Calcium Regulation of Connexin Hemichannels
Abstract
:1. Introduction
2. Extracellular Ca2+ Regulation of Connexin HCs
3. Cytosolic Ca2+ Regulation of Connexin HCs
4. Pathological Alterations of HC Gating by Ca2+
| Cx Isoform | Mutation | Cx Domain | Mutant HC Defective Properties | Linked Disease |
|---|---|---|---|---|
| Cx26 | G11E | NT | Increased Ca2+ leakage in normal [Ca2+]e [127]. | KID syndrome |
| G12R | NT | Increased dye uptake in both normal and [Ca2+]e-free solutions, halted [Ca2+]e-dependent deactivation kinetics, increased Ca2+ leakage [123,128,129]. | Syndromic deafness | |
| G12V | NT | Increased dye uptake in [Ca2+]e-free solution [129]. | Non-syndromic deafness | |
| N14K | NT | Reduced [Ca2+]e sensitivity leading to increased HC currents and slowed deactivation kinetics, increased Ca2+ leakage [123,130,131]. | Clouston syndrome/KID syndrome | |
| N14Y | NT | Increased dye uptake and HC currents in both normal and [Ca2+]e-free solutions, increased Ca2+ leakage [129]. | Syndromic deafness | |
| S17F | NT | Decreased dye uptake in both normal and [Ca2+]e-free solutions, leaky HCs when co-expressed with Cx30 [123,129,138]. | KID syndrome | |
| I30N | TM1 | Increased dye uptake in both normal and [Ca2+]e-free solutions, increased Ca2+ leakage [132]. | KID syndrome | |
| V37I | TM1 | Abolished dye uptake in [Ca2+]e-free solution [139]. | Deafness | |
| A40G | TM1 | Abolished dye uptake in [Ca2+]e-free solution [139]. | Deafness | |
| A40V | TM1 | Reduced [Ca2+]e sensitivity leading to increased HC currents [133]. | KID syndrome | |
| G45E | EL1 | Increased dye uptake at a normal [Ca2+]e and a reduced [Ca2+]e sensitivity, leading to increased HC currents and Ca2+ leakage [133,140]. | KID syndrome | |
| D46C | EL1 | Halted [Ca2+]e-dependent deactivation kinetics [73]. | No association | |
| E47K | EL1 | Halted dye uptake in [Ca2+]e-free solution [140]. | Deafness | |
| E47Q | EL1 | Reduced [Ca2+]e sensitivity, leading to increased HC currents and halted deactivation kinetics [73]. | No association | |
| Q48A | EL1 | Increased [Ca2+]e sensitivity, leading to decreased HC currents and reduced deactivation kinetics [137]. | No association | |
| D50A | EL1 | Reduced [Ca2+]e sensitivity, leading to increased HC currents and halted deactivation kinetics [134,137]. | KID syndrome | |
| D50N | EL1 | Reduced [Ca2+]e sensitivity, leading to increased HC currents and halted deactivation kinetics [73,78,123,127,130]. | KID syndrome | |
| D50Y | EL1 | Increased dye uptake in both normal and [Ca2+]e-free solutions, halted [Ca2+]e-dependent deactivation kinetics, increased Ca2+ leakage [78,132]. | KID syndrome | |
| N54K | EL1 | Decreased dye uptake in [Ca2+]e-free solution [130]. | Bart–Pumphrey syndrome | |
| R75W | TM2 | Decreased HC currents in both normal and [Ca2+]e-free solutions [135]. | Deafness | |
| A88V | TM2 | Increased HC currents in [Ca2+]e-free solution [134]. | KID syndrome | |
| S183F | EL2 | Halted dye uptake in [Ca2+]e-free solution [130]. | Palmoplantar keratoderma and hearing loss | |
| R184K | EL2 | Reduced [Ca2+]e sensitivity leading to both increased HC currents and increased deactivation kinetics [73]. | No association | |
| Cx30 | G11R | NT | Increased ATP release in normal [Ca2+]e solution [141]. | Clouston syndrome |
| G45E | EL1 | Reduced [Ca2+]e sensitivity, leading to dye uptake in both normal and [Ca2+]e-free solutions [92]. | No association | |
| A88V | TM2 | Increased ATP release in both normal and [Ca2+]e-free solutions, increased leakage of Ca2+ and other ions [58,141]. | Clouston syndrome | |
| Cx30.3 | G12D | NT | Increased dye uptake in [Ca2+]e-free solution [118]. | EKV |
| T85P | TM2 | Increased dye uptake in [Ca2+]e-free solution [118]. | EKV | |
| F189Y | TM4 | Increased dye uptake in both normal and [Ca2+]e-free solutions [118]. | EKV | |
| Cx32 | G45E | EL1 | Increased dye uptake in [Ca2+]e-free solution [92]. | No association |
| S85C | TM2 | Increased HC current in normal [Ca2+]e solution [142]. | CMT1X | |
| D169N | EL2 | Reduced [Ca2+]e sensitivity, leading to increased HC currents [72]. | No association | |
| D178N | EL2 | Reduced [Ca2+]e sensitivity, leading to increased HC currents [72]. | No association | |
| D178Y | EL2 | Reduced [Ca2+]e sensitivity, leading to increased HC currents [72]. | CMT1X | |
| R220X | CT | Reduced [Ca2+]c sensitivity, leading to halted HC current [74]. | CMT1X | |
| Cx43 | L7V | NT | Increased ATP release in normal [Ca2+]e solution [116]. | ODDD |
| G8V | NT | Increased HC current in [Ca2+]e-free solution [143]. | KHLS | |
| Y17S | NT | Decreased dye uptake in [Ca2+]e-free solution [144]. | ODDD | |
| G21R | TM1 | Decreased dye uptake in [Ca2+]e-free solution [144]. | ODDD | |
| I31M | TM1 | Increased ATP release in [Ca2+]e-free solution [122]. | ODDD | |
| A40V | TM1 | Decreased dye uptake in [Ca2+]e-free solution [144]. | ODDD | |
| A44V | TM1 | Increased HC current in [Ca2+]e-free solution [143]. | EKV | |
| G45E | EL1 | Increased dye uptake in [Ca2+]e-free solution [92]. | No association | |
| F52dup | EL1 | Decreased dye uptake in [Ca2+]e-free solution [144]. | ODDD | |
| L90V | TM2 | Decreased dye uptake in [Ca2+]e-free solution [144]. | ODDD | |
| I130T | CL | Decreased dye uptake in [Ca2+]e-free solution [144]. | ODDD | |
| G138R | CL | Increased ATP release in [Ca2+]e-free solution [122]. | ODDD | |
| G143S | CL | Increased ATP release in [Ca2+]e-free solution [122]. | ODDD | |
| E227D | CT | Increased HC current in [Ca2+]e-free solution [143]. | EKV | |
| M239X | CT | Abolished ATP release in [Ca2+]e-free solution and upon increased [Ca2+]c [145]. | No association | |
| Cx46 | D47N | EL1 | Increased [Ca2+]e sensitivity, leading to decreased HC currents [73]. | No association |
| E48Q | EL1 | Reduced [Ca2+]e sensitivity, leading to increased HC currents [73]. | No association | |
| D51N | EL1 | Reduced [Ca2+]e sensitivity, leading to increased HC currents [73]. | No association | |
| N63S | EL1 | Abolished HC currents that were restored in [Ca2+]e-free solution [146]. | Congenital cataracts | |
| G143R | CL | Increased dye uptake in both normal and [Ca2+]e-free solutions, decreased dye uptake upon increased [Ca2+]c [85,147]. | Congenital cataracts |
