Valpalf®: A New Nutraceutical Formulation Containing Bovine Lactoferrin That Exhibits Potentiated Biological Activity
Abstract
1. Introduction
2. Results
2.1. Iron Chelation Ability of Valpalf®
- -
- bLf in saline solution: 2.8 mg (maximum absorbance at 468 nm OD 0.151)
- -
- bLf in sodium citrate solution: 2.9 mg (maximum absorbance at 468 nm OD 0.157)
- -
- bLf in sodium bicarbonate solution: 2.9 mg (maximum absorbance at 468 nm OD 0.158)
- -
- Valpalf®: 10 mg (maximum absorbance at 468 nm OD 0.540)
2.2. Tryptic Resistance of Valpalf®
2.3. Lactoferrins Internalization and Subcellular Localization
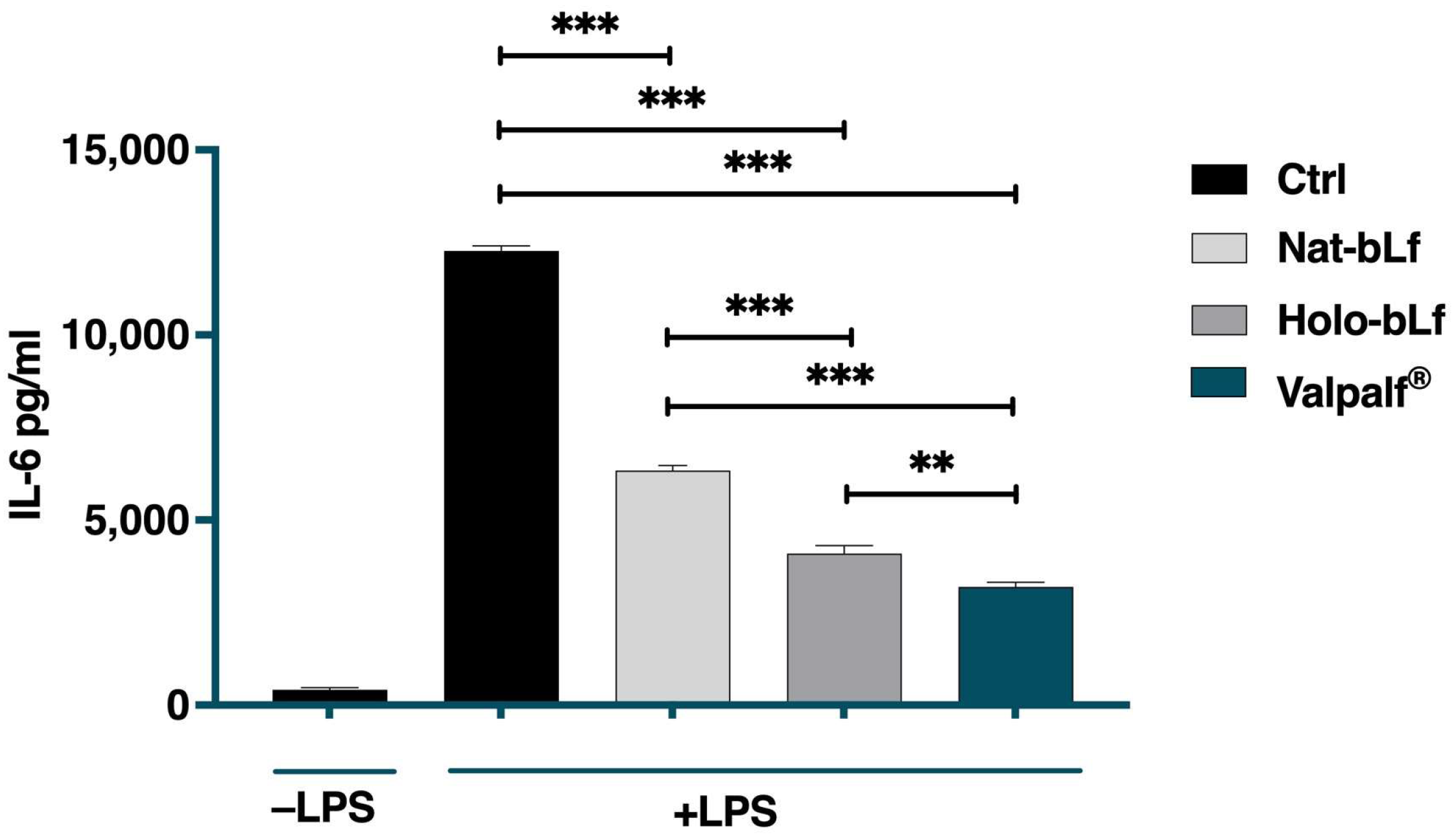
2.4. Anti-Inflammatory Activity of Valpalf®
2.5. Antioxidant Activity of Valpalf®
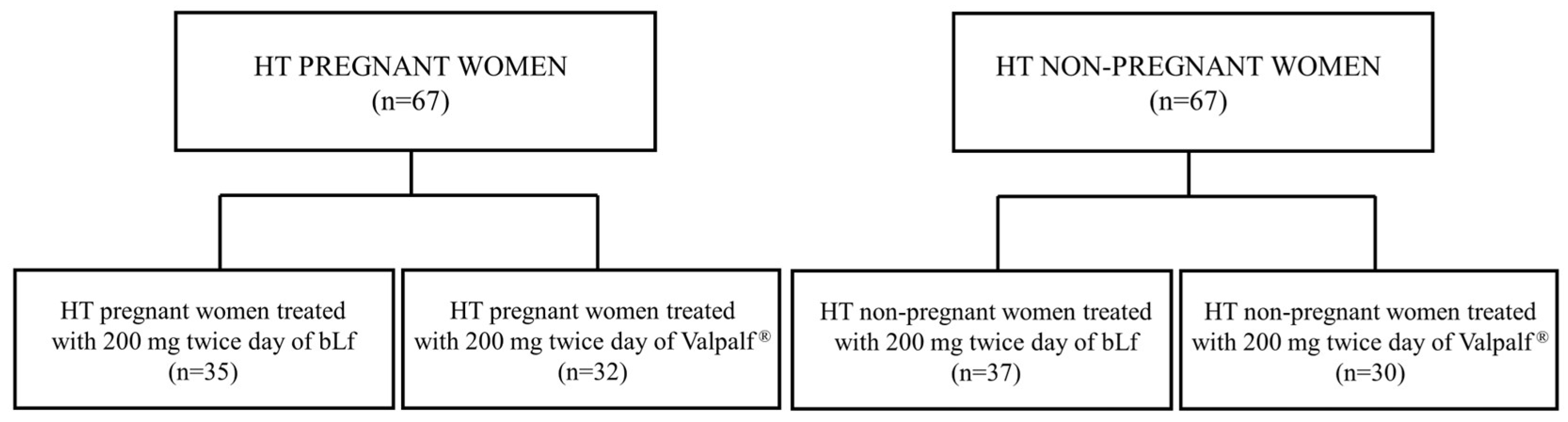
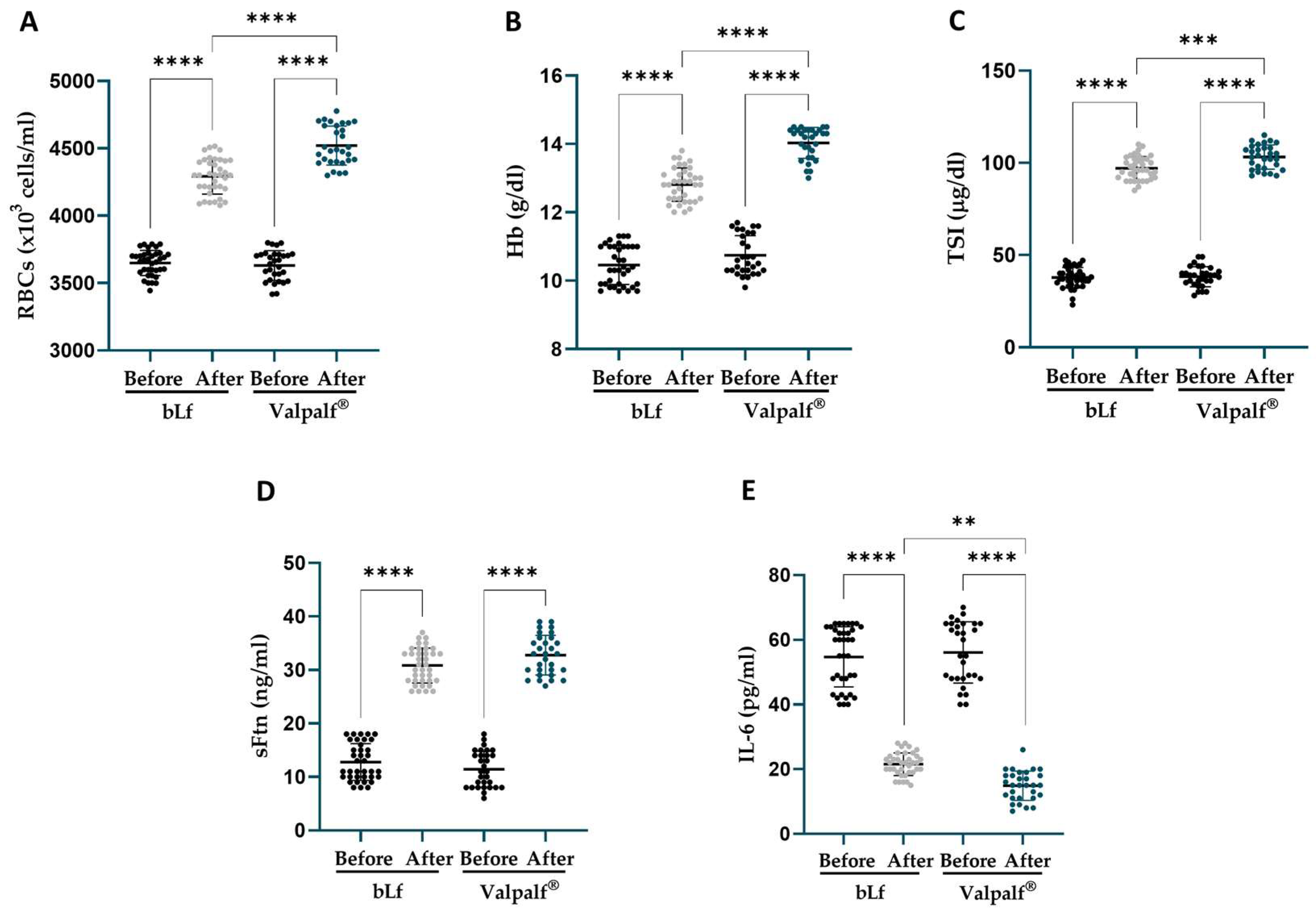
2.6. Retrospective Study: Efficacy of Valpalf® in the Treatment of Anemia of Inflammation in Hereditary Thrombophilic Pregnant and Non-Pregnant Women
3. Discussion
4. Materials and Methods
4.1. In Vitro Studies
4.1.1. Reagents
4.1.2. Bovine Lactoferrin
4.1.3. Iron Titration Assay
4.1.4. Tryptic Digestion
4.1.5. Cell Culture
4.1.6. Total and Nuclear Extracts
4.1.7. Anti-Inflammatory Activity
4.1.8. Antioxidant Proteins
4.1.9. Western Blot
4.2. Retrospective Study
4.2.1. Study Design
4.2.2. Patients
4.2.3. Laboratory Analyses
4.2.4. Study Population
4.2.5. Patients’ Treatments
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ianiro, G.; Niro, A.; Rosa, L.; Valenti, P.; Musci, G.; Cutone, A. To Boost or to Reset: The Role of Lactoferrin in Energy Metabolism. Int. J. Mol. Sci. 2023, 24, 15925. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; Rosa, L.; Bonaccorsi di Patti, M.C.; Valenti, P.; Musci, G.; Cutone, A. Lactoferrin: From the structure to the functional orchestration of iron homeostasis. Biometals 2023, 36, 391–416. [Google Scholar] [CrossRef]
