application in Pb2+ Removal from Contaminated Water. Polysaccharides 2021, 2, 245–256. [Google Scholar] [CrossRef]
- Hernández-Francisco, E.; Bonilla-Cruz, J.; Márquez-Lamas, U.; Suárez-Jacobo, Á.; Longoria-Rodríguez, F.; Rivera-Haro, J.; Russell, P.; Ali, Z.; Yin, C.-Y.; Lara-Ceniceros, T.E. Entangled cellulose nanofibrils/nanosheets derived from native mexican agave for lead(II) ion removal. Cellulose 2020, 27, 8785–8798. [Google Scholar] [CrossRef]
- Chen, H.; Sharma, S.K.; Sharma, P.R.; Chi, K.; Fung, E.; Aubrecht, K.; Keroletswe, N.; Chigome, S.; Hsiao, B.S. Nitro-oxidized carboxycellulose nanofibers from moringa plant: Effective bioadsorbent for mercury removal. Cellulose 2021, 28, 8611–8628. [Google Scholar] [CrossRef]
- Zhu, C.; Monti, S.; Mathew, A.P. Evaluation of nanocellulose interaction with water pollutants using nanocellulose colloidal probes and molecular dynamic simulations. Carbohydr. Polym. 2020, 229, 115510. [Google Scholar] [CrossRef] [PubMed]
- Kardam, A.; Raj, K.R.; Srivastava, S.; Srivastava, M.M. Nanocellulose fibers for biosorption of cadmium, nickel, and lead ions from aqueous solution. Clean Technol. Environ. Policy 2014, 16, 385–393. [Google Scholar] [CrossRef]
- Chen, B.; Zheng, Q.; Zhu, J.; Li, J.; Cai, Z.; Chen, L.; Gong, S. Mechanically strong fully biobased anisotropic cellulose aerogels. RSC Adv. 2016, 6, 96518–96526. [Google Scholar] [CrossRef]
- Karim, Z.; Monti, S. Microscopic Hybrid Membranes Made of Cellulose-Based Materials Tuned for Removing Metal Ions from Industrial Effluents. ACS Appl. Polym. Mater. 2021, 3, 3733–3746. [Google Scholar] [CrossRef]
- Liu, P.; Sehaqui, H.; Tingaut, P.; Wichser, A.; Oksman, K.; Mathew, A.P. Cellulose and chitin nanomaterials for capturing silver ions (Ag+) from water via surface adsorption. Cellulose 2014, 21, 449–461. [Google Scholar] [CrossRef]
- Yu, X.; Tong, S.; Ge, M.; Wu, L.; Zuo, J.; Cao, C.; Song, W. Adsorption of heavy metal ions from aqueous solution by carboxylated cellulose nanocrystals. J. Environ. Sci. 2013, 25, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Rocky, M.M.H.; Rahman, I.M.M.; Biswas, F.B.; Rahman, S.; Endo, M.; Wong, K.H.; Mashio, A.S.; Hasegawa, H. Cellulose-based materials for scavenging toxic and precious metals from water and wastewater: A review. Chem. Eng. J. 2023, 472, 144677. [Google Scholar] [CrossRef]
- Liu, S. Preparation of nanocellulose grafted molecularly imprinted polymer for selective adsorption Pb(II) and Hg(II). Chemosphere 2023, 316, 137832. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, F.H.A.; Attia, H.A.E.-A.; Yahya, R.; Elshaarawy, R.F.M.; Hassan, N. Cellulose microfibrils-embedded sulfonated polyethersulfone for efficient Zn2+ ions removal from aqueous effluents. Chem. Eng. Res. Des. 2022, 186, 374–386. [Google Scholar] [CrossRef]
