SCD2 Regulation Targeted by miR-200c-3p on Lipogenesis Alleviates Mesenchymal Stromal Cell Senescence
Abstract
1. Introduction
2. Results
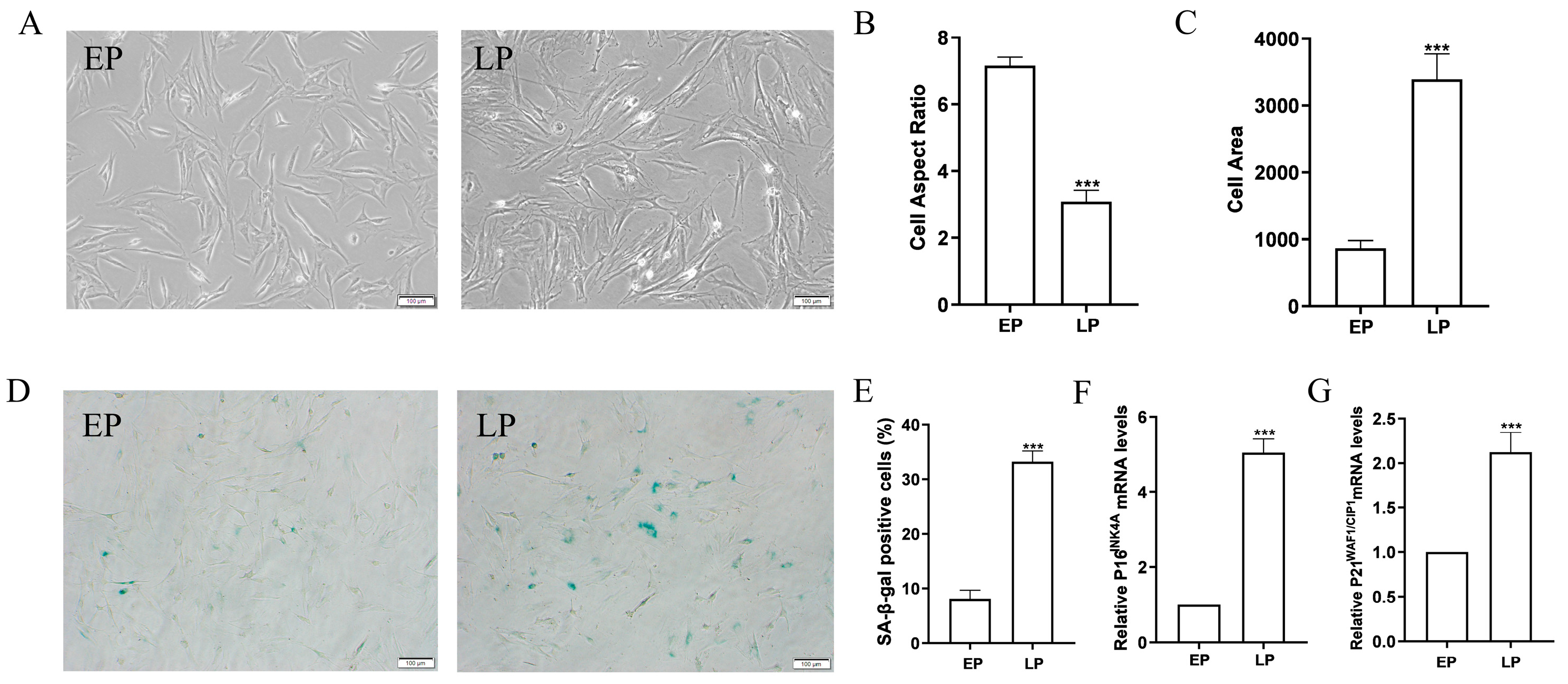
2.1. MSCs at Late Passage Present Obvious Senescent Phenotypes
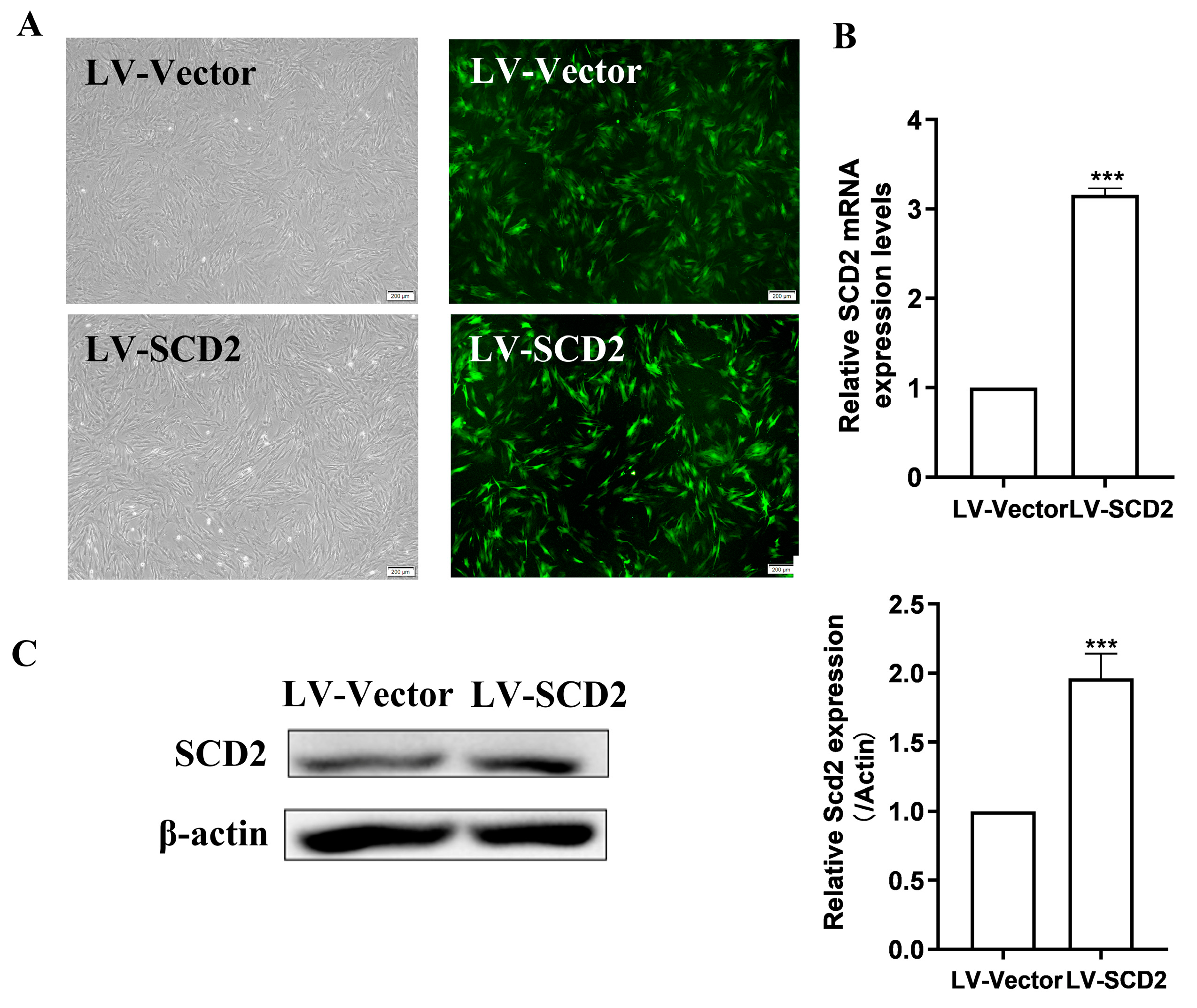
2.2. SCD2 Expression Is Enforced in Late Passage MSCs
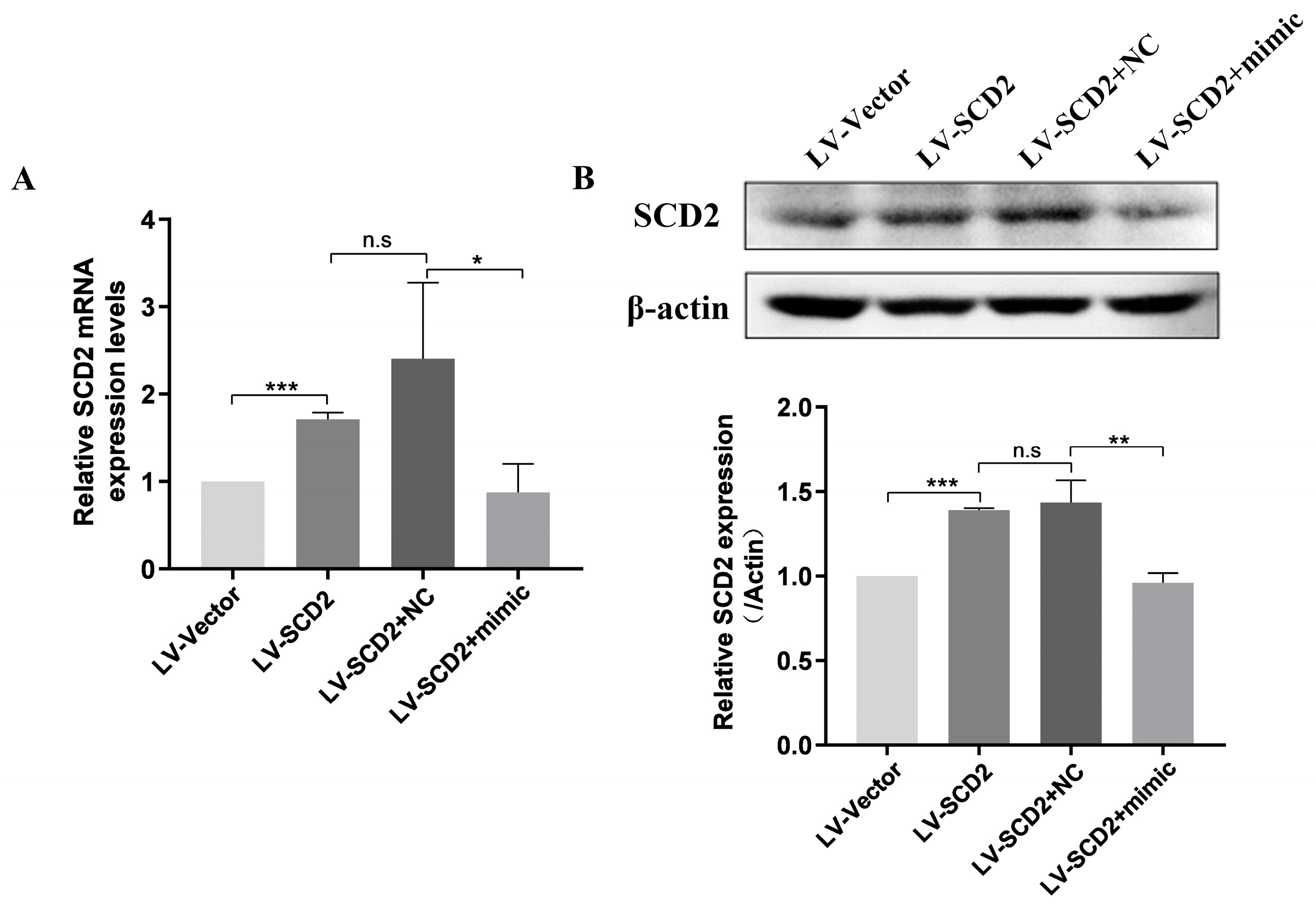
2.3. SCD2 Replenishment Alleviates MSC Senescence by Boosting Lipogenesis
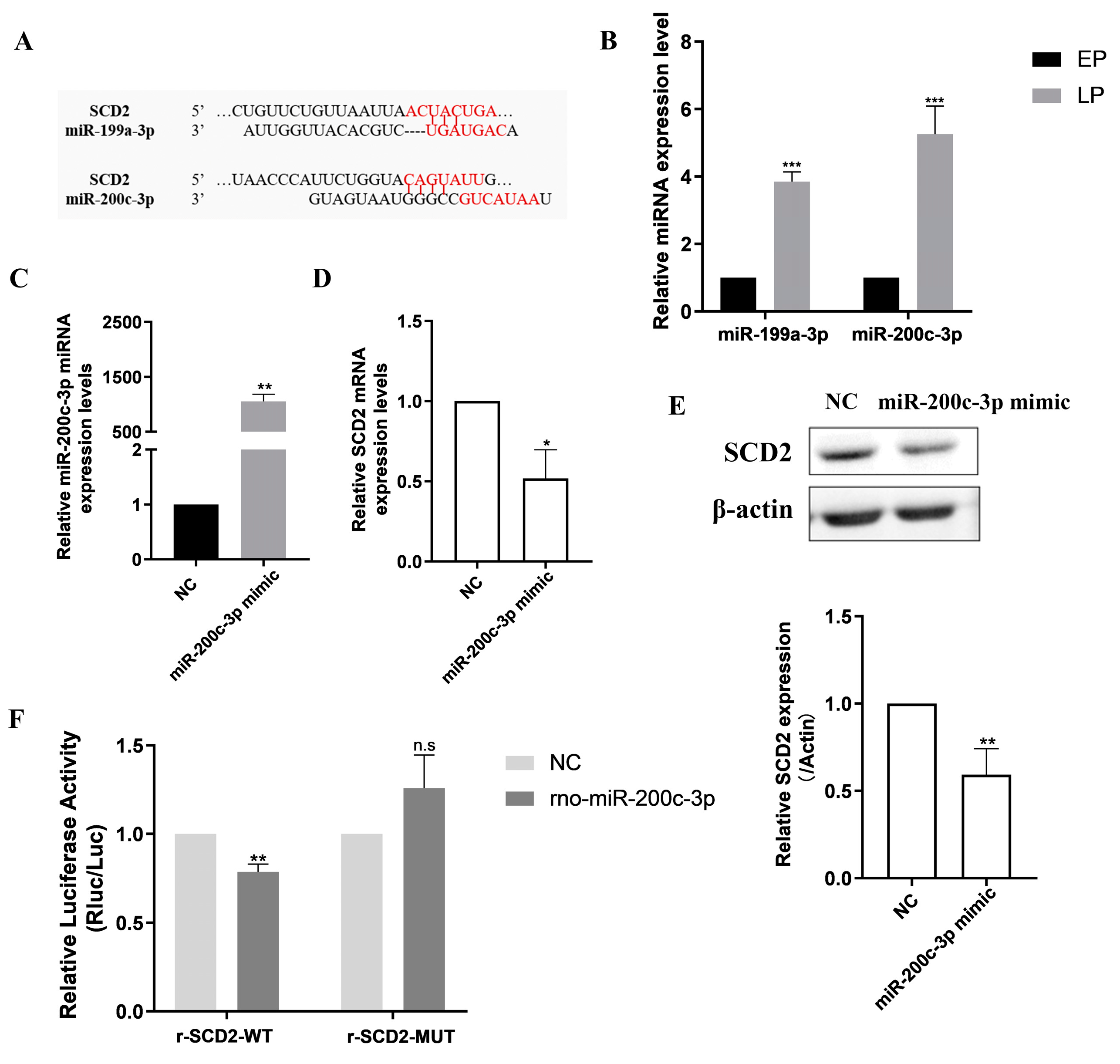
2.4. Prediction and Identification of Upstream miRNAs Targeting SCD2
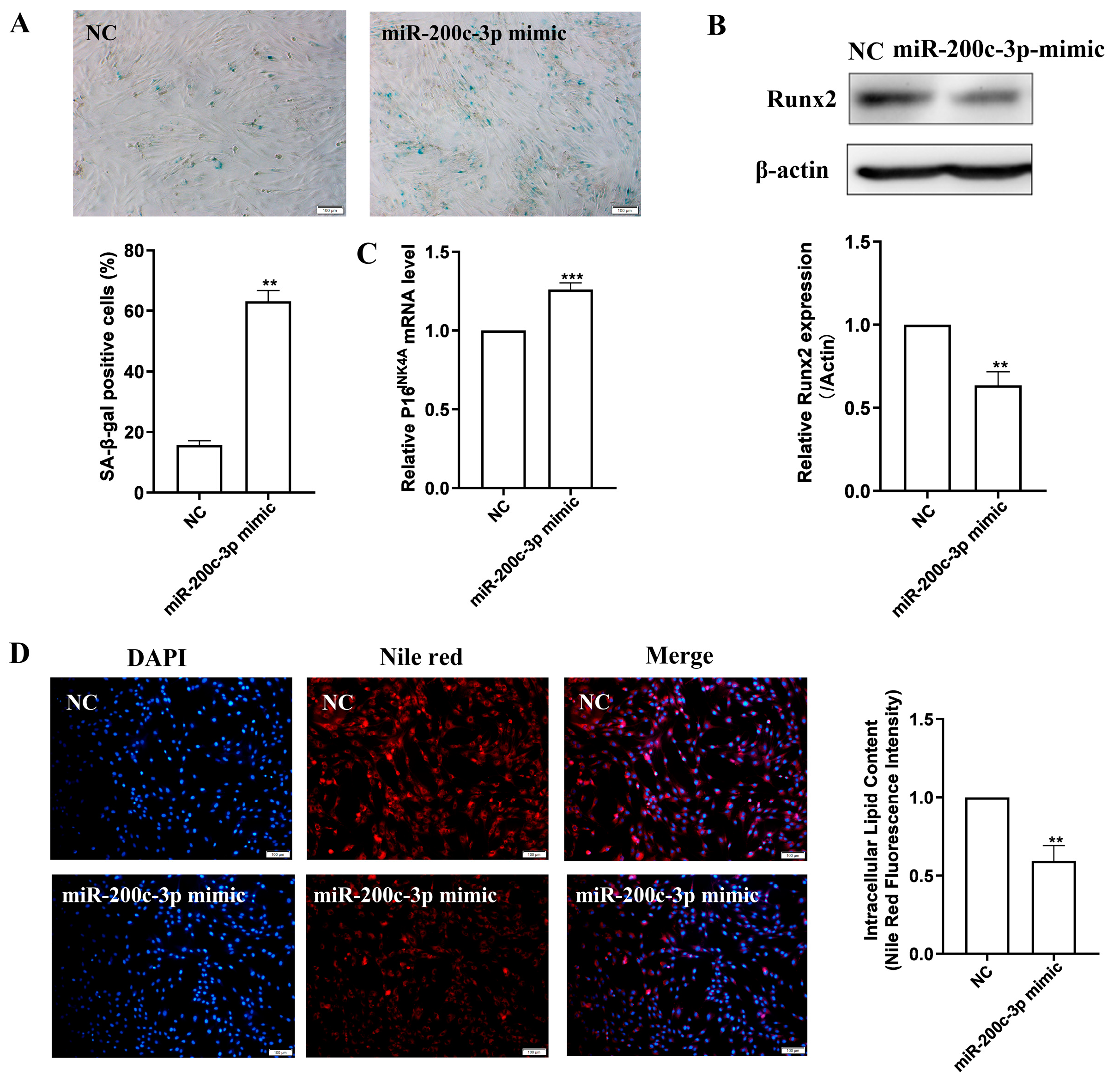
2.5. MiR-200c-3p Over-Expression Accelerates MSC Senescence and Eliminates Lipogenesis
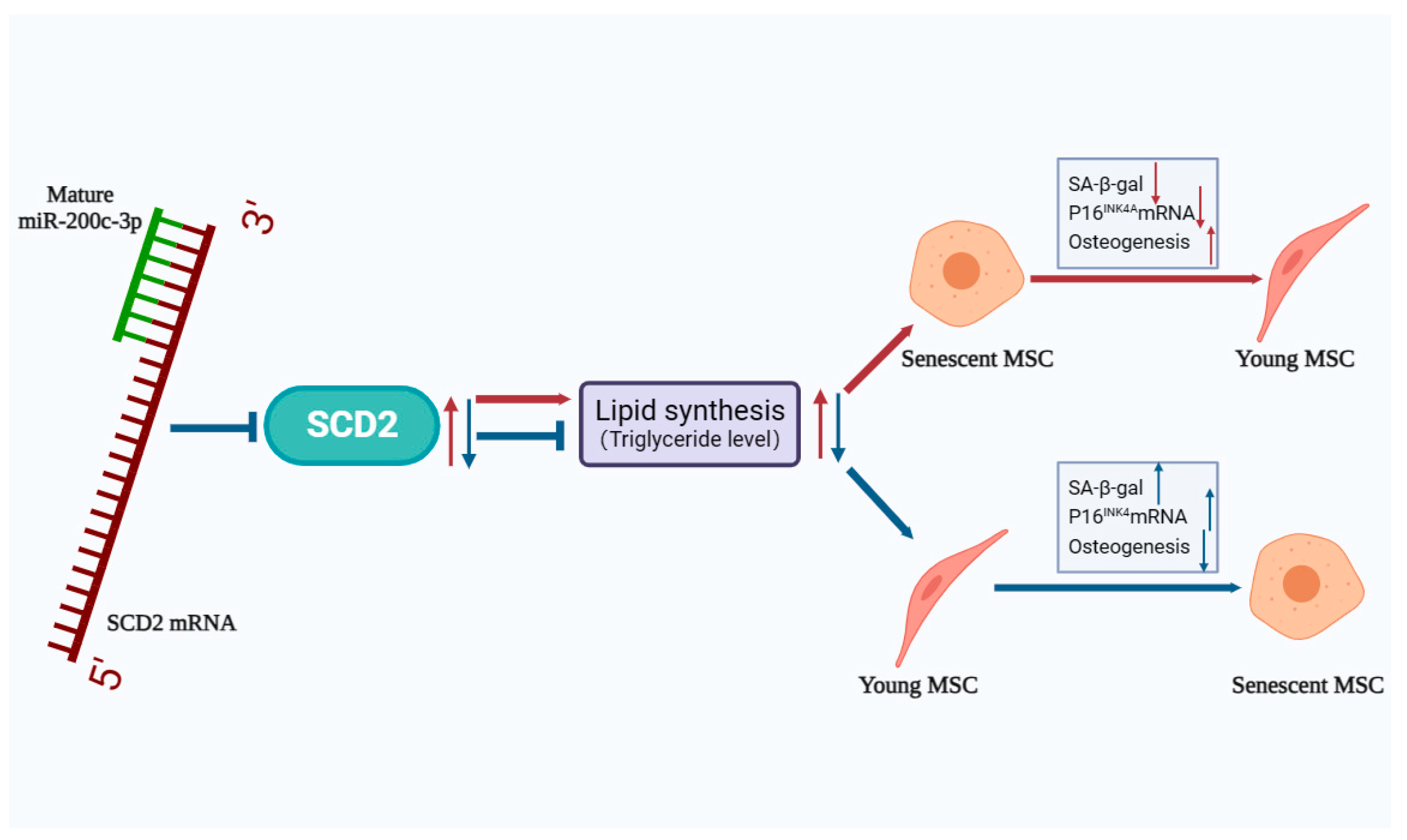
2.6. SCD2 Is Targeted by miR-200c-3p to Influence MSC Senescence via Regulating Lipogenesis
3. Discussion
4. Materials and Methods
4.1. MSC Isolation and Culture
4.2. Morphological Observation and Quantitative Analysis of MSCs
4.3. Senescence-Associated β-Galactosidase (SA-β-Gal) Staining
4.4. Construction of SCD2-Over-Expressed MSCs
4.5. Osteogenic Differentiation and Alizarin Red s (ARS) Staining
4.6. Nile Red Staining
4.7. Prediction for Upstream miRNAs of SCD2
4.8. Dual Luciferase Reporter Assay
4.9. Construction of miR-200c-3p-Over-Expressed MSCs
4.10. Gene Expression Analysis
4.11. Western Blot Analysis
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Bin-Jumah, M.N.; Nadeem, M.S.; Gilani, S.J.; Al-Abbasi, F.A.; Ullah, I.; Alzarea, S.I.; Ghoneim, M.M.; Alshehri, S.; Uddin, A.; Murtaza, B.N.; et al. Genes and Longevity of Lifespan. Int. J. Mol. Sci. 2022, 23, 1499. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Xing, L.; Zhang, L.; Liu, F.; Wang, S.; Xie, Y.; Wang, J.; Jiang, H.; Guo, J.; Li, X.; et al. NAP1L2 drives mesenchymal stem cell senescence and suppresses osteogenic differentiation. Aging Cell 2022, 21, e13551. [Google Scholar] [CrossRef]
