New Insights into Involvement of Low Molecular Weight Proteins in Complex Defense Mechanisms in Higher Plants
Abstract
1. Introduction
2. Dehydrins
3. Cyclotides
4. Heat Shock Proteins
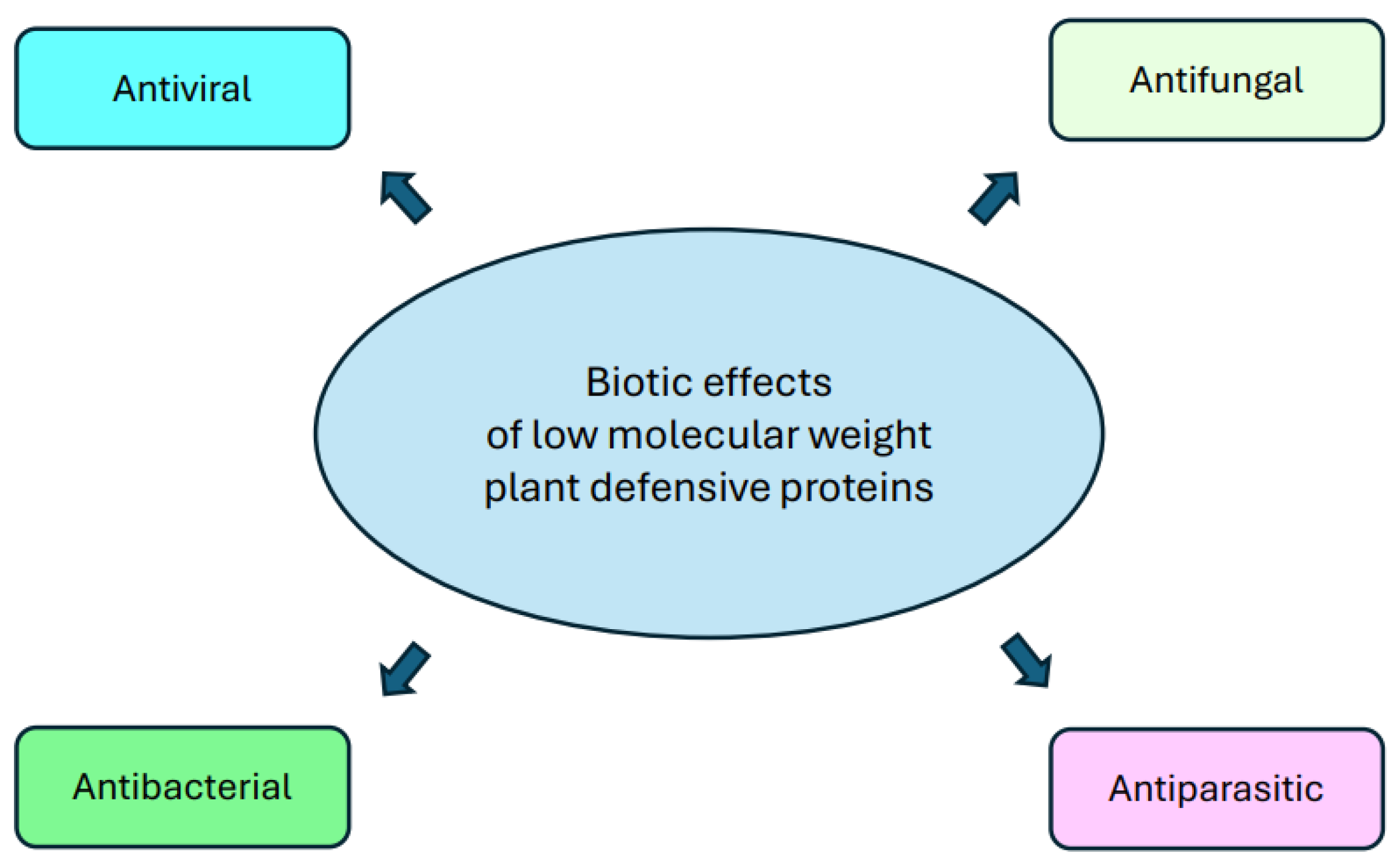
5. Pathogenesis-Related Proteins
5.1. Thionins
5.2. Defensins
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and Mechanism of Jasmonic Acid in Plant Responses to Abiotic and Biotic Stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Hernández, M.C.; Parola-Contreras, I.; Montoya-Gómez, L.M.; Torres-Pacheco, I.; Schwarz, D.; Guevara-González, R.G. Eustressors: Chemical and Physical Stress Factors Used to Enhance Vegetables Production. Sci. Hortic. 2019, 250, 223–229. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The Global Burden of Pathogens and Pests on Major Food Crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, N.W.; Singh, M.; Kumar, S.; Sangle, U.R.; Nityanand; Singh, R.; Sachitanand; Prasad, R.; Singh, S.S.; Singh, S.; et al. Trichoderma harzianum Improves the Performance of Stress-Tolerant Rice Varieties in Rainfed Ecologies of Bihar, India. Field Crops Res. 2018, 220, 97–104. [Google Scholar] [CrossRef]
- Macheroux, P. More Important than Ever: Understanding How Plants Cope with Stress. FEBS J. 2022, 289, 1720–1722. [Google Scholar] [CrossRef]
- Karlova, R.; Boer, D.; Hayes, S.; Testerink, C. Root Plasticity under Abiotic Stress. Plant Physiol. 2021, 187, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- West, G.; Inzé, D.; Beemster, G.T.S. Cell Cycle Modulation in the Response of the Primary Root of Arabidopsis to Salt Stress. Plant Physiol. 2004, 135, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of Plants to Water Stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Ahad, A.; Gul, A.; Batool, T.S.; Huda, N.; Naseeer, F.; Abdul Salam, U.; Abdul Salam, M.; Ilyas, M.; Turkyilmaz Unal, B.; Ozturk, M. Molecular and Genetic Perspectives of Cold Tolerance in Wheat. Mol. Biol. Rep. 2023, 50, 6997–7015. [Google Scholar] [CrossRef]
- Alsamir, M.; Mahmood, T.; Trethowan, R.; Ahmad, N. An Overview of Heat Stress in Tomato (Solanum lycopersicum L.). Saudi J. Biol. Sci. 2021, 28, 1654–1663. [Google Scholar] [CrossRef]
- Prasad, S.; Jaiswal, B.; Singh, S.; Rani, R.; Yadav, V.; Kumar, A.; Mishra, V.; Khan, N.; Yadav, R.; Singh, M. Evaluation of Wheat (Triticum aestivum L.) Varieties for Heat Tolerance at Grain Growth Stage by Physio-Molecular Approaches. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3745–3750. [Google Scholar]
- Chen, L.; Yun, M.; Cao, Z.; Liang, Z.; Liu, W.; Wang, M.; Yan, J.; Yang, S.; He, X.; Jiang, B.; et al. Phenotypic Characteristics and Transcriptome of Cucumber Male Flower Development Under Heat Stress. Front. Plant Sci. 2021, 12, 758976. [Google Scholar] [CrossRef] [PubMed]
- Nishad, A.; Nandi, A.K. Recent Advances in Plant Thermomemory. Plant Cell Rep. 2021, 40, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Holopainen, J.K.; Virjamo, V.; Ghimire, R.P.; Blande, J.D.; Julkunen-Tiitto, R.; Kivimäenpää, M. Climate Change Effects on Secondary Compounds of Forest Trees in the Northern Hemisphere. Front. Plant Sci. 2018, 9, 1445. [Google Scholar] [CrossRef]
- Coatsworth, P.; Gonzalez-Macia, L.; Collins, A.S.P.; Bozkurt, T.; Güder, F. Continuous Monitoring of Chemical Signals in Plants under Stress. Nat. Rev. Chem. 2023, 7, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Nishad, R.; Ahmed, T.; Rahman, V.J.; Kareem, A. Modulation of Plant Defense System in Response to Microbial Interactions. Front. Microbiol. 2020, 11, 1298. [Google Scholar] [CrossRef] [PubMed]
- Jain, D.; Khurana, J.P. Role of Pathogenesis-Related (PR) Proteins in Plant Defense Mechanism. In Molecular Aspects of Plant-Pathogen Interaction; Singh, A., Singh, I.K., Eds.; Springer: Singapore, 2018; pp. 265–281. [Google Scholar] [CrossRef]
- Malik, A.A.; Veltri, M.; Boddington, K.F.; Singh, K.K.; Graether, S.P. Genome Analysis of Conserved Dehydrin Motifs in Vascular Plants. Front. Plant Sci. 2017, 8, 709. [Google Scholar] [CrossRef] [PubMed]
- Ireland, D.C.; Colgrave, M.L.; Craik, D.J. A Novel Suite of Cyclotides from Viola odorata: Sequence Variation and the Implications for Structure, Function and Stability. Biochem. J. 2006, 400, 1–12. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, C.; Franco, O.L. Pathogenesis-Related Proteins (PRs) with Enzyme Activity Activating Plant Defense Responses. Plants 2023, 12, 2226. [Google Scholar] [CrossRef]
- Lay, F.T.; Anderson, M.A. Defensins—Components of the Innate Immune System in Plants. Curr. Protein Pept. Sci. 2005, 6, 85–101. [Google Scholar] [CrossRef]
- Höng, K.; Austerlitz, T.; Bohlmann, T.; Bohlmann, H. The Thionin Family of Antimicrobial Peptides. PLoS ONE 2021, 16, e0254549. [Google Scholar] [CrossRef]
- da Silva, P.B.; Vaz, T.A.A.; Acencio, M.L.; Bovolenta, L.A.; Hilhorst, H.W.M.; da Silva, E.A.A. Can Osmopriming Induce Cross-Tolerance for Abiotic Stresses in Solanum paniculatum L. Seeds? A Transcriptome Analysis Point of View. Seeds 2023, 2, 382–393. [Google Scholar] [CrossRef]
