Abstract
Melatonin regulates vital physiological processes in animals, such as the circadian cycle, sleep, locomotion, body temperature, food intake, and sexual and immune responses. In plants, melatonin modulates seed germination, longevity, circadian cycle, photoperiodicity, flowering, leaf senescence, postharvest fruit storage, and resistance against biotic and abiotic stresses. In plants, the effect of melatonin is mediated by various regulatory elements of the redox network, including RNS and ROS. Similarly, the radical gas NO mediates various physiological processes, like seed germination, flowering, leaf senescence, and stress responses. The biosynthesis of both melatonin and NO takes place in mitochondria and chloroplasts. Hence, both melatonin and nitric oxide are key signaling molecules governing their biological pathways independently. However, there are instances when these pathways cross each other and the two molecules interact with each other, resulting in the formation of N-nitrosomelatonin or NOMela, which is a nitrosated form of melatonin, discovered recently and with promising roles in plant development. The interaction between NO and melatonin is highly complex, and, although a handful of studies reporting these interactions have been published, the exact molecular mechanisms governing them and the prospects of NOMela as a NO donor have just started to be unraveled. Here, we review NO and melatonin production as well as RNS–melatonin interaction under normal and stressful conditions. Furthermore, for the first time, we provide highly sensitive, ozone-chemiluminescence-based comparative measurements of the nitric oxide content, as well as NO-release kinetics between NOMela and the commonly used NO donors CySNO and GSNO.
1. Introduction
Melatonin, or N-acetyl-5-methoxytryptamine, is a tryptophan derivative that was originally discovered in the bovine pineal gland [1]. In animals, melatonin plays an active role in regulating circadian rhythms, sleep, locomotor activities, body temperature, mood, and food intake, as well as sexual behavior and immune responses [2]. Melatonin was first isolated by Lerner et al. [3] from the pineal glands of cows in 1958. It is a hormone produced and released by the pineal gland in humans and most animals that are active during the day. A unique characteristic of melatonin is its diurnal fluctuations; that is, its secretion follows a circadian rhythm, with the highest levels in the blood occurring around 3 am to 4 am. The increase in daily melatonin secretion is associated with an increase in the desire to sleep about 2 h before the individual’s usual bedtime. This rhythmic release of melatonin is controlled by the body’s central circadian rhythm generator, called the “suprachiasmatic nucleus (SCN)”, which is located in the forward region of the brain called the hypothalamus [4]. Afterward, melatonin was reported in several other prokaryotes and eukaryotes, including plants.
In higher plants, melatonin was first detected in 1995 by the Dubbels and Hattori groups [5,6]. The magnitude of research on melatonin in plants is very limited compared to animals. The findings so far reveal that, in plants, melatonin functions in diverse physiological roles, from seed germination and longevity to plant growth regulation, circadian cycle, and photoperiodicity, which in turn affect flowering, leaf senescence, and postharvest fruit storage [7,8,9,10]. Melatonin is also involved in regulating plant resistance against pathogens [11], promotes root regeneration and embryogenesis, and curbs the damaging effects of toxic chemicals, salts, heavy metals, temperature fluctuations, and UV radiations [12,13,14]. Melatonin acts as an antioxidant, predominantly in the regulation of reactive nitrogen species (RNS) such as nitric oxide (NO) and peroxynitrite (ONOO¯), reactive oxygen species (ROS) like OH, H2O2, and other molecules, thus protecting plant tissues under stress [12,15]. In plants, the effect of melatonin is associated with various regulatory elements of the redox network through feedback mechanisms. Its roles in controlling plant stress acclimation are slowly emerging [16].
Nitric oxide (NO), a tiny molecule, was initially regarded as an air contaminant. This gas has since been revealed to exhibit an extensive variety of physiological roles in both plants and animals. In plants specifically, NO has been recognized as an important mediator in various physiological processes, like seed germination, flowering, leaf senescence, and different environmental stresses [17,18]. NO is one of the key signaling molecules and has both antioxidant and pro-oxidant properties. This dual action of NO is primarily determined by its local concentration and the mode of its spatial generation. Under biotic and abiotic stress, NO in plants may react with different redox molecules and regulate protein function through various mechanisms [19,20]. Moreover, nitric oxide plays a vital role in seed dormancy and germination, growth, development, and root morphogenesis, as well as regulation of stomata in plants [9,18,21]. It has been proven experimentally that NO influences chromatin remodeling [22]. Specifically, NO regulates histone acetylation via S-nitrosation and inhibits histone deacetylase (HDA) complex [22,23]. Hence, it appears that the effect of NO is dependent on cell chromatin status. In cells with high euchromatin entropy, NO acts as an oxidant, whereas in cells with regular euchromatin, it may act as an antioxidant because of different NO-mediated effects on auxin metabolism, where auxin acts as a main epigenetic regulator.
2. Biosynthesis and Metabolism of Melatonin
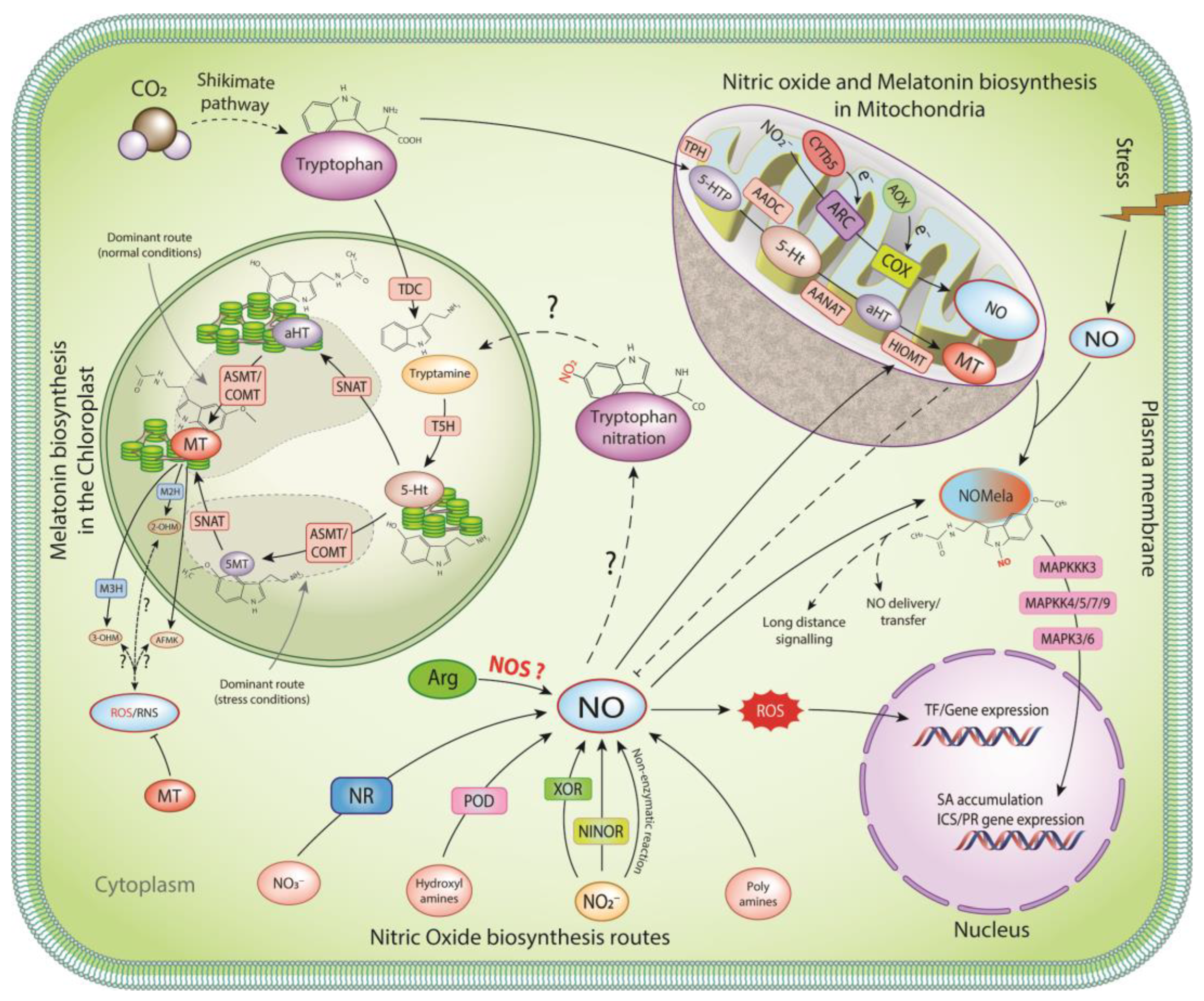
In plants, the synthesis of melatonin takes place in mitochondria and chloroplasts [24,25] through the specialized route known as the shikimate pathway (Figure 1), involving at least six distinct enzymes [tryptophan decarboxylase (TDC), tryptophan hydroxylase, tryptamine 5-hydroxylase (T5H), SNAT, ASMT, and caffeic acid O-methyltransferase (COMT)] [26,27]. Tryptophan is decarboxylated to tryptamine, catalyzed by the TDC enzyme. This is followed by rapid hydroxylation via tryptamine 5-hydroxylase (T5H), a P450 enzyme found in the endoplasmic reticulum. Abiotic stress induces the expression of ASMT/COMT, resulting in the formation of 5-methoxytryptamine by the ASMT, followed by acetylation to form melatonin [27]. However, under normal conditions, the dominant pathway involves the acetylation of serotonin (5-Ht) by the SNAT enzyme to form N-acetyl-5-hydroxytryptamine (aHT) and then O-methylation to form melatonin [28]. Similarly, melatonin biosynthesis follows the same route in the mitochondria, where the AADC, AANAT, and HIOMT convert tryptophan to melatonin (Figure 1).
