Connexin 43 Modulation in Human Chondrocytes, Osteoblasts and Cartilage Explants: Implications for Inflammatory Joint Disorders
Abstract
1. Introduction
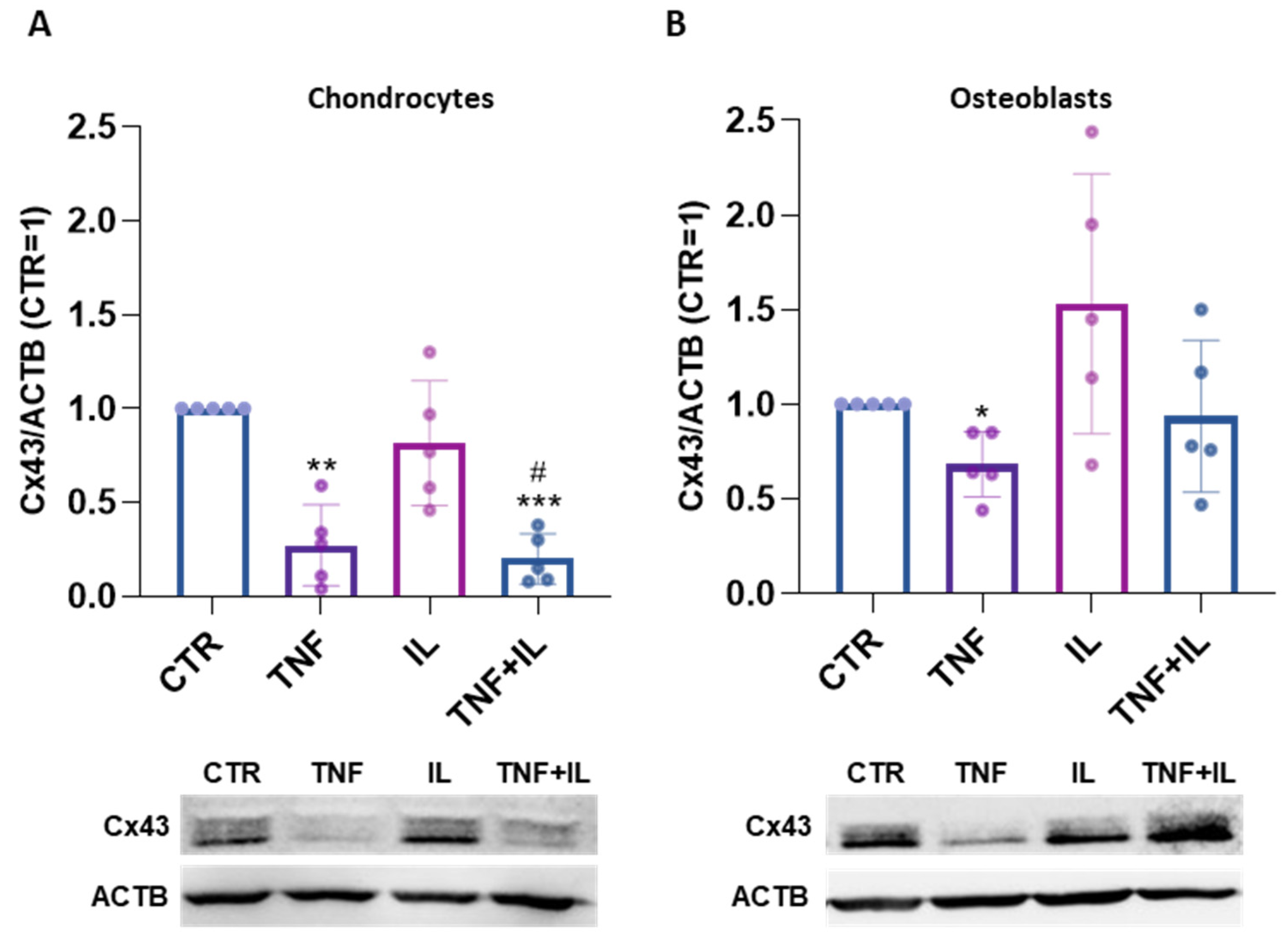
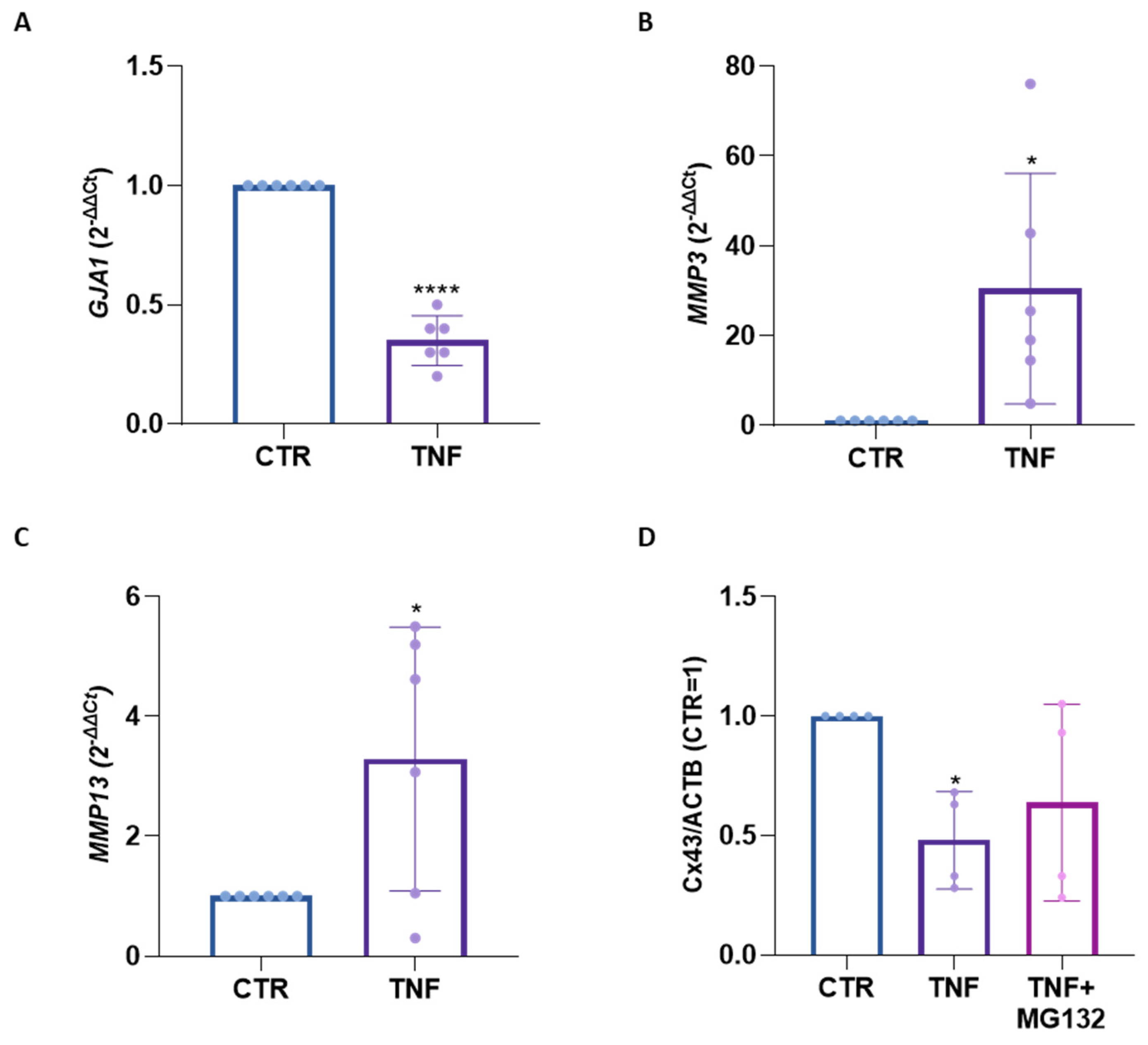
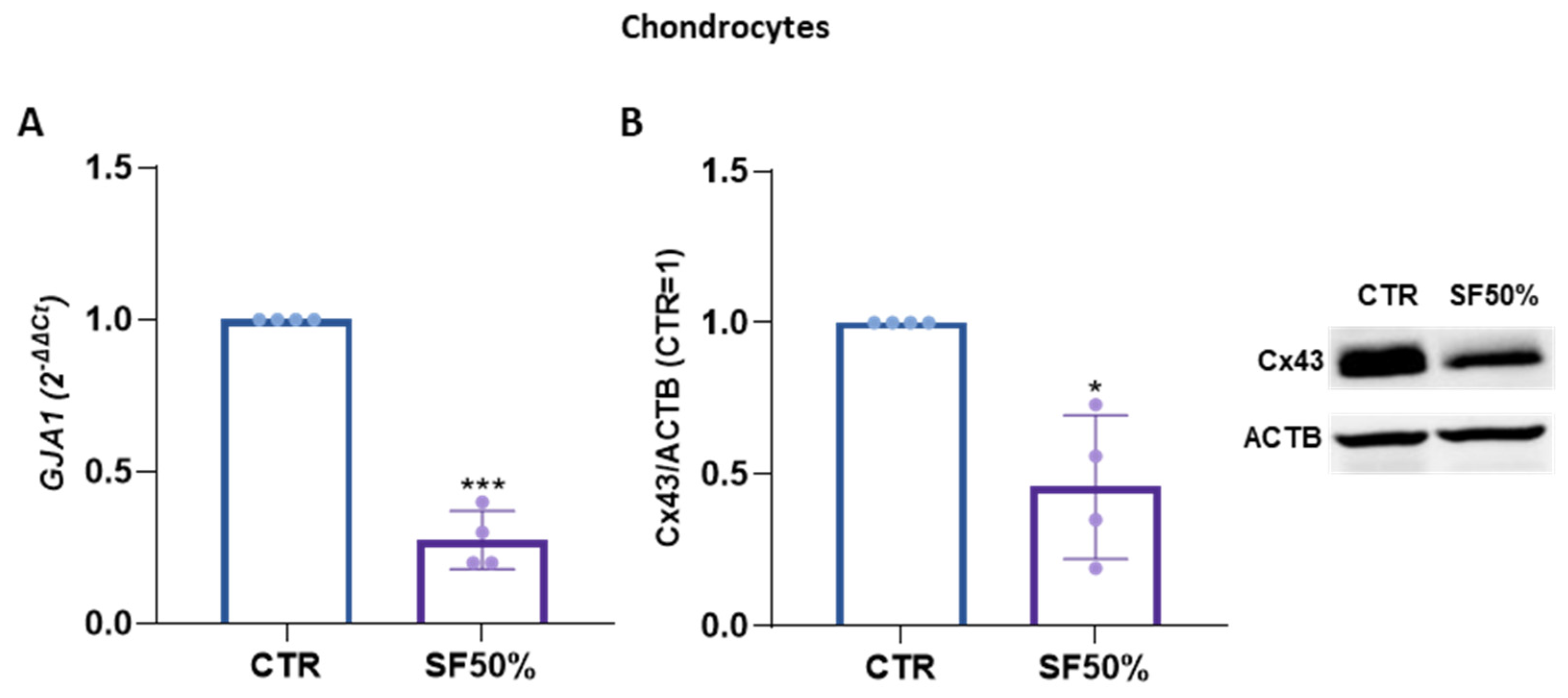
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell and Cartilage Explant Isolation and Cultures
4.2. Collection of Synovial Fluid
4.3. Treatments
4.4. Western Blotting
4.5. Gene Expression Analysis
4.6. Immunofluorescence
4.7. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fanani, M.L.; Wilke, N. Regulation of phase boundaries and phase-segregated patterns in model membranes. Biochim. Biophys. Acta Biomembr. 2018, 1860, 1972–1984. [Google Scholar] [CrossRef]
- Harris, A.L. Emerging issues of connexin channels: Biophysics fills the gap. Q. Rev. Biophys. 2001, 34, 325–472. [Google Scholar] [CrossRef] [PubMed]
- Zappalà, A.; Romano, I.R.; D’Angeli, F.; Musumeci, G.; Lo Furno, D.; Giuffrida, R.; Mannino, G. Functional Roles of Connexins and Gap Junctions in Osteo-Chondral Cellular Components. Int. J. Mol. Sci. 2023, 24, 4156. [Google Scholar] [CrossRef] [PubMed]
- Sedovy, M.W.; Leng, X.; Leaf, M.R.; Iqbal, F.; Payne, L.B.; Chappell, J.C.; Johnstone, S.R. Connexin 43 across the Vasculature: Gap Junctions and Beyond. J. Vasc. Res. 2023, 60, 101–113. [Google Scholar] [CrossRef]
- Batra, N.; Kar, R.; Jiang, J.X. Gap junctions and hemichannels in signal transmission, function and development of bone. Biochim. Biophys. Acta 2012, 1818, 1909–1918. [Google Scholar] [CrossRef]
- Chen, X.; Kong, X.; Zhuang, W.; Teng, B.; Yu, X.; Hua, S.; Wang, S.; Liang, F.; Ma, D.; Zhang, S.; et al. Dynamic changes in protein interaction between AKAP95 and Cx43 during cell cycle progression of A549 cells. Sci. Rep. 2016, 6, 21224. [Google Scholar] [CrossRef] [PubMed]
- Kotini, M.; Barriga, E.H.; Leslie, J.; Gentzel, M.; Rauschenberger, V.; Schambony, A.; Mayor, R. Gap junction protein Connexin-43 is a direct transcriptional regulator of N-cadherin in vivo. Nat. Commun. 2018, 9, 3846. [Google Scholar] [CrossRef]