- Athar, H.U.; Zulfiqar, F.; Moosa, A.; Ashraf, M.; Zafar, Z.U.; Zhang, L.; Ahmed, N.; Kalaji, H.M.; Nafees, M.; Hossain, M.A.; et al. Salt Stress Proteins in Plants: An overview. Front. Plant Sci. 2022, 13, 999058. [Google Scholar] [CrossRef]
- Foyer, C.H.; Rasool, B.; Davey, J.W.; Hancock, R.D. Cross-Tolerance To Biotic And Abiotic Stresses in Plants: A Focus on Resistance to Aphid Infestation. J. Exp. Bot. 2016, 67, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Katam, R.; Shokri, S.; Murthy, N.; Singh, S.K.; Suravajhala, P.; Khan, M.N.; Bahmani, M.; Sakata, K.; Reddy, K.R. Proteomics, Physiological, and Biochemical Analysis of Cross Tolerance Mechanisms in Response to Heat And Water Stresses in Soybean. PLoS ONE 2020, 15, e0233905. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.I.; Attia, K.A.; Ghazy, A.I.; Itoh, K.; Almajhdi, F.N.; Al-Doss, A.A. Molecular Characterization and Functional Localization of a Novel SUMOylation Gene in Oryza sativa. Biology 2022, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.I.; Baek, S.H.; Chung, C.H. Versatile Protein tag, SUMO: Its Enzymology and Biological Function. J. Cell. Physiol. 2002, 191, 257–268. [Google Scholar] [CrossRef]
- Srivastava, M.; Srivastava, A.K.; Orosa-Puente, B.; Campanaro, A.; Zhang, C.; Sadanandom, A. SUMO Conjugation to BZR1 Enables Brassinosteroid Signaling to Integrate Environmental Cues to Shape Plant Growth. Curr. Biol. 2020, 30, 1410–1423.e3. [Google Scholar] [CrossRef] [PubMed]
- Orosa, B.; Yates, G.; Verma, V.; Srivastava, A.K.; Srivastava, M.; Campanaro, A.; De Vega, D.; Fernandes, A.; Zhang, C.; Lee, J.; et al. SUMO Conjugation to the Pattern Recognition Receptor FLS2 Triggers Intracellular Signalling in Plant Innate Immunity. Nat. Commun. 2018, 9, 5185. [Google Scholar] [CrossRef]
- Srivastava, M.; Sadanandom, A.; Srivastava, A.K. Towards understanding the multifaceted role of SUMOylation in plant growth and development. Physiol. Plant. 2021, 171, 77–85. [Google Scholar] [CrossRef]
- Han, Z.-J.; Feng, Y.-H.; Gu, B.-H.; Li, Y.-M.; Chen, H. The Post-translational Modification, SUMOylation, and Cancer. Int. J. Oncol. 2018, 52, 1081–1094. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.-Q.; Xue, H.-W. The Ubiquitin-Proteasome System in Plant Responses to Environments. Plant Cell Environ. 2019, 42, 2931–2944. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.; Sadanandom, A. An Insight into the Factors Influencing Specificity of the SUMO System in Plants. Plants 2020, 9, 1788. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.L. Chapter Three—Role of the Ubiquitin Proteasome System in Plant Response to Abiotic Stress. Int. Rev. Cell Mol. Biol. 2019, 343, 65–110. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.R.; Graether, S.P. Physiological, Structural, and Functional Insights into the Cryoprotection of Membranes by the Dehydrins. Front. Plant Sci. 2022, 13, 886525. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Wang, X.; Zhang, L. Structural and Functional Dynamics of Dehydrins: A Plant Protector Protein under Abiotic Stress. Int. J. Mol. Sci. 2018, 19, 3420. [Google Scholar] [CrossRef] [PubMed]
- Graether, S.P.; Boddington, K.F. Disorder and Function: A Review of the Dehydrin Protein Family. Front. Plant Sci. 2014, 5, 576. [Google Scholar] [CrossRef]
- Szlachtowska, Z.; Rurek, M. Plant Dehydrins and Dehydrin-like Proteins: Characterization and Participation in Abiotic Stress Response. Front. Plant Sci. 2023, 14, 1213188. [Google Scholar] [CrossRef]
- Hernández-Sánchez, I.E.; Maruri-López, I.; Molphe-Balch, E.P.; Becerra-Flora, A.; Jaimes-Miranda, F.; Jiménez-Bremont, J.F. Evidence for in Vivo Interactions between Dehydrins and the Aquaporin AtPIP2B. Biochem. Biophys. Res. Commun. 2019, 510, 545–550. [Google Scholar] [CrossRef]
- Szabała, B.M. The Cationic Nature of Lysine-Rich Segments Modulates the Structural and Biochemical Properties of Wild Potato FSK3 Dehydrin. Plant Physiol. Biochem. 2023, 194, 480–488. [Google Scholar] [CrossRef]
- Riley, A.C.; Ashlock, D.A.; Graether, S.P. Evolution of the Modular, Disordered Stress Proteins Known as Dehydrins. PLoS ONE 2019, 14, e0211813. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, S.; Chen, W.; Zhang, J.; Zhang, L.; Sun, W.; Wang, Z. Plant Dehydrins: Expression, Regulatory Networks, and Protective Roles in Plants Challenged by Abiotic Stress. Int. J. Mol. Sci. 2021, 22, 12619. [Google Scholar] [CrossRef] [PubMed]
- Vítámvás, P.; Kosová, K.; Musilová, J.; Holková, L.; Mařík, P.; Smutná, P.; Klíma, M.; Prášil, I.T. Relationship Between Dehydrin Accumulation and Winter Survival in Winter Wheat and Barley Grown in the Field. Front. Plant Sci. 2019, 10, 7. [Google Scholar] [CrossRef]