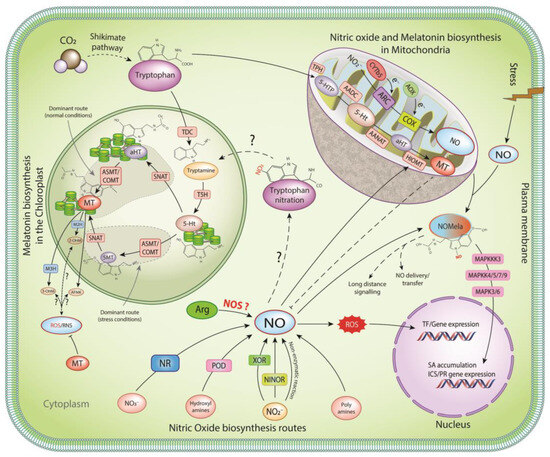
Figure 1.
NO and melatonin production and interaction in plants.
An important enzyme in melatonin degradation is melatonin 2-hydroxylase (M2H), an oxygenase enzyme that functions in the degradation of melatonin to its major metabolite, 2-hydroxymelatonin (2-OHM) [29]. M2H is located in the chloroplasts of the plant cells. Other important melatonin derivatives that are actively accumulated in plants include 3-hydroxymelatonin (3-OHM) catalyzed by melatonin 3-hydroxylase (M3H) in the cytoplasm [11], including cyclic melatonin, b-hydroxymelatonin, and cyclic b-hydroxymelatonin [30] (Figure 1). Although not clearly known, the generation of these metabolites may be due to the interaction of melatonin with oxidizing molecules, such as RNS and ROS [31].
Melatonin biosynthesis in plants occurs in mitochondria and chloroplasts [24] via the shikimate pathway, involving at least six distinct enzymes (tryptophan decarboxylase (TDC), tryptophan hydroxylase (TH), tryptamine 5-hydroxylase (T5H), serotonin N-acetyltransferase (SNAT), acetylserotonin O-methyltransferase (ASMT), and caffeic acid O-methyltransferase (COMT)) [27]. TDC converts tryptophan, which is hydroxylated by T5H, a P450 enzyme found in the endoplasmic reticulum. Stress induction of ASMT/COMT results in the formation of 5-methoxytryptamine, followed by acetylation to form melatonin [27]. Under optimal conditions, the dominant route involves the acetylation of serotonin (5-Ht) by the SNAT enzyme to form N-acetyl-5-hydroxytryptamine (aHT) and then O-methylation to form melatonin [28]. In mitochondria, aromatic L-amino acid decarboxylase (AADC), aralkylamine N-acetyltransferase (AANAT), and hydroxyindole O-methyltransferase (HIOMT) convert tryptophan to melatonin. Once formed, melatonin is converted to various metabolites, such as 2-OHM, 3-OHM, and N1-acetyl-N2-formyl-5-methoxykynuramine [AFMK]. Just like melatonin, NO is also produced in mitochondria and chloroplasts via multiple oxidative and reductive pathways [32]. NO production via the oxidation of L-arginine by a NO synthase-like enzyme and the oxidation of polyamine by polyamine synthase have been reported. Cytochrome oxidase and reductases generate NO from NADP and NADPH. Similarly, peroxidases convert N-omega hydroxyl-L-arginine (NOHA) to NO [33]. NO3− are reduced to NO2− by cytosolic nitrate reductase (NR), followed by NO via the action of xanthine oxidoreductase (XOR), plasma membrane-bound Nitrate-NO reductase (Ni-NOR), or NR in plastids [33]. NO production from nitrite occurs in mitochondria via the electron transport chain involving alternative oxidase (AOX) [34], the mitochondrial molybdopterin enzyme mARC, cytochrome b5 [35], and cytochrome-C oxidase (COX). NO and H2O2 levels rise during infection, leading to higher endogenous melatonin levels and activation of the MAPK cascade via OXI1/MAPKKK3, MAPKK4/5/7/9, and MAPK3/6 [36]. This activation of the MAP kinase cascade increases SA levels, inducing the expression of several defense-related genes, including PR1 [37]. The proximity of melatonin and nitric oxide in the same cellular organelles, as well as the published literature, suggests complex coordination between NO and melatonin to drive plant physiological responses under basal and stress conditions.
3. Relationship of Nitric Oxide and Melatonin in Plants
Just like melatonin, NO is known to be produced in mitochondria and chloroplasts. The NO production machinery of plants and animals has distinct differences, as NO production in plants occurs via multiple oxidative and reductive pathways [32]. One of the oxidative pathways leads to NO production via the oxidation of L-arginine by an NO synthase-like enzyme. Likewise, the oxidation of polyamine by polyamine synthase produces NO. Cytochrome oxidase and reductases can act upon NADP and NADPH, which leads to the production of NO. N-omega hydroxyl-L-arginine (NOHA) is converted to NO by peroxidase (POD) [33]. Alternatively, in the reductive pathway, nitrate reductase in the cytosol or plasma membrane reduces NO3− to NO2−. In the following step, a group of enzymes like nitrate reductase in plastids, xanthine oxidoreductase (XOR), and plasma membrane-bound nitrate-NO reductase (Ni-NOR) contribute to the conversion of NO2− to NO [33].
After decades of research, the most well-known route for the production of nitric oxide (NO) is perhaps the reduction of nitrite to NO through various nonenzymatic or enzymatic mechanisms. In plants, the primary enzymatic systems that carry out this reductive NO production are nitrate reductases (NRs), the mitochondrial electron transport chain [38], and a newly discovered complex between NR and NOFNiR (nitric oxide-forming nitrite reductase) in the unicellular alga Chlamydomonas reinhardtii [39]. Havemeyer, Bittner, Wollers, Mendel, Kunze, and Clement [39] showed that C. reinhardtii NR can interact with another partner protein from the amidoxime-reducing component (ARC) protein family, the NOFNiR, for the biosynthesis of NO from nitrite.
Mitochondria are an important source of NO in plants. NO production from nitrite can occur from electron pressure in the Q-cycle of Complex III. Alternative oxidase, which acts as a non-energy-conserving electron sink upstream of the Q-cycle, can reduce this electron pressure to produce NO [34]. The mitochondrial molybdopterin enzyme mARC reduces nitrite to NO using cytochrome b5 as an electron donor. The mARC proteins may represent a new pathway for hypoxic NO production in vivo [35] (Figure 1). However, cytochrome-C oxidase (COX) is both a source and a target of NO [40]. In addition to the reductive pathways, there is evidence to suggest the existence of an oxidative NO production route that is dependent on arginine, similar to the activity of nitric oxide synthase (NOS) in animals. However, no NOS homologs have been found in embryophyte genomes, and while there is increasing evidence for NOS-like activity in plants, the proteins involved have yet to be identified (Figure 1).
NO exerts its function via reversible posttranslational modification of cysteine thiols called S-nitrosothiols (SNOs). Depending on the cell chromatin status (stage/structure), NO can either be beneficial, promoting the survival and development of plants, or the opposite, as excessively high levels can cause oxidative injury in plant tissues. The global levels of SNOs in living organisms are thus maintained by the canonical antioxidant enzyme S-nitrosoglutathione reductase (GSNOR) [41].
3.1. NO, RNS, and Melatonin
As melatonin is produced in both animals and plants, Blask et al. [42] used the term phytomelatonin to differentiate between the two sources. Initially, it was shown to regulate plant growth, but is now known to play a key role in cell metabolism and responses to various biotic and abiotic stresses. It can act as a hormone as well as an antioxidant by scavenging various ROS and RNS. Furthermore, its amphiphilic characteristics make it easily permeable through membranes and enable it to move into the cytosol and organelles, further facilitating its regulatory role [43]. Melatonin and nitric oxide are both extremely important molecules required for normal physiology, governing their biochemical pathways independently. However, there are instances when these pathways cross each other and these two molecules interact with each other, resulting in the formation of N-nitrosomelatonin or NOMela. NOMela represents a nitrosated form of melatonin, discovered recently and with promising roles in plant physiology. The interaction between NO, other reactive nitrogen species (RNS), and melatonin exhibits a certain degree of complexity, since they act independently but also interact to regulate multiple signaling pathways [36]. However, extensive roles of phytomelatonin and NO in modulating various physiological pathways are emerging (reviewed by Mukherjee [16,44], Mukherjee [45]). Several studies [46,47,48,49,50,51] suggest interactions between phytomelatonin and NO or the putative plant NOS. Among the various S-nitrosated molecules, NOMela is emerging as an important signaling molecule in both animals and plants [52]. Aghdam et al. [53] reported that the exogenous application of melatonin promotes the accumulation of NO by triggering the activation of the arginine pathway in tomato fruits. Recently, Liu et al. [54] showed a significant delay in fruit senescence due to an increase in the NO content following melatonin application. On the other hand, the concentration of NO in tomato seedlings increases following the exogenous application of melatonin via the disruption of S-nitrosoglutathione reductase (GSNOR) activity and an increase in the expression of nitrate reductase (NR) [55].