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Delmar, M.; Laird, D.W.; Naus, C.C.; Nielsen, M.S.; Verselis, V.K.; White, T.W. Connexins and Disease. Cold Spring Harb. Perspect. Biol. 2018, 10, a029348. [Google Scholar] [CrossRef] [PubMed]
- Epifantseva, I.; Shaw, R.M. Intracellular trafficking pathways of Cx43 gap junction channels. Biochim. Biophys. Acta Biomembr. 2018, 1860, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Retamal, M.A.; Cortés, C.J.; Reuss, L.; Bennett, M.V.L.; Sáez, J.C. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: Induction by oxidant stress and reversal by reducing agents. Proc. Natl. Acad. Sci. USA 2006, 103, 4475–4480. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, H.A.; Orellana, J.A.; Verselis, V.K.; Saez, J.C. Metabolic inhibition increases activity of connexin-32 hemichannels permeable to Ca2+ in transfected HeLa cells. Am. J. Physiol. Cell Physiol. 2009, 297, C665–C678. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.L. Emerging issues of connexin channels: Biophysics fills the gap. Q. Rev. Biophys. 2001, 34, 325–472. [Google Scholar] [CrossRef] [PubMed]
- Alexander, D.B.; Goldberg, G.S. Transfer of biologically important molecules between cells through gap junction channels. Curr. Med. Chem. 2003, 10, 2045–2058. [Google Scholar] [CrossRef] [PubMed]
- Saez, J.C.; Schalper, K.A.; Retamal, M.A.; Orellana, J.A.; Shoji, K.F.; Bennett, M.V. Cell membrane permeabilization via connexin hemichannels in living and dying cells. Exp. Cell Res. 2010, 316, 2377–2389. [Google Scholar] [CrossRef] [PubMed]
- Anselmi, F.; Hernandez, V.H.; Crispino, G.; Seydel, A.; Ortolano, S.; Roper, S.D.; Kessaris, N.; Richardson, W.; Rickheit, G.; Filippov, M.A.; et al. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc. Natl. Acad. Sci. USA 2008, 105, 18770–18775. [Google Scholar] [CrossRef] [PubMed]
- Tran Van Nhieu, G.; Clair, C.; Bruzzone, R.; Mesnil, M.; Sansonetti, P.; Combettes, L. Connexin-dependent inter-cellular communication increases invasion and dissemination of Shigella in epithelial cells. Nat. Cell Biol. 2003, 5, 720–726. [Google Scholar] [CrossRef]
- Majumder, P.; Crispino, G.; Rodriguez, L.; Ciubotaru, C.D.; Anselmi, F.; Piazza, V.; Bortolozzi, M.; Mammano, F. ATP-mediated cell–cell signaling in the organ of Corti: The role of connexin channels. Purinergic Signal. 2010, 6, 167–187. [Google Scholar] [CrossRef]
- Sipos, A.; Vargas, S.L.; Toma, I.; Hanner, F.; Willecke, K.; Peti-Peterdi, J. Connexin 30 deficiency impairs renal tubular ATP release and pressure natriuresis. J. Am. Soc. Nephrol. 2009, 20, 1724–1732. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.G.; Su, C.C.; Nian, J.H.; Chiang, A.S.; Li, S.Y.; Yang, J.J. Human connexin30.2/31.3 (GJC3) does not form functional gap junction channels but causes enhanced ATP release in HeLa cells. Cell Biochem. Biophys. 2011, 61, 189–197. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, E.; Decrock, E.; Cabooter, L.; Dubyak, G.R.; Naus, C.C.; Evans, W.H.; Leybaert, L. Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J. 2006, 25, 34–44. [Google Scholar] [CrossRef]
- Schock, S.C.; Leblanc, D.; Hakim, A.M.; Thompson, C.S. ATP release by way of connexin 36 hemichannels mediates ischemic tolerance in vitro. Biochem. Biophys. Res. Commun. 2008, 368, 138–144. [Google Scholar] [CrossRef]
- Bahima, L.; Aleu, J.; Elias, M.; Martin-Satue, M.; Muhaisen, A.; Blasi, J.; Marsal, J.; Solsona, C. Endogenous hemichannels play a role in the release of ATP from Xenopus oocytes. J. Cell. Physiol. 2006, 206, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Toma, I.; Bansal, E.; Meer, E.J.; Kang, J.J.; Vargas, S.L.; Peti-Peterdi, J. Connexin 40 and ATP-dependent intercellular calcium wave in renal glomerular endothelial cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 294, R1769–R1776. [Google Scholar] [CrossRef] [PubMed]
- Cotrina, M.L.; Lin, J.H.; Alves-Rodrigues, A.; Liu, S.; Li, J.; Azmi-Ghadimi, H.; Kang, J.; Naus, C.C.; Nedergaard, M. Connexins regulate calcium signaling by controlling ATP release. Proc. Natl. Acad. Sci. USA 1998, 95, 15735–15740. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Kang, N.; Lovatt, D.; Torres, A.; Zhao, Z.; Lin, J.; Nedergaard, M. Connexin 43 hemichannels are permeable to ATP. J. Neurosci. 2008, 28, 4702–4711. [Google Scholar] [CrossRef] [PubMed]
- Lohman, A.W.; Billaud, M.; Isakson, B.E. Mechanisms of ATP release and signalling in the blood vessel wall. Cardiovasc. Res. 2012, 95, 269–280. [Google Scholar] [CrossRef]
- Stout, C.E.; Costantin, J.L.; Naus, C.C.; Charles, A.C. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J. Biol. Chem. 2002, 277, 10482–10488. [Google Scholar] [CrossRef]
- Gomes, P.; Srinivas, S.P.; Van Driessche, W.; Vereecke, J.; Himpens, B. ATP release through connexin hemichannels in corneal endothelial cells. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1208–1218. [Google Scholar] [CrossRef] [PubMed]
- Leybaert, L.; Sanderson, M.J. Intercellular Ca(2+) waves: Mechanisms and function. Physiol. Rev. 2012, 92, 1359–1392. [Google Scholar] [CrossRef] [PubMed]
- Valiunas, V. Cyclic nucleotide permeability through unopposed connexin hemichannels. Front. Pharmacol. 2013, 4, 75. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.C.; Wyeth, M.S.; Baltan-Tekkok, S.; Ransom, B.R. Functional hemichannels in astrocytes: A novel mechanism of glutamate release. J. Neurosci. 2003, 23, 3588–3596. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Jin, S.; Wang, J.; Zhang, G.; Kawanokuchi, J.; Kuno, R.; Sonobe, Y.; Mizuno, T.; Suzumura, A. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J. Biol. Chem. 2006, 281, 21362–21368. [Google Scholar] [CrossRef] [PubMed]
- Bruzzone, S.; Guida, L.; Zocchi, E.; Franco, L.; De Flora, A. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J. 2001, 15, 10–12. [Google Scholar] [CrossRef] [PubMed]
- Zocchi, E.; Podesta, M.; Pitto, A.; Usai, C.; Bruzzone, S.; Franco, L.; Guida, L.; Bacigalupo, A.; De Flora, A. Paracrinally stimulated expansion of early human hemopoietic progenitors by stroma-generated cyclic ADP-ribose. FASEB J. 2001, 15, 1610–1612. [Google Scholar] [CrossRef] [PubMed]
