The Exosomes of Stem Cells from Human Exfoliated Deciduous Teeth Suppress Inflammation in Osteoarthritis
Abstract
:1. Introduction
2. Results
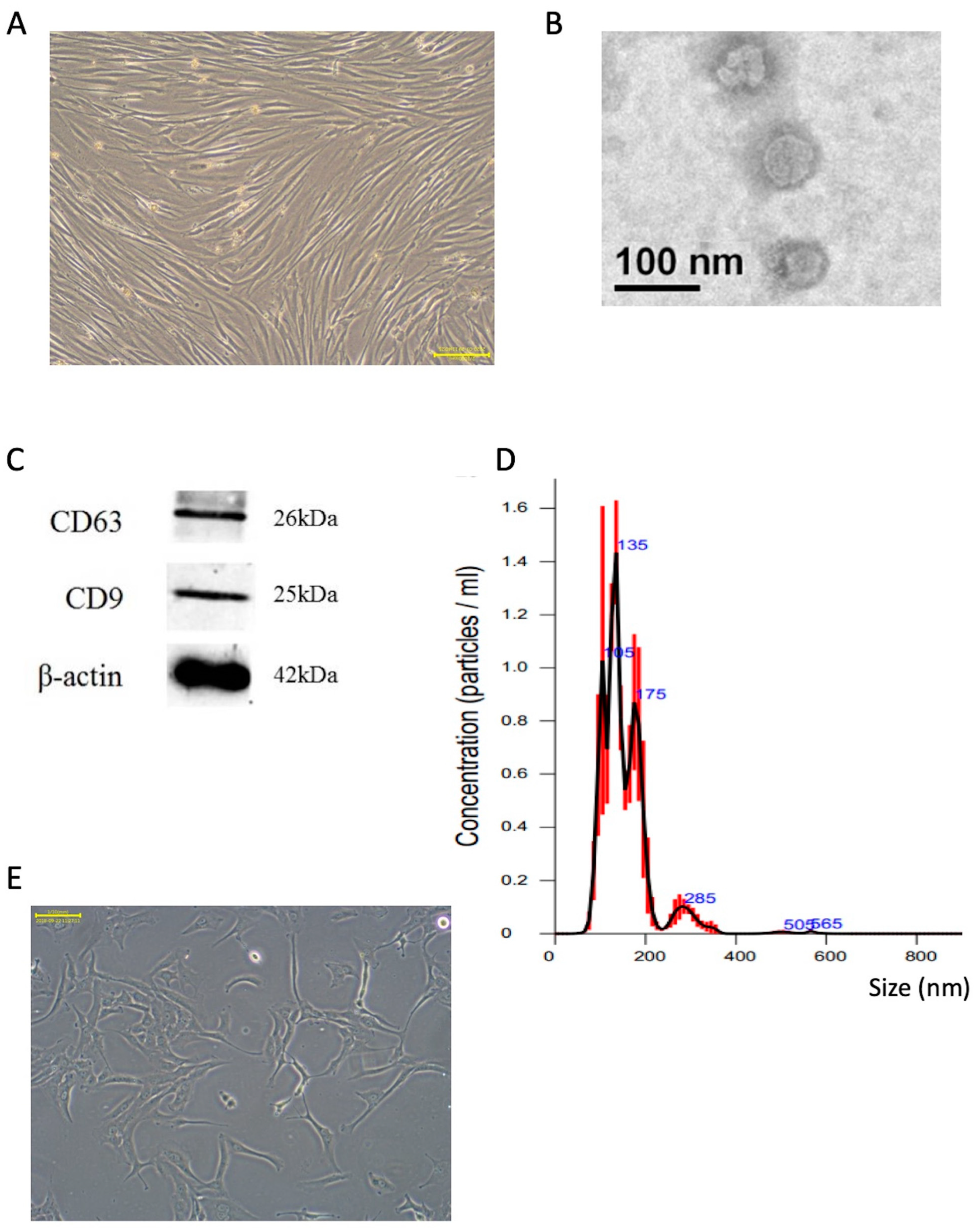
2.1. Characterization of Extracted Exosomes from SHED Cultured Medium
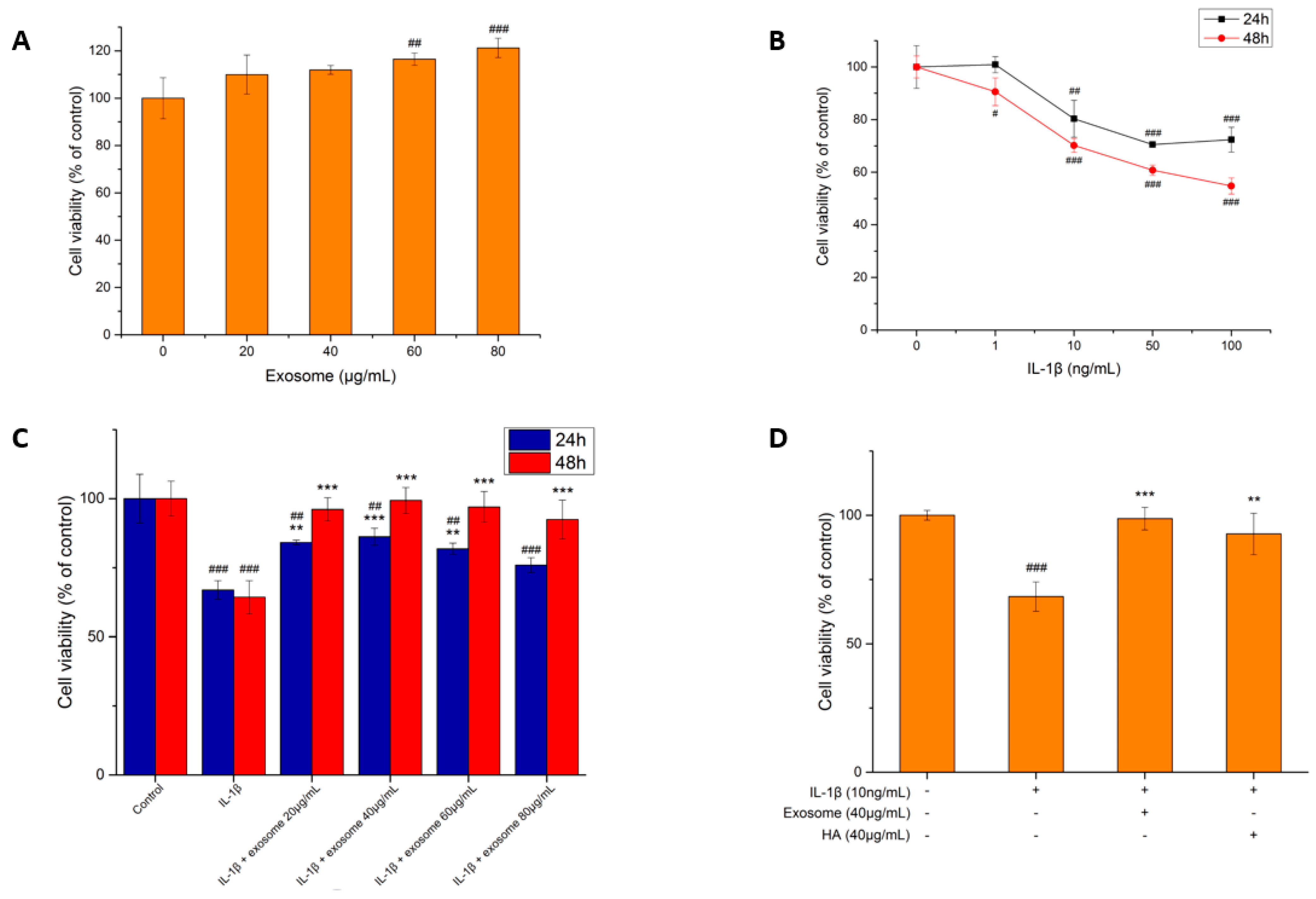
2.2. Cell Viability Test
2.2.1. Exosome Toxicity Tests
2.2.2. The Effect of IL-1β on the Cell Viability of SW1353 Cells
2.2.3. The Effect of Exosomes on the Survival of Inflammatory Cells
2.2.4. The Effect of Hyaluronic Acid on Inflammatory Cell Survival
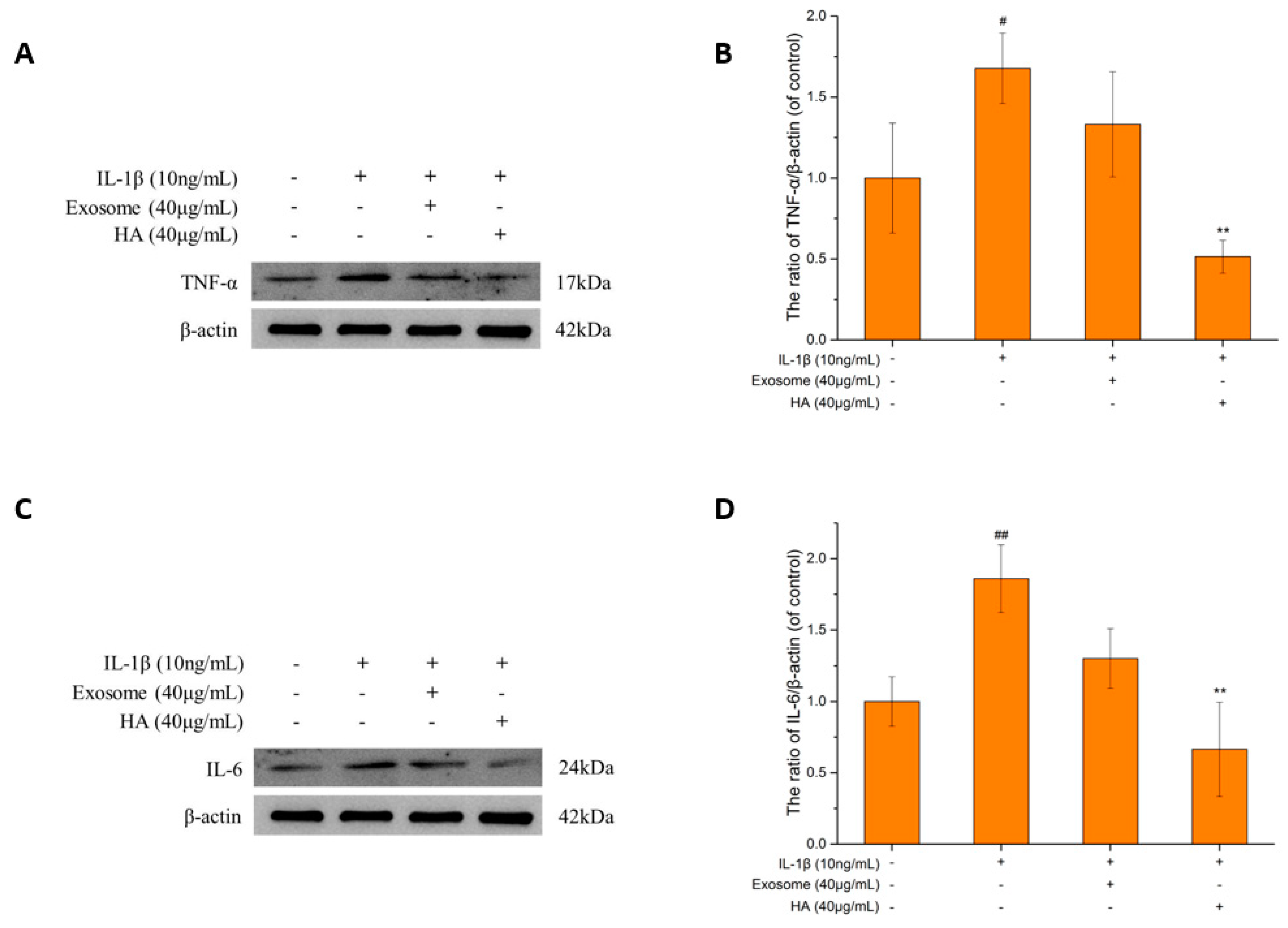
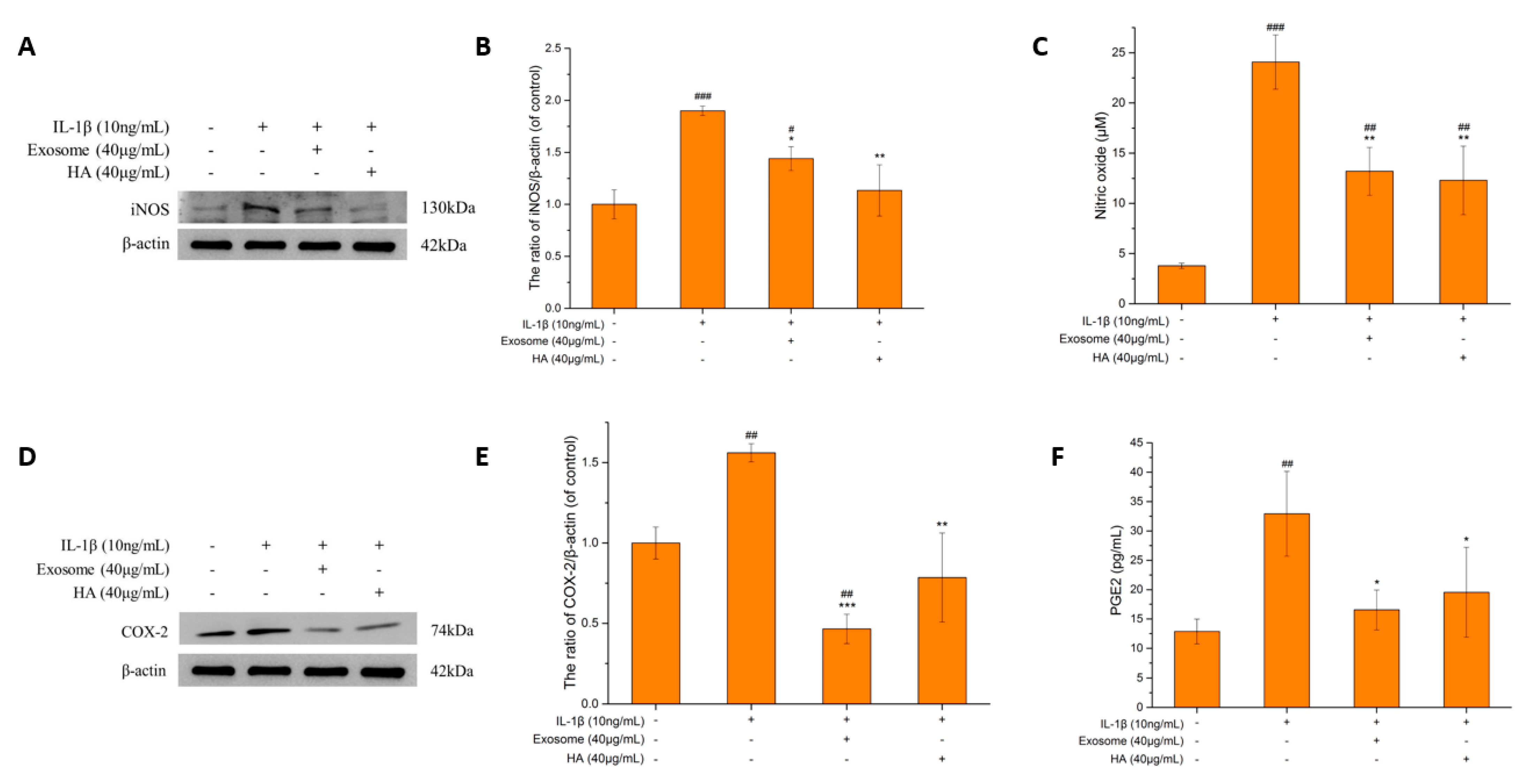
2.3. Inflammatory Markers
2.3.1. The TNF-α and IL-6
2.3.2. The iNOS and NO
2.3.3. COX-2 and PGE2
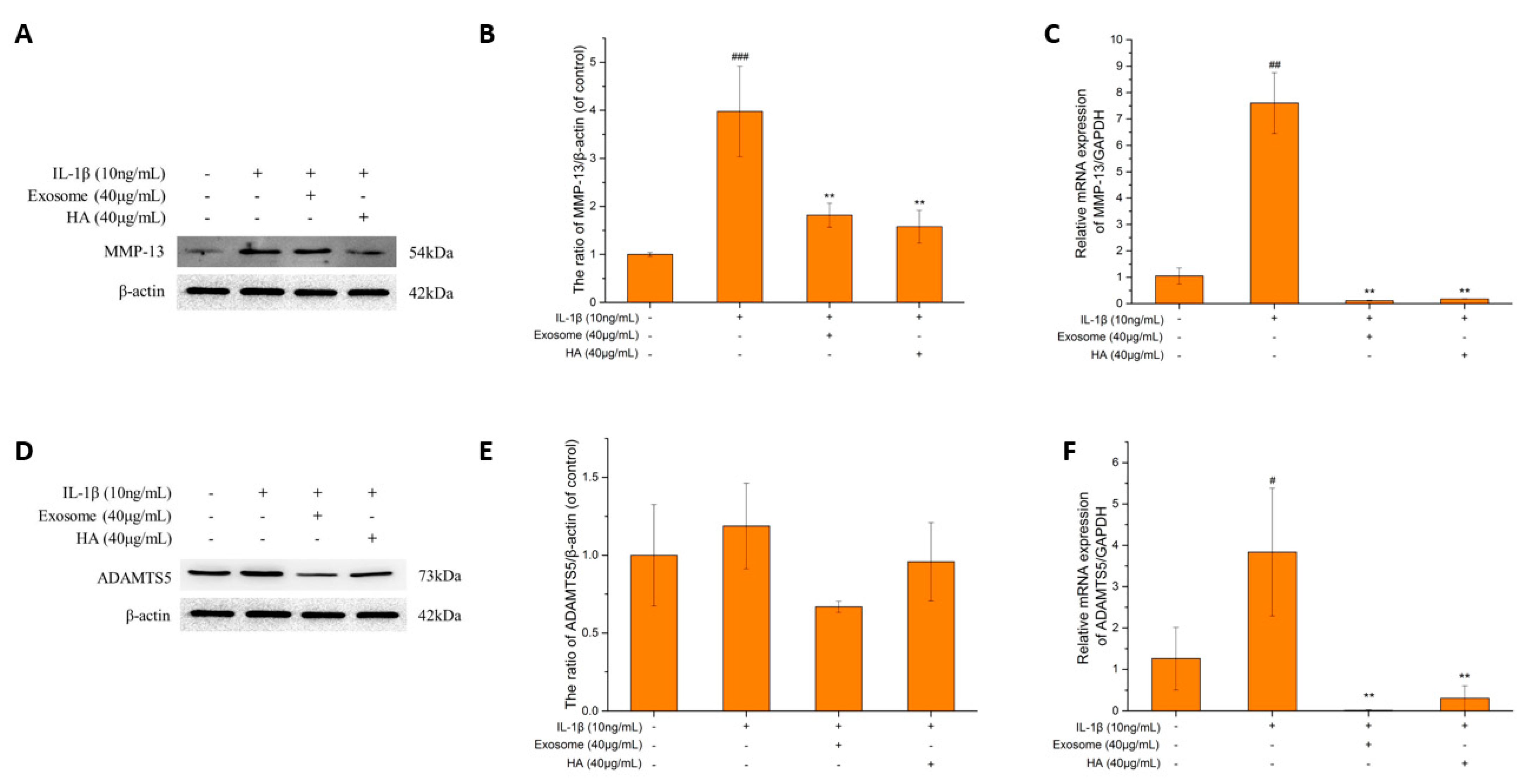
2.4. The Degrading Enzyme of Extrachondral Matrix
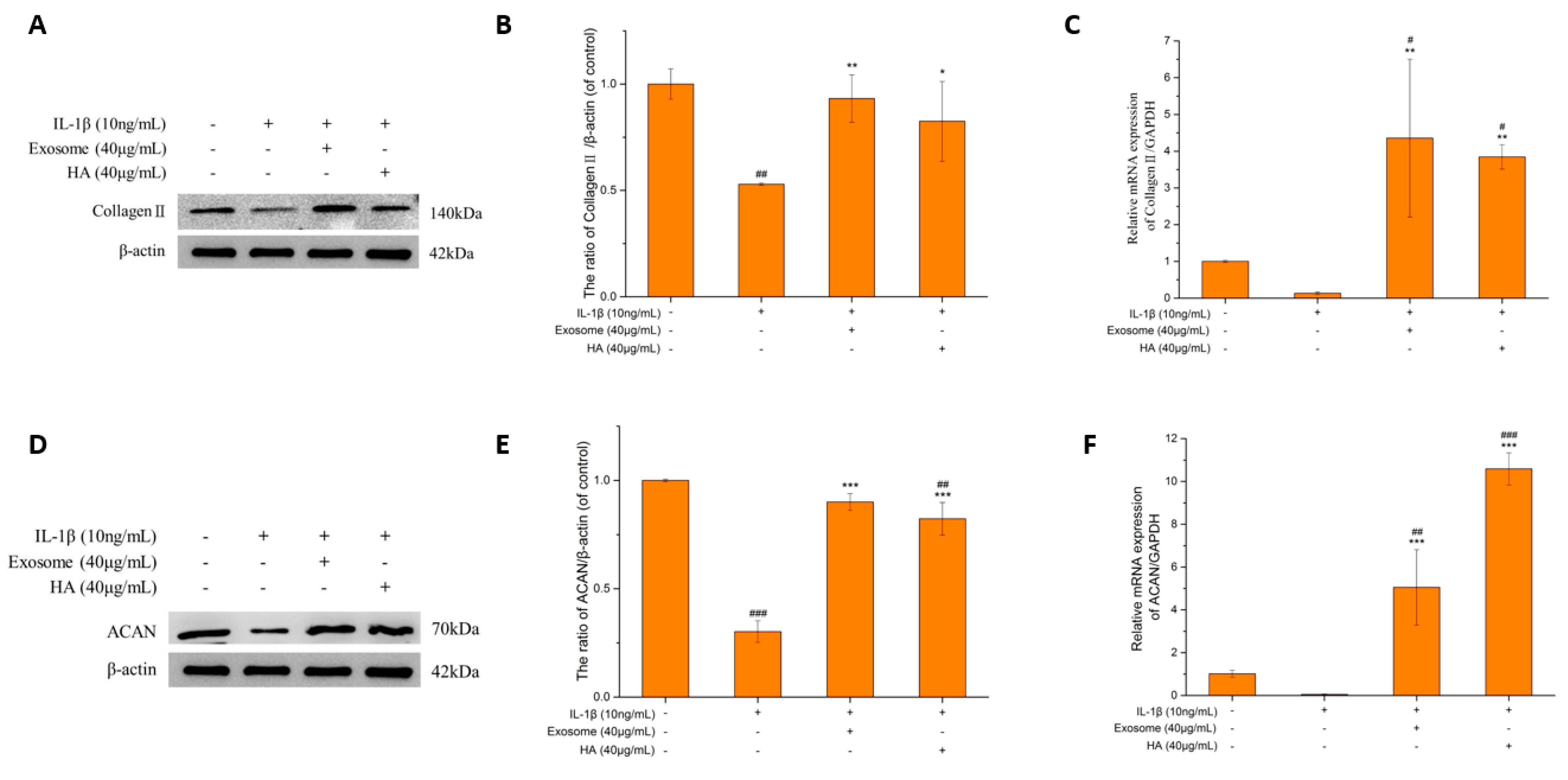
2.5. The Extrachondral Matrix
Collagen II and ACAN
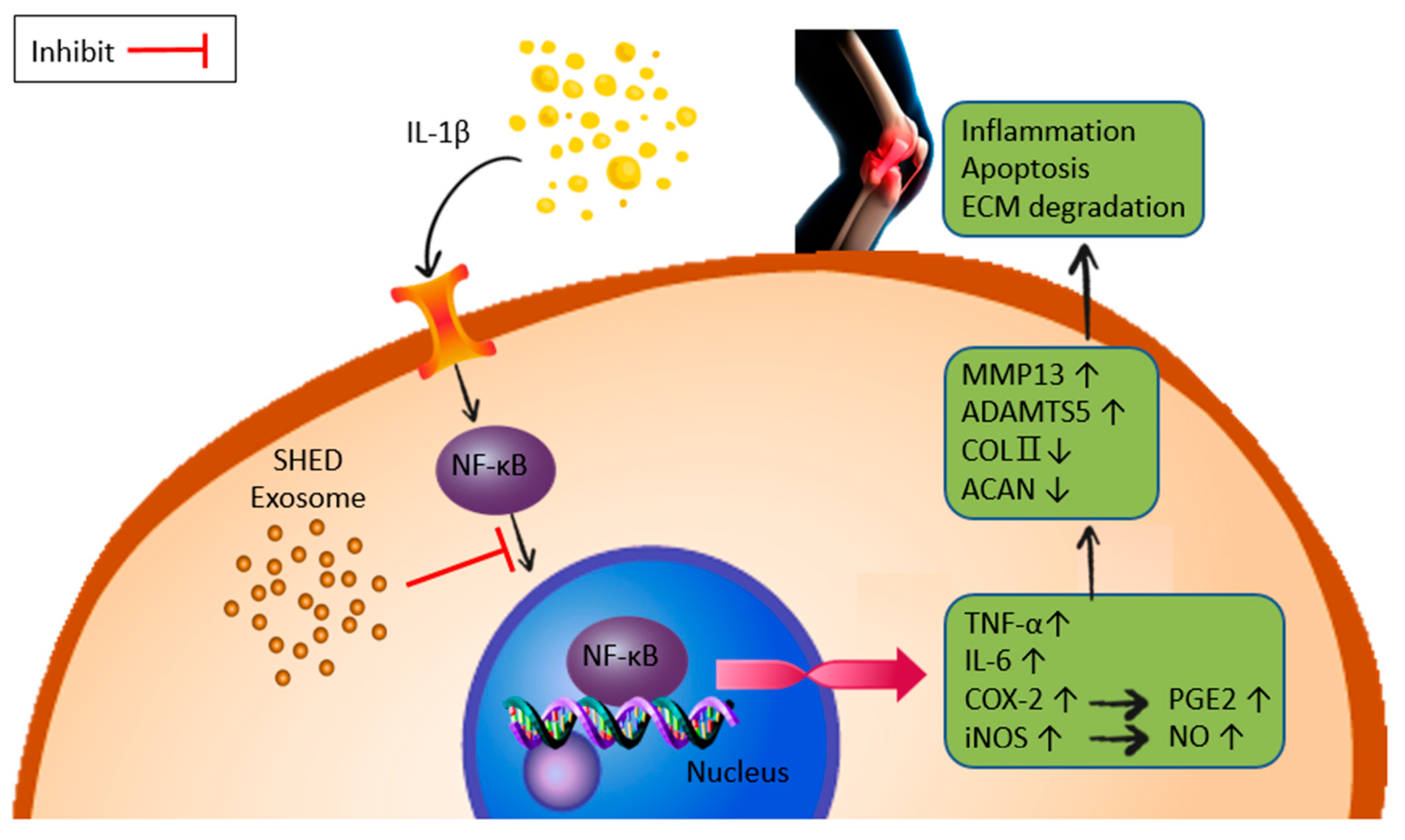
2.6. NF-κB Gene Expression and Activity
3. Discussion
Limitations of the Study
4. Materials and Methods
4.1. The Stem Cells from Human Exfoliated Deciduous Teeth (SHED) and SW1353
4.2. Harvest Exosomes
4.3. Exosome Extraction
4.4. Cell Viability Assay
4.5. Western Blotting
4.6. qPCR
4.7. Nitrite Detection
4.8. Determination of NF-κB Activity
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.L.; Im, H.J. Osteoarthritis: Toward a comprehensive understanding of pathological mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef]
- Kim, I.; Kim, H.A.; Seo, Y.I.; Song, Y.W.; Jeong, J.Y.; Kim, D.H. The prevalence of knee osteoarthritis in elderly community residents in Korea. J. Korean Med. Sci. 2010, 25, 293–298. [Google Scholar] [CrossRef]
- Akkiraju, H.; Nohe, A. Role of Chondrocytes in Cartilage Formation, Progression of Osteoarthritis and Cartilage Regeneration. J. Dev. Biol. 2015, 3, 177–192. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.-P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef]
- Rutjes, A.W.; Jüni, P.; da Costa, B.R.; Trelle, S.; Nüesch, E.; Reichenbach, S. Viscosupplementation for osteoarthritis of the knee: A systematic review and meta-analysis. Ann. Intern. Med. 2012, 157, 180–191. [Google Scholar] [CrossRef]