- Chaible, L.M.; Sanches, D.S.; Cogliati, B.; Mennecier, G.; Dagli, M.L. Delayed osteoblastic differentiation and bone development in Cx43 knockout mice. Toxicol. Pathol. 2011, 39, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Donahue, H.J.; Qu, R.W.; Genetos, D.C. Joint diseases: From connexins to gap junctions. Nat. Rev. Rheumatol. 2017, 14, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Gago-Fuentes, R.; Bechberger, J.F.; Varela-Eirin, M.; Varela-Vazquez, A.; Acea, B.; Fonseca, E.; Naus, C.C.; Mayan, M.D. The C-terminal domain of connexin43 modulates cartilage structure via chondrocyte phenotypic changes. Oncotarget 2016, 7, 73055–73067. [Google Scholar] [CrossRef]
- Della Morte, E.; Niada, S.; Giannasi, C.; Zagra, L.; Brini, A.T. Dynamics of Connexin 43 down Modulation in Human Articular Chondrocytes Stimulated by Tumor Necrosis Factor Alpha. Int. J. Mol. Sci. 2022, 23, 5575. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.; Ramos, E.; Branco, J. Osteoarthritis. Acta Med. Port. 2015, 28, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Maruotti, N.; Corrado, A.; Cantatore, F.P. Osteoblast role in osteoarthritis pathogenesis. J. Cell. Physiol. 2017, 232, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef] [PubMed]
- Tacheau, C.; Laboureau, J.; Mauviel, A.; Verrecchia, F. TNF-α represses connexin43 expression in HaCat keratinocytes via activation of JNK signaling. J. Cell. Physiol. 2008, 216, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Nishida, T. Role of the ubiquitin-proteasome pathway in downregulation of the gap-junction protein Connexin43 by TNF-α in human corneal fibroblasts. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1943–1947. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Orita, T.; Morishige, N.; Nishida, T.; Sonoda, K.H. Role of the JNK signaling pathway in downregulation of connexin43 by TNF-α in human corneal fibroblasts. Curr. Eye Res. 2013, 38, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, P.; Lamboux, A.; Albarède, F.; Miossec, P. Differential effects of TNF-α and IL-1β on the control of metal metabolism and cadmium-induced cell death in chronic inflammation. PLoS ONE 2018, 13, e0196285. [Google Scholar] [CrossRef]
- Alvarez, A.M.; DeOcesano-Pereira, C.; Teixeira, C.; Moreira, V. IL-1β and TNF-α Modulation of Proliferated and Committed Myoblasts: IL-6 and COX-2-Derived Prostaglandins as Key Actors in the Mechanisms Involved. Cells 2020, 9, 2005. [Google Scholar] [CrossRef]
- Ott, L.W.; Resing, K.A.; Sizemore, A.W.; Heyen, J.W.; Cocklin, R.R.; Pedrick, N.M.; Woods, H.C.; Chen, J.Y.; Goebl, M.G.; Witzmann, F.A.; et al. Tumor Necrosis Factor-alpha- and interleukin-1-induced cellular responses: Coupling proteomic and genomic information. J. Proteome Res. 2007, 6, 2176–2185. [Google Scholar] [CrossRef]
- Kono, J.; Ueda, M.; Sengiku, A.; Suadicani, S.O.; Woo, J.T.; Kobayashi, T.; Ogawa, O.; Negoro, H. Flavonoid Nobiletin Attenuates Cyclophosphamide-Induced Cystitis in Mice through Mechanisms That Involve Inhibition of IL-1β Induced Connexin 43 Upregulation and Gap Junction Communication in Urothelial Cells. Int. J. Mol. Sci. 2022, 23, 5037. [Google Scholar] [CrossRef] [PubMed]
- Su, X.L.; Wang, S.H.; Komal, S.; Cui, L.G.; Ni, R.C.; Zhang, L.R.; Han, S.N. The caspase-1 inhibitor VX765 upregulates connexin 43 expression and improves cell-cell communication after myocardial infarction via suppressing the IL-1β/p38 MAPK pathway. Acta Pharmacol. Sin. 2022, 43, 2289–2301. [Google Scholar] [CrossRef]
- Retamal, M.A.; Froger, N.; Palacios-Prado, N.; Ezan, P.; Sáez, P.J.; Sáez, J.C.; Giaume, C. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J. Neurosci. 2007, 27, 13781–13792. [Google Scholar] [CrossRef] [PubMed]
- De Bock, M.; Wang, N.; Decrock, E.; Bultynck, G.; Leybaert, L. Intracellular Cleavage of the Cx43 C-Terminal Domain by Matrix-Metalloproteases: A Novel Contributor to Inflammation? Mediat. Inflamm. 2015, 2015, 257471. [Google Scholar] [CrossRef]