- Liu, J.; Ding, Y.; Liu, Z.; Liang, X. Senescence in Mesenchymal Stem Cells: Functional Alterations, Molecular Mechanisms, and Rejuvenation Strategies. Front. Cell Dev. Biol. 2020, 8, 258. [Google Scholar] [CrossRef] [PubMed]
- Wechsler, M.E.; Rao, V.V.; Borelli, A.N.; Anseth, K.S. Engineering the Msc Secretome: A Hydrogel Focused Approach. Adv. Healthc. Mater. 2021, 10, e2001948. [Google Scholar] [CrossRef]
- Beane, O.S.; Fonseca, V.C.; Cooper, L.L.; Koren, G.; Darling, E.M. Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS ONE 2014, 9, e115963. [Google Scholar] [CrossRef]
- Vono, R.; Jover Garcia, E.; Spinetti, G.; Madeddu, P. Oxidative Stress in Mesenchymal Stem Cell Senescence: Regulation by Coding and Noncoding RNAs. Antioxid. Redox Signal. 2018, 29, 864–879. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, H.; Kusumoto, D.; Hashimoto, H.; Yuasa, S. Stem Cell Aging in Skeletal Muscle Regeneration and Disease. Int. J. Mol. Sci. 2020, 21, 1830. [Google Scholar] [CrossRef] [PubMed]
- Weichhart, T. mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology 2018, 64, 127–134. [Google Scholar] [CrossRef]
- Kersten, S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2001, 2, 282–286. [Google Scholar] [CrossRef]
- Knobloch, M.; Braun, S.M.; Zurkirchen, L.; von Schoultz, C.; Zamboni, N.; Araúzo-Bravo, M.J.; Kovacs, W.J.; Karalay, O.; Suter, U.; Machado, R.A.; et al. Metabolic control of adult neural stem cell activity by Fasn-dependent lipogenesis. Nature 2013, 493, 226–230. [Google Scholar] [CrossRef]