- Prasadam, I.; Crawford, R.; Xiao, Y. Aggravation of ADAMTS and matrix metalloproteinase production and role of ERK1/2 pathway in the interaction of osteoarthritic subchondral bone osteoblasts and articular cartilage chondrocytes–Possible pathogenic role in osteoarthritis. J. Rheumatol. 2012, 39, 621–634. [Google Scholar] [CrossRef]
- Sugita, R.; Kuwabara, H.; Kubota, K.; Sugimoto, K.; Kiho, T.; Tengeiji, A.; Kawakami, K.; Shimada, K. Simultaneous Inhibition of PGE2 and PGI2 Signals Is Necessary to Suppress Hyperalgesia in Rat Inflammatory Pain Models. Mediat. Inflamm. 2016, 2016, 9847840. [Google Scholar] [CrossRef]
- Bunning, R.A.; Russell, R.G. The effect of tumor necrosis factor alpha and gamma-interferon on the resorption of human articular cartilage and on the production of prostaglandin E and of caseinase activity by human articular chondrocytes. Arthritis Rheum. 1989, 32, 780–784. [Google Scholar] [CrossRef]
- Miwa, M.; Saura, R.; Hirata, S.; Hayashi, Y.; Mizuno, K.; Itoh, H. Induction of apoptosis in bovine articular chondrocyte by prostaglandin E(2) through cAMP-dependent pathway. Osteoarthr. Cartil. 2000, 8, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S.; Takahashi, K.; Amiel, D.; Coutts, R.D.; Lotz, M. Chondrocyte apoptosis and nitric oxide production during experimentally induced osteoarthritis. Arthritis Rheum. 1998, 41, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Marcu, K.B.; Otero, M.; Olivotto, E.; Borzi, R.M.; Goldring, M.B. NF-kappaB signaling: Multiple angles to target OA. Curr. Drug Targets 2010, 11, 599–613. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Ulivi, V.; Giannoni, P.; Gentili, C.; Cancedda, R.; Descalzi, F. p38/NF-kB-dependent expression of COX-2 during differentiation and inflammatory response of chondrocytes. J. Cell. Biochem. 2008, 104, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, X.; Yang, Y.; He, J.; Jiang, M.; Huang, Y.; Liu, X.; Liu, L.; Gu, H. Resveratrol Exerts Anti-Osteoarthritic Effect by Inhibiting TLR4/NF-κB Signaling Pathway via the TLR4/Akt/FoxO1 Axis in IL-1β-Stimulated SW1353 Cells. Drug Des. Dev. Ther. 2020, 14, 2079–2090. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zeng, L.; Wang, Z.M.; Zhang, S.; Rong, X.F.; Li, R.H. Ginsenoside Rb1 inhibits matrix metalloproteinase 13 through down-regulating Notch signaling pathway in osteoarthritis. Exp. Biol. Med. 2015, 240, 1614–1621. [Google Scholar] [CrossRef] [PubMed]