- Ouyang, L.; Leus, L.; De Keyser, E.; Van Labeke, M.-C. Cold Acclimation and Deacclimation of Two Garden Rose Cultivars Under Controlled Daylength and Temperature. Front. Plant Sci. 2020, 11, 327. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, S.; Eremina, N.; Barth, A.; Danielsson, J.; Harryson, P. Membrane-Induced Folding of the Plant Stress Dehydrin Lti30. Plant Physiol. 2016, 171, 932–943. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Marzinek, J.K.; Jefferies, D.; Bond, P.J.; Harryson, P.; Wohland, T. The Disordered Plant Dehydrin Lti30 Protects the Membrane during Water-Related Stress by Cross-Linking Lipids. J. Biol. Chem. 2019, 294, 6468–6482. [Google Scholar] [CrossRef]
- Stival Sena, J.; Giguère, I.; Rigault, P.; Bousquet, J.; Mackay, J. Expansion of the Dehydrin Gene Family in the Pinaceae Is Associated with Considerable Structural Diversity and Drought-Responsive Expression. Tree Physiol. 2018, 38, 442–456. [Google Scholar] [CrossRef]
- Shakirova, F.; Allagulova, C.; Maslennikova, D.; Fedorova, K.; Yuldashev, R.; Lubyanova, A.; Bezrukova, M.; Avalbaev, A. Involvement of Dehydrins in 24-Epibrassinolide-Induced Protection of Wheat Plants against Drought Stress. Plant Physiol. Biochem. 2016, 108, 539–548. [Google Scholar] [CrossRef]
- Habibpourmehraban, F.; Atwell, B.J.; Haynes, P.A. Unique and Shared Proteome Responses of Rice Plants (Oryza sativa) to Individual Abiotic Stresses. Int. J. Mol. Sci. 2022, 23, 15552. [Google Scholar] [CrossRef]
- Ghanmi, S.; Graether, S.P.; Hanin, M. The Halophyte Dehydrin Sequence Landscape. Biomolecules 2022, 12, 330. [Google Scholar] [CrossRef]
- Cheng, B.; Li, Z.; Liang, L.; Cao, Y.; Zeng, W.; Zhang, X.; Ma, X.; Huang, L.; Nie, G.; Liu, W.; et al. The γ-Aminobutyric Acid (GABA) Alleviates Salt Stress Damage during Seeds Germination of White Clover Associated with Na+/K+ Transportation, Dehydrins Accumulation, and Stress-Related Genes Expression in White Clover. Int. J. Mol. Sci. 2018, 19, 2520. [Google Scholar] [CrossRef] [PubMed]
- López-Cristoffanini, C.; Bundó, M.; Serrat, X.; San Segundo, B.; López-Carbonell, M.; Nogués, S. A Comprehensive Study of the Proteins Involved in Salinity Stress Response in Roots and Shoots of the FL478 Genotype of Rice (Oryza sativa L. ssp. Indica). Crop J. 2021, 9, 1154–1168. [Google Scholar] [CrossRef]
- Lv, A.; Fan, N.; Xie, J.; Yuan, S.; An, Y.; Zhou, P. Expression of CdDHN4, a Novel YSK2-Type Dehydrin Gene from Bermudagrass, Responses to Drought Stress through the ABA-Dependent Signal Pathway. Front. Plant Sci. 2017, 8, 748. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Du, D.; An, Y.; Yang, W.; Wang, J.; Cheng, T.; Zhang, Q. Overexpression of Prunus mume Dehydrin Genes in Tobacco Enhances Tolerance to Cold and Drought. Front. Plant Sci. 2017, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Hou, X.; Zhang, Y.; Meng, Y.; Zhang, H.; Liu, S.; Wang, X.; Chen, R. CaDHN5, a Dehydrin Gene from Pepper, Plays an Important Role in Salt and Osmotic Stress Responses. Int. J. Mol. Sci. 2019, 20, 1989. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.-C.; Zhang, H.-F.; Pan, X.-X.; Chen, N.; Hu, H.-F.; Haq, S.U.; Khan, A.; Chen, R.-G. CaDHN3, a Pepper (Capsicum annuum L.) Dehydrin Gene Enhances the Tolerance against Salt and Drought Stresses by Reducing ROS Accumulation. Int. J. Mol. Sci. 2021, 22, 3205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, S.; Ma, J.; Wang, X.; Haq, S.U.; Meng, Y.; Zhang, Y.; Chen, R. CaDHN4, a Salt and Cold Stress-Responsive Dehydrin Gene from Pepper Decreases Abscisic Acid Sensitivity in Arabidopsis. Int. J. Mol. Sci. 2020, 21, 26. [Google Scholar] [CrossRef] [PubMed]
- Halder, T.; Upadhyaya, G.; Ray, S. YSK2 Type Dehydrin (SbDhn1) from Sorghum bicolor Showed Improved Protection under High Temperature and Osmotic Stress Condition. Front. Plant Sci. 2017, 8, 918. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Zhang, L.; Wang, X.; Zhang, M.; Xi, Y.; Wang, A.; Zhu, J. Overexpression of Saussurea involucrata Dehydrin Gene SiDHN Promotes Cold and Drought Tolerance in Transgenic Tomato Plants. PLoS ONE 2019, 14, e0225090. [Google Scholar] [CrossRef] [PubMed]
- Slazak, B.; Jędrzejska, A.; Badyra, B.; Shariatgorji, R.; Nilsson, A.; Andrén, P.E.; Göransson, U. The Influence of Plant Stress Hormones and Biotic Elicitors on Cyclotide Production in Viola uliginosa Cell Suspension Cultures. Plants 2022, 11, 1876. [Google Scholar] [CrossRef]
- Slazak, B.; Kapusta, M.; Malik, S.; Bohdanowicz, J.; Kuta, E.; Malec, P.; Göransson, U. Immunolocalization of Cyclotides in Plant Cells, Tissues and Organ Supports Their Role in Host Defense. Planta 2016, 244, 1029–1040. [Google Scholar] [CrossRef] [PubMed]
- Salehi, H.; Bahramnejad, B.; Majdi, M. Induction of Two Cyclotide-like Genes Zmcyc1 and Zmcyc5 by Abiotic and Biotic Stresses in Zea mays. Acta Physiol. Plant 2017, 39, 131. [Google Scholar] [CrossRef]
- Andrási, N.; Pettkó-Szandtner, A.; Szabados, L. Diversity of Plant Heat Shock Factors: Regulation, Interactions, and Functions. J. Exp. Bot. 2021, 72, 1558–1575. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Shahwar, D. Role of Heat Shock Proteins (HSPs) and Heat Stress Tolerance in Crop Plants. In Sustainable Agriculture in the Era of Climate Change; Roychowdhury, R., Choudhury, S., Hasanuzzaman, M., Srivastava, S., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 211–234. [Google Scholar] [CrossRef]