- Patel, K.; Sutar, A.K.; Maharana, T. Synthesis of carboxylic graphene oxide-carboxymethyl chitosan composite and its applications toward the remediation of U6+, Pb2+, Cr6+, and Cd2+ ions from aqueous solutions. J. Chin. Chem. Soc. 2022, 69, 2027–2041. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhou, J.; Fan, L.; Li, Y.; He, Y.; Wang, Y.; Li, L. Controllable Preparation of Cu-MOF-Coated Carboxyl Filter Paper for Simultaneous Removal of Organic Dye and Metal Ions. Ind. Eng. Chem. Res. 2021, 60, 7311–7319. [Google Scholar] [CrossRef]
- Ashour, R.M.; Abdel-Magied, A.F.; Wu, Q.; Olsson, R.T.; Forsberg, K. Green Synthesis of Metal-Organic Framework Bacterial Cellulose Nanocomposites for Separation Applications. Polymers 2020, 12, 1104. [Google Scholar] [CrossRef]
- Schelling, M.; Kim, M.; Otal, E.; Aguirre, M.; Hinestroza, J.P. Synthesis of a zinc–imidazole metal–organic framework (ZIF-8) using ZnO rods grown on cotton fabrics as precursors: Arsenate absorption studies. Cellulose 2020, 27, 6399–6410. [Google Scholar] [CrossRef]
- Wang, N.; Ouyang, X.-K.; Yang, L.-Y.; Omer, A.M. Fabrication of a Magnetic Cellulose Nanocrystal/Metal–Organic Framework Composite for Removal of Pb(II) from Water. ACS Sustain. Chem. Eng. 2017, 5, 10447–10458. [Google Scholar] [CrossRef]
- Ma, X.; Lou, Y.; Chen, X.-B.; Shi, Z.; Xu, Y. Multifunctional flexible composite aerogels constructed through in-situ growth of metal-organic framework nanoparticles on bacterial cellulose. Chem. Eng. J. 2019, 356, 227–235. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, M.; Nie, J.; Tan, J.; Song, S.; Luo, Y. Lightweight and porous cellulose-based foams with high loadings of zeolitic imidazolate frameworks-8 for adsorption applications. Carbohydr. Polym. 2019, 208, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Ren, W.; Lei, C.; Xie, Y.; Cai, Y.; Wang, S.; Gao, J.; Ni, Q.; Yao, J. Flexible and porous cellulose aerogels/zeolitic imidazolate framework (ZIF-8) hybrids for adsorption removal of Cr(IV) from water. J. Solid State Chem. 2018, 262, 135–141. [Google Scholar] [CrossRef]
- Bahmani, E.; Seyyed Zonouzi, H.; Koushkbaghi, S.; Keyvani Hafshejani, F.; Fassadi Chimeh, A.; Irani, M. Electrospun polyacrylonitrile/cellulose acetate/MIL-125/TiO2 composite nanofibers as an efficient photocatalyst and anticancer drug delivery system. Cellulose 2020, 27, 10029–10045. [Google Scholar] [CrossRef]
- Zhu, H.; Yang, X.; Cranston, E.D.; Zhu, S. Flexible and Porous Nanocellulose Aerogels with High Loadings of Metal-Organic-Framework Particles for Separations Applications. Adv. Mater. 2016, 28, 7652–7657. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Gao, J.; Ren, W.; Xie, Y.; Abdalkarim, S.Y.H.; Wang, S.; Ni, Q.; Yao, J. Fabrication of metal-organic frameworks@cellulose aerogels composite materials for removal of heavy metal ions in water. Carbohydr. Polym. 2019, 205, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tan, S.; Xu, Z. Anisotropic Nanocellulose Aerogel Loaded with Modified UiO-66 as Efficient Adsorbent for Heavy Metal Ions Removal. Nanomaterials 2020, 10, 1114. [Google Scholar] [CrossRef] [PubMed]