- Zhao, Y.; Rivieccio, M.A.; Lutz, S.; Scemes, E.; Brosnan, C.F. The TLR3 ligand polyI: C downregulates connexin 43 expression and function in astrocytes by a mechanism involving the NF-κB and PI3 kinase pathways. Glia 2006, 54, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Peng, X.; Wang, F.; You, Z.; Dong, Y.; Wang, S. Effects of tumor necrosis factor-alpha on the 26S proteasome and 19S regulator in skeletal muscle of severely scalded mice. J. Burn Care Res. 2006, 27, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Hallermalm, K.; Seki, K.; Wei, C.; Castelli, C.; Rivoltini, L.; Kiessling, R.; Levitskaya, J. Tumor necrosis factor-alpha induces coordinated changes in major histocompatibility class I presentation pathway, resulting in increased stability of class I complexes at the cell surface. Blood 2001, 98, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Quan, R.; Huang, Z.; Yue, Z.; Xin, D.; Yang, D.; Pan, J.; Zhang, L. Effects of a proteasome inhibitor on the NF-κB signalling pathway in experimental osteoarthritis. Scand. J. Rheumatol. 2013, 42, 400–407. [Google Scholar] [CrossRef]
- Radwan, M.; Wilkinson, D.J.; Hui, W.; Destrument, A.P.; Charlton, S.H.; Barter, M.J.; Gibson, B.; Coulombe, J.; Gray, D.A.; Rowan, A.D.; et al. Protection against murine osteoarthritis by inhibition of the 26S proteasome and lysine-48 linked ubiquitination. Ann. Rheum. Dis. 2015, 74, 1580–1587. [Google Scholar] [CrossRef]
- Zhan, J.; Yan, Z.; Kong, X.; Liu, J.; Lin, Z.; Qi, W.; Wu, Y.; Lin, J.; Pan, X.; Xue, X. Lycopene inhibits IL-1β-induced inflammation in mouse chondrocytes and mediates murine osteoarthritis. J. Cell. Mol. Med. 2021, 25, 3573–3584. [Google Scholar] [CrossRef]