- Guo, H.; Zhang, H.; Wang, G.; Wang, C.; Wang, Y.; Liu, X.; Ji, W. Identification and Expression Analysis of Heat-Shock Proteins in Wheat Infected with Powdery Mildew and Stripe Rust. Plant Genome 2021, 14, e20092. [Google Scholar] [CrossRef] [PubMed]
- ul Haq, S.; Khan, A.; Ali, M.; Khattak, A.M.; Gai, W.-X.; Zhang, H.-X.; Wei, A.-M.; Gong, Z.-H. Heat Shock Proteins: Dynamic Biomolecules to Counter Plant Biotic and Abiotic Stresses. Int. J. Mol. Sci. 2019, 20, 5321. [Google Scholar] [CrossRef]
- Taghavizadeh Yazdi, M.E.; Amiri, M.S.; Nourbakhsh, F.; Rahnama, M.; Forouzanfar, F.; Mousavi, S.H. Bio-Indicators in Cadmium Toxicity: Role of HSP27 and HSP70. Environ. Sci. Pollut. Res. 2021, 28, 26359–26379. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zheng, Y.; Lei, K.; Tao, W.; Zhou, N. Systematic Analysis of Heat Shock Protein 70 (HSP70) Gene Family in Radish and Potential Roles in Stress Tolerance. BMC Plant Biol. 2024, 24, 2. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.; Yu, T.-F.; Wang, C.-X.; Wei, J.-T.; Zhang, S.-X.; Liu, Y.-W.; Chen, J.; Zhou, Y.-B.; Chen, M.; Ma, Y.-Z.; et al. Heat Shock Protein TaHSP17.4, a TaHOP Interactor in Wheat, Improves Plant Stress Tolerance. Int. J. Biol. Macromol. 2023, 246, 125694. [Google Scholar] [CrossRef] [PubMed]
- Al Khateeb, W.; Muhaidat, R.; Alahmed, S.; Al Zoubi, M.S.; Al-Batayneh, K.M.; El-Oqlah, A.; Abo Gamar, M.; Hussein, E.; Aljabali, A.A.; Alkaraki, A.K. Heat Shock Proteins Gene Expression and Physiological Responses in Durum Wheat (Triticum durum) under Salt Stress. Physiol. Mol. Biol. Plants 2020, 26, 1599–1608. [Google Scholar] [CrossRef]
- Wu, J.; Gao, T.; Hu, J.; Zhao, L.; Yu, C.; Ma, F. Research Advances in Function and Regulation Mechanisms of Plant Small Heat Shock Proteins (sHSPs) under Environmental Stresses. Sci. Total Environ. 2022, 825, 154054. [Google Scholar] [CrossRef]
- Chen, S.; Qiu, G. Overexpression of Zostera japonica Heat Shock Protein Gene ZjHsp70 Enhances the Thermotolerance of Transgenic Arabidopsis. Mol. Biol. Rep. 2022, 49, 6189–6197. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.-H.; Zhang, H.-X.; Ali, M.; Gai, W.-X.; Cheng, G.-X.; Yu, Q.-H.; Yang, S.-B.; Li, X.-X.; Gong, Z.-H. A Small Heat Shock Protein CaHsp25.9 Positively Regulates Heat, Salt, and Drought Stress Tolerance in Pepper (Capsicum annuum L.). Plant Physiol. Biochem. 2019, 142, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhu, J.; Li, X.; Li, Z.; Han, L.; Luo, H. AsHSP26.8a, a Creeping Bentgrass Small Heat Shock Protein Integrates Different Signaling Pathways to Modulate Plant Abiotic Stress Response. BMC Plant Biol. 2020, 20, 184. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dong, K.; Du, H.; Wang, X.; Sun, J.; Hu, Q.; Luo, H.; Sun, X. AsHSP26.2, a Creeping Bentgrass Chloroplast Small Heat Shock Protein Positively Regulates Plant Development. Plant Cell Rep. 2024, 43, 32. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Wu, T.; Shalmani, A.; Xu, L.; Li, C.; Zhang, W.; Pan, R. Heat Shock Protein HvHSP16.9 from Wild Barley Enhances Tolerance to Salt Stress. Physiol. Mol. Biol. Plants 2024, 30, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, J.; Li, M.; Zhang, S.; Song, S.; Wang, W.; Wang, S.; Chang, J.; Xia, Z.; Zhang, S.; et al. NtHSP70-8b Positively Regulates Heat Tolerance and Seed Size in Nicotiana tabacum. Plant Physiol. Biochem. 2023, 201, 107901. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Lv, M.; Liu, Y.; Guo, Q.; Gai, J.; Yang, S. A Small Heat Shock Protein GmHSP18.5a Improves the Male Fertility Restorability of Cytoplasmic Male Sterility-Based Restorer Line under High Temperature Stress in Soybean. Plant Sci. 2023, 337, 111867. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Kaur, S.; Kaur, A.; Sarao, N.K.; Sharma, D.; Kaur, A.; Kaur, S.; Kaur, A.; Sarao, N.K.; Sharma, D. Pathogenesis-Related Proteins and Their Transgenic Expression for Developing Disease-Resistant Crops: Strategies Progress and Challenges. In Case Studies of Breeding Strategies in Major Plant Species; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Patil, S.V.; Jayamohan, N.S.; Kumudini, B.S. Strategic Assessment of Multiple Plant Growth Promotion Traits for Shortlisting of Fluorescent Pseudomonas spp. and Seed Priming against Ragi Blast Disease. Plant Growth Regul. 2016, 80, 47–58. [Google Scholar] [CrossRef]
- Van loon, L.C.; Van Strien, E.A. The Families of Pathogenesis-Related Proteins, Their Activities, and Comparative Analysis of PR-1 Type Proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- Sooriyaarachchi, S.; Jaber, E.; Covarrubias, A.S.; Ubhayasekera, W.; Asiegbu, F.O.; Mowbray, S.L. Expression and β-Glucan Binding Properties of Scots Pine (Pinus sylvestris L.) Antimicrobial Protein (Sp-AMP). Plant Mol. Biol. 2011, 77, 33–45. [Google Scholar] [CrossRef]
- Anil Kumar, S.; Hima Kumari, P.; Shravan Kumar, G.; Mohanalatha, C.; Kavi Kishor, P.B. Osmotin: A Plant Sentinel and a Possible Agonist of Mammalian Adiponectin. Front Plant Sci. 2015, 6, 163. [Google Scholar] [CrossRef]