- Baker, E.N.; Anderson, B.F.; Baker, H.M.; Day, C.L.; Haridas, M.; Norris, G.E.; Rumball, S.V.; Smith, C.A.; Thomas, D.H. Three-dimensional structure of lactoferrin in various functional states. Adv. Exp. Med. Biol. 1994, 357, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Baker, H.M.; Baker, E.N. Lactoferrin and iron: Structural and dynamic aspects of binding and release. Biometals 2004, 17, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Timilsena, Y.P.; Blanch, E.; Adhikari, B. Lactoferrin: Structure, function, denaturation and digestion. Crit. Rev. Food Sci. Nutr. 2019, 59, 580–596. [Google Scholar] [CrossRef] [PubMed]
- Anderson, B.F.; Baker, H.M.; Norris, G.E.; Rice, D.W.; Baker, E.N. Structure of human lactoferrin: Crystallographic structure analysis and refinement at 2.8 Å resolution. J. Mol. Biol. 1989, 209, 711–734. [Google Scholar] [CrossRef] [PubMed]
- Brock, J.H.; Arzabe, F.; Lampreave, F.; Piñeiro, A. The effect of trypsin on bovine transferrin and lactoferrin. Biochim. Biophys. Acta 1976, 446, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Brines, R.; Brock, J. The effect of trypsin and chymotrypsin on the in vitro antimicrobial and iron-binding properties of lactoferrin in human milk and bovine colostrum: Unusual resistance of human apolactoferrin to proteolytic digestion. Biochim. Biophys. Acta 1983, 759, 229–235. [Google Scholar] [CrossRef]
- Takeuchi, T.; Kitagawa, H.; Harada, E. Evidence of lactoferrin transportation into blood circulation from intestine via lymphatic pathway in adult rats. Exp. Physiol. 2004, 89, 263–270. [Google Scholar] [CrossRef]
- Fischer, R.; Debbabi, H.; Blais, A.; Dubarry, M.; Rautureau, M.; Boyaka, P.N.; Tome, D. Uptake of ingested bovine lactoferrin and its accumulation in adult mouse tissues. Int. Immunopharmacol. 2007, 7, 1387–1393. [Google Scholar] [CrossRef]