- Hashem, T.; Ibrahim, A.H.; Wöll, C.; Alkordi, M.H. Grafting Zirconium-Based Metal–Organic Framework UiO-66-NH2 Nanoparticles on Cellulose Fibers for the Removal of Cr(VI) Ions and Methyl Orange from Water. ACS Appl. Nano Mater. 2019, 2, 5804–5808. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, Y.; Li, H.; Qin, W.; Zhang, H.; Wang, G.; Zhang, Y.; Zhao, H. Fabrication of hierarchically porous NH2-MIL-53/wood-carbon hybrid membrane for highly effective and selective sequestration of Pb2+. Chem. Eng. J. 2020, 387, 124141. [Google Scholar] [CrossRef]
- Yang, W.; Wang, J.; Yang, Q.; Pei, H.; Hu, N.; Suo, Y.; Li, Z.; Zhang, D.; Wang, J. Facile fabrication of robust MOF membranes on cloth via a CMC macromolecule bridge for highly efficient Pb(II) removal. Chem. Eng. J. 2018, 339, 230–239. [Google Scholar] [CrossRef]
- Liu, J.; Hao, D.; Sun, H.; Li, Y.; Han, J.; Fu, B.; Zhou, J. Integration of MIL-101-NH2 into Cellulosic Foams for Efficient Cr(VI) Reduction under Visible Light. Ind. Eng. Chem. Res. 2021, 60, 12220–12227. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A. Cellulose-Metal Organic Frameworks (CelloMOFs) Hybrid Materials and their Multifaceted Applications: A Review. Coord. Chem. Rev. 2022, 451, 214263. [Google Scholar] [CrossRef]
- Baruah, J.; Chaliha, C.; Kalita, E.; Nath, B.K.; Field, R.A.; Deb, P. Modelling and optimization of factors influencing adsorptive performance of agrowaste-derived Nanocellulose Iron Oxide Nanobiocomposites during remediation of Arsenic contaminated groundwater. Int. J. Biol. Macromol. 2020, 164, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Xu, X.; Shaghaleh, H.; Guo, J.; Guo, L.; Qian, Y.; Liu, H.; Wang, S. Factors influencing the morphology and adsorption performance of cellulose nanocrystal/iron oxide nanorod composites for the removal of arsenic during water treatment. Int. J. Biol. Macromol. 2020, 156, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cui, J.; Xu, X.; Sun, B.; Zhang, L.; Dong, W.; Chen, C.; Sun, D. Bacterial cellulose/attapulgite magnetic composites as an efficient adsorbent for heavy metal ions and dye treatment. Carbohydr. Polym. 2020, 229, 115512. [Google Scholar] [CrossRef] [PubMed]
- Haniffa, M.A.C.M.; Munawar, K.; Chee, C.Y.; Pramanik, S.; Halilu, A.; Illias, H.A.; Rizwan, M.; Senthilnithy, R.; Mahanama, K.R.R.; Tripathy, A.; et al. Cellulose supported magnetic nanohybrids: Synthesis, physicomagnetic properties and biomedical applications—A review. Carbohydr. Polym. 2021, 267, 118136. [Google Scholar] [CrossRef] [PubMed]
- Onwumere, J.; Pia̧tek, J.; Budnyak, T.; Chen, J.; Budnyk, S.; Karim, Z.; Thersleff, T.; Kuśtrowski, P.; Mathew, A.P.; Slabon, A. CelluPhot: Hybrid Cellulose−Bismuth Oxybromide Membrane for Pollutant Removal. ACS Appl. Mater. Interfaces 2020, 12, 42891–42901. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.B.; Alamry, K.A.; Marwani, H.M.; Asiri, A.M.; Rahman, M.M. Synthesis and environmental applications of cellulose/ZrO2 nanohybrid as a selective adsorbent for nickel ion. Compos. Part B Eng. 2013, 50, 253–258. [Google Scholar] [CrossRef]