- Hao, G.; Bakker, M.G.; Kim, H.-S. Enhanced Resistance to Fusarium graminearum in Transgenic Arabidopsis Plants Expressing a Modified Plant Thionin. Phytopathology 2020, 110, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Leybourne, D.J.; Valentine, T.A.; Binnie, K.; Taylor, A.; Karley, A.J.; Bos, J.I.B. Drought Stress Increases the Expression of Barley Defence Genes with Negative Consequences for Infesting Cereal Aphids. J. Exp. Bot. 2022, 73, 2238–2250. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gong, X.; Zhou, D.; Jiang, Q.; Liang, Y.; Ye, R.; Zhang, S.; Wang, Y.; Tang, X.; Li, F.; et al. Plant Defensin-Dissimilar Thionin OsThi9 Alleviates Cadmium Toxicity in Rice Plants and Reduces Cadmium Accumulation in Rice Grains. J. Agric. Food Chem. 2023, 71, 8367–8380. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Lamotte, O.; Alsulaiman, M.; Ruffel, S.; Krouk, G.; Berger, N.; Demolombe, V.; Nespoulous, C.; Dang, T.M.N.; Aimé, S.; et al. Reduction in PLANT DEFENSIN 1 Expression in Arabidopsis thaliana Results in Increased Resistance to Pathogens and Zinc Toxicity. J. Exp. Bot. 2023, 74, 5374–5393. [Google Scholar] [CrossRef] [PubMed]
- dos Santos-Silva, C.A.; Vilela, L.M.B.; de Oliveira-Silva, R.L.d.; da Silva, J.B.; Machado, A.R.; Bezerra-Neto, J.P.; Crovella, S.; Benko-Iseppon, A.M. Cassava (Manihot esculenta) Defensins: Prospection, Structural Analysis and Tissue-Specific Expression under Biotic/Abiotic Stresses. Biochimie 2021, 186, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Boonpa, K.; Tantong, S.; Weerawanich, K.; Panpetch, P.; Pringsulaka, O.; Roytrakul, S.; Sirikantaramas, S. In Silico Analyses of Rice Thionin Genes and the Antimicrobial Activity of OsTHION15 Against Phytopathogens. Phytopathology 2019, 109, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Yusuf, M.A.; Yadav, P.; Narayan, S.; Kumar, M. Overexpression of Chickpea Defensin Gene Confers Tolerance to Water-Deficit Stress in Arabidopsis thaliana. Front. Plant Sci. 2019, 10, 290. [Google Scholar] [CrossRef]
- Craik, D.J.; Malik, U. Cyclotide Biosynthesis. Curr. Opin. Chem. Biol. 2013, 17, 546–554. [Google Scholar] [CrossRef]
- Li, Y.; Bi, T.; Camarero, J.A. Chapter Nine—Chemical and Biological Production of Cyclotides. In Advances in Botanical Research; Craik, D.J., Ed.; Plant Cyclotides; Academic Press: Cambridge, MA, USA, 2015; Volume 76, pp. 271–303. [Google Scholar] [CrossRef]
- de Veer, S.J.; Kan, M.-W.; Craik, D.J. Cyclotides: From Structure to Function. Chem. Rev. 2019, 119, 12375–12421. [Google Scholar] [CrossRef]
- Slazak, B.; Haugmo, T.; Badyra, B.; Göransson, U. The Life Cycle of Cyclotides: Biosynthesis and Turnover in Plant Cells. Plant Cell Rep. 2020, 39, 1359–1367. [Google Scholar] [CrossRef]
- Thorstholm, L.; Craik, D.J. Discovery and Applications of Naturally Occurring Cyclic Peptides. Drug Discov. Today Technol. 2012, 9, e13–e21. [Google Scholar] [CrossRef]
- Burman, R.; Yeshak, M.Y.; Larsson, S.; Craik, D.J.; Rosengren, K.J.; Göransson, U. Distribution of Circular Proteins in Plants: Large-Scale Mapping of Cyclotides in the Violaceae. Front. Plant Sci. 2015, 6, 855. [Google Scholar] [CrossRef]
- Poth, A.G.; Mylne, J.S.; Grassl, J.; Lyons, R.E.; Millar, A.H.; Colgrave, M.L.; Craik, D.J. Cyclotides Associate with Leaf Vasculature and Are the Products of a Novel Precursor in Petunia (Solanaceae). J. Biol. Chem. 2012, 287, 27033–27046. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.-F.; Gagnon, J.; Chiche, L.; Nguyen, T.M.; Andrieu, J.-P.; Heitz, A.; Trinh Hong, T.; Pham, T.T.C.; Le Nguyen, D. Squash Trypsin Inhibitors from Momordica cochinchinensis Exhibit an Atypical Macrocyclic Structure. Biochemistry 2000, 39, 5722–5730. [Google Scholar] [CrossRef] [PubMed]
- Poon, S.; Harris, K.S.; Jackson, M.A.; McCorkelle, O.C.; Gilding, E.K.; Durek, T.; van der Weerden, N.L.; Craik, D.J.; Anderson, M.A. Co-Expression of a Cyclizing Asparaginyl Endopeptidase Enables Efficient Production of Cyclic Peptides in Planta. J. Exp. Bot. 2018, 69, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Gilding, E.K.; Jackson, M.A.; Poth, A.G.; Henriques, S.T.; Prentis, P.J.; Mahatmanto, T.; Craik, D.J. Gene Coevolution and Regulation Lock Cyclic Plant Defence Peptides to Their Targets. New Phytol. 2016, 210, 717–730. [Google Scholar] [CrossRef]
- Slazak, B.; Jędrzejska, A.; Badyra, B.; Sybilska, A.; Lewandowski, M.; Kozak, M.; Kapusta, M.; Shariatgorji, R.; Nilsson, A.; Andrén, P.E.; et al. The Involvement of Cyclotides in Mutual Interactions of Violets and the Two-Spotted Spider Mite. Sci. Rep. 2022, 12, 1914. [Google Scholar] [CrossRef]
- Ho, T.N.T.; Pham, S.H.; Nguyen, L.T.T.; Nguyen, H.T.; Nguyen, L.T.; Dang, T.T. Insights into the Synthesis Strategies of Plant-Derived Cyclotides. Amino Acids 2023, 55, 713–729. [Google Scholar] [CrossRef]
- Strömstedt, A.A.; Park, S.; Burman, R.; Göransson, U. Bactericidal Activity of Cyclotides Where Phosphatidylethanolamine-Lipid Selectivity Determines Antimicrobial Spectra. Biochim. Biophys. Acta Biomembr. 2017, 1859, 1986–2000. [Google Scholar] [CrossRef]