Similarly, NO application increases the level of melatonin by inducing the expression of tryptophan decarboxylase (TDC), tryptamine 5-hydroxylase (T5H), serotonin N-acetyltransferase (SNAT), and caffeic acid O-methyltransferase (COMT) via a cyclic guanosine monophosphate (cGMP) pathway. The cyclic 3-hydroxymelatonin (C3HOM) is an immediate product of melatonin’s interaction with various reactive oxygen species (ROS). In addition, other forms of melatonin metabolites have also been reported in animals and plants, such as 3OHM, 2OHM, and N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK—the first melatonin reported from plants [56]). These melatonin metabolites are also known to react with NO in the course of stress signaling. In this regard, 2-hydroxymelatonin (2OHM), in particular, could be an active molecule in the NO-mediated signaling pathways. Exogenous application of 2OHM enhances plant tolerance to cold, drought [57], and cadmium stress [58]. NO combines with melatonin to form N-nitrosomelatonin (NO-Mela) via nitrosation. NO can nitrosate melatonin via a radical mechanism or an ionic mechanism under different pH conditions. NO-Mela is an effective NO donor in cultured cells, especially in the presence of serotonin and its derivatives [59]. NO donor sodium nitroprusside (SNP) can mimic the effects of light on photoreceptor melatonin synthesis [60]. NO–melatonin crosstalk is particularly important during stress responses. Melatonin triggers NO accumulation via NR modulating the activity of nitrate reductase (NR) and nitric oxide synthase (NOS) enzymes [53,61,62]. However, NO-melatonin antagonistic interactions have also been described, such as NOS inhibition by melatonin [63]. This shows that melatonin can induce NO production but can also induce NO scavenging mechanisms. Thus, it is a key player in NO regulation at the cellular level.
3.2. NO, Melatonin, and Antioxidative Defense
Melatonin is fundamentally considered an antioxidant with an imperative role in regulating reactive nitrogen and reactive oxygen species. Melatonin has a high scavenging potential toward·OH, ·NO, ·NO2, ·ONOO-, and N3. A decline in oxidative damage has been observed in the animal system as a result of the inhibition of NO synthase activity and NO biosynthesis caused by the regulatory action of melatonin [63]. Furthermore, melatonin is capable of minimizing the detrimental effects of several xenobiotics in both plants and animal cells [9]. There are two underlying mechanisms involved in this process: (1) melatonin acts directly on RNS and ROS generated by the xenobiotics, and (2) melatonin acts indirectly to initiate the antioxidant enzyme gene expression, such as catalases, halo-peroxidases, glutathione-, glutathione synthases, glutathione reductases, glutathione S-transferases, ascorbate-, ascorbate oxidases, monodehydro- and dehydroascorbate reductases, thioredoxins, peroxiredoxins, etc., all geared towards curtailing ROS toxicity [9]. Likewise, the three principal melatonin derivatives, AMK, AFMK, and 3OHM, play an additional significant role in lowering oxidative distress in plants and animal systems [64,65]. Similarly, NO plays an important function in counteracting ROS-mediated damage during stress [66]. For instance, the application of NO to maize plants leads to a decline in the H2O2 content amid the activation of the antioxidative defense of maize. Similarly, in Brassica juncea, the exogenous NO significantly overcomes the oxidative injury produced by salt stress [67].
3.3. NO, Melatonin, and Stress Response
Plants produce variable levels of melatonin under different circumstances [12]. However, its production is increased under a variety of stress conditions, with its highest concentration in the flowers, followed by the leaves and seeds [68]. Information regarding the interaction of melatonin and NO in plants is limited. Only a handful of studies reporting these interactions have been published over the past decade, and the exact molecular mechanisms governing these interactions have just started to be unraveled. Nonetheless, melatonin treatment is known to alter endogenous NO levels in plants. A brief account of the published literature reporting NO–melatonin interaction under different physiological conditions is shown in Table 1. Melatonin is known to scavenge H2O2, OH, NO, ONOO-, HOCl, and 1O2, thereby providing protection under biotic and abiotic stress conditions [69]. Foliar treatment with melatonin protects Silybum marianum plants against salt stress by modulating photosynthesis and activating the antioxidant machinery [70]. NO, on the other hand, may act downstream of melatonin to promote plant defense against a variety of biotic and abiotic stresses [71].
Recently, Khan et al. [72] showed that melatonin-regulated heat shock proteins and mitochondrial ATP synthase confer drought tolerance via the maintenance of ROS homeostasis in a hydrogen sulfide-dependent manner, whereas [73] reported melatonin-mediated enhancement of photosynthesis and salt tolerance in wheat. Similarly, the exogenous application of melatonin has been shown to mitigate chilling stress in mango [74], drought tolerance in tomato [75], salt stress in Ranunculus asiaticus [76], polyethylene (PEG)-induced osmotic stress in soybean [77], salt stress in tomato plants via modulation of carbohydrate and nitrogen metabolism [78], waterlogging stress in Zanthoxylum armatum via enhancement of photosynthesis and increase in carotenoid content [79], and to protect rice plants against rice blast disease by acting synergistically with the fungicide isoprothiolane to interfere with lipid metabolism by targeting the isocitrate lyase-encoding gene MoICL1 [80]. Xu et al. [81] reported that exogenous melatonin application protected the ornamental woody plant rhododendron against extended heat stress by improving the melatonin contents, electron transport rate, photosystem II and I activities, rubisco activity, and ATP content. Furthermore, they performed transcriptomic analysis to identify several heat-induced differentially expressed genes associated with photosynthetic activity.
One very interesting and recent study (preprint) highlighted a key aspect of the melatonin and nitric oxide interaction in tomato, where Gong et al. [82] showed that saline-alkali stress induced the S-nitrosation of the plasma membrane H+-ATPase 2 (HA2) at Cys206 aggravated by GSNOR knockdown but alleviated by COMT-overexpression. The COMT was found to enhance melatonin synthesis and NO scavenging to improve saline-alkali tolerance. HA2 S-nitrosation suppressed its interaction with the 14-3-3 protein 1 (TFT1), resulting in the inhibition of its enzymatic activity and saline–alkali tolerance. This indicates that melatonin relieves the S-nitrosation of plasma membrane H+-ATPase 2 to enhance saline–alkali tolerance in tomato. They concluded that, under physiological status, melatonin and NO act jointly as a redox switch of HA2 to regulate root H+ and Na+ efflux to affect saline–alkali tolerance. Once published, this may be the first convincing study showing the molecular basis of melatonin and nitric oxide interaction under saline–alkali stress in tomato through the melatonin-HA2-S-nitrosation module.
Endogenous melatonin content in plants varies with circadian rhythms, indicating a central role for melatonin in plant development [10]. The melatonin receptor CAND2 mediates H2O2 and Ca2+ signaling to regulate melatonin-induced stomatal closure in Arabidopsis [83]. Exogenous application of melatonin increases endogenous NO levels via inhibition of GSNOR activity [55]. Corpas et al. [84] suggested that melatonin accumulation may act as a NO scavenger during tomato fruit ripening. Similarly, nitrosated melatonin (NOMela) mediates the crosstalk between NO and ethylene during fruit ripening [45]. NO–melatonin interaction regulates a plethora of plant responses under a variety of stress conditions (reviewed by Mukherjee and He and He [85]).
In plants, melatonin may be implicated in day–night cycles. Plants maintain a balance between melatonin biosynthesis and degradation. However, the concentration of melatonin may increase in response to environmental stress. Furthermore, information regarding the concentration of melatonin during various growth stages is largely unknown. For instance, Arnao and Hernandez-Ruiz [86] reported that the levels of melatonin in tomato plants in vitro were highest in leaves followed by roots and stem, whereas, in the open field, the melatonin content of tomato plants was in the order of leaf > stem > root. This may have been the result of variations in the duration and intensity of light in the field and in controlled conditions. Likewise, it is induced by NaCl in barley roots [87]. High-intensity UVB radiation for a short period was also found to induce a greater concentration of melatonin in Glycyrrhiza uralensis roots [88].
Melatonin is a multifunctional signaling molecule with diverse roles in plant physiology. These roles are often orchestrated via intricate networks that influence other phytohormones [89,90]. For example, similar to auxin, melatonin promotes root generation and lateral/adventitious roots [91]. Melatonin is also known to affect gibberellin metabolism and cytokinin levels and modulate the expression of ABA and ethylene pathway genes, thereby affecting fruit ripening and postharvest processes [92,93]. Melatonin also contributes to plant immunity alongside salicylic acid (SA) and jasmonic acid (JA). Studies have shown that melatonin protects chlorophyll and promotes cellular integrity by delaying senescence [92,93]. On the other hand, the role of NO in regulating phytohormones is well known. Several key proteins involved in phytohormone signaling are known to be modulated by NO via S-nitrosation. For example, the S-nitrosation of ABI5 triggers its degradation, promoting seed germination and seedling growth [94]. COP1 S-nitrosation inhibits its function during photomorphogenesis [95]. The auxin receptor transport inhibitor response 1 (TIR1) undergoes S-nitrosation, which enhances its interaction with Aux/IAA to form a coreceptor crucial for auxin responses in plants [96]. Similarly, other proteins, such as JAZ (JA pathway) and NPR1 (SA-pathway) proteins, are known to be regulated by NO via S-nitrosation [97]. As recent studies (including this one) propose NOMela as a new and versatile NO donor, it would be interesting to explore the impact of this new NO donor on phytohormonal networks in plants.
Plants are frequently exposed to phytopathogenic bacteria, viruses, fungi, and herbivores. Melatonin is emerging as a plant defense regulator, as the exogenous application of melatonin improves the resistance of Malus prunifolia to Marssonina apple blotch [98]. The expression of defense genes PR1, ICS1, and PDF1.2 decreased after infection with the avirulent pathogen Pseudomonas syringae pv. tomato (PstDC3000-avrRpt2) [99]. External application of melatonin increased plant resistance to pathogens such as Fusarium oxysporum, Penicillium spp., Phytophthora infestans, Botrytis cinerea, and Rhizopus stolonifera [100]. In cotton, the external application of melatonin increased the expression of genes involved in the phenylpropanoid, mevalonate (MVA), and gossypol pathways following Verticillium dahliae inoculation [101]. As described, NO is a signaling molecule that plays a key role in plant responses to pathogen invasion and is upstream of the innate immune system in plants. Many studies have investigated NO-mediated plant disease responses and the relationship between NO and SA [19]. Plants deficient in SA (for example, the NahG-overexpressing line) and NO (noa1 and nia1nia2 mutant lines) are highly sensitive to bacterial pathogens. Both SA and NO regulate Arabidopsis resistance to pathogenic bacteria, with synergy between the two compounds playing an important role in natural immunity [102]. Treatment of Arabidopsis with melatonin followed by Pseudomonas syringe pv. tomato (Pst) DC3000 infection rapidly increased NO levels in leaves [103].