- Klatt, A.R.; Klinger, G.; Neumüller, O.; Eidenmüller, B.; Wagner, I.; Achenbach, T.; Aigner, T.; Bartnik, E. TAK1 downregulation reduces IL-1beta induced expression of MMP13, MMP1 and TNF-alpha. Biomed. Pharmacother. 2006, 60, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Pattoli, M.A.; MacMaster, J.F.; Gregor, K.R.; Burke, J.R. Collagen and aggrecan degradation is blocked in interleukin-1-treated cartilage explants by an inhibitor of IkappaB kinase through suppression of metalloproteinase expression. J. Pharmacol. Exp. Ther. 2005, 315, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Yang, H.; Bau, B.; Söder, S.; Aigner, T. Role of mitogen-activated protein kinases and NFkappaB on IL-1beta-induced effects on collagen type II, MMP-1 and 13 mRNA expression in normal articular human chondrocytes. Rheumatol. Int. 2006, 26, 900–903. [Google Scholar] [CrossRef] [PubMed]
- Mautner, K.; Gottschalk, M.; Boden, S.D.; Akard, A.; Bae, W.C.; Black, L.; Boggess, B.; Chatterjee, P.; Chung, C.B.; Easley, K.A.; et al. Cell-based versus corticosteroid injections for knee pain in osteoarthritis: A randomized phase 3 trial. Nat. Med. 2023, 29, 3120–3126. [Google Scholar] [CrossRef]
- Kim, K.-I.; Kim, M.-S.; Kim, J.-H. Intra-articular Injection of Autologous Adipose-Derived Stem Cells or Stromal Vascular Fractions: Are They Effective for Patients With Knee Osteoarthritis? A Systematic Review With Meta-analysis of Randomized Controlled Trials. Am. J. Sports Med. 2022, 51, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Fuentes, D.E.; Fernández-Garza, L.E.; Samia-Meza, J.A.; Barrera-Barrera, S.A.; Caplan, A.I.; Barrera-Saldaña, H.A. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Arch. Med. Res. 2021, 52, 93–101. [Google Scholar] [CrossRef]
- Nishino, Y.; Yamada, Y.; Ebisawa, K.; Nakamura, S.; Okabe, K.; Umemura, E.; Hara, K.; Ueda, M. Stem cells from human exfoliated deciduous teeth (SHED) enhance wound healing and the possibility of novel cell therapy. Cytotherapy 2011, 13, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Kim, H.; Tsuboi, N.; Yamamoto, A.; Akiyama, S.; Shi, Y.; Katsuno, T.; Kosugi, T.; Ueda, M.; Matsuo, S.; et al. Therapeutic Potential of Stem Cells from Human Exfoliated Deciduous Teeth in Models of Acute Kidney Injury. PLoS ONE 2015, 10, e0140121. [Google Scholar] [CrossRef]