- Wang, B.; Rong, X.; Palladino, E.N.D.; Wang, J.; Fogelman, A.M.; Martín, M.G.; Alrefai, W.A.; Ford, D.A.; Tontonoz, P. Phospholipid Remodeling and Cholesterol Availability Regulate Intestinal Stemness and Tumorigenesis. Cell Stem Cell 2018, 22, 206–220.e4. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Lu, Y.; Wang, Y.; Du, L.; Zhang, Y.; Tao, J. Let-7c regulates proliferation and osteodifferentiation of human adipose-derived mesenchymal stem cells under oxidative stress by targeting SCD-1. Am. J. Physiol. Cell Physiol. 2019, 316, C57–C69. [Google Scholar] [CrossRef]
- Chen, Y.S.; Kang, X.R.; Zhou, Z.H.; Yang, J.; Xin, Q.; Ying, C.T.; Zhang, Y.P.; Tao, J. MiR-1908/EXO1 and MiR-203a/FOS, regulated by scd1, are associated with fracture risk and bone health in postmenopausal diabetic women. Aging 2020, 12, 9549–9584. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, Y.; Wang, H.; Bai, Y.; Zhao, J.; Zhang, X.; Liang, L.; Chen, Y.; Ye, C.; Li, Y.; et al. Integrated Lipidomics and Transcriptomics Characterization upon Aging-Related Changes of Lipid Species and Pathways in Human Bone Marrow Mesenchymal Stem Cells. J. Proteome Res. 2019, 18, 2065–2077. [Google Scholar] [CrossRef]
- Yu, X.; Sun, H.; Gao, X.; Zhang, C.; Sun, Y.; Wang, H.; Zhang, H.; Shi, Y.; He, X. A comprehensive analysis of age-related metabolomics and transcriptomics reveals metabolic alterations in rat bone marrow mesenchymal stem cells. Aging 2022, 14, 1014–1032. [Google Scholar] [CrossRef] [PubMed]
- Rykova, E.; Ershov, N.; Damarov, I.; Merkulova, T. SNPs in 3′UTR miRNA Target Sequences Associated with Individual Drug Susceptibility. Int. J. Mol. Sci. 2022, 23, 13725. [Google Scholar] [CrossRef]
- Potter, M.L.; Hill, W.D.; Isales, C.M.; Hamrick, M.W.; Fulzele, S. MicroRNAs are critical regulators of senescence and aging in mesenchymal stem cells. Bone 2021, 142, 115679. [Google Scholar] [CrossRef]