- Slazak, B.; Kapusta, M.; Strömstedt, A.A.; Słomka, A.; Krychowiak, M.; Shariatgorji, M.; Andrén, P.E.; Bohdanowicz, J.; Kuta, E.; Göransson, U. How Does the Sweet Violet (Viola odorata L.) Fight Pathogens and Pests—Cyclotides as a Comprehensive Plant Host Defense System. Front. Plant Sci. 2018, 9, 1296. [Google Scholar] [CrossRef]
- Svangård, E.; Burman, R.; Gunasekera, S.; Lövborg, H.; Gullbo, J.; Göransson, U. Mechanism of Action of Cytotoxic Cyclotides: Cycloviolacin O2 Disrupts Lipid Membranes. J. Nat. Prod. 2007, 70, 643–647. [Google Scholar] [CrossRef]
- Eliasen, R.; Daly, N.L.; Wulff, B.S.; Andresen, T.L.; Conde-Frieboes, K.W.; Craik, D.J. Design, Synthesis, Structural and Functional Characterization of Novel Melanocortin Agonists Based on the Cyclotide Kalata B1. J. Biol. Chem. 2012, 287, 40493–40501. [Google Scholar] [CrossRef]
- Kalmankar, N.V.; Hari, H.; Sowdhamini, R.; Venkatesan, R. Disulfide-Rich Cyclic Peptides from Clitoria ternatea Protect against β-Amyloid Toxicity and Oxidative Stress in Transgenic Caenorhabditis elegans. J. Med. Chem. 2021, 64, 7422–7433. [Google Scholar] [CrossRef]
- Huynh, N.T.; Ho, T.N.T.; Pham, Y.N.D.; Dang, L.H.; Pham, S.H.; Dang, T.T. Immunosuppressive Cyclotides: A Promising Approach for Treating Autoimmune Diseases. Protein J. 2024, 43, 159–170. [Google Scholar] [CrossRef]
- Dayani, L.; Dinani, M.S.; Aliomrani, M.; Hashempour, H.; Varshosaz, J.; Taheri, A. Immunomodulatory Effects of Cyclotides Isolated from Viola odorata in an Experimental Autoimmune Encephalomyelitis Animal Model of Multiple Sclerosis. Mult. Scler. Relat. Disord. 2022, 64, 103958. [Google Scholar] [CrossRef]
- Troeira Henriques, S.; Craik, D.J. Cyclotide Structure and Function: The Role of Membrane Binding and Permeation. Biochem. 2017, 56, 669–682. [Google Scholar] [CrossRef]
- Tran, G.-H.; Tran, T.-H.; Pham, S.H.; Xuan, H.L.; Dang, T.T. Cyclotides: The next Generation in Biopesticide Development for Eco-Friendly Agriculture. J. Pept. Sci. 2024, 30, e3570. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant Adaptations to the Combination of Drought and High Temperatures. Physiol. Plant. 2018, 162, 2–12. [Google Scholar] [CrossRef]
- Sharma, L.; Priya, M.; Kaushal, N.; Bhandhari, K.; Chaudhary, S.; Dhankher, O.P.; Prasad, P.V.V.; Siddique, K.H.M.; Nayyar, H. Plant Growth-Regulating Molecules as Thermoprotectants: Functional Relevance and Prospects for Improving Heat Tolerance in Food Crops. J. Exp. Bot. 2020, 71, 569–594. [Google Scholar] [CrossRef] [PubMed]
- Saini, N.; Nikalje, G.C.; Zargar, S.M.; Suprasanna, P. Molecular Insights into Sensing, Regulation and Improving of Heat Tolerance in Plants. Plant Cell Rep. 2022, 41, 799–813. [Google Scholar] [CrossRef]
- Tian, F.; Hu, X.-L.; Yao, T.; Yang, X.; Chen, J.-G.; Lu, M.-Z.; Zhang, J. Recent Advances in the Roles of HSFs and HSPs in Heat Stress Response in Woody Plants. Front. Plant Sci. 2021, 12, 704905. [Google Scholar] [CrossRef]
- Namazi, F.; Bordbar, E.; Bakhshaei, F.; Nazifi, S. The Effect of Urtica Dioica Extract on Oxidative Stress, Heat Shock Proteins, and Brain Histopathology in Multiple Sclerosis Model. Physiol. Rep. 2022, 10, e15404. [Google Scholar] [CrossRef]
- Qin, F.; Yu, B.; Li, W. Heat Shock Protein 101 (HSP101) Promotes Flowering under Nonstress Conditions. Plant Physiol. 2021, 186, 407–419. [Google Scholar] [CrossRef]
- Majee, A.; Kumari, D.; Sane, V.A.; Singh, R.K. Novel Roles of HSFs and HSPs, Other than Relating to Heat Stress, in Temperature-Mediated Flowering. Ann. Bot. 2023, 132, 1103–1106. [Google Scholar] [CrossRef]
- Guz, N.; Dageri, A.; Altincicek, B.; Aksoy, S. Molecular Characterization and Expression Patterns of Heat Shock Proteins in Spodoptera littoralis, Heat Shock or Immune Response? Cell Stress Chaperon. 2021, 26, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Liu, X.-Y.; Li, B.; Li, M.-Y.; Li, S.-G.; Liu, S. A Heat Shock Protein Protects against Oxidative Stress Induced by Lambda-Cyhalothrin in the Green Peach Aphid Myzus persicae. Pestic. Biochem. Physiol. 2022, 181, 104995. [Google Scholar] [CrossRef]
- Sandner, G.; Mueller, A.S.; Zhou, X.; Stadlbauer, V.; Schwarzinger, B.; Schwarzinger, C.; Wenzel, U.; Maenner, K.; van der Klis, J.D.; Hirtenlehner, S.; et al. Ginseng Extract Ameliorates the Negative Physiological Effects of Heat Stress by Supporting Heat Shock Response and Improving Intestinal Barrier Integrity: Evidence from Studies with Heat-Stressed Caco-2 Cells, C. elegans and Growing Broilers. Molecules 2020, 25, 835. [Google Scholar] [CrossRef] [PubMed]
- Madkour, M.; Alaqaly, A.M.; Soliman, S.S.; Ali, S.I.; Aboelazab, O. Growth Performance, Blood Biochemistry, and mRNA Expression of Hepatic Heat Shock Proteins of Heat-Stressed Broilers in Response to Rosemary and Oregano Extracts. J. Therm. Biol. 2024, 119, 103791. [Google Scholar] [CrossRef]