- Yamaza, T.; Alatas, F.S.; Yuniartha, R.; Yamaza, H.; Fujiyoshi, J.K.; Yanagi, Y.; Yoshimaru, K.; Hayashida, M.; Matsuura, T.; Aijima, R.; et al. In vivo hepatogenic capacity and therapeutic potential of stem cells from human exfoliated deciduous teeth in liver fibrosis in mice. Stem Cell Res. Ther. 2015, 6, 171. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zheng, Z.; Yuan, Y.; Pathak, J.L.; Yang, X.; Wang, L.; Ye, Z.; Cho, W.C.; Zeng, M.; Wu, L. The Emerging Role of Exosomes in Oral Squamous Cell Carcinoma. Front. Cell Dev. Biol. 2021, 9, 628103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Chu, W.C.; Lai, R.C.; Lim, S.K.; Hui, J.H.; Toh, W.S. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr. Cartil. 2016, 24, 2135–2140. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, J.Y.; Peng, W.M.; Yuan, B.; Bi, Q.; Xu, Y.J. Exosomes from adipose-derived stem cells promote chondrogenesis and suppress inflammation by upregulating miR-145 and miR-221. Mol. Med. Rep. 2020, 21, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, L.; Ma, C.; Wang, G.; Zhang, Y.; Sun, S. Exosomes derived from platelet-rich plasma present a novel potential in alleviating knee osteoarthritis by promoting proliferation and inhibiting apoptosis of chondrocyte via Wnt/β-catenin signaling pathway. J. Orthop. Surg. Res. 2019, 14, 470. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, N.; Tripathi, R.M. Short interfering RNA therapeutics: Nanocarriers, prospects and limitations. IET Nanobiotechnol. 2015, 9, 386–395. [Google Scholar] [CrossRef]
- Jiang, L.; Vader, P.; Schiffelers, R.M. Extracellular vesicles for nucleic acid delivery: Progress and prospects for safe RNA-based gene therapy. Gene Ther. 2017, 24, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Liu, D.-P.; Xiao, D.-W.; Tian, D.-C.; Su, Y.-W.; Jin, S.-F. Exosomes derived from mesenchymal stem cells inhibit mitochondrial dysfunction-induced apoptosis of chondrocytes via p38, ERK, and Akt pathways. In Vitro Cell. Dev. Biol. Anim. 2019, 55, 203–210. [Google Scholar] [CrossRef]