- Wagschal, A.; Najafi-Shoushtari, S.H.; Wang, L.; Goedeke, L.; Sinha, S.; deLemos, A.S.; Black, J.C.; Ramírez, C.M.; Li, Y.; Tewhey, R.; et al. Genome-wide identification of microRNAs regulating cholesterol and triglyceride homeostasis. Nat. Med. 2015, 21, 1290–1297. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, J.; Wang, C.; Li, K.; Liu, B.; Du, Y.; Xiao, F.; Chen, S.; Guo, F. miR-212-5p suppresses lipid accumulation by targeting FAS and SCD1. J. Mol. Endocrinol. 2017, 59, 205–217. [Google Scholar] [CrossRef]
- Nishiga, M.; Horie, T.; Kuwabara, Y.; Nagao, K.; Baba, O.; Nakao, T.; Nishino, T.; Hakuno, D.; Nakashima, Y.; Nishi, H.; et al. MicroRNA-33 Controls Adaptive Fibrotic Response in the Remodeling Heart by Preserving Lipid Raft Cholesterol. Circ. Res. 2017, 120, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Docrat, T.F.; Nagiah, S.; Krishnan, A.; Naidoo, D.B.; Chuturgoon, A.A. Atorvastatin induces MicroRNA-145 expression in HEPG2 cells via regulation of the PI3K/AKT signalling pathway. Chem.-Biol. Interact. 2018, 287, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Capri, M.; Olivieri, F.; Lanzarini, C.; Remondini, D.; Borelli, V.; Lazzarini, R.; Graciotti, L.; Albertini, M.C.; Bellavista, E.; Santoro, A.; et al. Identification of miR-31-5p, miR-141-3p, miR-200c-3p, and GLT1 as human liver aging markers sensitive to donor-recipient age-mismatch in transplants. Aging Cell 2017, 16, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Magenta, A.; Cencioni, C.; Fasanaro, P.; Zaccagnini, G.; Greco, S.; Sarra-Ferraris, G.; Antonini, A.; Martelli, F.; Capogrossi, M.C. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ. 2011, 18, 1628–1639. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Sohn, J.; Shen, H.; Langhans, M.T.; Tuan, R.S. Bone marrow mesenchymal stem cells: Aging and tissue engineering applications to enhance bone healing. Biomaterials 2019, 203, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.M.; Hong, S.M.; Lee, Y.K.; Min, S.; Yoon, G. Metabolic