- Suzuki, Y.A.; Lopez, V.; Lönnerdal, B. Mammalian lactoferrin receptors: Structure and function. Cell. Mol. Life Sci. 2005, 62, 2560–2575. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Oshima, K.; Kuhara, T.; Shin, K.; Abe, F.; Iwatsuki, K.; Nadano, D.; Matsuda, T. A lactoferrin-receptor, intelectin 1, affects uptake, sub-cellular localization and release of immunochemically detectable lactoferrin by intestinal epithelial Caco-2 cells. J. Biochem. 2013, 154, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Legrand, D. Overview of Lactoferrin as a Natural Immune Modulator. J. Pediatr. 2016, 173, S10–S15. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.A.; Wong, H.; Ashida, K.-Y.; Schryvers, A.B.; Lönnerdal, B. The N1 Domain of Human Lactoferrin Is Required for Internalization by Caco-2 Cells and Targeting to the Nucleus. Biochemistry 2008, 47, 10915–10920. [Google Scholar] [CrossRef]
- Lepanto, M.S.; Rosa, L.; Paesano, R.; Valenti, P.; Cutone, A. Lactoferrin in Aseptic and Septic Inflammation. Molecules 2019, 24, 1323. [Google Scholar] [CrossRef]
- Jiang, R.; Lopez, V.; Kelleher, S.L.; Lönnerdal, B. Apo- and Holo-lactoferrin are both internalized by lactoferrin receptor via clathrin-mediated endocytosis but differentially affect ERK-signaling and cell proliferation in Caco-2 cells. J. Cell. Physiol. 2011, 226, 3022–3031. [Google Scholar] [CrossRef]
- Superti, F. Lactoferrin from Bovine Milk: A Protective Companion for Life. Nutrients 2020, 12, 2562. [Google Scholar] [CrossRef]
- Legrand, D.; Elass, E.; Carpentier, M.; Mazurier, J. Lactoferrin: A Modulator of Immune and Inflammatory Responses. Cell. Mol. Life Sci. 2005, 62, 2549–2559. [Google Scholar] [CrossRef] [PubMed]
- Giansanti, F.; Panella, G.; Leboffe, L.; Antonini, G. Lactoferrin from Milk: Nutraceutical and Pharmacological Properties. Pharmaceuticals 2016, 9, 61. [Google Scholar] [CrossRef]
- Ellison, R.T.; Giehl, T.J.; LaForce, F.M. Damage of the outer membrane of enteric Gram-negative bacteria by lactoferrin and transferrin. Infect. Immun. 1988, 56, 2774–2781. [Google Scholar] [CrossRef]