After infection of Arabidopsis with PstDC3000, melatonin treatment induces transcription of CBF/DREB1s, leading to increased accumulation of soluble sugars [104] with an increase in the endogenous soluble sugars, glycerol, SA, and NO, resulting in enhanced resistance against pathogens [105]. Infection by pathogenic bacteria may also cause increased levels of H2O2 and NO, leading to enhanced endogenous melatonin level activation of the MAPK cascade via OXI1/MAPKKK3, MAPKK4/5/7/9 and MAPK3/6 [36]. This melatonin-mediated MAPK cascade activation has been shown to increase SA levels, inducing the expression of several defense-related genes, including PR1 [37] (Figure 1). From the above literature, it is clear that melatonin plays a vital role in the regulation of RNS, ultimately affecting physiological processes under stress. Furthermore, complex coordination between NO and melatonin drives plant responses against pathogen invasion. Further studies are required to understand the relationship between melatonin and NO via genomics, proteomics, transcriptomics, metabolomics, PTMomics, interactomics, proxiomics, etc.

Table 1.
Nitric oxide–melatonin interaction in plants.
Table 1.
Nitric oxide–melatonin interaction in plants.
| S. No. | Plant Species/Tissue | Plant Physiological Processes | NO Content | Melatonin Content | References |
|---|---|---|---|---|---|
| Biotic stress | |||||
| 1 | Arabidopsis | Pathogen infection | + | + | [37,102,105] |
| 2 | Bacterial infection | + | + | [102,103,105] | |
| 3 | Tomato | Pathogen infection | + | + | [106] |
| Abiotic stress | |||||
| 4 | Tomato | Cold stress | + | + | [53] |
| 5 | Capsicum | Salt stress | + | + | [107] |
| 6 | Alfalfa | Drought stress | + | + | [61] |
| 7 | Sunflower | Salt stress | + | + | [108,109] |
| 8 | Rapeseed | Salt stress | + | + | [110] |
| 9 | Arabidopsis | Aluminum/Cd toxicity | + | − | [111] |
| 10 | Iron deficiency | + | + | [112] | |
| 11 | Wheat | Aluminum/Cd toxicity | + | + | [113] |
| 12 | Cucumber | Cold stress | + | + | [114] |
| Growth and Development | |||||
| 13 | Tomato | Root development | + | + | [55] |
| 14 | Response to alkalinity | + | + | [115] | |
| 15 | Saline-alkali stress | + | + | [82] | |
| 16 | Capsicum | Fruit ripening | + | + | [44] |
| 17 | Pear | Fruit senescence | + | + | [54] |
+ = increase. − = decrease.
3.4. NOMela as a Nitric Oxide Donor
The roles and effects of nitric oxide and melatonin have been established, and much research has been conducted over the past few decades on the use of these important signaling molecules, especially nitric oxide, in both animal and plant systems. However, research involving N-nitrosomelatonin (NOMela) is very limited. NOMela is well known for its ability to transnitrosylate nucleophiles, including ascorbate and thiols [116]. Blanchard-Fillion et al. [117] showed that NOMela releases NO, thereby acting as an efficient NO donor. Afterward, other studies have reported the shift of NO moiety from NOMela to other biological macromolecules, including various activated hydroxy compounds like vitamin C [118,119], vitamin E [120], catechol [121], serotonin [59], and protein thiols [122]. Berchner-Pfannschmidt, Tug, Trinidad, Becker, Oehme, Flamme, Fandrey, and Kirsch [116] used NOMela as a NO donor in cell culture experiments and recorded significantly higher viability of the cells, likely due to the antioxidant properties of NOMela. From studies in animal systems, it appears that NOMela has prominent melatonin-like effects, as NOMela is known to enhance photic synchronization of mammalian circadian rhythms correlated with high suprachiasmatic immunoreactivity of the proto-oncogene cFOS and period circadian regulator 1 (PER1) [123]. In animals, NOMela and the melatonin derivative NOM (1-nitroso melatonin) are NO as well as melatonin donors and are considered new potential drugs, particularly in neurological diseases [124].
As described earlier, studies involving the use of NOMela as an NO donor are very limited, particularly in plant sciences. Recently, Singh et al. [125] reported a preferential uptake of NOMela by Arabidopsis roots compared to the commonly used NO donor GSNO. They also recorded a long-distance transport of NOMela via the vascular bundles, releasing 52.8% more NO compared to GSNO. Using confocal laser scanning microscopy, they showed a strong NO signal generated in the mitochondria. They concluded that NOMela is a more efficient NO donor than an equimolar concentration of GSNO. Apart from this finding, no other studies have been published involving the use of NOMela in plants. One interesting reason for this (at least in our hands) is the apparent difficulty in synthesizing N-nitrosomelatonin, and the commercially available stocks are currently too expensive. Melatonin can be converted to NOMela in the presence of oxygen and acidic pH conditions [119,126]. Melatonin and nitric oxide are both produced in the same cellular compartments, and there is considerable evidence for the physical and chemical interaction between the two signaling molecules [53,61,107,108,109,110,113,115,127,128].
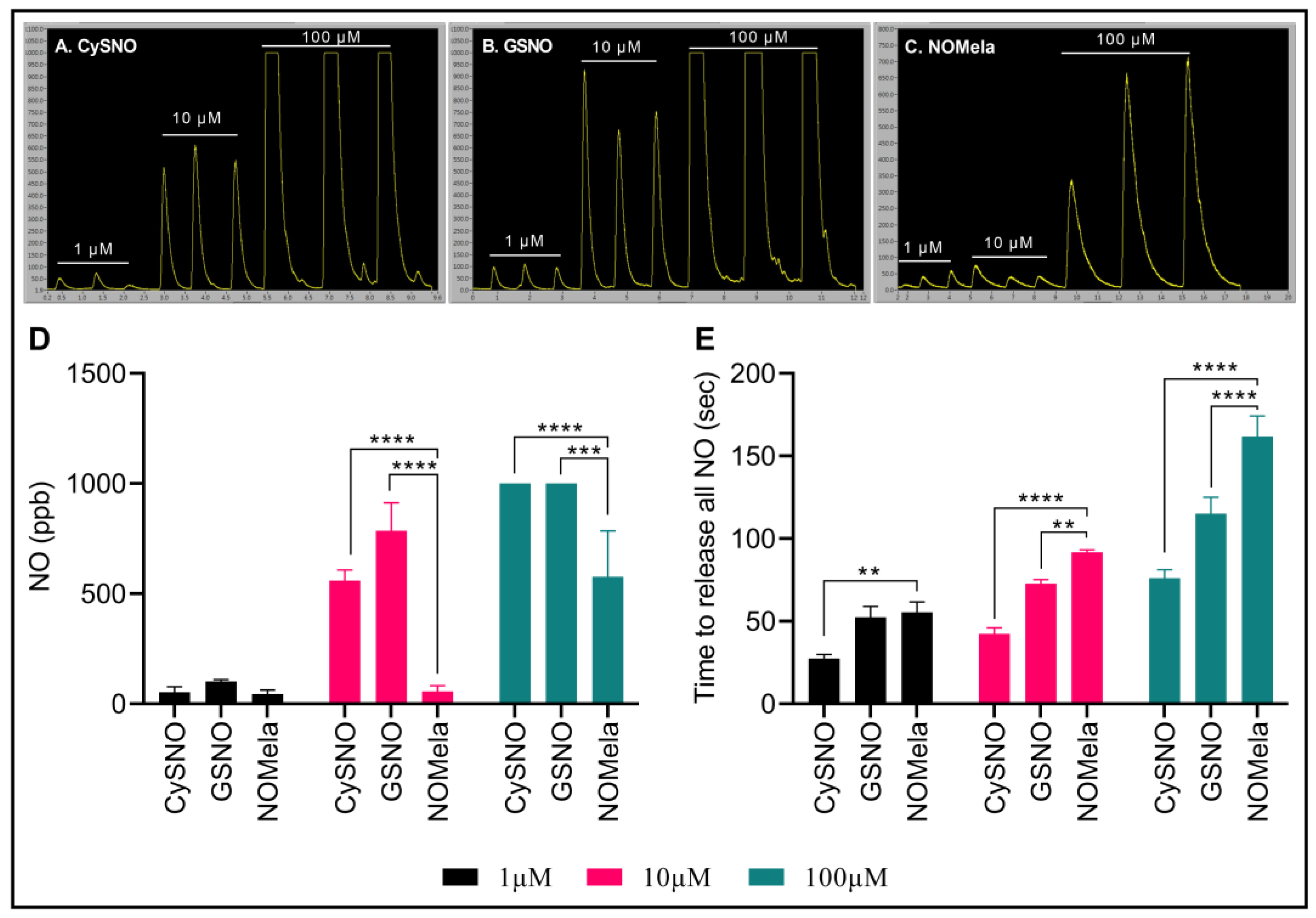
Here, we synthesized NOMela according to Kirsch and De Groot [52] at 4 °C in the dark and measured its NO-releasing capacity efficiency compared to the commonly used NO donors CySNO and GSNO using highly sensitive, ozone-chemiluminescence-based comparative measurements through the Sievers NA280i NO Analyzer with a CuCl2 reducing buffer system [129]. We compared three different concentrations of NOMela (1 µM, 10 µM, and 100 µM) with equimolar concentrations of CySNO and GSNO. Interestingly, our results indicated that NOMela released significantly less NO compared to GSNO and CySNO (Figure 2A–D). More precisely, 1 µM NOMela released an average of 16.9% and 56.8% less NO than equimolar concentrations of CySNO and GSNO, respectively. This pattern was more pronounced in the higher concentrations, where 10 µM NOMela released an average of 90% and 72.9% less NO than equimolar concentrations of GSNO, and CySNO, respectively. However, it was interesting to note that, under the same equimolar concentrations of the NO donors and reducing buffer conditions, NOMela took a significantly longer time to release all the NO than CySNO and GSNO (Figure 2A–C,E). More precisely, the time taken for complete reduction and release of NO from 100 µL of NOMela was approximately double the time taken by the same volume of equimolar concentrations of CySNO and GSNO. This may be a highly important attribute of NOMela, particularly as an NO donor, as the slow and consistent release of NO can have long-lasting effects under physiological conditions. Considering this aspect of NOMela as an NO donor, our results corroborated with the findings of Singh, Jain, Gupta, Khurana, and Bhatla [125] who described NOMela as a better NO donor than GSNO. To our knowledge, this is the first report of highly sensitive ozone-chemiluminescence-based measurement of NO from NOMela. More work is needed to understand the rates of absorption, diffusion, release of NO and melatonin, and chemical kinetics of the newly discovered signaling molecule NOMela and its interaction with other components, such as phytohormones, metabolites, ROS, and RNS.