- Brown, A.; Bozman, M.; Hickman, T.; Hossain, M.I.; Glover, T.G.; West, K.N.; West, C.W. Superhydrophobic Functionalization of Cotton Fabric via Reactive Dye Chemistry and a Thiol-ene Click Reaction. Ind. Eng. Chem. Res. 2019, 58, 22534–22540. [Google Scholar] [CrossRef]
- He, X.; Kim, H.; Dong, T.G.; Gates, I.; Lu, Q. Green synthesis of Ag/lignin nanoparticle-loaded cellulose aerogel for catalytic degradation and antimicrobial applications. Cellulose 2022, 29, 9341–9360. [Google Scholar] [CrossRef]
- Soliman, A.I.A.; Díaz Baca, J.A.; Fatehi, P. One-pot synthesis of magnetic cellulose nanocrystal and its post-functionalization for doxycycline adsorption. Carbohydr. Polym. 2023, 308, 120619. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Natarajan, T.S.; Sheikh, M.U.D.; Bano, M.; Khan, F. Self-organized graphene oxide and TiO2 nanoparticles incorporated alginate/carboxymethyl cellulose nanocomposites with efficient photocatalytic activity under direct sunlight. J. Photochem. Photobiol. A Chem. 2017, 346, 113–125. [Google Scholar] [CrossRef]
- Sun, X.; Danumah, C.; Liu, Y.; Boluk, Y. Flocculation of bacteria by depletion interactions due to rod-shaped cellulose nanocrystals. Chem. Eng. J. 2012, 198–199, 476–481. [Google Scholar] [CrossRef]
- Eyley, S.; Vandamme, D.; Lama, S.; Van den Mooter, G.; Muylaert, K.; Thielemans, W. CO2 controlled flocculation of microalgae using pH responsive cellulose nanocrystals. Nanoscale 2015, 7, 14413–14421. [Google Scholar] [CrossRef] [PubMed]
- Pei, A.; Butchosa, N.; Berglund, L.A.; Zhou, Q. Surface quaternized cellulose nanofibrils with high water absorbency and adsorption capacity for anionic dyes. Soft Matter 2013, 9, 2047. [Google Scholar] [CrossRef]
- Sehaqui, H.; Mautner, A.; Perez de Larraya, U.; Pfenninger, N.; Tingaut, P.; Zimmermann, T. Cationic cellulose nanofibers from waste pulp residues and their nitrate, fluoride, sulphate and phosphate adsorption properties. Carbohydr. Polym. 2016, 135, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Zhu, X.; Wang, M.; Qiu, Z.; Zhang, T.; Qiu, F. Controlled Fabrication of the Biomass Cellulose–CeO2 Nanocomposite Membrane as Efficient and Recyclable Adsorbents for Fluoride Removal. Ind. Eng. Chem. Res. 2021, 60, 5914–5923. [Google Scholar] [CrossRef]
- Yu, X.; Tong, S.; Ge, M.; Zuo, J. Removal of fluoride from drinking water by cellulose@hydroxyapatite nanocomposites. Carbohydr. Polym. 2013, 92, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, G.; Liu, B.; Kong, H.; Xiong, Z.; Guo, L.; Wei, G. Biomineralization of ZrO2 nanoparticles on graphene oxide-supported peptide/cellulose binary nanofibrous membranes for high-performance removal of fluoride ions. Chem. Eng. J. 2022, 430, 132721. [Google Scholar] [CrossRef]
- Darabitabar, F.; Yavari, V.; Hedayati, A.; Zakeri, M.; Yousefi, H. Novel cellulose nanofiber aerogel for aquaculture wastewater treatment. Environ. Technol. Innov. 2020, 18, 100786. [Google Scholar] [CrossRef]
- Robledo-Peralta, A.; Torres-Castañón, L.A.; Rodríguez-Beltrán, R.I.; Reynoso-Cuevas, L. Lignocellulosic Biomass as Sorbent for Fluoride Removal in Drinking Water. Polymers 2022, 14, 5219. [Google Scholar] [CrossRef] [PubMed]