- He, L.; He, T.; Xing, J.; Zhou, Q.; Fan, L.; Liu, C.; Chen, Y.; Wu, D.; Tian, Z.; Liu, B.; et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res. Ther. 2020, 11, 276. [Google Scholar] [CrossRef]
- Yasui, T.; Akatsuka, M.; Tobetto, K.; Hayaishi, M.; Ando, T. The effect of hyaluronan on interleukin-1α-induced prostaglandin E2 production in human osteoarthritic synovial cells. Agents Actions 1992, 37, 155–156. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.-H.; Liu, S.-Q.; Peng, H. The effect of hyaluronic acid on IL-1β-induced chondrocyte apoptosis in a rat model of osteoarthritis. J. Orthop. Res. 2008, 26, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Song, J.H.; Yoon, J.H.; Park, Y.G.; Lee, S.W.; Choi, Y.J.; Nam, S.W.; Lee, J.Y.; Park, W.S. TNF-α and TNF-β Polymorphisms are Associated with Susceptibility to Osteoarthritis in a Korean Population. Korean J. Pathol. 2012, 46, 30–37. [Google Scholar] [CrossRef]
- Livshits, G.; Zhai, G.; Hart, D.J.; Kato, B.S.; Wang, H.; Williams, F.M.K.; Spector, T.D. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: The Chingford study. Arthritis Rheum. 2009, 60, 2037–2045. [Google Scholar] [CrossRef]
- Wehling, N.; Palmer, G.D.; Pilapil, C.; Liu, F.; Wells, J.W.; Müller, P.E.; Evans, C.H.; Porter, R.M. Interleukin-1β and tumor necrosis factor α inhibit chondrogenesis by human mesenchymal stem cells through NF-κB–dependent pathways. Arthritis Rheum. 2009, 60, 801–812. [Google Scholar] [CrossRef]
- Stannus, O.; Jones, G.; Cicuttini, F.; Parameswaran, V.; Quinn, S.; Burgess, J.; Ding, C. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthr. Cartil. 2010, 18, 1441–1447. [Google Scholar] [CrossRef]
- Catrina, A.I.; Lampa, J.; Ernestam, S.; af Klint, E.; Bratt, J.; Klareskog, L.; Ulfgren, A.K. Anti-tumour necrosis factor (TNF)-α therapy (etanercept) down-regulates serum matrix metalloproteinase (MMP)-3 and MMP-1 in rheumatoid arthritis. Rheumatology 2002, 41, 484–489. [Google Scholar] [CrossRef]