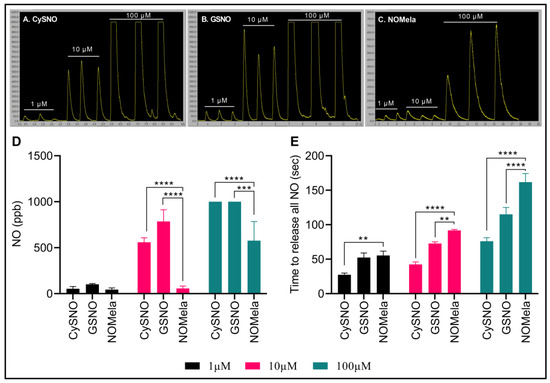
Figure 2.
Comparison of CySNO, GSNO, and NOMela as NO donors. (A–C) Comparative ozone-chemiluminescence-based comparative NO measurements via Sievers NA 280i NO Analyzer. (D) Comparison of the nitric oxide content (ppb) in 100 µL of equimolar concentrations (1 µM, 10 µM, and 100 µM) of CySNO, GSNO, and NOMela. (E) Kinetics of NO released from 100 µL of equimolar concentrations of CySNO, GSNO, and NOMela. Each data point represents the mean of three independent replications, error bars represent standard deviation, and asterisks represent significant differences among the means determined via student’s t-test at p = 0.05 (**), 0.01 (***), and 0.001 (****) in Microsoft Excel. Standard and sample solutions were prepared, and NO measurements were performed in an NA280i NO Analyzer with a CuCl2 reducing buffer system, as described by Hussain, Yun, and Loake [129].
Author Contributions
A.H.: Conceptualization, methodology, investigation, writing—original draft preparation, B.F.: Original draft preparation, review, and editing, H.-S.J., D.-S.L. and B.-G.M.: Software, data curation, investigation, N.K.R. and B.-W.Y.: supervision, visualization, validation, reviewing, editing, and funding. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Basic Science Research Program through the National Research Foundation (NRF) of Korea, by the Ministry of Education through Grant Number 2021R1A6C101A416, the Biological Materials Specialized Graduate Program through the Korea Environmental Industry & Technology Institute (KEITI) funded by the Ministry of Environment (MOE), and a research grant from Chungbuk National University, Republic of Korea.
Data Availability Statement
All relevant data are available within the manuscript.
Acknowledgments
Quantitative PCR was performed at the KNU NGS center (Daegu, Republic of Korea).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hardeland, R.; Cardinali, D.P.; Srinivasan, V.; Spence, D.W.; Brown, G.M.; Pandi-Perumal, S.R. Melatonin—A pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 2011, 93, 350–384. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Vico, A.; Lardone, P.J.; Alvarez-Sanchez, N.; Rodriguez-Rodriguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of Melatonin, the Pineal Gland Factor that Lightens Melanocytes1. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Gillette, M.U.; Tischkau, S.A. Suprachiasmatic nucleus: The brain’s circadian clock. Recent Prog. Horm. Res. 1999, 54, 33–58; discussion 58-9. [Google Scholar] [PubMed]
- Dubbels, R.; Reiter, R.J.; Klenke, E.; Goebel, A.; Schnakenberg, E.; Ehlers, C.; Schiwara, H.W.; Schloot, W. Melatonin in edible plants identified by radioimmunoassay and by high performance liquid chromatography-mass spectrometry. J. Pineal Res. 1995, 18, 28–31. [Google Scholar] [CrossRef] [PubMed]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar]
- Golembeski, G.S.; Kinmonth-Schultz, H.A.; Song, Y.H.; Imaizumi, T. Photoperiodic flowering regulation in Arabidopsis thaliana. Adv. Bot. Res. 2014, 72, 1–28. [Google Scholar]
- Johansson, M.; Staiger, D. Time to flower: Interplay between photoperiod and the circadian clock. J. Exp. Bot. 2015, 66, 719–730. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin: A New Plant Hormone and/or a Plant Master Regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Functions of melatonin in plants: A review. J. Pineal Res. 2015, 59, 133–150. [Google Scholar] [CrossRef]
- Lee, H.Y.; Back, K. Mitogen-activated protein kinase pathways are required for melatonin-mediated defense responses in plants. J. Pineal Res. 2016, 60, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin in Plants—Diversity of Levels and Multiplicity of Functions. Front. Plant Sci. 2016, 7, 198. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhang, N.; Yang, R.C.; Wang, L.; Sun, Q.Q.; Li, D.B.; Cao, Y.Y.; Weeda, S.; Zhao, B.; Ren, S.; et al. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA(4) interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef]
- Wei, Z.; Li, C.; Gao, T.; Zhang, Z.; Liang, B.; Lv, Z.; Zou, Y.; Ma, F. Melatonin increases the performance of Malus hupehensis after UV-B exposure. Plant Physiol. Biochem. 2019, 139, 630–641. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.B.; Karbownik, M.; Calvo, J.R. Significance of melatonin in antioxidative defense system: Reactions and products. Biol. Signals Recept. 2000, 9, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S. Novel perspectives on the molecular crosstalk mechanisms of serotonin and melatonin in plants. Plant Physiol. Biochem. 2018, 132, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Mur, L.A.; Mandon, J.; Persijn, S.; Cristescu, S.M.; Moshkov, I.E.; Novikova, G.V.; Hall, M.A.; Harren, F.J.; Hebelstrup, K.H.; Gupta, K.J. Nitric oxide in plants: An assessment of the current state of knowledge. AoB Plants 2013, 5, pls052. [Google Scholar] [CrossRef]
- Hussain, A.; Shah, F.; Ali, F.; Yun, B.-W. Role of Nitric Oxide in plant senescence. Front. Plant Sci. 2022, 13, 851631. [Google Scholar] [CrossRef] [PubMed]
- Sami, F.; Faizan, M.; Faraz, A.; Siddiqui, H.; Yusuf, M.; Hayat, S. Nitric oxide-mediated integrative alterations in plant metabolism to confer abiotic stress tolerance, NO crosstalk with phytohormones and NO-mediated post translational modifications in modulating diverse plant stress. Nitric Oxide 2018, 73, 22–38. [Google Scholar] [CrossRef]
- Hussain, A.; Mun, B.-G.; Imran, Q.M.; Lee, S.-U.; Adamu, T.A.; Shahid, M.; Kim, K.-M.; Yun, B.-W. Nitric Oxide Mediated Transcriptome Profiling Reveals Activation of Multiple Regulatory Pathways in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 975. [Google Scholar] [CrossRef]
- Nabi, R.B.S.; Tayade, R.; Hussain, A.; Kulkarni, K.P.; Imran, Q.M.; Mun, B.-G.; Yun, B.-W. Nitric oxide regulates plant responses to drought, salinity, and heavy metal stress. Environ. Exp. Bot. 2019, 161, 120–133. [Google Scholar] [CrossRef]
- Ageeva-Kieferle, A.; Rudolf, E.E.; Lindermayr, C. Redox-Dependent Chromatin Remodeling: A New Function of Nitric Oxide as Architect of Chromatin Structure in Plants. Front. Plant Sci. 2019, 10, 625. [Google Scholar] [CrossRef] [PubMed]
- Mengel, A.; Ageeva, A.; Georgii, E.; Bernhardt, J.; Wu, K.; Durner, J.; Lindermayr, C. Nitric Oxide Modulates Histone Acetylation at Stress Genes by Inhibition of Histone Deacetylases. Plant Physiol. 2016, 173, 1434–1452. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Tan, D.X.; Allan, A.C.; Zuo, B.; Zhao, Y.; Reiter, R.J.; Wang, L.; Wang, Z.; Guo, Y.; Zhou, J.; et al. Chloroplastic biosynthesis of melatonin and its involvement in protection of plants from salt stress. Sci. Rep. 2017, 7, 41236. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Liu, X.; Rosales-Corral, S.A.; Acuna-Castroviejo, D.; Reiter, R.J. Mitochondria and chloroplasts as the original sites of melatonin synthesis: A hypothesis related to melatonin’s primary function and evolution in eukaryotes. J. Pineal Res. 2013, 54, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Back, K.; Tan, D.-X.; Reiter, R.J. Melatonin biosynthesis in plants: Multiple pathways catalyze tryptophan to melatonin in the cytoplasm or chloroplasts. J. Pineal Res. 2016, 61, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Mostafa, S.; Lu, Z.; Jin, B. Melatonin-Mediated Abiotic Stress Tolerance in Plants. Front. Plant Sci. 2022, 13, 847175. [Google Scholar] [CrossRef]
- Ye, T.; Yin, X.; Yu, L.; Zheng, S.-J.; Cai, W.-J.; Wu, Y.; Feng, Y.-Q. Metabolic analysis of the melatonin biosynthesis pathway using chemical labeling coupled with liquid chromatography-mass spectrometry. J. Pineal Res. 2019, 66, e12531. [Google Scholar] [CrossRef]
- Byeon, Y.; Choi, G.H.; Lee, H.Y.; Back, K. Melatonin biosynthesis requires N-acetylserotonin methyltransferase activity of caffeic acid O-methyltransferase in rice. J. Exp. Bot. 2015, 66, 6917–6925. [Google Scholar] [CrossRef]