- Raval, P.; Thomas, N.; Hamdouna, L.; Delevoye, L.; Lafon, O.; Manjunatha Reddy, G.N. Boron Adsorption Kinetics of Microcrystalline Cellulose and Polymer Resin. Langmuir 2023, 39, 5384–5395. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Guan, K.; Mai, Z.; Zhou, S.; Song, Q.; Li, Z.; Xu, P.; Hu, M.; Chiao, Y.-H.; Zhang, P.; et al. Complexation of cellulose nanocrystals and amine monomer for improved interfacial polymerization of nanofiltration membrane. J. Memb. Sci. 2023, 687, 122048. [Google Scholar] [CrossRef]
- Wang, S.; Chen, X.; Li, Z.; Zeng, W.; Meng, D.; Wang, Y.; Xiao, Z.; Wang, H.; Liang, D.; Xie, Y. Click chemistry-induced selective adsorption of cationic and anionic dyes using functionalized cellulose methacrylate hydrogels. Cellulose 2022, 29, 8843–8861. [Google Scholar] [CrossRef]
- Qiu, C.; Li, Y.; Liu, H.; Wang, X.; Hu, S.; Qi, H. A novel crosslinking strategy on functional cellulose-based aerogel for effective and selective removal of dye. Chem. Eng. J. 2023, 463, 142404. [Google Scholar] [CrossRef]
- Goetz, L.A.; Naseri, N.; Nair, S.S.; Karim, Z.; Mathew, A.P. All cellulose electrospun water purification membranes nanotextured using cellulose nanocrystals. Cellulose 2018, 25, 3011–3023. [Google Scholar] [CrossRef]
- Yu, S.; Liu, M.; Ma, M.; Qi, M.; Lü, Z.; Gao, C. Impacts of membrane properties on reactive dye removal from dye/salt mixtures by asymmetric cellulose acetate and composite polyamide nanofiltration membranes. J. Memb. Sci. 2010, 350, 83–91. [Google Scholar] [CrossRef]
- Zhou, C.; Wu, Q.; Lei, T.; Negulescu, I.I. Adsorption kinetic and equilibrium studies for methylene blue dye by partially hydrolyzed polyacrylamide/cellulose nanocrystal nanocomposite hydrogels. Chem. Eng. J. 2014, 251, 17–24. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, H.; Pan, Y.; Wang, L. Novel Composite Adsorbent Consisting of Dissolved Cellulose Fiber/Microfibrillated Cellulose for Dye Removal from Aqueous Solution. ACS Sustain. Chem. Eng. 2018, 6, 6994–7002. [Google Scholar] [CrossRef]
- Kaushik, M.; Moores, A. Review: Nanocelluloses as versatile supports for metal nanoparticles and their applications in catalysis. Green Chem. 2016, 18, 622–637. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Z.; Cui, Z.; Luo, L.; Lin, P.; Xie, M.; Zhang, Q.; Sa, B.; Wen, C. Enhanced interfacial interactions and enriched active sites in self-assembly amino-functionalized bacterial cellulose/MXene composite for wastewater treatment. Chem. Eng. J. 2024, 488, 151078. [Google Scholar] [CrossRef]
- Zhang, W.; Ji, X.-X.; Ma, M.-G. Emerging MXene/cellulose composites: Design strategies and diverse applications. Chem. Eng. J. 2023, 458, 141402. [Google Scholar] [CrossRef]
- Almeida da Silva, T.C.; Marchiori, L.; Oliveira Mattos, B.; Ullah, S.; Barud, H.d.S.; Romano Domeneguetti, R.; Rojas-Mantilla, H.D.; Boldrin Zanoni, M.V.; Rodrigues-Filho, U.P.; Ferreira-Neto, E.P.; et al. Designing Highly Photoactive Hybrid Aerogels for In-Flow Photocatalytic Contaminant Removal Using Silica-Coated Bacterial Nanocellulose Supports. ACS Appl. Mater. Interfaces 2023, 15, 23146–23159. [Google Scholar] [CrossRef]