- Bellamy, W.; Takase, M.; Wakabayashi, H.; Kawase, K.; Tomita, M. Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J. Appl. Bacteriol. 1992, 73, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Drago-Serrano, M.E.; Campos-Rodríguez, R.; Carrero, J.C.; De la Garza, M. Lactoferrin: Balancing Ups and Downs of Inflammation Due to Microbial Infections. Int. J. Mol. Sci. 2017, 18, 501. [Google Scholar] [CrossRef]
- Yami, H.A.; Tahmoorespur, M.; Javadmanesh, A.; Tazarghi, A.; Sekhavati, M.H. The immunomodulatory effects of lactoferrin and its derived peptides on NF-kappaB signaling pathway: A systematic review and meta-analysis. Immun. Inflamm. Dis. 2023, 11, e972. [Google Scholar] [CrossRef] [PubMed]
- Salaris, C.; Scarpa, M.; Elli, M.; Bertolini, A.; Guglielmetti, S.; Pregliasco, F.; Blandizzi, C.; Brun, P.; Castagliuolo, I. Protective Effects of Lactoferrin against SARS-CoV-2 Infection In Vitro. Nutrients 2021, 13, 328. [Google Scholar] [CrossRef] [PubMed]
- Hering, N.A.; Luettig, J.; Krug, S.M.; Wiegand, S.; Gross, G.; Van Tol, E.A.; Schulzke, J.D.; Rosenthal, R. Lactoferrin protects against intestinal inflammation and bacteria-induced barrier dysfunction in vitro. Ann. N. Y. Acad. Sci. 2017, 1405, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Zong, Q.; Zhao, Y.; Gu, H.; Liu, Y.; Gu, F.; Liu, H.-Y.; Ahmed, A.A.; Bao, W.; Cai, D. Lactoferrin Attenuates Intestinal Barrier Dysfunction and Inflammation by Modulating the MAPK Pathway and Gut Microbes in Mice. J. Nutr. 2022, 152, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Togawa, J.; Nagase, H.; Tanaka, K.; Inamori, M.; Umezawa, T.; Nakajima, A.; Naito, M.; Sato, S.; Saito, T.; Sekihara, H. Lactoferrin reduces colitis in rats via modulation of the immune system and correction of cytokine imbalance. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G187–G195. [Google Scholar] [CrossRef]
- Anand, N.; Sehgal, R.; Kanwar, R.K.; Dubey, M.L.; Vasishta, R.K.; Kanwar, J.R. Oral administration of encapsulated bovine lactoferrin protein nanocapsules against intracellular parasite toxoplasma gondii. Int. J. Nanomed. 2015, 10, 6355. [Google Scholar]