- Rana, S.; Dringen, R. Gap junction hemichannel-mediated release of glutathione from cultured rat astrocytes. Neurosci. Lett. 2007, 415, 45–48. [Google Scholar] [CrossRef]
- Linsambarth, S.; Carvajal, F.J.; Moraga-Amaro, R.; Mendez, L.; Tamburini, G.; Jimenez, I.; Verdugo, D.A.; Gomez, G.I.; Jury, N.; Martinez, P.; et al. Astroglial gliotransmitters released via Cx43 hemichannels regulate NMDAR-dependent transmission and short-term fear memory in the basolateral amygdala. FASEB J. 2022, 36, e22134. [Google Scholar] [CrossRef]
- Cherian, P.P.; Siller-Jackson, A.J.; Gu, S.; Wang, X.; Bonewald, L.F.; Sprague, E.; Jiang, J.X. Mechanical strain opens connexin 43 hemichannels in osteocytes: A novel mechanism for the release of prostaglandin. Mol. Biol. Cell 2005, 16, 3100–3106. [Google Scholar] [CrossRef]
- Burra, S.; Jiang, J.X. Connexin 43 hemichannel opening associated with Prostaglandin E(2) release is adaptively regulated by mechanical stimulation. Commun. Integr. Biol. 2009, 2, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Liu, X.; Liu, R.; Yang, L.; Zuo, J.; Liu, W. Connexin 43 hemichannel regulates H9c2 cell proliferation by modulating intracellular ATP and [Ca2+]. Acta Biochim. Biophys. Sin. 2010, 42, 472–482. [Google Scholar] [CrossRef] [PubMed]
- Cotrina, M.L.; Lin, J.H.; Nedergaard, M. Adhesive properties of connexin hemichannels. Glia 2008, 56, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Kamermans, M.; Fahrenfort, I.; Schultz, K.; Janssen-Bienhold, U.; Sjoerdsma, T.; Weiler, R. Hemichannel-mediated inhibition in the outer retina. Science 2001, 292, 1178–1180. [Google Scholar] [CrossRef] [PubMed]
- Mathias, R.T.; White, T.W.; Gong, X. Lens gap junctions in growth, differentiation, and homeostasis. Physiol. Rev. 2010, 90, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Beyer, E.C.; Berthoud, V.M. Connexin hemichannels in the lens. Front. Physiol. 2014, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Ceriani, F.; Pozzan, T.; Mammano, F. Critical role of ATP-induced ATP release for Ca2+ signaling in nonsensory cell networks of the developing cochlea. Proc. Natl. Acad. Sci. USA 2016, 113, E7194–E7201. [Google Scholar] [CrossRef] [PubMed]
- Tritsch, N.X.; Bergles, D.E. Developmental regulation of spontaneous activity in the Mammalian cochlea. J. Neurosci. 2010, 30, 1539–1550. [Google Scholar] [CrossRef] [PubMed]
- Kar, R.; Riquelme, M.A.; Werner, S.; Jiang, J.X. Connexin 43 channels protect osteocytes against oxidative stress-induced cell death. J. Bone Miner. Res. 2013, 28, 1611–1621. [Google Scholar] [CrossRef]
- Lecanda, F.; Warlow, P.M.; Sheikh, S.; Furlan, F.; Steinberg, T.H.; Civitelli, R. Connexin43 deficiency causes delayed ossification, craniofacial abnormalities, and osteoblast dysfunction. J. Cell Biol. 2000, 151, 931–944. [Google Scholar] [CrossRef]
- Riquelme, M.A.; Jiang, J.X. Elevated Intracellular Ca2+ Signals by Oxidative Stress Activate Connexin 43 Hemichannels in Osteocytes. Bone Res. 2013, 1, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Riquelme, M.A.; Hua, R.; Zhang, J.; Acosta, F.M.; Gu, S.; Jiang, J.X. Mechanosensitive piezo1 calcium channel activates connexin 43 hemichannels through PI3K signaling pathway in bone. Cell Biosci. 2022, 12, 191. [Google Scholar] [CrossRef] [PubMed]
- Quist, A.P.; Rhee, S.K.; Lin, H.; Lal, R. Physiological role of gap-junctional hemichannels. Extracellular calcium-dependent isosmotic volume regulation. J. Cell Biol. 2000, 148, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- De Bock, M.; Culot, M.; Wang, N.; Bol, M.; Decrock, E.; De Vuyst, E.; da Costa, A.; Dauwe, I.; Vinken, M.; Simon, A.M.; et al. Connexin channels provide a target to manipulate brain endothelial calcium dynamics and blood-brain barrier permeability. J. Cereb. Blood Flow. Metab. 2011, 31, 1942–1957. [Google Scholar] [CrossRef] [PubMed]
- Bosch, M.; Kielian, T. Hemichannels in neurodegenerative diseases: Is there a link to pathology? Front. Cell. Neurosci. 2014, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Crunelli, V.; Carmignoto, G.; Steinhauser, C. Novel astrocyte targets: New avenues for the therapeutic treatment of epilepsy. Neuroscientist 2015, 21, 62–83. [Google Scholar] [CrossRef] [PubMed]
- Retamal, M.A.; Reyes, E.P.; Garcia, I.E.; Pinto, B.; Martinez, A.D.; Gonzalez, C. Diseases associated with leaky hemichannels. Front. Cell. Neurosci. 2015, 9, 267. [Google Scholar] [CrossRef] [PubMed]
- Orellana, J.A.; Stehberg, J. Hemichannels: New roles in astroglial function. Front. Physiol. 2014, 5, 193. [Google Scholar] [CrossRef] [PubMed]
- Stehberg, J.; Moraga-Amaro, R.; Salazar, C.; Becerra, A.; Echeverria, C.; Orellana, J.A.; Bultynck, G.; Ponsaerts, R.; Leybaert, L.; Simon, F.; et al. Release of gliotransmitters through astroglial connexin 43 hemichannels is necessary for fear memory consolidation in the basolateral amygdala. FASEB J. 2012, 26, 3649–3657. [Google Scholar] [CrossRef]
- Meunier, C.; Wang, N.; Yi, C.; Dallerac, G.; Ezan, P.; Koulakoff, A.; Leybaert, L.; Giaume, C. Contribution of Astroglial Cx43 Hemichannels to the Modulation of Glutamatergic Currents by D-Serine in the Mouse Prefrontal Cortex. J. Neurosci. 2017, 37, 9064–9075. [Google Scholar] [CrossRef]
- Bejarano, E.; Yuste, A.; Patel, B.; Stout, R.F., Jr.; Spray, D.C.; Cuervo, A.M. Connexins modulate autophagosome biogenesis. Nat. Cell Biol. 2014, 16, 401–414. [Google Scholar] [CrossRef]
- Schalper, K.A.; Carvajal-Hausdorf, D.; Oyarzo, M.P. Possible role of hemichannels in cancer. Front. Physiol. 2014, 5, 237. [Google Scholar] [CrossRef] [PubMed]
- Gadicherla, A.K.; Wang, N.; Bulic, M.; Agullo-Pascual, E.; Lissoni, A.; De Smet, M.; Delmar, M.; Bultynck, G.; Krysko, D.V.; Camara, A.; et al. Mitochondrial Cx43 hemichannels contribute to mitochondrial calcium entry and cell death in the heart. Basic Res. Cardiol. 2017, 112, 27. [Google Scholar] [CrossRef] [PubMed]
- Miro-Casas, E.; Ruiz-Meana, M.; Agullo, E.; Stahlhofen, S.; Rodriguez-Sinovas, A.; Cabestrero, A.; Jorge, I.; Torre, I.; Vazquez, J.; Boengler, K.; et al. Connexin43 in cardiomyocyte mitochondria contributes to mitochondrial potassium uptake. Cardiovasc. Res. 2009, 83, 747–756. [Google Scholar] [CrossRef]