- Zou, M.; Tan, C.; Yuan, Z.; Zhang, L.; Wu, M.; Hu, J.; Ma, Z.; Zhou, H. In situ generated CdS on mixed cellulose membrane matrix as an easily recyclable photocatalyst for mineral flotation wastewater treatment. Cellulose 2024, 31, 1089–1104. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.-F.; Shu, L.; Yao, J. Copper sulfide integrated functional cellulose hydrogel for efficient solar water purification. Carbohydr. Polym. 2023, 319, 121161. [Google Scholar] [CrossRef]
- Zhu, L.; Zong, L.; Wu, X.; Li, M.; Wang, H.; You, J.; Li, C. Shapeable Fibrous Aerogels of Metal–Organic-Frameworks Templated with Nanocellulose for Rapid and Large-Capacity Adsorption. ACS Nano 2018, 12, 4462–4468. [Google Scholar] [CrossRef]
- Vatanpour, V.; Yuksekdag, A.; Ağtaş, M.; Mehrabi, M.; Salehi, E.; Castro-Muñoz, R.; Koyuncu, I. Zeolitic imidazolate framework (ZIF-8) modified cellulose acetate NF membranes for potential water treatment application. Carbohydr. Polym. 2023, 299, 120230. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Jeon, Y.; Kwon, G.; Lee, S.; Ko, Y.; Park, J.; Kim, J.; You, J. Multiporous ZIF-8 carbon/cellulose composite beads: Highly efficient and scalable adsorbents for water treatment. Carbohydr. Polym. 2024, 335, 122047. [Google Scholar] [CrossRef]
- Uchiyama, K.; Asamoto, H.; Minamisawa, H.; Yamada, K. Quaternization of Porous Cellulose Beads and Their Use for Removal of Humic Acid from Aqueous Medium. Physchem 2023, 3, 61–76. [Google Scholar] [CrossRef]
- Vandamme, D.; Eyley, S.; Van den Mooter, G.; Muylaert, K.; Thielemans, W. Highly charged cellulose-based nanocrystals as flocculants for harvesting Chlorella vulgaris. Bioresour. Technol. 2015, 194, 270–275. [Google Scholar] [CrossRef]
- Mousa, H.M.; Fahmy, H.S.; Ali, G.A.M.; Abdelhamid, H.N.; Ateia, M. Membranes for Oil/Water Separation: A Review. Adv. Mater. Interfaces 2022, 9, 2200557. [Google Scholar] [CrossRef]
- Yin, Z.; Li, Z.; Deng, Y.; Xue, M.; Chen, Y.; Ou, J.; Xie, Y.; Luo, Y.; Xie, C.; Hong, Z. Multifunctional CeO2-coated pulp/cellulose nanofibers (CNFs) membrane for wastewater treatment: Effective oil/water separation, organic contaminants photodegradation, and anti-bioadhesion activity. Ind. Crop. Prod. 2023, 197, 116672. [Google Scholar] [CrossRef]
- Abbas, S.M.; Al-Jubouri, S.M. High performance and antifouling zeolite@polyethersulfone/cellulose acetate asymmetric membrane for efficient separation of oily wastewater. J. Environ. Chem. Eng. 2024, 12, 112775. [Google Scholar] [CrossRef]
- Elgueta, E.; Becerra, Y.; Martínez, A.; Pereira, M.; Carrillo-Varela, I.; Sanhueza, F.; Nuñez, D.; Rivas, B.L. Adsorbents Derived from Xylan Hemicellulose with Removal Properties of Pollutant Metals. Chin. J. Polym. Sci. 2023, 41, 874–886. [Google Scholar] [CrossRef]