- Kołodziejczyk, I.; Bałabusta, M.; Szewczyk, R.; Posmyk, M.M. The levels of melatonin and its metabolites in conditioned corn (Zea mays L.) and cucumber (Cucumis sativus L.) seeds during storage. Acta Physiol. Plant. 2015, 37, 105. [Google Scholar] [CrossRef]
- Manchester, L.C.; Coto-Montes, A.; Boga, J.A.; Andersen, L.P.; Zhou, Z.; Galano, A.; Vriend, J.; Tan, D.X.; Reiter, R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015, 59, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Imran, Q.M.; Shahid, M.; Yun, B.-W. 2—Nitric oxide synthase in the plant kingdom. In Nitric Oxide in Plant Biology; Pratap Singh, V., Singh, S., Tripathi, D.K., Romero-Puertas, M.C., Sandalio, L.M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 43–52. [Google Scholar]
- Corpas, F.J.; González-Gordo, S.; Cañas, A.; Palma, J.M. Nitric oxide and hydrogen sulfide in plants: Which comes first? J. Exp. Bot. 2019, 70, 4391–4404. [Google Scholar] [CrossRef] [PubMed]
- Alber, N.A.; Sivanesan, H.; Vanlerberghe, G.C. The occurrence and control of nitric oxide generation by the plant mitochondrial electron transport chain. Plant Cell Environ. 2017, 40, 1074–1085. [Google Scholar] [CrossRef] [PubMed]
- Sparacino-Watkins, C.E.; Tejero, J.; Sun, B.; Gauthier, M.C.; Thomas, J.; Ragireddy, V.; Merchant, B.A.; Wang, J.; Azarov, I.; Basu, P.; et al. Nitrite Reductase and Nitric-oxide Synthase Activity of the Mitochondrial Molybdopterin Enzymes mARC1 and mARC2*. J. Biol. Chem. 2014, 289, 10345–10358. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Gao, H.; Lu, M.; Hao, C.; Pu, Z.; Guo, M.; Hou, D.; Chen, L.-Y.; Huang, X. Melatonin-Nitric Oxide Crosstalk and Their Roles in the Redox Network in Plants. Int. J. Mol. Sci. 2019, 20, 6200. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Back, K. Melatonin is required for H2O2- and NO-mediated defense signaling through MAPKKK3 and OXI1 in Arabidopsis thaliana. J. Pineal Res. 2017, 62, e12379. [Google Scholar] [CrossRef] [PubMed]
- Astier, J.; Gross, I.; Durner, J. Nitric oxide production in plants: An update. J. Exp. Bot. 2017, 69, 3401–3411. [Google Scholar] [CrossRef] [PubMed]
- Havemeyer, A.; Bittner, F.; Wollers, S.; Mendel, R.; Kunze, T.; Clement, B. Identification of the Missing Component in the Mitochondrial Benzamidoxime Prodrug-converting System as a Novel Molybdenum Enzyme *. J. Biol. Chem. 2006, 281, 34796–34802. [Google Scholar] [CrossRef] [PubMed]
- Igamberdiev, A.U.; Ratcliffe, R.G.; Gupta, K.J. Plant mitochondria: Source and target for nitric oxide. Mitochondrion 2014, 19, 329–333. [Google Scholar] [CrossRef]
- Hussain, A.; Yun, B.-W.; Kim, J.H.; Gupta, K.J.; Hyung, N.-I.; Loake, G.J. Novel and conserved functions of S-nitrosoglutathione reductase in tomato. J. Exp. Bot. 2019, 70, 4877–4886. [Google Scholar] [CrossRef]
- Blask, D.E.; Dauchy, R.T.; Sauer, L.A.; Krause, J.A. Melatonin uptake and growth prevention in rat hepatoma 7288CTC in response to dietary melatonin: Melatonin receptor-mediated inhibition of tumor linoleic acid metabolism to the growth signaling molecule 13-hydroxyoctadecadienoic acid and the potential role of phytomelatonin. Carcinogenesis 2004, 25, 951–960. [Google Scholar] [PubMed]
- Martínez-Lorente, S.E.; Pardo-Hernández, M.; Martí-Guillén, J.M.; López-Delacalle, M.; Rivero, R.M. Interaction between melatonin and NO: Action mechanisms, main targets, and putative roles of the emerging molecule NOmela. Int. J. Mol. Sci. 2022, 23, 6646. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S. Recent advancements in the mechanism of nitric oxide signaling associated with hydrogen sulfide and melatonin crosstalk during ethylene-induced fruit ripening in plants. Nitric Oxide 2019, 82, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S. Insights into nitric oxide-melatonin crosstalk and N-nitrosomelatonin functioning in plants. J. Exp. Bot. 2019, 70, 6035–6047. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Terron, M.P.; Flores, L.J.; Reiter, R.J. One molecule, many derivatives: A never-ending interaction of melatonin with reactive oxygen and nitrogen species? J. Pineal Res. 2007, 42, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gu, Q.; Yu, X.; Huang, L.; Xu, S.; Wang, R.; Shen, W.; Shen, W. Hydrogen peroxide acts downstream of melatonin to induce lateral root formation. Ann. Bot. 2018, 121, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, H.; Yang, K.; Wang, Y.; Yang, L.; Hu, L.; Liu, R.; Shi, Z. Melatonin facilitates lateral root development by coordinating PAO-derived hydrogen peroxide and Rboh-derived superoxide radical. Free Radic. Biol. Med. 2019, 143, 534–544. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xie, Y.; Gu, Q.; Zhao, G.; Zhang, Y.; Cui, W.; Xu, S.; Wang, R.; Shen, W. The AtrbohF-dependent regulation of ROS signaling is required for melatonin-induced salinity tolerance in Arabidopsis. Free Radic. Biol. Med. 2017, 108, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Yan, Y.; Wen, D.; Shi, Q. Hydrogen peroxide produced by NADPH oxidase: A novel downstream signaling pathway in melatonin-induced stress tolerance in Solanum lycopersicum. Physiol. Plant 2017, 160, 396–409. [Google Scholar] [CrossRef]
- Noda, Y.; Mori, A.; Liburdy, R.; Packer, L. Melatonin and its precursors scavenge nitric oxide. J. Pineal Res. 1999, 27, 159–163. [Google Scholar] [CrossRef]
- Kirsch, M.; De Groot, H. N-Nitrosomelatonin: Synthesis, chemical properties, potential prodrug. J. Pineal Res. 2009, 46, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.S.; Luo, Z.; Jannatizadeh, A.; Sheikh-Assadi, M.; Sharafi, Y.; Farmani, B.; Fard, J.R.; Razavi, F. Employing exogenous melatonin applying confers chilling tolerance in tomato fruits by upregulating ZAT2/6/12 giving rise to promoting endogenous polyamines, proline, and nitric oxide accumulation by triggering arginine pathway activity. Food Chem. 2019, 275, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yang, J.; Zhang, H.; Cong, L.; Zhai, R.; Yang, C.; Wang, Z.; Ma, F.; Xu, L. Melatonin Inhibits Ethylene Synthesis via Nitric Oxide Regulation To Delay Postharvest Senescence in Pears. J. Agric. Food Chem. 2019, 67, 2279–2288. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Gong, B.; Sun, S.; Liu, S.; Wang, X.; Wei, M.; Yang, F.; Li, Y.; Shi, Q. Promoting Roles of Melatonin in Adventitious Root Development of Solanum lycopersicum L. by Regulating Auxin and Nitric Oxide Signaling. Front. Plant Sci. 2016, 7, 718. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Di Mascio, P.; Martinez, G.R.; Prado, F.M.; Reiter, R.J. Novel rhythms of N1-acetyl-N2-formyl-5-methoxykynuramine and its precursor melatonin in water hyacinth: Importance for phytoremediation. FASEB J. 2007, 21, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Back, K. 2-Hydroxymelatonin confers tolerance against combined cold and drought stress in tobacco, tomato, and cucumber as a potent anti-stress compound in the evolution of land plants. Mela Res. 2019, 2, 35–46. [Google Scholar] [CrossRef]
- Shah, A.A.; Ahmed, S.; Ali, A.; Yasin, N.A. 2-Hydroxymelatonin mitigates cadmium stress in cucumis sativus seedlings: Modulation of antioxidant enzymes and polyamines. Chemosphere 2020, 243, 125308. [Google Scholar] [CrossRef] [PubMed]
- Kopczak, A.; Korth, H.G.; de Groot, H.; Kirsch, M. N-nitroso-melatonin releases nitric oxide in the presence of serotonin and its derivatives. J. Pineal Res. 2007, 43, 343–350. [Google Scholar] [CrossRef]
- Wellard, J.W.; Morgan, I.G. Nitric oxide donors mimic the effects of light on photoreceptor melatonin synthesis. Aust. N. Z. J. Ophthalmol. 1996, 24 (Suppl. 2), 61–63. [Google Scholar] [CrossRef]
- Antoniou, C.; Chatzimichail, G.; Xenofontos, R.; Pavlou, J.J.; Panagiotou, E.; Christou, A.; Fotopoulos, V. Melatonin systemically ameliorates drought stress-induced damage in Medicago sativa plants by modulating nitro-oxidative homeostasis and proline metabolism. J. Pineal Res. 2017, 62, e12401. [Google Scholar] [CrossRef]
- Aghdam, M.S.; Mukherjee, S.; Flores, F.B.; Arnao, M.B.; Luo, Z.; Corpas, F.J. Functions of melatonin during postharvest of horticultural crops. Plant Cell Physiol. 2021, 63, 1764–1786. [Google Scholar] [CrossRef] [PubMed]