- Bukauskas, F.F.; Kreuzberg, M.M.; Rackauskas, M.; Bukauskiene, A.; Bennett, M.V.; Verselis, V.K.; Willecke, K. Properties of mouse connexin 30.2 and human connexin 31.9 hemichannels: Implications for atrioventricular conduction in the heart. Proc. Natl. Acad. Sci. USA 2006, 103, 9726–9731. [Google Scholar] [CrossRef] [PubMed]
- De Smet, M.A.J.; Lissoni, A.; Nezlobinsky, T.; Wang, N.; Dries, E.; Pérez-Hernández, M.; Lin, X.; Amoni, M.; Vervliet, T.; Witschas, K.; et al. Cx43 hemichannel microdomain signaling at the intercalated disc enhances cardiac excitability. J. Clin. Investig. 2021, 131, e137752. [Google Scholar] [CrossRef]
- Bol, M.; Wang, N.; De Bock, M.; Wacquier, B.; Decrock, E.; Gadicherla, A.; Decaluwe, K.; Vanheel, B.; van Rijen, H.V.; Krysko, D.V.; et al. At the cross-point of connexins, calcium, and ATP: Blocking hemichannels inhibits vasoconstriction of rat small mesenteric arteries. Cardiovasc. Res. 2017, 113, 195–206. [Google Scholar] [CrossRef]
- Kuang, Y.; Zorzi, V.; Buratto, D.; Ziraldo, G.; Mazzarda, F.; Peres, C.; Nardin, C.; Salvatore, A.M.; Chiani, F.; Scavizzi, F.; et al. A potent antagonist antibody targeting connexin hemichannels alleviates Clouston syndrome symptoms in mutant mice. EBioMedicine 2020, 57, 102825. [Google Scholar] [CrossRef]
- Contreras, J.E.; Saez, J.C.; Bukauskas, F.F.; Bennett, M.V. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc. Natl. Acad. Sci. USA 2003, 100, 11388–11393. [Google Scholar] [CrossRef]
- Valiunas, V.; Weingart, R. Electrical properties of gap junction hemichannels identified in transfected HeLa cells. Pflugers Arch. 2000, 440, 366–379. [Google Scholar] [CrossRef]
- Retamal, M.A. Connexin and Pannexin hemichannels are regulated by redox potential. Front. Physiol. 2014, 5, 80. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, E.; Decrock, E.; De Bock, M.; Yamasaki, H.; Naus, C.C.; Evans, W.H.; Leybaert, L. Connexin hemichannels and gap junction channels are differentially influenced by lipopolysaccharide and basic fibroblast growth factor. Mol. Biol. Cell 2007, 18, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, T.F.; Lazrak, A.; Peracchia, C.; Goldberg, G.S.; Lampe, P.D.; Johnson, R.G. Properties and regulation of gap junctional hemichannels in the plasma membranes of cultured cells. J. Cell Biol. 1996, 134, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Sachs, F.; Dahl, G. Connexins are mechanosensitive. Am. J. Physiol. Cell Physiol. 2004, 287, C1389–C1395. [Google Scholar] [CrossRef] [PubMed]
- Svenningsen, P.; Burford, J.L.; Peti-Peterdi, J. ATP releasing connexin 30 hemichannels mediate flow-induced calcium signaling in the collecting duct. Front. Physiol. 2013, 4, 292. [Google Scholar] [CrossRef] [PubMed]
- Bevans, C.G.; Harris, A.L. Regulation of connexin channels by pH. Direct action of the protonated form of taurine and other aminosulfonates. J. Biol. Chem. 1999, 274, 3711–3719. [Google Scholar] [CrossRef] [PubMed]
- Huckstepp, R.T.; Eason, R.; Sachdev, A.; Dale, N. CO2-dependent opening of connexin 26 and related beta connexins. J. Physiol. 2010, 588 Pt 20, 3921–3931. [Google Scholar] [CrossRef] [PubMed]
- Trexler, E.B.; Bukauskas, F.F.; Bennett, M.V.; Bargiello, T.A.; Verselis, V.K. Rapid and direct effects of pH on connexins revealed by the connexin46 hemichannel preparation. J. Gen. Physiol. 1999, 113, 721–742. [Google Scholar] [CrossRef] [PubMed]
- Delmar, M.; Coombs, W.; Sorgen, P.; Duffy, H.S.; Taffet, S.M. Structural bases for the chemical regulation of Connexin43 channels. Cardiovasc. Res. 2004, 62, 268–275. [Google Scholar] [CrossRef]
- Srinivas, M.; Calderon, D.P.; Kronengold, J.; Verselis, V.K. Regulation of connexin hemichannels by monovalent cations. J. Gen. Physiol. 2006, 127, 67–75. [Google Scholar] [CrossRef]
- Ebihara, L.; Liu, X.; Pal, J.D. Effect of external magnesium and calcium on human connexin46 hemichannels. Biophys. J. 2003, 84, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Hernandez, J.M.; de Miguel, M.; Larrosa, B.; Gonzalez, D.; Barrio, L.C. Molecular basis of calcium regulation in connexin-32 hemichannels. Proc. Natl. Acad. Sci. USA 2003, 100, 16030–16035. [Google Scholar] [CrossRef] [PubMed]
- Lopez, W.; Ramachandran, J.; Alsamarah, A.; Luo, Y.; Harris, A.L.; Contreras, J.E. Mechanism of gating by calcium in connexin hemichannels. Proc. Natl. Acad. Sci. USA 2016, 113, E7986–E7995. [Google Scholar] [CrossRef] [PubMed]
- Carrer, A.; Leparulo, A.; Crispino, G.; Ciubotaru, C.D.; Marin, O.; Zonta, F.; Bortolozzi, M. Cx32 hemichannel opening by cytosolic Ca2+ is inhibited by the R220X mutation that causes Charcot-Marie-Tooth disease. Hum. Mol. Genet. 2018, 27, 80–94. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, E.; Wang, N.; Decrock, E.; De Bock, M.; Vinken, M.; Van Moorhem, M.; Lai, C.; Culot, M.; Rogiers, V.; Cecchelli, R.; et al. Ca2+ regulation of connexin 43 hemichannels in C6 glioma and glial cells. Cell Calcium 2009, 46, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Breitwieser, G.E. Extracellular calcium as an integrator of tissue function. Int. J. Biochem. Cell Biol. 2008, 40, 1467–1480. [Google Scholar] [CrossRef]
- Bosher, S.K.; Warren, R.L. Very low calcium content of cochlear endolymph, an extracellular fluid. Nature 1978, 273, 377–378. [Google Scholar] [CrossRef]
- Lopez, W.; Gonzalez, J.; Liu, Y.; Harris, A.L.; Contreras, J.E. Insights on the mechanisms of Ca2+ regulation of connexin26 hemichannels revealed by human pathogenic mutations (D50N/Y). J. Gen. Physiol. 2013, 142, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Puljung, M.C.; Berthoud, V.M.; Beyer, E.C.; Hanck, D.A. Polyvalent Cations Constitute the Voltage Gating Particle in Human Connexin37 Hemichannels. J. Gen. Physiol. 2004, 124, 587–603. [Google Scholar] [CrossRef]
- Vargas, A.A.; Cisterna, B.A.; Saavedra-Leiva, F.; Urrutia, C.; Cea, L.A.; Vielma, A.H.; Gutierrez-Maldonado, S.E.; Martin, A.J.; Pareja-Barrueto, C.; Escalona, Y.; et al. On Biophysical Properties and Sensitivity to Gap Junction Blockers of Connexin 39 Hemichannels Expressed in HeLa Cells. Front. Physiol. 2017, 8, 38. [Google Scholar] [CrossRef]