features and regulation in cell senescence. BMB Rep. 2019, 52, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Pi, C.; Ma, C.; Wang, H.; Sun, H.; Yu, X.; Gao, X.; Yang, Y.; Sun, Y.; Zhang, H.; Shi, Y.; et al. MiR-34a suppression targets Nampt to ameliorate bone marrow mesenchymal stem cell senescence by regulating NAD(+)-Sirt1 pathway. Stem Cell Res. Ther. 2021, 12, 271. [Google Scholar] [CrossRef] [PubMed]
- Otte, M.; Stachelscheid, J.; Glaß, M.; Wahnschaffe, L.; Jiang, Q.; Lone, W.; Ianevski, A.; Aittokallio, T.; Iqbal, J.; Hallek, M.; et al. The miR-141/200c-STAT4 Axis Contributes to Leukemogenesis by Enhancing Cell Proliferation in T-PLL. Cancers 2023, 15, 2527. [Google Scholar] [CrossRef] [PubMed]
- Sikora, E. Rejuvenation of senescent cells-the road to postponing human aging and age-related disease? Exp. Gerontol. 2013, 48, 661–666. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Lin, K.; Jiang, T.; Shao, W.; Huang, C.; Jiang, B.; Li, Q.; Lin, D. NMR-based metabonomic analysis of HUVEC cells during replicative senescence. Aging 2020, 12, 3626–3646. [Google Scholar] [CrossRef]
- Hochmuth, C.E.; Biteau, B.; Bohmann, D.; Jasper, H. Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell 2011, 8, 188–199. [Google Scholar] [CrossRef]
- Rera, M.; Bahadorani, S.; Cho, J.; Koehler, C.L.; Ulgherait, M.; Hur, J.H.; Ansari, W.S.; Lo, T., Jr.; Jones, D.L.; Walker, D.W. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 2011, 14, 623–634. [Google Scholar] [CrossRef]
- Azzu, V.; Valencak, T.G. Energy Metabolism and Ageing in the Mouse: A Mini-Review. Gerontology 2017, 63, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Bazan, H.E.; Bazan, N.G.; Feeney-Burns, L.; Berman, E.R. Lipids in human lipofuscin-enriched subcellular fractions of two age populations. Comparison with rod outer segments and neural retina. Investig. Ophthalmol. Vis. Sci. 1990, 31, 1433–1443. [Google Scholar]