- López-Armada, M.J.; Caramés, B.; Martín, M.A.; Cillero-Pastor, B.; Lires-Dean, M.; Fuentes-Boquete, I.; Arenas, J.; Blanco, F.J. Mitochondrial activity is modulated by TNFα and IL-1β in normal human chondrocyte cells. Osteoarthr. Cartil. 2006, 14, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Porée, B.; Kypriotou, M.; Chadjichristos, C.; Beauchef, G.; Renard, E.; Legendre, F.; Melin, M.; Gueret, S.; Hartmann, D.-J.; Malléin-Gerin, F.; et al. Interleukin-6 (IL-6) and/or Soluble IL-6 Receptor Down-regulation of Human Type II Collagen Gene Expression in Articular Chondrocytes Requires a Decrease of Sp1·Sp3 Ratio and of the Binding Activity of Both Factors to the COL2A1 Promoter*. J. Biol. Chem. 2008, 283, 4850–4865. [Google Scholar] [CrossRef] [PubMed]
- Leonidou, A.; Lepetsos, P.; Mintzas, M.; Kenanidis, E.; Macheras, G.; Tzetis, M.; Potoupnis, M.; Tsiridis, E. Inducible nitric oxide synthase as a target for osteoarthritis treatment. Expert Opin. Ther. Targets 2018, 22, 299–318. [Google Scholar] [CrossRef]
- Ahmad, N.; Ansari, M.Y.; Haqqi, T.M. Role of iNOS in osteoarthritis: Pathological and therapeutic aspects. J. Cell. Physiol. 2020, 235, 6366–6376. [Google Scholar] [CrossRef]
- Abramson, S.B. Nitric oxide in inflammation and pain associated with osteoarthritis. Arthritis Res. Ther. 2008, 10, S2. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Boileau, C.; Pelletier, J.-P.; Roughley, P.J. Cartilage in normal and osteoarthritis conditions. Best Pract. Res. Clin. Rheumatol. 2008, 22, 351–384. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Ni, D.; Rosenkrans, Z.T.; Cai, W. Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res. 2018, 11, 4955–4984. [Google Scholar] [CrossRef]
- Sadowski, T.; Steinmeyer, J. Effects of non-steroidal antiinflammatory drugs and dexamethasone on the activity and expression of matrix metalloproteinase-1, matrix metalloproteinase-3 and tissue inhibitor of metalloproteinases-1 by bovine articular chondrocytes. Osteoarthr. Cartil. 2001, 9, 407–415. [Google Scholar] [CrossRef]
- Hardy, M.M.; Seibert, K.; Manning, P.T.; Currie, M.G.; Woerner, B.M.; Edwards, D.; Koki, A.; Tripp, C.S. Cyclooxygenase 2-dependent prostaglandin E2 modulates cartilage proteoglycan degradation in human osteoarthritis explants. Arthritis Rheum. 2002, 46, 1789–1803. [Google Scholar] [CrossRef] [PubMed]
- Martel-Pelletier, J.; Pelletier, J.-P.; Fahmi, H. Cyclooxygenase-2 and prostaglandins in articular tissues. Semin. Arthritis Rheum. 2003, 33, 155–167. [Google Scholar] [CrossRef]