- Allen, M.J.; Gemel, J.; Beyer, E.C.; Lal, R. Atomic force microscopy of Connexin40 gap junction hemichannels reveals calcium-dependent three-dimensional molecular topography and open-closed conformations of both the extracellular and cytoplasmic faces. J. Biol. Chem. 2011, 286, 22139–22146. [Google Scholar] [CrossRef] [PubMed]
- Bader, P.; Weingart, R.; Egger, M. Regulation of Cx45 hemichannels mediated by extracellular and intracellular calcium. Pflugers Arch. 2012, 464, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Pfahnl, A.; Dahl, G. Gating of cx46 gap junction hemichannels by calcium and voltage. Pflügers Arch. 1999, 437, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zou, T.; Liu, Y.; Qi, Y. The gating effect of calmodulin and calcium on the connexin50 hemichannel. Biol. Chem. 2006, 387, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Riquelme, M.A.; Wang, B.; Bugay, V.; Brenner, R.; Gu, S.; Jiang, J.X. Cataract-associated Connexin 46 Mutation Alters Its Interaction with Calmodulin and Function of Hemichannels. J. Biol. Chem. 2018, 293, 2573–2585. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Wang, F.; Xu, Q.; Fujita, T.; Dobrowolski, R.; Willecke, K.; Takano, T.; Nedergaard, M. Extracellular Ca2+ Acts as a Mediator of Communication from Neurons to Glia. Science Signal. 2012, 5, ra8. [Google Scholar] [CrossRef] [PubMed]
- Ceriani, F.; Hendry, A.; Jeng, J.Y.; Johnson, S.L.; Stephani, F.; Olt, J.; Holley, M.C.; Mammano, F.; Engel, J.; Kros, C.J.; et al. Coordinated calcium signalling in cochlear sensory and non-sensory cells refines afferent innervation of outer hair cells. EMBO J. 2019, 38, e99839. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Lee, S.H. Skin Barrier and Calcium. Ann. Dermatol. 2018, 30, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Muller, D.J.; Hand, G.M.; Engel, A.; Sosinsky, G.E. Conformational changes in surface structures of isolated connexin 26 gap junctions. EMBO J. 2002, 21, 3598–3607. [Google Scholar] [CrossRef]
- Ripps, H.; Qian, H.; Zakevicius, J. Properties of connexin26 hemichannels expressed in Xenopus oocytes. Cell. Mol. Neurobiol. 2004, 24, 647–665. [Google Scholar] [CrossRef]
- Zonta, F.; Mammano, F.; Torsello, M.; Fortunati, N.; Orian, L.; Polimeno, A. Role of gamma carboxylated Glu47 in connexin 26 hemichannel regulation by extracellular Ca2+: Insight from a local quantum chemistry study. Biochem. Biophys. Res. Commun. 2014, 445, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hao, H. Conserved glycine at position 45 of major cochlear connexins constitutes a vital component of the Ca2+ sensor for gating of gap junction hemichannels. Biochem. Biophys. Res. Commun. 2013, 436, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Pinto, B.I.; Pupo, A.; Garcia, I.E.; Mena-Ulecia, K.; Martinez, A.D.; Latorre, R.; Gonzalez, C. Calcium binding and voltage gating in Cx46 hemichannels. Sci. Rep. 2017, 7, 15851. [Google Scholar] [CrossRef] [PubMed]
- Zonta, F.; Polles, G.; Zanotti, G.; Mammano, F. Permeation pathway of homomeric connexin 26 and connexin 30 channels investigated by molecular dynamics. J. Biomol. Struct. Dyn. 2012, 29, 985–998. [Google Scholar] [CrossRef] [PubMed]
- Fasciani, I.; Temperan, A.; Perez-Atencio, L.F.; Escudero, A.; Martinez-Montero, P.; Molano, J.; Gomez-Hernandez, J.M.; Paino, C.L.; Gonzalez-Nieto, D.; Barrio, L.C. Regulation of connexin hemichannel activity by membrane potential and the extracellular calcium in health and disease. Neuropharmacology 2013, 75, 479–490. [Google Scholar] [CrossRef]
- Choi, E.J.; Palacios-Prado, N.; Saez, J.C.; Lee, J. Identification of Cx45 as a Major Component of GJs in HeLa Cells. Biomolecules 2020, 10, 1389. [Google Scholar]
- Verselis, V.K.; Srinivas, M. Divalent cations regulate connexin hemichannels by modulating intrinsic voltage-dependent gating. J. Gen. Physiol. 2008, 132, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Beahm, D.L.; Hall, J.E. Hemichannel and junctional properties of connexin 50. Biophys. J. 2002, 82, 2016–2031. [Google Scholar] [CrossRef] [PubMed]
- Bootman, M.D.; Bultynck, G. Fundamentals of Cellular Calcium Signaling: A Primer. Cold Spring Harb. Perspect. Biol. 2020, 12, a038802. [Google Scholar] [CrossRef]
- Bagur, R.; Hajnoczky, G. Intracellular Ca2+ Sensing: Its Role in Calcium Homeostasis and Signaling. Mol. Cell 2017, 66, 780–788. [Google Scholar] [CrossRef]
- Lurtz, M.M.; Louis, C.F. Intracellular calcium regulation of connexin43. Am. J. Physiol. Cell Physiol. 2007, 293, C1806–C1813. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, V.; Saez, P.J.; Salas, J.D.; Salas, D.; Jara, O.; Martinez, A.D.; Saez, J.C.; Retamal, M.A. Linoleic acid induces opening of connexin26 hemichannels through a PI3K/Akt/Ca2+-dependent pathway. Biochim. Biophys. Acta 2013, 1828, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Schalper, K.A.; Palacios-Prado, N.; Retamal, M.A.; Shoji, K.F.; Martinez, A.D.; Saez, J.C. Connexin hemichannel composition determines the FGF-1-induced membrane permeability and free [Ca2+]i responses. Mol. Biol. Cell 2008, 19, 3501–3513. [Google Scholar] [CrossRef] [PubMed]
- Lagos-Cabre, R.; Brenet, M.; Diaz, J.; Perez, R.D.; Perez, L.A.; Herrera-Molina, R.; Quest, A.F.G.; Leyton, L. Intracellular Ca2+ Increases and Connexin 43 Hemichannel Opening Are Necessary but Not Sufficient for Thy-1-Induced Astrocyte Migration. Int. J. Mol. Sci. 2018, 19, 2179. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.A.; Dale, N.; Llaudet, E.; Mobbs, P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron 2005, 46, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; De Vuyst, E.; Ponsaerts, R.; Boengler, K.; Palacios-Prado, N.; Wauman, J.; Lai, C.P.; De Bock, M.; Decrock, E.; Bol, M.; et al. Selective inhibition of Cx43 hemichannels by Gap19 and its impact on myocardial ischemia/reperfusion injury. Basic Res. Cardiol. 2013, 108, 309. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; De Bock, M.; Antoons, G.; Gadicherla, A.K.; Bol, M.; Decrock, E.; Evans, W.H.; Sipido, K.R.; Bukauskas, F.F.; Leybaert, L. Connexin mimetic peptides inhibit Cx43 hemichannel opening triggered by voltage and intracellular Ca2+ elevation. Basic. Res. Cardiol. 2012, 107, 304. [Google Scholar] [CrossRef] [PubMed]