- Loiselle, A.E.; Paul, E.M.; Lewis, G.S.; Donahue, H.J. Osteoblast and osteocyte-specific loss of Connexin43 results in delayed bone formation and healing during murine fracture healing. J. Orthop. Res. 2013, 31, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Hua, R.; Gu, S.; Jiang, J.X. Connexin 43 Hemichannels Regulate Osteoblast to Osteocyte Differentiation. Front. Cell Dev. Biol. 2022, 10, 892229. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhang, L.; Lu, Z.; Yang, B.; Yang, H.; Shang, P.; Jiang, J.X.; Wang, D.; Xu, H. Connexin 43 Channels in Osteocytes Are Necessary for Bone Mass and Skeletal Muscle Function in Aged Male Mice. Int. J. Mol. Sci. 2022, 23, 13506. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, L.I.; Bellido, T. Beyond gap junctions: Connexin43 and bone cell signaling. Bone 2013, 52, 157–166. [Google Scholar] [CrossRef]
- Leithe, E.; Mesnil, M.; Aasen, T. The connexin 43 C-terminus: A tail of many tales. Biochim. Biophys. Acta Biomembr. 2018, 1860, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Tamer, T.M. Hyaluronan and synovial joint: Function, distribution and healing. Interdiscip. Toxicol. 2013, 6, 111–125. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, W.; Yong, H.; He, M.; Yang, Y.; Deng, Z.; Li, Y. Macrophages in osteoarthritis: Pathophysiology and therapeutics. Am. J. Transl. Res. 2020, 12, 261–268. [Google Scholar] [PubMed]
- Ragni, E.; Perucca Orfei, C.; Valli, F.; Zagra, L.; de Girolamo, L. Molecular Characterization of Secreted Factors and Extracellular Vesicles-Embedded miRNAs from Bone Marrow-Derived Mesenchymal Stromal Cells in Presence of Synovial Fluid from Osteoarthritis Patients. Biology 2022, 11, 1632. [Google Scholar] [CrossRef]
- Ragni, E.; De Luca, P.; Valli, F.; Zagra, L.; de Girolamo, L. Inflammatory Treatment Used to Mimic Osteoarthritis and Patients’ Synovial Fluid Have Divergent Molecular Impact on Chondrocytes In Vitro. Int. J. Mol. Sci. 2023, 24, 2625. [Google Scholar] [CrossRef]
- Sun, E.Y.; Fleck, A.K.M.; Abu-Hakmeh, A.E.; Kotsakis, A.; Leonard, G.R.; Wan, L.Q. Cartilage Metabolism is Modulated by Synovial Fluid through Metalloproteinase Activity. Ann. Biomed. Eng. 2018, 46, 810–818. [Google Scholar] [CrossRef]