- Vincenti, M.P.; Brinckerhoff, C.E. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: Integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. Ther. 2002, 4, 157. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, T.; Lemaître, V.; D’Armiento, J.; Okada, Y. Matrix metalloproteinases, a disintegrin and metalloproteinases, and a disintegrin and metalloproteinases with thrombospondin motifs in non-neoplastic diseases. Pathol. Int. 2010, 60, 477–496. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, M.D.; Burn, T.C.; Pratta, M.A.; Abbaszade, I.; Hollis, J.M.; Liu, R.; Rosenfeld, S.A.; Copeland, R.A.; Decicco, C.P.; Wynn, R.; et al. Purification and Cloning of Aggrecanase-1: A Member of the ADAMTS Family of Proteins. Science 1999, 284, 1664–1666. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, M.D.; Pratta, M.; Liu, R.-Q.; Austin, J.; Ross, O.H.; Abbaszade, I.; Burn, T.; Arner, E. Sites of Aggrecan Cleavage by Recombinant Human Aggrecanase-1 (ADAMTS-4)*. J. Biol. Chem. 2000, 275, 18566–18573. [Google Scholar] [CrossRef] [PubMed]
- Fushimi, K.; Troeberg, L.; Nakamura, H.; Lim, N.H.; Nagase, H. Functional Differences of the Catalytic and Non-catalytic Domains in Human ADAMTS-4 and ADAMTS-5 in Aggrecanolytic Activity*. J. Biol. Chem. 2008, 283, 6706–6716. [Google Scholar] [CrossRef]
- Liu, C.C.; Zhang, Y.; Dai, B.L.; Ma, Y.J.; Zhang, Q.; Wang, Y.; Yang, H. Chlorogenic acid prevents inflammatory responses in IL-1β-stimulated human SW-1353 chondrocytes, a model for osteoarthritis. Mol. Med. Rep. 2017, 16, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Cecen, B.; Keles, D.; Oktay, G.; Kozaci, L.D. Effects of simvastatin on matrix metalloproteinase regulation in IL-1β-induced SW1353 cells. Chem.-Biol. Interact. 2019, 310, 108730. [Google Scholar] [CrossRef] [PubMed]
- Zuliani-Alvarez, L.; Piccinini, A.M.; Midwood, K.S. Screening for Novel Endogenous Inflammatory Stimuli Using the Secreted Embryonic Alkaline Phosphatase NF-κB Reporter Assay. Bio-Protoc. 2017, 7, e2220. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.A.; Park, B.-R.; Moon, S.-M.; Hong, J.H.; Kim, D.K.; Kim, C.S. Chondroprotective Effect of Cynaroside in IL-1β-Induced Primary Rat Chondrocytes and Organ Explants via NF-κB and MAPK Signaling Inhibition. Oxidative Med. Cell. Longev. 2020, 2020, 9358080. [Google Scholar] [CrossRef]