- Braet, K.; Vandamme, W.; Martin, P.E.; Evans, W.H.; Leybaert, L. Photoliberating inositol-1,4,5-trisphosphate triggers ATP release that is blocked by the connexin mimetic peptide gap 26. Cell Calcium 2003, 33, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Arcuino, G.; Lin, J.H.; Takano, T.; Liu, C.; Jiang, L.; Gao, Q.; Kang, J.; Nedergaard, M. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc. Natl. Acad. Sci. USA 2002, 99, 9840–9845. [Google Scholar] [CrossRef]
- Van Campenhout, R.; Gomes, A.R.; De Groof, T.W.M.; Muyldermans, S.; Devoogdt, N.; Vinken, M. Mechanisms Underlying Connexin Hemichannel Activation in Disease. Int. J. Mol. Sci. 2021, 22, 3503. [Google Scholar] [CrossRef]
- Guo, A.; Zhang, H.; Li, H.; Chiu, A.; Garcia-Rodriguez, C.; Lagos, C.F.; Saez, J.C.; Lau, C.G. Inhibition of connexin hemichannels alleviates neuroinflammation and hyperexcitability in temporal lobe epilepsy. Proc. Natl. Acad. Sci. USA 2022, 119, e2213162119. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Jamett, A.; Vasquez, W.; Cifuentes-Riveros, G.; Martinez-Pando, R.; Saez, J.C.; Cardenas, A.M. Oxidative Stress, Inflammation and Connexin Hemichannels in Muscular Dystrophies. Biomedicines 2022, 10, 507. [Google Scholar] [CrossRef] [PubMed]
- Verselis, V.K. Connexin hemichannels and cochlear function. Neurosci. Lett. 2019, 695, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.E.; Bosen, F.; Mujica, P.; Pupo, A.; Flores-Munoz, C.; Jara, O.; Gonzalez, C.; Willecke, K.; Martinez, A.D. From Hyperactive Connexin26 Hemichannels to Impairments in Epidermal Calcium Gradient and Permeability Barrier in the Keratitis-Ichthyosis-Deafness Syndrome. J. Investig. Dermatol. 2016, 136, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Bortolozzi, M. What’s the Function of Connexin 32 in the Peripheral Nervous System? Front. Mol. Neurosci. 2018, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.J.; Esseltine, J.L.; Shao, Q.; Jabs, E.W.; Sampson, J.; Auranen, M.; Bai, D.; Laird, D.W. Specific functional pathologies of Cx43 mutations associated with oculodentodigital dysplasia. Mol. Biol. Cell 2016, 27, 2172–2185. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cao, X.; Lin, Z.; Lee, M.; Jia, X.; Ren, Y.; Dai, L.; Guan, L.; Zhang, J.; Lin, X.; et al. Exome sequencing reveals mutation in GJA1 as a cause of keratoderma-hypotrichosis-leukonychia totalis syndrome. Hum. Mol. Genet. 2015, 24, 6564. [Google Scholar] [CrossRef]
- Lucaciu, S.A.; Figliuzzi, R.; Neumann, R.; Nazarali, S.; Del Sordo, L.; Leighton, S.E.; Hauser, A.; Shao, Q.; Johnston, D.; Bai, D.; et al. GJB4 variants linked to skin disease exhibit a trafficking deficiency en route to gap junction formation that can be restored by co-expression of select connexins. Front. Cell Dev. Biol. 2023, 11, 1073805. [Google Scholar] [CrossRef] [PubMed]
- Beyer, E.C.; Ebihara, L.; Berthoud, V.M. Connexin mutants and cataracts. Front. Pharmacol. 2013, 4, 43. [Google Scholar] [CrossRef]
- Prieto-Villalobos, J.; Alvear, T.F.; Liberona, A.; Lucero, C.M.; Martinez-Araya, C.J.; Balmazabal, J.; Inostroza, C.A.; Ramirez, G.; Gomez, G.I.; Orellana, J.A. Astroglial Hemichannels and Pannexons: The Hidden Link between Maternal Inflammation and Neurological Disorders. Int. J. Mol. Sci. 2021, 22, 9503. [Google Scholar] [CrossRef]
- Lucero, C.M.; Prieto-Villalobos, J.; Marambio-Ruiz, L.; Balmazabal, J.; Alvear, T.F.; Vega, M.; Barra, P.; Retamal, M.A.; Orellana, J.A.; Gomez, G.I. Hypertensive Nephropathy: Unveiling the Possible Involvement of Hemichannels and Pannexons. Int. J. Mol. Sci. 2022, 23, 15936. [Google Scholar] [CrossRef]
- Dobrowolski, R.; Sommershof, A.; Willecke, K. Some oculodentodigital dysplasia-associated Cx43 mutations cause increased hemichannel activity in addition to deficient gap junction channels. J. Membr. Biol. 2007, 219, 9–17. [Google Scholar] [CrossRef]
- Lee, J.R.; Derosa, A.M.; White, T.W. Connexin mutations causing skin disease and deafness increase hemichannel activity and cell death when expressed in Xenopus oocytes. J. Investig. Dermatol. 2009, 129, 870–878. [Google Scholar] [CrossRef]
- Ebihara, L.; Korzyukov, Y.; Kothari, S.; Tong, J.J. Cx46 hemichannels contribute to the sodium leak conductance in lens fiber cells. Am. J. Physiol. Cell Physiol. 2014, 306, C506–C513. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.D.; Acuna, R.; Figueroa, V.; Maripillan, J.; Nicholson, B. Gap-junction channels dysfunction in deafness and hearing loss. Antioxid. Redox Signal. 2009, 11, 309–322. [Google Scholar] [CrossRef]
- Xu, J.; Nicholson, B.J. The role of connexins in ear and skin physiology-functional insights from disease-associated mutations. Biochim. Biophys. Acta 2013, 1828, 167–178. [Google Scholar] [CrossRef]
- Terrinoni, A.; Codispoti, A.; Serra, V.; Didona, B.; Bruno, E.; Nistico, R.; Giustizieri, M.; Alessandrini, M.; Campione, E.; Melino, G. Connexin 26 (GJB2) mutations, causing KID Syndrome, are associated with cell death due to calcium gating deregulation. Biochem. Biophys. Res. Commun. 2010, 394, 909–914. [Google Scholar] [CrossRef]
- Garcia, I.E.; Villanelo, F.; Contreras, G.F.; Pupo, A.; Pinto, B.I.; Contreras, J.E.; Perez-Acle, T.; Alvarez, O.; Latorre, R.; Martinez, A.D.; et al. The syndromic deafness mutation G12R impairs fast and slow gating in Cx26 hemichannels. J. Gen. Physiol. 2018, 150, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.E.; Maripillan, J.; Jara, O.; Ceriani, R.; Palacios-Munoz, A.; Ramachandran, J.; Olivero, P.; Perez-Acle, T.; Gonzalez, C.; Saez, J.C.; et al. Keratitis-ichthyosis-deafness syndrome-associated Cx26 mutants produce nonfunctional gap junctions but hyperactive hemichannels when co-expressed with wild type Cx43. J. Investig. Dermatol. 2015, 135, 1338–1347. [Google Scholar] [CrossRef]
- Press, E.R.; Shao, Q.; Kelly, J.J.; Chin, K.; Alaga, A.; Laird, D.W. Induction of cell death and gain-of-function properties of connexin26 mutants predict severity of skin disorders and hearing loss. J. Biol. Chem. 2017, 292, 9721–9732. [Google Scholar] [CrossRef]
- Valdez Capuccino, J.M.; Chatterjee, P.; Garcia, I.E.; Botello-Smith, W.M.; Zhang, H.; Harris, A.L.; Luo, Y.; Contreras, J.E. The connexin26 human mutation N14K disrupts cytosolic intersubunit interactions and promotes channel opening. J. Gen. Physiol. 2019, 151, 328–341. [Google Scholar] [CrossRef]