- Aydogan, S.; Yerer, M.B.; Goktas, A. Melatonin and nitric oxide. J. Endocrinol. Investig. 2006, 29, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Galano, A.; Tan, D.X.; Reiter, R.J. Cyclic 3-hydroxymelatonin, a key metabolite enhancing the peroxyl radical scavenging activity of melatonin. RSC Adv. 2014, 4, 5220–5227. [Google Scholar] [CrossRef]
- Galano, A.; Reiter, R.J. Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal Res. 2018, 65, e12514. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Soares, C.; Sousa, B.; Martins, M.; Kumar, V.; Shahzad, B.; Sidhu, G.P.S.; Bali, A.S.; Asgher, M.; Bhardwaj, R.; et al. Nitric oxide-mediated regulation of oxidative stress in plants under metal stress: A review on molecular and biochemical aspects. Physiolog. Plant 2020, 168, 318–344. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.L.; Liu, L.; Wang, B.R.; Wu, X.M.; Zhou, Y. Physiological effects of exogenous nitric oxide on Brassica juncea seedlings under NaCl stress. Bio. Plant 2011, 55, 345–348. [Google Scholar] [CrossRef]
- Mannino, G.; Pernici, C.; Serio, G.; Gentile, C.; Bertea, C.M. Melatonin and phytomelatonin: Chemistry, biosynthesis, metabolism, distribution and bioactivity in plants and animals-An overview. Int. J. Mol. Sci. 2021, 22, 9996. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Hardeland, R.; Manchester, L.C.; Paredes, S.D.; Korkmaz, A.; Sainz, R.M.; Mayo, J.C.; Fuentes-Broto, L.; Reiter, R.J. The changing biological roles of melatonin during evolution: From an antioxidant to signals of darkness, sexual selection and fitness. Biol. Rev. Camb. Philos. Soc. 2010, 85, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-M.; Shaffique, S.; Injamum-Ul-Hoque, M.; Alomrani, S.O.; Park, Y.-S.; Lee, I.-J. Foliar treatment with melatonin modulates photosynthetic and antioxidant responses in Silybum marianum L. under salt stress. Sci. Hortic. Amst. 2024, 325, 112664. [Google Scholar] [CrossRef]
- Rodriguez, C.; Mayo, J.C.; Sainz, R.M.; Antolin, I.; Herrera, F.; Martin, V.; Reiter, R.J. Regulation of antioxidant enzymes: A significant role for melatonin. J. Pineal Res. 2004, 36, 1–9. [Google Scholar] [CrossRef]
- Khan, M.N.; Siddiqui, M.H.; AlSolami, M.A.; Siddiqui, Z.H. Melatonin-regulated heat shock proteins and mitochondrial ATP synthase induce drought tolerance through sustaining ROS homeostasis in H2S-dependent manner. Plant Physiol. Biochem. 2023, 206, 108231. [Google Scholar] [CrossRef]
- Yan, D.; Wang, J.; Lu, Z.; Liu, R.; Hong, Y.; Su, B.; Wang, Y.; Peng, Z.; Yu, C.; Gao, Y.; et al. Melatonin-Mediated Enhancement of Photosynthetic Capacity and Photoprotection Improves Salt Tolerance in Wheat. Plants 2023, 12, 3984. [Google Scholar] [CrossRef] [PubMed]
- Kebbeh, M.; Dong, J.-x.; Huan, C.; Liu, Y.; Zheng, X.-L. Melatonin treatment alleviates chilling injury in mango fruit ‘Keitt’ by modulating proline metabolism under chilling stress. J. Integr. Agric. 2023, 22, 935–944. [Google Scholar] [CrossRef]
- Huang, Q.; Yan, H.; You, M.; Duan, J.; Chen, M.; Xing, Y.; Hu, X.; Li, X. Enhancing Drought Tolerance and Fruit Characteristics in Tomato through Exogenous Melatonin Application. Horticulturae 2023, 9, 1083. [Google Scholar] [CrossRef]
- Eisa, E.A.; Honfi, P.; Tilly-Mándy, A.; Mirmazloum, I. Exogenous Melatonin Application Induced Morpho-Physiological and Biochemical Regulations Conferring Salt Tolerance in Ranunculus asiaticus L. Horticulturae 2023, 9, 228. [Google Scholar] [CrossRef]
- Jahan, M.S.; Zhao, C.J.; Shi, L.B.; Liang, X.R.; Jabborova, D.; Nasar, J.; Zhou, X.B. Physiological mechanism of melatonin attenuating to osmotic stress tolerance in soybean seedlings. Front. Plant Sci. 2023, 14, 1193666. [Google Scholar] [CrossRef]
- Jahan, M.S.; Li, G.; Xie, D.; Farag, R.; Hasan, M.M.; Alabdallah, N.M.; Al-Harbi, N.A.; Al-Qahtani, S.M.; Zeeshan, M.; Nasar, J.; et al. Melatonin Mitigates Salt-Induced Growth Inhibition through the Regulation of Carbohydrate and Nitrogen Metabolism in Tomato Seedlings. J. Soil Sci. Plant Nutr. 2023, 23, 4290–4308. [Google Scholar] [CrossRef]
- Su, C.; Wang, P.; Wu, J.; Wang, H.; Fan, J.; Gong, W.; Hui, W.; Wang, J. Effects of Melatonin on the Photosynthetic Characteristics of Zanthoxylum armatum under Waterlogging Stress. Russ. J. Plant Physiol. 2023, 70, 82. [Google Scholar] [CrossRef]
- Bi, R.; Li, R.; Xu, Z.; Cai, H.; Zhao, J.; Zhou, Y.; Wu, B.; Sun, P.; Yang, W.; Zheng, L.; et al. Melatonin targets MoIcl1 and works synergistically with fungicide isoprothiolane in rice blast control. J. Pineal Res. 2023, 75, e12896. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, J.; Wan, Z.-y.; Huang, S.; Di, H.; He, Y.; Jin, S. Physiological and transcriptome analyses provide new insights into the mechanism mediating the enhanced tolerance of melatonin-treated rhododendron plants to heat stress1. J. Integr. Agric. 2023, 22, 2397–2411. [Google Scholar] [CrossRef]
- Gong, B.; Wei, J.-W.; Liu, M.; Cao, B.; Shan, Q.; Liu, X.; Liu, W.; Shi, Q.; Liu, D. Melatonin relieves the S-nitrosylation of plasma membrane H+-ATPase 2 to enhance saline-alkali tolerance in tomato. Res. Sq. 2023. [Google Scholar] [CrossRef]
- Wei, J.; Li, D.X.; Zhang, J.R.; Shan, C.; Rengel, Z.; Song, Z.B.; Chen, Q. Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar] [CrossRef] [PubMed]
- Corpas, F.J.; Freschi, L.; Rodríguez-Ruiz, M.; Mioto, P.T.; González-Gordo, S.; Palma, J.M. Nitro-oxidative metabolism during fruit ripening. J. Exp. Bot. 2018, 69, 3449–3463. [Google Scholar] [CrossRef] [PubMed]
- He, H.; He, L.-F. Crosstalk between melatonin and nitric oxide in plant development and stress responses. Physiol. Plant. 2020, 170, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernandez-Ruiz, J. Growth conditions influence the melatonin content of tomato plants. Food Chem. 2013, 138, 1212–1214. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernandez-Ruiz, J. Chemical stress by different agents affects the melatonin content of barley roots. J. Pineal Res. 2009, 46, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Afreen, F.; Zobayed, S.M.; Kozai, T. Melatonin in Glycyrrhiza uralensis: Response of plant roots to spectral quality of light and UV-B radiation. J. Pineal Res. 2006, 41, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.X.; Manchester, L.C.; Hardeland, R.; Lopez-Burillo, S.; Mayo, J.C.; Sainz, R.M.; Reiter, R.J. Melatonin: A hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J. Pineal Res. 2003, 34, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Murch, S.J.; Erland, L.A.E. A Systematic Review of Melatonin in Plants: An Example of Evolution of Literature. Front. Plant Sci. 2021, 12, 683047. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef]
- Wei, W.; Li, Q.T.; Chu, Y.N.; Reiter, R.J.; Yu, X.M.; Zhu, D.H.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, J.S.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Chen, Y.; Dong, W.; Li, S.; Chen, W.; Yang, Z.; Cao, S. Melatonin delayed senescence by modulating the contents of plant signalling molecules in postharvest okras. Front. Plant Sci. 2024, 15, 1304913. [Google Scholar] [CrossRef] [PubMed]
- Albertos, P.; Romero-Puertas, M.C.; Tatematsu, K.; Mateos, I.; Sanchez-Vicente, I.; Nambara, E.; Lorenzo, O. S-nitrosylation triggers ABI5 degradation to promote seed germination and seedling growth. Nat. Commun. 2015, 6, 1304913. [Google Scholar]
- Zhang, Q.; Cai, X.; Wu, B.; Tong, B.; Xu, D.; Wang, J.; Cui, B.; Yin, R.; Lin, L. S-nitrosylation may inhibit the activity of COP1 in plant photomorphogenesis. Biochem. Biophys. Res. Commun. 2024, 719, 150096. [Google Scholar] [CrossRef] [PubMed]