- Paesano, R.; Berlutti, F.; Pietropaoli, M.; Pantanella, F.; Pacifici, E.; Goolsbee, W.; Valenti, P. Lactoferrin efficacy versus ferrous sulfate in curing iron deficiency and iron deficiency anemia in pregnant women. Biometals 2014, 27, 999–1006. [Google Scholar] [CrossRef]
- Lepanto, M.S.; Rosa, L.; Cutone, A.; Conte, M.P.; Paesano, R.; Valenti, P. Efficacy of Lactoferrin Oral Administration in the Treatment of Anemia and Anemia of Inflammation in Pregnant and Non-pregnant Women: An Interventional Study. Front. Immunol. 2018, 9, 2123. [Google Scholar] [CrossRef]
- Okazaki, Y.; Kono, I.; Kuriki, T.; Funahashi, S.; Fushimi, S.; Iqbal, M.; Okada, S.; Toyokuni, S. Bovine Lactoferrin Ameliorates Ferric Nitrilotriacetate-Induced Renal Oxidative Damage in Rats. J. Clin. Biochem. Nutr. 2012, 51, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Kruzel, M.L.; Actor, J.K.; Zimecki, M.; Wise, J.; Płoszaj, P.; Mirza, S.; Kruzel, M.; Hwang, S.-A.; Ba, X.; Boldogh, I. Novel Recombinant Human Lactoferrin: Differential Activation of Oxidative Stress Related Gene Expression. J. Biotechnol. 2013, 168, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Ianiro, G.; D’Ezio, V.; Carpinelli, L.; Casella, C.; Bonaccorsi di Patti, M.C.; Rosa, L.; Valenti, P.; Colasanti, M.; Musci, G.; Cutone, A.; et al. Iron Saturation Drives Lactoferrin Effects on Oxidative Stress and Neurotoxicity Induced by HIV-1 Tat. Int. J. Mol. Sci. 2023, 24, 7947. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.Y.; Kim, S.; Park, H.M.; Lim, C.M.; Kim, J.; Park, J.Y.; Jeon, K.B.; Lee, H.P.; Oh, S.R.; Ahn, J.; et al. Cinnamomum verum extract inhibits NOX2/ROS and PKCδ/JNK/AP-1/NF-κB pathway-mediated inflammatory response in PMA-stimulated THP-1 monocytes. Phytomedicine 2023, 112, 154685. [Google Scholar] [CrossRef] [PubMed]
- Kell, D.; Heyden, E.; Pretorius, E. The biology of lactoferrin, an iron-binding protein that can help defend against viruses and bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef] [PubMed]