- Yang, K.G.; Saris, D.B.; Verbout, A.J.; Creemers, L.B.; Dhert, W.J. The effect of synovial fluid from injured knee joints on in vitro chondrogenesis. Tissue Eng. 2006, 12, 2957–2964. [Google Scholar] [CrossRef] [PubMed]
- Hegewald, A.A.; Ringe, J.; Bartel, J.; Krüger, I.; Notter, M.; Barnewitz, D.; Kaps, C.; Sittinger, M. Hyaluronic acid and autologous synovial fluid induce chondrogenic differentiation of equine mesenchymal stem cells: A preliminary study. Tissue Cell 2004, 36, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Mayan, M.D.; Carpintero-Fernandez, P.; Gago-Fuentes, R.; Martinez-de-Ilarduya, O.; Wang, H.Z.; Valiunas, V.; Brink, P.; Blanco, F.J. Human articular chondrocytes express multiple gap junction proteins: Differential expression of connexins in normal and osteoarthritic cartilage. Am. J. Pathol. 2013, 182, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhai, C.; Shen, K.; Liu, G.; Liu, L.; He, J.; Chen, J.; Xu, Y. miR-1 Inhibits the Ferroptosis of Chondrocyte by Targeting CX43 and Alleviates Osteoarthritis Progression. J. Immunol. Res. 2023, 2023, 2061071. [Google Scholar] [CrossRef] [PubMed]
- Kohn, M.D.; Sassoon, A.A.; Fernando, N.D. Classifications in Brief: Kellgren-Lawrence Classification of Osteoarthritis. Clin. Orthop. Relat. Res. 2016, 474, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Cadelano, F.; Della Morte, E.; Niada, S.; Anzano, F.; Zagra, L.; Giannasi, C.; Brini, A.T.M. Cartilage responses to inflammatory stimuli and adipose stem/stromal cell-derived conditioned medium: Results from an ex vivo model. Regen. Ther. 2024, 26, 346–353. [Google Scholar] [CrossRef]
- Casnici, C.; Lattuada, D.; Tonna, N.; Crotta, K.; Storini, C.; Bianco, F.; Truzzi, M.C.; Corradini, C.; Marelli, O. Optimized “in vitro” culture conditions for human rheumatoid arthritis synovial fibroblasts. Mediat. Inflamm. 2014, 2014, 702057. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]


| Age | Sex | BMI | |||