- Terrile, M.C.; París, R.; Calderón-Villalobos, L.I.; Iglesias, M.J.; Lamattina, L.; Estelle, M.; Casalongué, C.A. Nitric oxide influences auxin signaling through S-nitrosylation of the Arabidopsis TRANSPORT INHIBITOR RESPONSE 1 auxin receptor. Plant J. 2012, 70, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Huang, D.; Wang, C.; Wang, B.; Fang, H.; Huo, J.; Liao, W. Recent Progress in Protein S-Nitrosylation in Phytohormone Signaling. Plant Cell Physiol. 2019, 60, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Wang, P.; Li, M.; Ke, X.; Li, C.; Liang, D.; Wu, S.; Ma, X.; Li, C.; Zou, Y.; et al. Exogenous melatonin improves Malus resistance to Marssonina apple blotch. J. Pineal Res. 2013, 54, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Byeon, Y.; Tan, D.X.; Reiter, R.J.; Back, K. Arabidopsis serotonin N-acetyltransferase knockout mutant plants exhibit decreased melatonin and salicylic acid levels resulting in susceptibility to an avirulent pathogen. J. Pineal Res. 2015, 58, 291–299. [Google Scholar] [CrossRef]
- Debnath, B.; Islam, W.; Li, M.; Sun, Y.; Lu, X.; Mitra, S.; Hussain, M.; Liu, S.; Qiu, D. Melatonin Mediates Enhancement of Stress Tolerance in Plants. Int. J. Mol. Sci. 2019, 20, 1040. [Google Scholar] [CrossRef]
- Li, C.; He, Q.; Zhang, F.; Yu, J.; Li, C.; Zhao, T.; Zhang, Y.; Xie, Q.; Su, B.; Mei, L.; et al. Melatonin enhances cotton immunity to Verticillium wilt via manipulating lignin and gossypol biosynthesis. Plant J. 2019, 100, 784–800. [Google Scholar] [CrossRef]
- Shi, H.; Chen, Y.; Tan, D.-X.; Reiter, R.J.; Chan, Z.; He, C. Melatonin induces nitric oxide and the potential mechanisms relate to innate immunity against bacterial pathogen infection in Arabidopsis. J. Pineal Res. 2015, 59, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.-T.; Li, R.-J.; Cai, W.; Liu, W.; Wang, C.-L.; Lu, Y.-T. Increasing Nitric Oxide Content in Arabidopsis thaliana by Expressing Rat Neuronal Nitric Oxide Synthase Resulted in Enhanced Stress Tolerance. Plant Cell Physiol. 2011, 53, 344–357. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Qian, Y.; Tan, D.-X.; Reiter, R.J.; He, C. Melatonin induces the transcripts of CBF/DREB1s and their involvement in both abiotic and biotic stresses in Arabidopsis. J. Pineal Res. 2015, 59, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Tan, D.-X.; Reiter, R.J.; Shi, H. Comparative metabolomic analysis highlights the involvement of sugars and glycerol in melatonin-mediated innate immunity against bacterial pathogen in Arabidopsis. Sci. Rep. 2015, 5, 15815. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, L.; Gu, P.; Zhan, X.; Zhang, Y.; Hou, C.; Wu, Z.; Wu, Y.-F.; Wang, Q.-C. Exogenous application of melatonin improves plant resistance to virus infection. Plant Pathol. 2019, 68, 1287–1295. [Google Scholar] [CrossRef]
- Kaya, C.; Higgs, D.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Integrative roles of nitric oxide and hydrogen sulfide in melatonin-induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physiol. Plant 2020, 168, 256–277. [Google Scholar] [CrossRef]
- Kaur, H.; Bhatla, S.C. Melatonin and nitric oxide modulate glutathione content and glutathione reductase activity in sunflower seedling cotyledons accompanying salt stress. Nitric Oxide 2016, 59, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Arora, D.; Bhatla, S.C. Melatonin and nitric oxide regulate sunflower seedling growth under salt stress accompanying differential expression of Cu/Zn SOD and Mn SOD. Free Radic. Biol. Med. 2017, 106, 315–328. [Google Scholar] [CrossRef]
- Zhao, G.; Zhao, Y.; Yu, X.; Kiprotich, F.; Han, H.; Guan, R.; Wang, R.; Shen, W. Nitric Oxide Is Required for Melatonin-Enhanced Tolerance against Salinity Stress in Rapeseed (Brassica napus L.) Seedlings. Int. J. Mol. Sci. 2018, 19, 1912. [Google Scholar] [CrossRef]
- Zhang, J.; Li, D.; Wei, J.; Ma, W.; Kong, X.; Rengel, Z.; Chen, Q. Melatonin alleviates aluminum-induced root growth inhibition by interfering with nitric oxide production in Arabidopsis. Environ. Exp. Bot. 2019, 161, 157–165. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, Z.; Zhu, L.; Ma, Z.; Wang, J.; Zhu, J. Exogenous Melatonin Improves Plant Iron Deficiency Tolerance via Increased Accumulation of Polyamine-Mediated Nitric Oxide. Int. J. Mol. Sci. 2016, 17, 1777. [Google Scholar] [CrossRef]
- Kaya, C.; Okant, M.; Ugurlar, F.; Alyemeni, M.N.; Ashraf, M.; Ahmad, P. Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere 2019, 225, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Fu, X.; Han, L.; Xu, C.; Liu, C.; Bi, H.; Ai, X. Nitric Oxide Functions as a Downstream Signal for Melatonin-Induced Cold Tolerance in Cucumber Seedlings. Front. Plant Sci. 2021, 12, 686545. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Gong, B.; Jin, Z.; Wang, X.; Wei, M.; Yang, F.; Li, Y.; Shi, Q. Sodic alkaline stress mitigation by exogenous melatonin in tomato needs nitric oxide as a downstream signal. J. Plant Physiol. 2015, 186–187, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Berchner-Pfannschmidt, U.; Tug, S.; Trinidad, B.; Becker, M.; Oehme, F.; Flamme, I.; Fandrey, J.; Kirsch, M. The impact of N-nitrosomelatonin as nitric oxide donor in cell culture experiments. J. Pineal Res. 2008, 45, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Blanchard-Fillion, B.; Servy, C.; Ducrocq, C. 1-Nitrosomelatonin is a spontaneous NO-releasing compound. Free Radic. Res. 2001, 35, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, M.; Fuchs, A.; de Groot, H. Regiospecific Nitrosation of N-terminal-blocked Tryptophan Derivatives by N2O3 at Physiological pH*. J. Biol. Chem. 2003, 278, 11931–11936. [Google Scholar] [CrossRef] [PubMed]
- Kytzia, A.; Korth, H.G.; Sustmann, R.; de Groot, H.; Kirsch, M. On the mechanism of the ascorbic acid-induced release of nitric oxide from N-nitrosated tryptophan derivatives: Scavenging of NO by ascorbyl radicals. Chemistry 2006, 12, 8786–8797. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Korth, H.-G.; de Groot, H.; Kirsch, M. Reaction of Vitamin E Compounds with N-Nitrosated Tryptophan Derivatives and Its Analytical Use. Chem. A Eur. J. 2007, 13, 7532–7542. [Google Scholar] [CrossRef]
- Kytzia, A.; Korth, H.G.; de Groot, H.; Kirsch, M. Catecholamine-induced release of nitric oxide from N-nitrosotryptophan derivatives: A non-enzymatic method for catecholamine oxidation. Org. Biomol. Chem. 2006, 4, 257–267. [Google Scholar] [CrossRef]
- Sonnenschein, K.; de Groot, H.; Kirsch, M. Formation of S-nitrosothiols from regiospecific reaction of thiols with N-nitrosotryptophan derivatives. J. Biol. Chem. 2004, 279, 45433–45440. [Google Scholar] [CrossRef] [PubMed]
- Baidanoff, F.M.; Plano, S.A.; Doctorovich, F.; Suárez, S.A.; Golombek, D.A.; Chiesa, J.J. N-nitrosomelatonin enhances photic synchronization of mammalian circadian rhythms. J. Neurochem. 2014, 129, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Peyrot, F.; Grillon, C.; Vergely, C.; Rochette, L.; Ducrocq, C. Pharmacokinetics of 1-nitrosomelatonin and detection by EPR using iron dithiocarbamate complex in mice. Biochem. J. 2005, 387 Pt 2, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Jain, P.; Gupta, S.; Khurana, J.M.; Bhatla, S.C. N-Nitrosomelatonin, an efficient nitric oxide donor and transporter in Arabidopsis seedlings. Nitric Oxide 2021, 113–114, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Bonnett, R.; Holleyhead, R. Reaction of tryptophan derivatives with nitrite. J. Chem. Soc. Perkin Trans. 1974, 1, 962–964. [Google Scholar] [CrossRef]
- Jahan, M.S.; Shu, S.; Wang, Y.; Chen, Z.; He, M.; Tao, M.; Sun, J.; Guo, S. Melatonin alleviates heat-induced damage of tomato seedlings by balancing redox homeostasis and modulating polyamine and nitric oxide biosynthesis. BMC Plant Biol. 2019, 19, 414. [Google Scholar] [CrossRef]
- Yan, F.; Wei, H.; Li, W.; Liu, Z.; Tang, S.; Chen, L.; Ding, C.; Jiang, Y.; Ding, Y.; Li, G. Melatonin improves K(+) and Na(+) homeostasis in rice under salt stress by mediated nitric oxide. Ecotox. Environ. Saf. 2020, 206, 111358. [Google Scholar] [CrossRef]
- Hussain, A.; Yun, B.-W.; Loake, G.J. Nitric Oxide Analyzer Quantification of Plant S-Nitrosothiols. In Nitric Oxide: Methods and Protocols; Mengel, A., Lindermayr, C., Eds.; Springer New York: New York, NY, USA, 2018; pp. 223–230. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).