- Cheng, C.; Geng, F.; Cheng, X.; Guo, D. Lipid metabolism reprogramming and its potential targets in cancer. Cancer Commun. 2018, 38, 27. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Schroeder, E.A.; Silva-García, C.G.; Hebestreit, K.; Mair, W.B.; Brunet, A. Mono-unsaturated fatty acids link H3K4me3 modifiers to C. elegans lifespan. Nature 2017, 544, 185–190. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, W.; Lu, X.; Ma, Y.; Zhang, P.; Wang, Z.; Cui, Z.; Xia, Q. AUP1 regulates lipid metabolism and induces lipid accumulation to accelerate the progression of renal clear cell carcinoma. Cancer Sci. 2022, 113, 2600–2615. [Google Scholar] [CrossRef]
- Warensjö, E.; Rosell, M.; Hellenius, M.L.; Vessby, B.; De Faire, U.; Risérus, U. Associations between estimated fatty acid desaturase activities in serum lipids and adipose tissue in humans: Links to obesity and insulin resistance. Lipids Health Dis. 2009, 8, 37. [Google Scholar] [CrossRef]
- Lin, J.B.; Moolani, H.V.; Sene, A.; Sidhu, R.; Kell, P.; Lin, J.B.; Dong, Z.; Ban, N.; Ory, D.S.; Apte, R.S. Macrophage microRNA-150 promotes pathological angiogenesis as seen in age-related macular degeneration. JCI Insight 2018, 3, e120157. [Google Scholar] [CrossRef]
- Tracz-Gaszewska, Z.; Dobrzyn, P. Stearoyl-CoA Desaturase 1 as a Therapeutic Target for the Treatment of Cancer. Cancers 2019, 11, 948. [Google Scholar] [CrossRef]
- Kanno, T.; Nakajima, T.; Yokoyama, S.; Asou, H.K.; Sasamoto, S.; Kamii, Y.; Hayashizaki, K.; Ouchi, Y.; Onodera, T.; Takahashi, Y.; et al. SCD2-mediated monounsaturated fatty acid metabolism regulates cGAS-STING-dependent type I IFN responses in CD4(+) T cells. Commun. Biol. 2021, 4, 820. [Google Scholar] [CrossRef]