- Aypek, H.; Bay, V.; Mese, G. Altered cellular localization and hemichannel activities of KID syndrome associated connexin26 I30N and D50Y mutations. BMC Cell Biol. 2016, 17, 5. [Google Scholar] [CrossRef]
- Sanchez, H.A.; Mese, G.; Srinivas, M.; White, T.W.; Verselis, V.K. Differentially altered Ca2+ regulation and Ca2+ permeability in Cx26 hemichannels formed by the A40V and G45E mutations that cause keratitis ichthyosis deafness syndrome. J. Gen. Physiol. 2010, 136, 47–62. [Google Scholar] [CrossRef]
- Mhaske, P.V.; Levit, N.A.; Li, L.; Wang, H.Z.; Lee, J.R.; Shuja, Z.; Brink, P.R.; White, T.W. The human Cx26-D50A and Cx26-A88V mutations causing keratitis-ichthyosis-deafness syndrome display increased hemichannel activity. Am. J. Physiol. Cell Physiol. 2013, 304, C1150–C1158. [Google Scholar] [CrossRef]
- Chen, Y.; Deng, Y.; Bao, X.; Reuss, L.; Altenberg, G.A. Mechanism of the defect in gap-junctional communication by expression of a connexin 26 mutant associated with dominant deafness. FASEB J. 2005, 19, 1516–1518. [Google Scholar] [CrossRef]
- Bosen, F.; Celli, A.; Crumrine, D.; vom Dorp, K.; Ebel, P.; Jastrow, H.; Dormann, P.; Winterhager, E.; Mauro, T.; Willecke, K. Altered epidermal lipid processing and calcium distribution in the KID syndrome mouse model Cx26S17F. FEBS Lett. 2015, 589, 1904–1910. [Google Scholar] [CrossRef]
- Lopez, W.; Liu, Y.; Harris, A.L.; Contreras, J.E. Divalent regulation and intersubunit interactions of human Connexin26 (Cx26) hemichannels. Channels 2014, 8, 1–4. [Google Scholar] [CrossRef]
- Abbott, A.C.; Garcia, I.E.; Villanelo, F.; Flores-Munoz, C.; Ceriani, R.; Maripillan, J.; Novoa-Molina, J.; Figueroa-Cares, C.; Perez-Acle, T.; Saez, J.C.; et al. Expression of KID syndromic mutation Cx26S17F produces hyperactive hemichannels in supporting cells of the organ of Corti. Front. Cell Dev. Biol. 2022, 10, 1071202. [Google Scholar] [CrossRef]
- Jara, O.; Acuna, R.; Garcia, I.E.; Maripillan, J.; Figueroa, V.; Saez, J.C.; Araya-Secchi, R.; Lagos, C.F.; Perez-Acle, T.; Berthoud, V.M.; et al. Critical role of the first transmembrane domain of Cx26 in regulating oligomerization and function. Mol. Biol. Cell 2012, 23, 3299–3311. [Google Scholar] [CrossRef]
- Stong, B.C.; Chang, Q.; Ahmad, S.; Lin, X. A novel mechanism for connexin 26 mutation linked deafness: Cell death caused by leaky gap junction hemichannels. Laryngoscope 2006, 116, 2205–2210. [Google Scholar] [CrossRef]
- Essenfelder, G.M.; Bruzzone, R.; Lamartine, J.; Charollais, A.; Blanchet-Bardon, C.; Barbe, M.T.; Meda, P.; Waksman, G. Connexin30 mutations responsible for hidrotic ectodermal dysplasia cause abnormal hemichannel activity. Hum. Mol. Genet. 2004, 13, 1703–1714. [Google Scholar] [CrossRef]
- Abrams, C.K.; Bennett, M.V.; Verselis, V.K.; Bargiello, T.A. Voltage opens unopposed gap junction hemichannels formed by a connexin 32 mutant associated with X-linked Charcot-Marie-Tooth disease. Proc. Natl. Acad. Sci. USA 2002, 99, 3980–3984. [Google Scholar] [CrossRef]
- Srinivas, M.; Jannace, T.F.; Cocozzelli, A.G.; Li, L.; Slavi, N.; Sellitto, C.; White, T.W. Connexin43 mutations linked to skin disease have augmented hemichannel activity. Sci. Rep. 2019, 9, 19. [Google Scholar] [CrossRef]
- Lai, A.; Le, D.N.; Paznekas, W.A.; Gifford, W.D.; Jabs, E.W.; Charles, A.C. Oculodentodigital dysplasia connexin43 mutations result in non-functional connexin hemichannels and gap junctions in C6 glioma cells. J. Cell Sci. 2006, 119 Pt 3, 532–541. [Google Scholar] [CrossRef]
- Ponsaerts, R.; De Vuyst, E.; Retamal, M.; D’Hondt, C.; Vermeire, D.; Wang, N.; De Smedt, H.; Zimmermann, P.; Himpens, B.; Vereecke, J.; et al. Intramolecular loop/tail interactions are essential for connexin 43-hemichannel activity. FASEB J. 2010, 24, 4378–4395. [Google Scholar] [CrossRef]
- Pal, J.D.; Liu, X.; Mackay, D.; Shiels, A.; Berthoud, V.M.; Beyer, E.C.; Ebihara, L. Connexin46 mutations linked to congenital cataract show loss of gap junction channel function. Am. J. Physiol. Cell Physiol. 2000, 279, C596–C602. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Riquelme, M.A.; Xu, J.; Yan, X.; Nicholson, B.J.; Gu, S.; Jiang, J.X. Cataract-causing mutation of human connexin 46 impairs gap junction, but increases hemichannel function and cell death. PLoS ONE 2013, 8, e74732. [Google Scholar] [CrossRef] [PubMed]
- Bergoffen, J.; Scherer, S.S.; Wang, S.; Scott, M.O.; Bone, L.J.; Paul, D.L.; Chen, K.; Lensch, M.W.; Chance, P.F.; Fischbeck, K.H. Connexin mutations in X-linked Charcot-Marie-Tooth disease. Science 1993, 262, 2039–2042. [Google Scholar] [CrossRef]
- Zheng, L.; Chenavas, S.; Kieken, F.; Trease, A.; Brownell, S.; Anbanandam, A.; Sorgen, P.L.; Spagnol, G. Calmodulin Directly Interacts with the Cx43 Carboxyl-Terminus and Cytoplasmic Loop Containing Three ODDD-Linked Mutants (M147T, R148Q, and T154A) that Retain alpha-Helical Structure, but Exhibit Loss-of-Function and Cellular Trafficking Defects. Biomolecules 2020, 10, 1452. [Google Scholar] [CrossRef]
- Jiang, J.X. Gap junctions or hemichannel-dependent and independent roles of connexins in cataractogenesis and lens development. Curr. Mol. Med. 2010, 10, 851–863. [Google Scholar] [CrossRef]
- Villanelo, F.; Carrasco, J.; Jensen-Flores, J.; Garate, J.A.; Perez-Acle, T. Simulations on Simple Models of Connexin Hemichannels Indicate That Ca2+ Blocking Is Not a Pure Electrostatic Effect. Membranes 2021, 11, 372. [Google Scholar] [CrossRef] [PubMed]
- Jagielnicki, M.; Kucharska, I.; Bennett, B.C.; Harris, A.L.; Yeager, M. Connexin Gap Junction Channels and Hemichannels: Insights from High-Resolution Structures. Biology 2024, 13, 298. [Google Scholar] [CrossRef] [PubMed]