- Barros, C.A.; Sanches, D.; Marques de Carvalho, C.A.; Santos, R.A.; Ferraz de Souza, T.L.; Macena Leite, V.L.; Pereira da Costa Campos, S.; Cheble de Oliveira, A.; Gonçalves, R.B. Influence of iron binding in the structural stability and cellular internalization of bovine lactoferrin. Heliyon 2021, 7, e08087. [Google Scholar] [CrossRef] [PubMed]
- Spiro, T.G.; Pape, L.; Saltman, P. The hydrolytic polymerization of ferric citrate I. The chemistry of the polymer. J. Am. Chem. Soc. 1967, 89, 5555–5559. [Google Scholar] [CrossRef]
- Spiro, T.G.; Bates, G.; Saltman, P. The hydrolytic polymerization of ferric citrate II. The influence of excess citrate. J. Am. Chem. Soc. 1967, 89, 5559–5562. [Google Scholar] [CrossRef]
- Ganz, T.; Bino, A.; Salusky, I.B. Mechanism of Action and Clinical Attributes of Auryxia® (Ferric Citrate). Drugs 2019, 79, 957–968. [Google Scholar] [CrossRef]
- Schade, A.L.; Reinhart, R.W.; Levy, H. Carbon dioxide and oxygen in complex formation with iron and siderophilin, the iron-binding component of human plasma. Arch. Biochem. 1949, 20, 170–172. [Google Scholar]
- Baker, E.N. Structure and reactivity of transferrins. Adv. Inorg. Chem. 1994, 41, 389–463. [Google Scholar]
- Baker, E.N.; Baker, H.M.; Kidd, R.D. Lactoferrin and transferrin: Functional variations on a common structural framework. Biochem. Cell Biol. 2002, 80, 27–34. [Google Scholar] [CrossRef] [PubMed]
- MacGillivray, R.T.A.; Moore, S.A.; Chen, J.; Anderson, B.F.; Baker, H.; Luo, Y.; Bewley, M.; Smith, C.A.; Murphy, M.E.P.; Wang, Y.; et al. Two high-resolution crystal structures of the recombinant N-lobe of human transferrin reveal a structural change implicated in iron release. Biochemistry 1998, 37, 7919–7931. [Google Scholar] [CrossRef] [PubMed]