|---|---|---|---|---|---|
| Range | Mean ± SD | Female | Male | Mean ± SD | |
| Cartilage explants (n = 8) | 60–88 | 72 ± 10 | 2 | 6 | 27.5 ± 4.3 |
| Osteoblasts (n = 11) | 53–83 | 70 ± 10 | 3 | 8 | 26.2 ± 3.2 |
| Chondrocytes (n = 11) | 53–83 | 66 ± 10 | 5 | 6 | 27.5 ± 4.3 |
| Age | Sex | BMI | |||
|---|---|---|---|---|---|
| Range | Mean ± SD | Female | Male | Mean ± SD | |
| Pool 1 (n = 14) | 50–88 | 69 ± 11 | 7 | 7 | 26.0 ± 3.9 |
| Pool 2 (n = 13) | 50–88 | 70 ± 10 | 6 | 7 | 26.7 ± 4.6 |
| Pool 3 (n = 15) | 42–88 | 68 ± 14 | 7 | 8 | 26.5 ± 4.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Della Morte, E.; Giannasi, C.; Valenza, A.; Cadelano, F.; Aldegheri, A.; Zagra, L.; Niada, S.; Brini, A.T. Connexin 43 Modulation in Human Chondrocytes, Osteoblasts and Cartilage Explants: Implications for Inflammatory Joint Disorders. Int. J. Mol. Sci. 2024, 25, 8547. https://doi.org/10.3390/ijms25158547
Della Morte E, Giannasi C, Valenza A, Cadelano F, Aldegheri A, Zagra L, Niada S, Brini AT. Connexin 43 Modulation in Human Chondrocytes, Osteoblasts and Cartilage Explants: Implications for Inflammatory Joint Disorders. International Journal of Molecular Sciences. 2024; 25(15):8547. https://doi.org/10.3390/ijms25158547
Chicago/Turabian StyleDella Morte, Elena, Chiara Giannasi, Alice Valenza, Francesca Cadelano, Alessandro Aldegheri, Luigi Zagra, Stefania Niada, and Anna Teresa Brini. 2024. "Connexin 43 Modulation in Human Chondrocytes, Osteoblasts and Cartilage Explants: Implications for Inflammatory Joint Disorders" International Journal of Molecular Sciences 25, no. 15: 8547. https://doi.org/10.3390/ijms25158547
APA StyleDella Morte, E., Giannasi, C., Valenza, A., Cadelano, F., Aldegheri, A., Zagra, L., Niada, S., & Brini, A. T. (2024). Connexin 43 Modulation in Human Chondrocytes, Osteoblasts and Cartilage Explants: Implications for Inflammatory Joint Disorders. International Journal of Molecular Sciences, 25(15), 8547. https://doi.org/10.3390/ijms25158547