- Zonta, F.; Buratto, D.; Crispino, G.; Carrer, A.; Bruno, F.; Yang, G.; Mammano, F.; Pantano, S. Cues to Opening Mechanisms From in Silico Electric Field Excitation of Cx26 Hemichannel and in Vitro Mutagenesis Studies in HeLa Transfectans. Front. Mol. Neurosci. 2018, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jeong, H.; Hyun, J.; Ryu, B.; Park, K.; Lim, H.H.; Yoo, J.; Woo, J.S. Cryo-EM structure of human Cx31.3/GJC3 connexin hemichannel. Sci. Adv. 2020, 6, eaba4996. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Hulpiau, P.; Wagner, L.E.; Witschas, K.; Yule, D.I.; Bultynck, G.; Leybaert, L. IP3RPEP6, a novel peptide inhibitor of IP(3) receptor channels that does not affect connexin-43 hemichannels. Acta Physiol. 2024, 240, e14086. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, C.; Wang, X.G.; Peracchia, L.L. Chemical gating of gap junction channels. Methods 2000, 20, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.G.; Peracchia, C. Molecular dissection of a basic COOH-terminal domain of Cx32 that inhibits gap junction gating sensitivity. Am. J. Physiol. 1998, 275 Pt 1, C1384–C1390. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, W.; Lurtz, M.M.; Chen, Y.; Jiang, J.; Huang, Y.; Louis, C.F.; Yang, J.J. Calmodulin mediates the Ca2+-dependent regulation of Cx44 gap junctions. Biophys. J. 2009, 96, 2832–2848. [Google Scholar] [CrossRef]
- Peracchia, C.; Wang, X.G.; Peracchia, L.L. Behavior of chemical- and slow voltage-sensitive gating of connexin channels: The “cork” gating hypothesis. In Gap Junctions. Molecular Basis of Cell Communication in Health and Disease; Peracchia, C., Ed.; Academic Press: San Diego, CA, USA, 2000; pp. 271–295. [Google Scholar]
- Lissoni, A.; Wang, N.; Nezlobinskii, T.; De Smet, M.; Panfilov, A.V.; Vandersickel, N.; Leybaert, L.; Witschas, K. Gap19, a Cx43 Hemichannel Inhibitor, Acts as a Gating Modifier That Decreases Main State Opening While Increasing Substate Gating. Int. J. Mol. Sci. 2020, 21, 7340. [Google Scholar] [CrossRef]
- Ponsaerts, R.; Wang, N.; Himpens, B.; Leybaert, L.; Bultynck, G. The contractile system as a negative regulator of the connexin 43 hemichannel. Biol. Cell 2012, 104, 367–377. [Google Scholar] [CrossRef]
- Peracchia, C.; Peracchia, L.M.L. Calmodulin-Connexin Partnership in Gap Junction Channel Regulation-Calmodulin-Cork Gating Model. Int. J. Mol. Sci. 2021, 22, 13055. [Google Scholar] [CrossRef] [PubMed]
- Tran, O.; Kerruth, S.; Coates, C.; Kaur, H.; Peracchia, C.; Carter, T.; Torok, K. Ca2+-Dependent and -Independent Calmodulin Binding to the Cytoplasmic Loop of Gap Junction Connexins. Int. J. Mol. Sci. 2023, 24, 4153. [Google Scholar] [CrossRef] [PubMed]
- Torok, K.; Stauffer, K.; Evans, W.H. Connexin 32 of gap junctions contains two cytoplasmic calmodulin-binding domains. Biochem. J. 1997, 326 Pt 2, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Martin, P.E.; Evans, W.H. Assembly of gap junction channels: Mechanism, effects of calmodulin antagonists and identification of connexin oligomerization determinants. Eur. J. Biochem. 2001, 268, 4544–4552. [Google Scholar] [CrossRef] [PubMed]
- Mones, S.; Bordignon, B.; Peiretti, F.; Landrier, J.F.; Gess, B.; Bourguignon, J.J.; Bihel, F.; Fontes, M. CamKII inhibitors reduce mitotic instability, connexon anomalies and progression of the in vivo behavioral phenotype in transgenic animals expressing a mutated Gjb1 gene. Front. Neurosci. 2014, 8, 151. [Google Scholar] [CrossRef] [PubMed]
- Bihel, F.; Gess, B.; Fontes, M. CMTX Disorder and CamKinase. Front. Cell. Neurosci. 2016, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Caufriez, A.; Bock, D.; Martin, C.; Ballet, S.; Vinken, M. Peptide-based targeting of connexins and pannexins for therapeutic purposes. Expert. Opin. Drug Discov. 2020, 15, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- Buratto, D.; Donati, V.; Zonta, F.; Mammano, F. Harnessing the therapeutic potential of antibodies targeting connexin hemichannels. Biochim. Biophys. Acta Mol. Basis Dis. 2021, 1867, 166047. [Google Scholar] [CrossRef]
- AlFindee, M.N.; Subedi, Y.P.; Fiori, M.C.; Krishnan, S.; Kjellgren, A.; Altenberg, G.A.; Chang, C.T. Inhibition of Connexin Hemichannels by New Amphiphilic Aminoglycosides without Antibiotic Activity. ACS Med. Chem. Lett. 2018, 9, 697–701. [Google Scholar] [CrossRef]
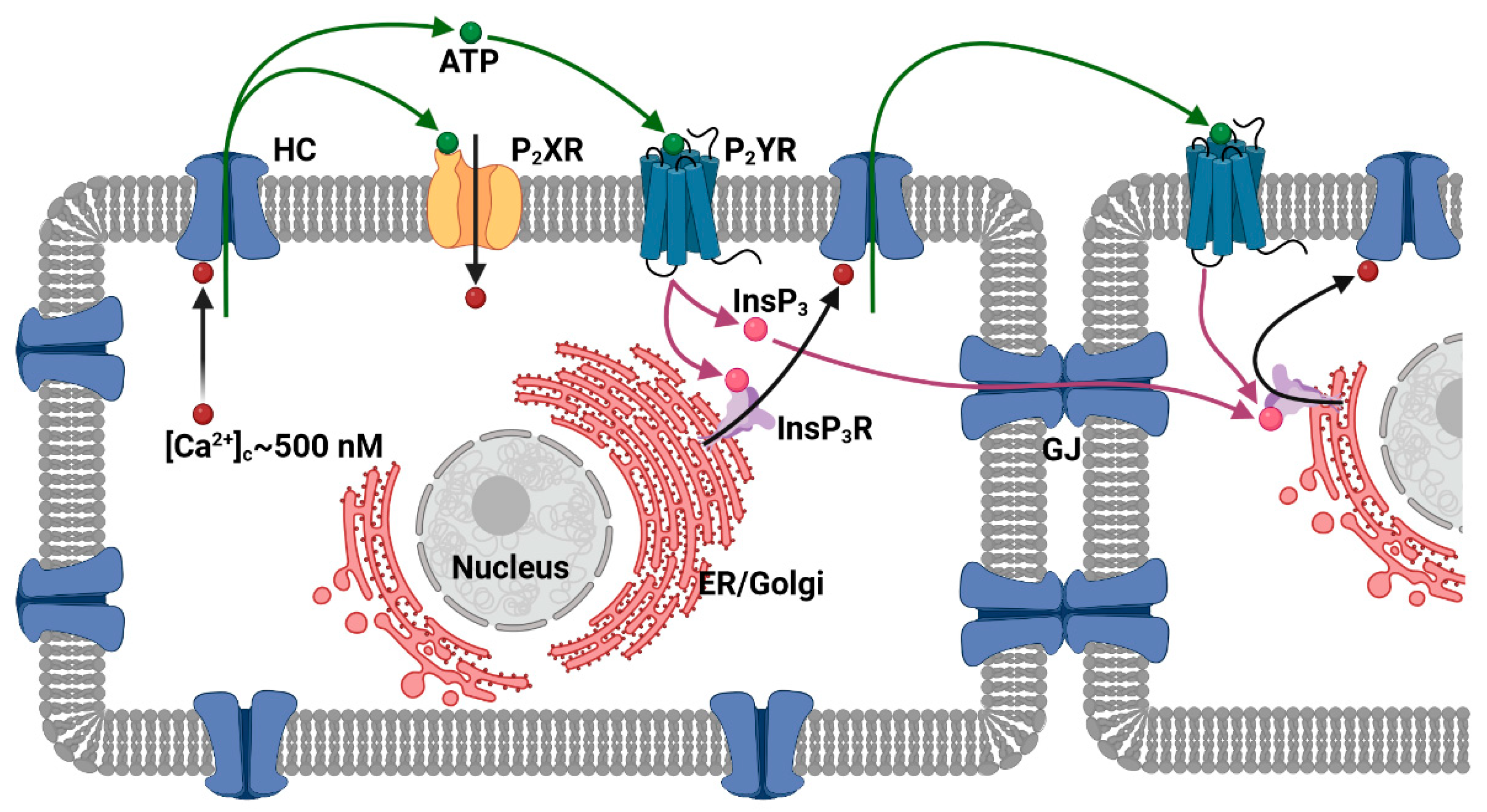
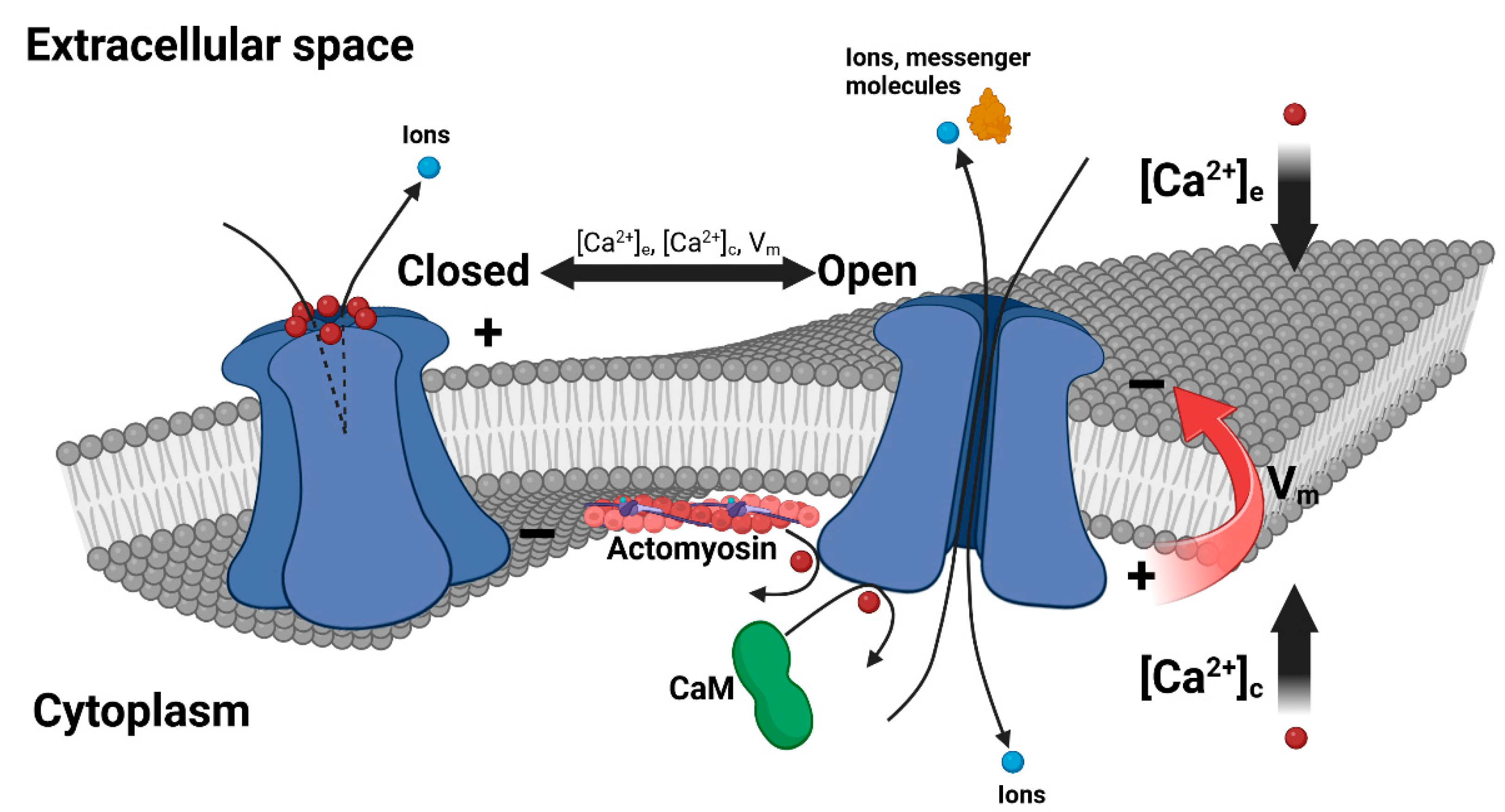
| Cx Isoform | HC Regulation by [Ca2+]e | HC Regulation by [Ca2+]c |
|---|---|---|
| Cx26 | Current ranging from a maximum at 0.01 mM to a minimum value at 10 mM [Ca2+]e, with an EC50 around 0.25 mM [78]. | ATP release increases with [Ca2+]c around 500 nM [8]. |
| Cx30 | Current increases in [Ca2+]e-free solution [60]. | ATP release and dye uptake increases with [Ca2+]c around 500 nM [8]. |
| Cx30.2/31.3 | ATP release increases in [Ca2+]e-free solution [12]. | Information not available. |
| Cx32 | Current ranging from a maximum at 0.5 mM to a minimum value at 5 mM [Ca2+]e, with an EC50 around 1.3 mM [72]. | Bell-shaped dependence of ATP release on [Ca2+]c, peaking at 500 nM [Ca2+]c [13]. |
| Cx37 | Current ranging from a maximum at 0.02 mM to a minimum value at 1 mM [Ca2+]e, with an EC50 around 0.1 mM [79]. | Information not available. |
| Cx39 | Dye uptake increases in [Ca2+]e-free solution [80]. | Information not available. |
| Cx40 | HC pore size increases at [Ca2+]e < 10 μM [81]. | Information not available. |
| Cx43 | Dye uptake ranging from a maximum at 0.01 mM [Ca2+]e to a minimum value at 1 mM [Ca2+]e [63]. | Bell-shaped dependence of ATP release on [Ca2+]c, peaking at 500 nM [Ca2+]c [75]. |
| Cx45 | Bi-sigmoidal dependence of the current on [Ca2+]e, with EC50 around 1 μM [Ca2+]e [82]. | Current increases with [Ca2+]c around 100 nM [82]. |
| Cx46 | Current ranging from a maximum at 0.01 mM to a minimum value at 1 mM [Ca2+]e [71]. | Dye uptake increases upon [Ca2+]c elevation [85]. |
| Cx50 | Dye leakage increases in [Ca2+]e-free solution [84]. | Information not available. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayraktar, E.; Lopez-Pigozzi, D.; Bortolozzi, M. Calcium Regulation of Connexin Hemichannels. Int. J. Mol. Sci. 2024, 25, 6594. https://doi.org/10.3390/ijms25126594
Bayraktar E, Lopez-Pigozzi D, Bortolozzi M. Calcium Regulation of Connexin Hemichannels. International Journal of Molecular Sciences. 2024; 25(12):6594. https://doi.org/10.3390/ijms25126594
Chicago/Turabian StyleBayraktar, Erva, Diego Lopez-Pigozzi, and Mario Bortolozzi. 2024. "Calcium Regulation of Connexin Hemichannels" International Journal of Molecular Sciences 25, no. 12: 6594. https://doi.org/10.3390/ijms25126594