- Phelps, C.F.; Antonini, E. A Study of the Kinetics of Iron and Copper Binding to Hen Ovotransferrin. Biochem. J. 1975, 147, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Hanudel, M.R.; Czaya, B.; Wong, S.; Rappaport, M.; Namjoshi, S.; Chua, K.; Jung, G.; Gabayan, V.; Qiao, B.; Nemeth, E.; et al. Enteral ferric citrate absorption is dependent on the iron transport protein ferroportin. Kidney Int. 2022, 101, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Kıraç, F.S. Is ethics approval necessary for all trials? A clear but not certain process. Mol. Imaging Radionucl. Ther. 2013, 22, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Kaandorp, S.; Di Nisio, M.; Goddijn, M.; Middeldorp, S. Aspirin or anticoagulants for treating recurrent miscarriage in women without antiphospholipid syndrome. Cochrane Database Syst. Rev. 2009, 1, CD004734. [Google Scholar] [CrossRef]
- Meier, P.R.; Nickerson, H.J.; Olson, K.A.; Berg, R.L.; Meyer, J.A. Prevention of iron deficiency anemia in adolescent and adult pregnancies. Clin. Med. Res. 2003, 1, 29–36. [Google Scholar] [CrossRef]
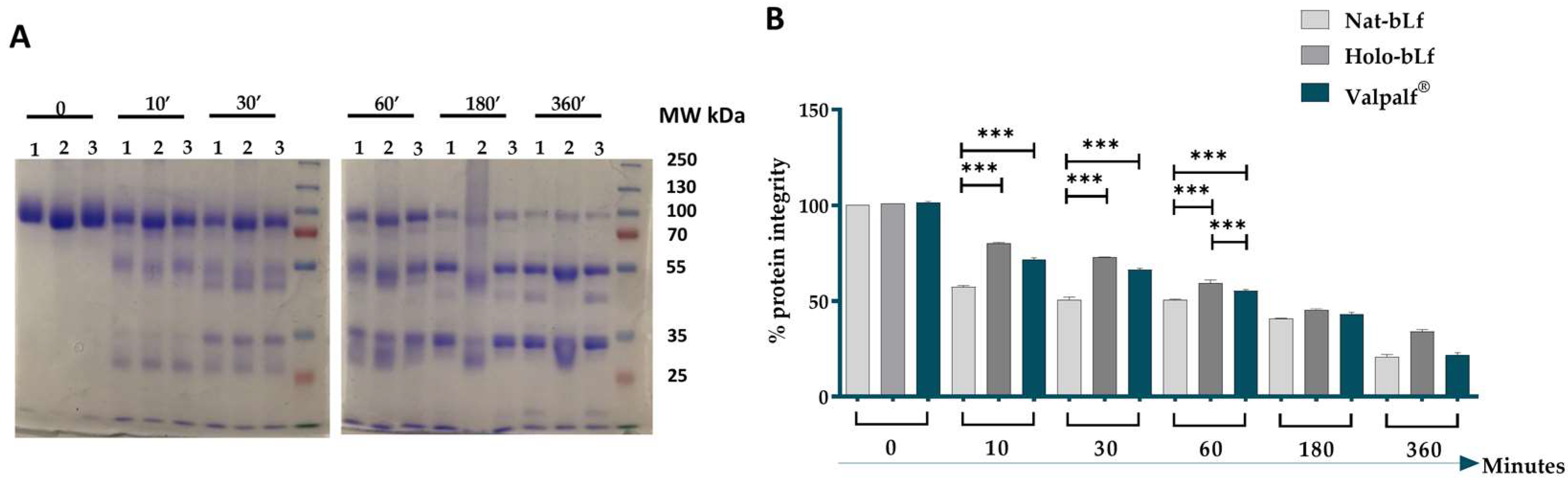



| Reagent | OD AT 468 nm (% Iron Saturation) | |||
|---|---|---|---|---|
| Ferric Chloride | bLf in Sodium Chloride | bLf in Sodium Citrate | bLf in Sodium Bicarbonate | Valpalf® in dH2O |
| - | 0.050 (9.3) | 0.052 (9.6) | 0.051 (9.4) | 0.050 (9.3) |
| 2.5 µL | 0.068 (12.6) | 0.067 (12.4) | 0.069 (12.8) | 0.105 (19.4) |
| 2.5 µL | 0.083 (15.4) | 0.098 (18.1) | 0.099 (18.3) | 0.168 (31.1) |
| 2.5 µL | 0.096 (17.8) | 0.107 (19.8) | 0.103 (19.1) | 0.256 (47.4) |
| 2.5 µL | 0.103 (19.1) | 0.115 (21.3) | 0.114 (21.1) | 0.330 (61.1) |
| 2.5 µL | 0.110 (20.4) | 0.122 (22.6) | 0.129 (23.9) | 0.411 (76.1) |
| 2.5 µL | 0.123 (22.8) | 0.131 (24.3) | 0.131 (24.3) | 0.470 (87.0) |
| 2.5 µL | 0.127 (23.5) | 0.139 (25.7) | 0.135 (25.0) | 0.498 (92.2) |
| 2.5 µL | 0.142 (26.3) | 0.146 (27.0) | 0.142 (26.3) | 0.540 (100) |
| 25 µL | 0.151 (28.0) | 0.157 (29.1) | 0.158 (29.3) | 0.540 (100) |
| Parameters | HT Pregnant Women (n = 35) before bLf Treatment | HT Pregnant Women (n = 32) before Valpalf® Treatment | p-Value |
|---|---|---|---|
| Age | 28.6 ± 6.3 | 28.4 ± 7.1 | 0.9989 |