- Kim, D.; Monaco, E.; Maki, A.; de Lima, A.S.; Kong, H.J.; Hurley, W.L.; Wheeler, M.B. Morphologic and transcriptomic comparison of adipose- and bone-marrow-derived porcine stem cells cultured in alginate hydrogels. Cell Tissue Res. 2010, 341, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Shehzad, A.; Khan, T.; Kim, Y.Y. MicroRNAs: Synthesis, mechanism, function, and recent clinical trials. Biochim. Et Biophys. Acta 2010, 1803, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Periyasamy-Thandavan, S.; Burke, J.; Mendhe, B.; Kondrikova, G.; Kolhe, R.; Hunter, M.; Isales, C.M.; Hamrick, M.W.; Hill, W.D.; Fulzele, S. MicroRNA-141-3p Negatively Modulates SDF-1 Expression in Age-Dependent Pathophysiology of Human and Murine Bone Marrow Stromal Cells. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2019, 74, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Tahara, H. The role of exosomes and microRNAs in senescence and aging. Adv. Drug Deliv. Rev. 2013, 65, 368–375. [Google Scholar] [CrossRef]
- Fan, L.; Wang, C.; Zhan, P.; Liu, Y. miR-141-3p is Poorly Expressed in Polycystic Ovary Syndrome and Correlates with Glucose and Lipid Metabolism. Int. J. Endocrinol. 2021, 2021, 2022938. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Chen, G.; Xia, Q.; Shao, S.; Fang, H. Exosomal miR-200 family as serum biomarkers for early detection and prognostic prediction of cholangiocarcinoma. Int. J. Clin. Exp. Pathol. 2019, 12, 3870–3876. [Google Scholar] [PubMed]
- Anastasiadou, E.; Ceccarelli, S.; Messina, E.; Gerini, G.; Megiorni, F.; Pontecorvi, P.; Camero, S.; Onesti, M.G.; Trivedi, P.; Faenza, M.; et al. MiR-200c-3p maintains stemness and proliferative potential in adipose-derived stem cells by counteracting senescence mechanisms. PLoS ONE 2021, 16, e0257070. [Google Scholar] [CrossRef]