- Kausar, A.; Zohra, S.T.; Ijaz, S.; Iqbal, M.; Iqbal, J.; Bibi, I.; Nouren, S.; El Messaoudi, N.; Nazir, A. Cellulose-based materials and their adsorptive removal efficiency for dyes: A review. Int. J. Biol. Macromol. 2023, 224, 1337–1355. [Google Scholar] [CrossRef] [PubMed]
- Morsi, R.E.; Corticelli, F.; Morandi, V.; Gentili, D.; Cavallini, M.; Figoli, A.; Russo, F.; Galiano, F.; Aluigi, A.; Ventura, B. Influence of the Fabrication Conditions on the Physical Properties and Water Treatment Efficiency of Cellulose Acetate Porous Membranes. Water 2023, 15, 1061. [Google Scholar] [CrossRef]
- Hazarika, T.; Kakati, B.; Pal, D.; Saikia, R.; Rawal, A.; Mahanta, M.K.; Biswas, S. Role of plasma process gas on permeate flux augmentation of cellulose nitrate membrane for mud water treatment. Sci. Rep. 2024, 14, 6585. [Google Scholar] [CrossRef]
- Namasivayam, S.K.R.; Prakash, P.; Babu, V.; Paul, E.J.; Bharani, R.S.A.; Kumar, J.A.; Kavisri, M.; Moovendhan, M. Aquatic biomass cellulose fabrication into cellulose nanocomposite and its application in water purification. J. Clean. Prod. 2023, 396, 136386. [Google Scholar] [CrossRef]
- Spinu, M.; Dos Santos, N.; Le Moigne, N.; Navard, P. How does the never-dried state influence the swelling and dissolution of cellulose fibres in aqueous solvent? Cellulose 2011, 18, 247–256. [Google Scholar] [CrossRef]
- Zhang, Z.; Ahmed, A.I.S.; Malik, M.Z.; Ali, N.; Khan, A.; Ali, F.; Hassan, M.O.; Mohamed, B.A.; Zdarta, J.; Bilal, M. Cellulose/inorganic nanoparticles-based nano-biocomposite for abatement of water and wastewater pollutants. Chemosphere 2023, 313, 137483. [Google Scholar] [CrossRef]
- Azimi, B.; Sepahvand, S.; Ismaeilimoghadam, S.; Kargarzadeh, H.; Ashori, A.; Jonoobi, M.; Danti, S. Application of Cellulose-Based Materials as Water Purification Filters; A State-of-the-Art Review. J. Polym. Environ. 2024, 32, 345–366. [Google Scholar] [CrossRef]


| Name | Diameter (nm) | Length (µm) | Aspect Ratio (L/D) |
|---|---|---|---|
| Cellulose Nanofibrils (CNFs) | 2–20 | 100–1000 | 50–500 |
| Cellulose Nanocrystals (CNCs) | 5–20 | 100–500 | 20–50 |
| Bacterial Nanocellulose (BNC) | 10–100 | 1–100 | 10–100 |
| Enzymatic Nanocellulose (ENC) | 5–50 | 100–500 | 20–100 |
| TEMPO-oxidized cellulose nanofibrils (TOCNFs) | 2–10 | 100–1000 | 50–500 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelhamid, H.N. Nanocellulose-Based Materials for Water Pollutant Removal: A Review. Int. J. Mol. Sci. 2024, 25, 8529. https://doi.org/10.3390/ijms25158529
Abdelhamid HN. Nanocellulose-Based Materials for Water Pollutant Removal: A Review. International Journal of Molecular Sciences. 2024; 25(15):8529. https://doi.org/10.3390/ijms25158529
Chicago/Turabian StyleAbdelhamid, Hani Nasser. 2024. "Nanocellulose-Based Materials for Water Pollutant Removal: A Review" International Journal of Molecular Sciences 25, no. 15: 8529. https://doi.org/10.3390/ijms25158529
APA StyleAbdelhamid, H. N. (2024). Nanocellulose-Based Materials for Water Pollutant Removal: A Review. International Journal of Molecular Sciences, 25(15), 8529. https://doi.org/10.3390/ijms25158529