- Finkina, E.I.; Melnikova, D.N.; Bogdanov, I.V.; Ovchinnikova, T.V. Plant Pathogenesis-Related Proteins PR-10 and PR-14 as Components of Innate Immunity System and Ubiquitous Allergens. Curr. Med. Chem. 2017, 24, 1772–1787. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Kumar, A.; Singh, I.K.; Singh, A. Pathogenesis Related Proteins: A Defensin for Plants but an Allergen for Humans. Int. J. Biol. Macromol. 2020, 157, 659–672. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Xu, Y.; Cao, J.; Zhu, Z.; Jiao, Y.; Wang, Y.; Guan, X.; Yang, Y.; Xu, W.; Fu, Z. Subcellular Localization and Functional Analyses of a PR10 Protein Gene from Vitis pseudoreticulata in Response to Plasmopara viticola Infection. Protoplasma 2013, 250, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Tan, J.; Lu, S.; Lin, C.; Hu, Y.; Guo, Z. Increased Tolerance to Oxidative Stress in Transgenic Tobacco Expressing a Wheat Oxalate Oxidase Gene via Induction of Antioxidant Enzymes Is Mediated by H2O2. Physiol. Plant. 2009, 136, 30–44. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P.; et al. Pathogenesis-Related Proteins and Peptides as Promising Tools for Engineering Plants with Multiple Stress Tolerance. Microbiol. Res. 2018, 212–213, 29–37. [Google Scholar] [CrossRef]
- Okushima, Y.; Koizumi, N.; Kusano, T.; Sano, H. Secreted Proteins of Tobacco Cultured BY2 Cells: Identification of a New Member of Pathogenesis-Related Proteins. Plant Mol. Biol. 2000, 42, 479–488. [Google Scholar] [CrossRef]
- Anisimova, O.K.; Shchennikova, A.V.; Kochieva, E.Z.; Filyushin, M.A. Pathogenesis-Related Genes of PR1, PR2, PR4, and PR5 Families Are Involved in the Response to Fusarium Infection in Garlic (Allium sativum L.). Int. J. Mol. Sci. 2021, 22, 6688. [Google Scholar] [CrossRef]
- Fu, Z.Q.; Dong, X. Systemic Acquired Resistance: Turning Local Infection into Global Defense. Annu. Rev. Plant Biol. 2013, 64, 839–863. [Google Scholar] [CrossRef]
- Backer, R.; Naidoo, S.; van den Berg, N. The NONEXPRESSOR OF PATHOGENESIS-RELATED GENES 1 (NPR1) and Related Family: Mechanistic Insights in Plant Disease Resistance. Front. Plant Sci. 2019, 10, 102. [Google Scholar] [CrossRef]
- Camagna, M.; Takemoto, D. Hypersensitive Response in Plants. In eLS; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 1–7. [Google Scholar] [CrossRef]
- Li, J.; Hu, S.; Jian, W.; Xie, C.; Yang, X. Plant Antimicrobial Peptides: Structures, Functions, and Applications. Bot. Stud. 2021, 62, 5. [Google Scholar] [CrossRef]
- Slezina, M.P.; Odintsova, T.I. Plant Antimicrobial Peptides: Insights into Structure-Function Relationships for Practical Applications. Curr. Issues Mol. Biol. 2023, 45, 3674–3704. [Google Scholar] [CrossRef]
- Lima, A.M.; Azevedo, M.I.G.; Sousa, L.M.; Oliveira, N.S.; Andrade, C.R.; Freitas, C.D.T.; Souza, P.F.N. Plant Antimicrobial Peptides: An Overview about Classification, Toxicity and Clinical Applications. Int. J. Biol. Macromol. 2022, 214, 10–21. [Google Scholar] [CrossRef] [PubMed]
- van der Weerden, N.L.; Bleackley, M.R.; Anderson, M.A. Properties and Mechanisms of Action of Naturally Occurring Antifungal Peptides. Cell Mol. Life Sci. 2013, 70, 3545–3570. [Google Scholar] [CrossRef]
- Muramoto, N.; Tanaka, T.; Shimamura, T.; Mitsukawa, N.; Hori, E.; Koda, K.; Otani, M.; Hirai, M.; Nakamura, K.; Imaeda, T. Transgenic Sweet Potato Expressing Thionin from Barley Gives Resistance to Black Rot Disease Caused by Ceratocystis fimbriata in Leaves and Storage Roots. Plant Cell Rep. 2012, 31, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Oard, S.V. Deciphering a Mechanism of Membrane Permeabilization by α-Hordothionin Peptide. Biochim. Biophys. Acta Biomembr. 2011, 1808, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Barashkova, A.S.; Sadykova, V.S.; Salo, V.A.; Zavriev, S.K.; Rogozhin, E.A. Nigellothionins from Black Cumin (Nigella sativa L.) Seeds Demonstrate Strong Antifungal and Cytotoxic Activity. Antibiotics 2021, 10, 166. [Google Scholar] [CrossRef]
- Sewelam, N.; El-Shetehy, M.; Mauch, F.; Maurino, V.G. Combined Abiotic Stresses Repress Defense and Cell Wall Metabolic Genes and Render Plants More Susceptible to Pathogen Infection. Plants 2021, 10, 1946. [Google Scholar] [CrossRef] [PubMed]
- Hammad, I.A.; Razek, A.; Soliman, E.; Tawfik, E. Transgenic Potato (Solanum tuberosum) Expressing Two Antifungal Thionin Genes Confer Resistance to Fusarium spp. J. Pharm. Biol. Sci. 2017, 12, 69–79. [Google Scholar] [CrossRef]
- Hao, G.; Stover, E.; Gupta, G. Overexpression of a Modified Plant Thionin Enhances Disease Resistance to Citrus Canker and Huanglongbing (HLB). Front. Plant Sci. 2016, 7, 1078. [Google Scholar] [CrossRef] [PubMed]
- Odintsova, T.I.; Slezina, M.P.; Istomina, E.A. Defensins of Grasses: A Systematic Review. Biomolecules 2020, 10, 1029. [Google Scholar] [CrossRef]
- Tam, J.P.; Wang, S.; Wong, K.H.; Tan, W.L. Antimicrobial Peptides from Plants. Pharmaceuticals 2015, 8, 711–757. [Google Scholar] [CrossRef]
- Kovaleva, V.; Bukhteeva, I.; Kit, O.Y.; Nesmelova, I.V. Plant Defensins from a Structural Perspective. Int. J. Mol. Sci. 2020, 21, 5307. [Google Scholar] [CrossRef]