- Beji, S.; Milano, G.; Scopece, A.; Cicchillitti, L.; Cencioni, C.; Picozza, M.; D’Alessandra, Y.; Pizzolato, S.; Bertolotti, M.; Spaltro, G.; et al. Doxorubicin upregulates CXCR4 via miR-200c/ZEB1-dependent mechanism in human cardiac mesenchymal progenitor cells. Cell Death Dis. 2017, 8, e3020. [Google Scholar] [CrossRef] [PubMed]
- Carlomosti, F.; D’Agostino, M.; Beji, S.; Torcinaro, A.; Rizzi, R.; Zaccagnini, G.; Maimone, B.; Di Stefano, V.; De Santa, F.; Cordisco, S.; et al. Oxidative stress-induced miR-200c disrupts the regulatory loop among SIRT1, FOXO1, and eNOS. Antioxid. Redox Signal. 2017, 27, 328–344. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Zhang, C.; Ma, Q.; Gao, X.; Sun, H.; Sun, Y.; Wang, Y.; Zhang, H.; Shi, Y.; Meng, X.; et al. SCD2 Regulation Targeted by miR-200c-3p on Lipogenesis Alleviates Mesenchymal Stromal Cell Senescence. Int. J. Mol. Sci. 2024, 25, 8538. https://doi.org/10.3390/ijms25158538
Yu X, Zhang C, Ma Q, Gao X, Sun H, Sun Y, Wang Y, Zhang H, Shi Y, Meng X, et al. SCD2 Regulation Targeted by miR-200c-3p on Lipogenesis Alleviates Mesenchymal Stromal Cell Senescence. International Journal of Molecular Sciences. 2024; 25(15):8538. https://doi.org/10.3390/ijms25158538
Chicago/Turabian StyleYu, Xiao, Chang Zhang, Qianhui Ma, Xingyu Gao, Hui Sun, Yanan Sun, Yuezeng Wang, Haiying Zhang, Yingai Shi, Xiaoting Meng, and et al. 2024. "SCD2 Regulation Targeted by miR-200c-3p on Lipogenesis Alleviates Mesenchymal Stromal Cell Senescence" International Journal of Molecular Sciences 25, no. 15: 8538. https://doi.org/10.3390/ijms25158538
APA StyleYu, X., Zhang, C., Ma, Q., Gao, X., Sun, H., Sun, Y., Wang, Y., Zhang, H., Shi, Y., Meng, X., & He, X. (2024). SCD2 Regulation Targeted by miR-200c-3p on Lipogenesis Alleviates Mesenchymal Stromal Cell Senescence. International Journal of Molecular Sciences, 25(15), 8538. https://doi.org/10.3390/ijms25158538