- Smyth, T.; Kullberg, M.; Malik, N.; Smith-Jones, P.; Graner, M.W.; Anchordoquy, T.J. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Control Release 2015, 199, 145–155. [Google Scholar] [CrossRef]
- Wei, J.; Song, Y.; Du, Z.; Yu, F.; Zhang, Y.; Jiang, N.; Jiang, N.; Ge, X. Exosomes derived from human exfoliated deciduous teeth ameliorate adult bone loss in mice through promoting osteogenesis. J. Mol. Histol. 2020, 51, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, F.; Fan, C.; Wang, C.; Ruan, H. Effects and relationship of ERK1 and ERK2 in interleukin-1β-induced alterations in MMP3, MMP13, type II collagen and aggrecan expression in human chondrocytes. Int. J. Mol. Med. 2011, 27, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; He, C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef] [PubMed]
- Bryniarska-Kubiak, N.; Basta-Kaim, A.; Kubiak, A. Mechanobiology of Dental Pulp Cells. Cells 2024, 13, 375. [Google Scholar] [CrossRef] [PubMed]
- Bryniarska, N.; Kubiak, A.; Łabędź-Masłowska, A.; Zuba-Surma, E. Impact of developmental origin, niche mechanics and oxygen availability on osteogenic differentiation capacity of mesenchymal stem/stromal cells. Acta Biochim. Pol. 2019, 66, 491–498. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Naruphontjirakul, P.; Huang, T.-Y.; Wu, Y.-C.; Cheng, W.-H.; Su, W.-T. The Exosomes of Stem Cells from Human Exfoliated Deciduous Teeth Suppress Inflammation in Osteoarthritis. Int. J. Mol. Sci. 2024, 25, 8560. https://doi.org/10.3390/ijms25168560
Lin C-Y, Naruphontjirakul P, Huang T-Y, Wu Y-C, Cheng W-H, Su W-T. The Exosomes of Stem Cells from Human Exfoliated Deciduous Teeth Suppress Inflammation in Osteoarthritis. International Journal of Molecular Sciences. 2024; 25(16):8560. https://doi.org/10.3390/ijms25168560
Chicago/Turabian StyleLin, Chuang-Yu, Parichart Naruphontjirakul, Te-Yang Huang, Yi-Chia Wu, Wei-Hsuan Cheng, and Wen-Ta Su. 2024. "The Exosomes of Stem Cells from Human Exfoliated Deciduous Teeth Suppress Inflammation in Osteoarthritis" International Journal of Molecular Sciences 25, no. 16: 8560. https://doi.org/10.3390/ijms25168560