| RBCs (×103 cells) | 3643 ± 107.9 | 3630 ± 136 | 0.9762 |
| Hb (g/dL) | 10.3 ± 0.5 | 10.5 ± 0.5 | 0.1188 |
| TSI (μg/dL) | 36.3 ± 7.0 | 36.8 ± 7.4 | 0.9933 |
| sFtn (ng/mL) | 11.4 ± 5.3 | 11.1 ± 5.1 | 0.9935 |
| IL-6 (pg/mL) | 89.4 ± 8.6 | 92.9 ± 8.5 | 0.1987 |
| Parameters | HT Non-Pregnant Women (n = 37) before bLf Treatment | HT Non-Pregnant Women (n = 30) before Valpalf® Treatment | p-Value |
|---|---|---|---|
| Age | 36 ± 9 | 37.1 ± 8.1 | 0.9703 |
| RBCs (×103 cells) | 3649 ± 93.5 | 3631 ± 111.1 | 0.9215 |
| Hb (g/dL) | 10.5 ± 0.6 | 10.7 ± 0.6 | 0.1212 |
| TSI (μg/dL) | 37.8 ± 5.6 | 38.3 ± 5.4 | 0.9855 |
| sFtn (ng/mL) | 12.8 ± 3.4 | 11.4 ± 3.4 | 0.3851 |
| IL-6 (pg/mL) | 54.7 ± 9.3 | 56.1 ± 9.5 | 0.8662 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, L.; Ianiro, G.; Niro, A.; Musci, G.; Paesano, R.; Cutone, A.; Valenti, P. Valpalf®: A New Nutraceutical Formulation Containing Bovine Lactoferrin That Exhibits Potentiated Biological Activity. Int. J. Mol. Sci. 2024, 25, 8559. https://doi.org/10.3390/ijms25168559
Rosa L, Ianiro G, Niro A, Musci G, Paesano R, Cutone A, Valenti P. Valpalf®: A New Nutraceutical Formulation Containing Bovine Lactoferrin That Exhibits Potentiated Biological Activity. International Journal of Molecular Sciences. 2024; 25(16):8559. https://doi.org/10.3390/ijms25168559
Chicago/Turabian StyleRosa, Luigi, Giusi Ianiro, Antonella Niro, Giovanni Musci, Rosalba Paesano, Antimo Cutone, and Piera Valenti. 2024. "Valpalf®: A New Nutraceutical Formulation Containing Bovine Lactoferrin That Exhibits Potentiated Biological Activity" International Journal of Molecular Sciences 25, no. 16: 8559. https://doi.org/10.3390/ijms25168559
APA StyleRosa, L., Ianiro, G., Niro, A., Musci, G., Paesano, R., Cutone, A., & Valenti, P. (2024). Valpalf®: A New Nutraceutical Formulation Containing Bovine Lactoferrin That Exhibits Potentiated Biological Activity. International Journal of Molecular Sciences, 25(16), 8559. https://doi.org/10.3390/ijms25168559