- Bleackley, M.R.; Vasa, S.; Harvey, P.J.; Shafee, T.M.A.; Kerenga, B.K.; Soares da Costa, T.P.; Craik, D.J.; Lowe, R.G.T.; Anderson, M.A. Histidine-Rich Defensins from the Solanaceae and Brasicaceae Are Antifungal and Metal Binding Proteins. J. Fungi 2020, 6, 145. [Google Scholar] [CrossRef]
- Shafee, T.M.A.; Lay, F.T.; Phan, T.K.; Anderson, M.A.; Hulett, M.D. Convergent Evolution of Defensin Sequence, Structure and Function. Cell Mol. Life Sci. 2017, 74, 663–682. [Google Scholar] [CrossRef]
- Parisi, K.; Shafee, T.M.A.; Quimbar, P.; van der Weerden, N.L.; Bleackley, M.R.; Anderson, M.A. The Evolution, Function and Mechanisms of Action for Plant Defensins. Semin. Cell Dev. Biol. 2019, 88, 107–118. [Google Scholar] [CrossRef]
- Luo, J.-S.; Gu, T.; Yang, Y.; Zhang, Z. A Non-Secreted Plant Defensin AtPDF2.6 Conferred Cadmium Tolerance via Its Chelation in Arabidopsis. Plant Mol. Biol. 2019, 100, 561–569. [Google Scholar] [CrossRef]
- Gu, T.-Y.; Qi, Z.-A.; Chen, S.-Y.; Yan, J.; Fang, Z.-J.; Wang, J.-M.; Gong, J.-M. Dual-Function DEFENSIN 8 Mediates Phloem Cadmium Unloading and Accumulation in Rice Grains. Plant Physiol. 2023, 191, 515–527. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, D.; Yue, N.; Song, H.; Luo, J.; Zhang, Z. PDF1.5 Enhances Adaptation to Low Nitrogen Levels and Cadmium Stress. Int. J. Mol. Sci. 2021, 22, 10455. [Google Scholar] [CrossRef]
- Sher Khan, R.; Iqbal, A.; Malak, R.; Shehryar, K.; Attia, S.; Ahmed, T.; Ali Khan, M.; Arif, M.; Mii, M. Plant Defensins: Types, Mechanism of Action and Prospects of Genetic Engineering for Enhanced Disease Resistance in Plants. 3 Biotech 2019, 9, 192. [Google Scholar] [CrossRef]
- Wang, X.; Zafar, J.; Yang, X.; De Mandal, S.; Hong, Y.; Jin, F.; Xu, X. Gut Bacterium Burkholderia cepacia (BsNLG8) and Immune Gene Defensin a Contribute to the Resistance against Nicotine-Induced Stress in Nilaparvata lugens (Stål). Ecotoxicol. Environ. Saf. 2024, 277, 116371. [Google Scholar] [CrossRef] [PubMed]
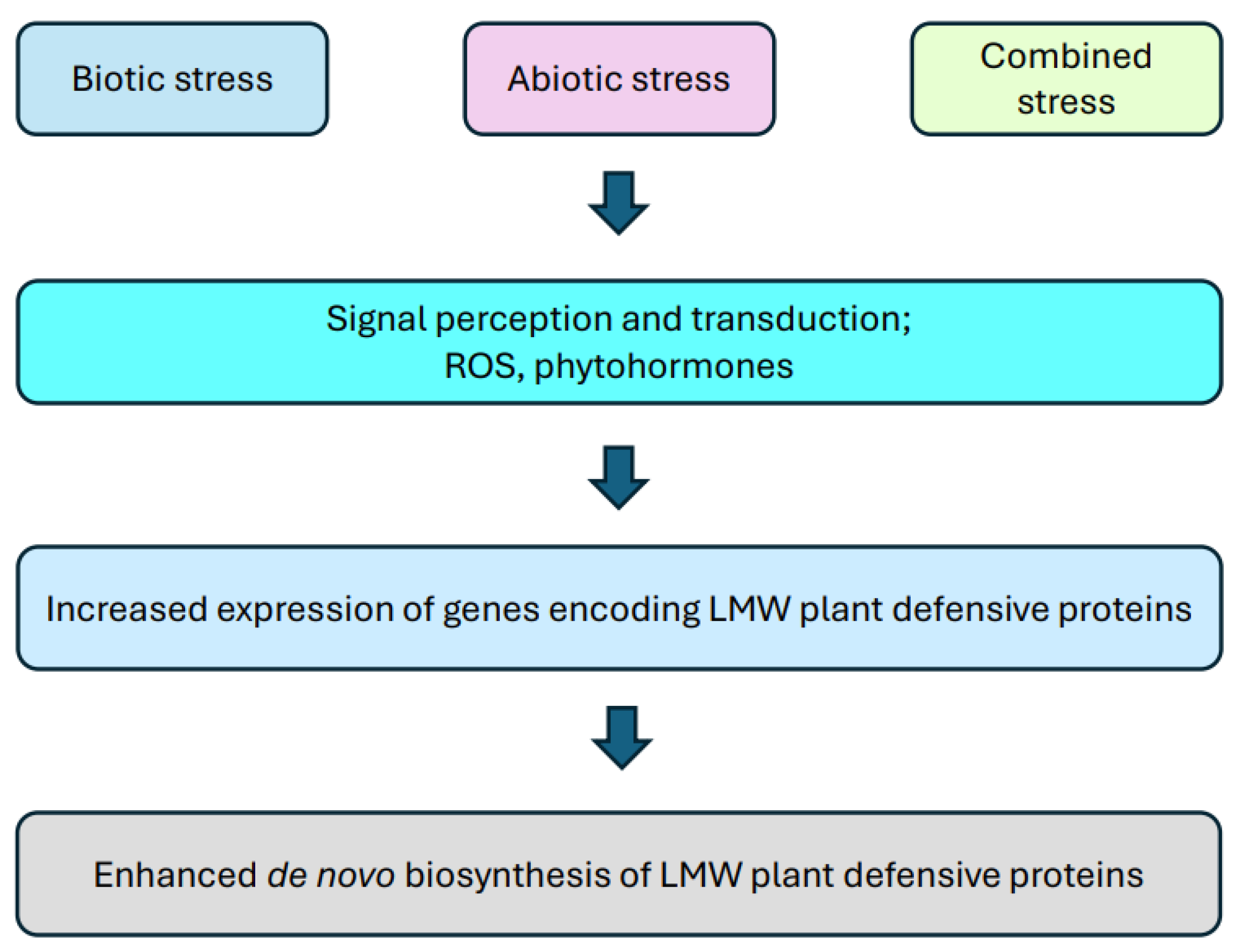
| Groups of Proteins | Stress Factors Affecting Plants | References |
|---|---|---|
| Dehydrins | Drought; Low temperature; Osmotic stress; Salt stress. | [44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60] |
| Cyclotides | Jasmonic acid, methyl jasmonate and salicylic acid treatments; Pathogen attack; Pest infestation; Mechanical injury. | [61,62,63] |
| Heat shock proteins | Drought; Heavy metal stress; Heat stress; Low temperature; Salt stress; UV radiation; Mechanical injury; Pathogen attack. | [64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79] |
| Pathogenesis-related proteins | Heavy metal stress; Mechanical injury; Pathogen attack; Pest infestation. | [80,81,82,83,84,85,86,87,88,89] |
| Cloned Gene | Source Plant Species | Target Plant Species | Increased Tolerance to the Specific Abiotic and Biotic Stresses | References |
|---|---|---|---|---|
| CaDHN5 (dehydrin) | Capsicum annuum L. | Arabidopsis thaliana L. | Salt and osmotic stress | [56] |
| CaDHN4 (dehydrin) | Capsicum annuum L. | Arabidopsis thaliana L. | Salt and cold stress | [58] |
| CaDHN3 (dehydrin) | Capsicum annuum L. | Arabidopsis thaliana L. | Osmotic stress | [57] |
| SbDHN1 (dehydrin) | Sorghum bicolor (L.) Moench | Nicotiana tabacum L. | High-temperature and osmotic stress | [59] |
| SiDHN (dehydrin) | Saussurea involucrate Kar. and Kir. | Solanum lycopersicum L. | Drought and cold stress | [60] |
| ZjHsp70 (heat shock protein) | Zostera japonica Asch. and Graebn | Arabidopsis thaliana L. | High-temperature stress | [73] |
| CaHsp25.9 (heat shock protein) | Capsicum annuum L. | Arabidopsis thaliana L. | High-temperature, salt, and drought stress | [74] |
| HvHSP16.9 (heat shock protein) | Hordeum spontaneum (Koch) Thell | Arabidopsis thaliana L. | Salt stress | [77] |
| OsTHION15 (thionin) | Oryza sativa L. | Nicotiana benthamiana Domin | Bacterial and fungal infection | [90] |
| Ca-AFP (defensin) | Cicer arietinum L. | Arabidopsis thaliana L. | Water-deficit stress | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruszczyńska, M.; Sytykiewicz, H. New Insights into Involvement of Low Molecular Weight Proteins in Complex Defense Mechanisms in Higher Plants. Int. J. Mol. Sci. 2024, 25, 8531. https://doi.org/10.3390/ijms25158531
Ruszczyńska M, Sytykiewicz H. New Insights into Involvement of Low Molecular Weight Proteins in Complex Defense Mechanisms in Higher Plants. International Journal of Molecular Sciences. 2024; 25(15):8531. https://doi.org/10.3390/ijms25158531
Chicago/Turabian StyleRuszczyńska, Magdalena, and Hubert Sytykiewicz. 2024. "New Insights into Involvement of Low Molecular Weight Proteins in Complex Defense Mechanisms in Higher Plants" International Journal of Molecular Sciences 25, no. 15: 8531. https://doi.org/10.3390/ijms25158531
APA StyleRuszczyńska, M., & Sytykiewicz, H. (2024). New Insights into Involvement of Low Molecular Weight Proteins in Complex Defense Mechanisms in Higher Plants. International Journal of Molecular Sciences, 25(15), 8531. https://doi.org/10.3390/ijms25158531






