Increased Homer Activity and NMJ Localization in the Vestibular Lesion het−/− Mouse soleus Muscle
Abstract
:1. Introduction
2. Results
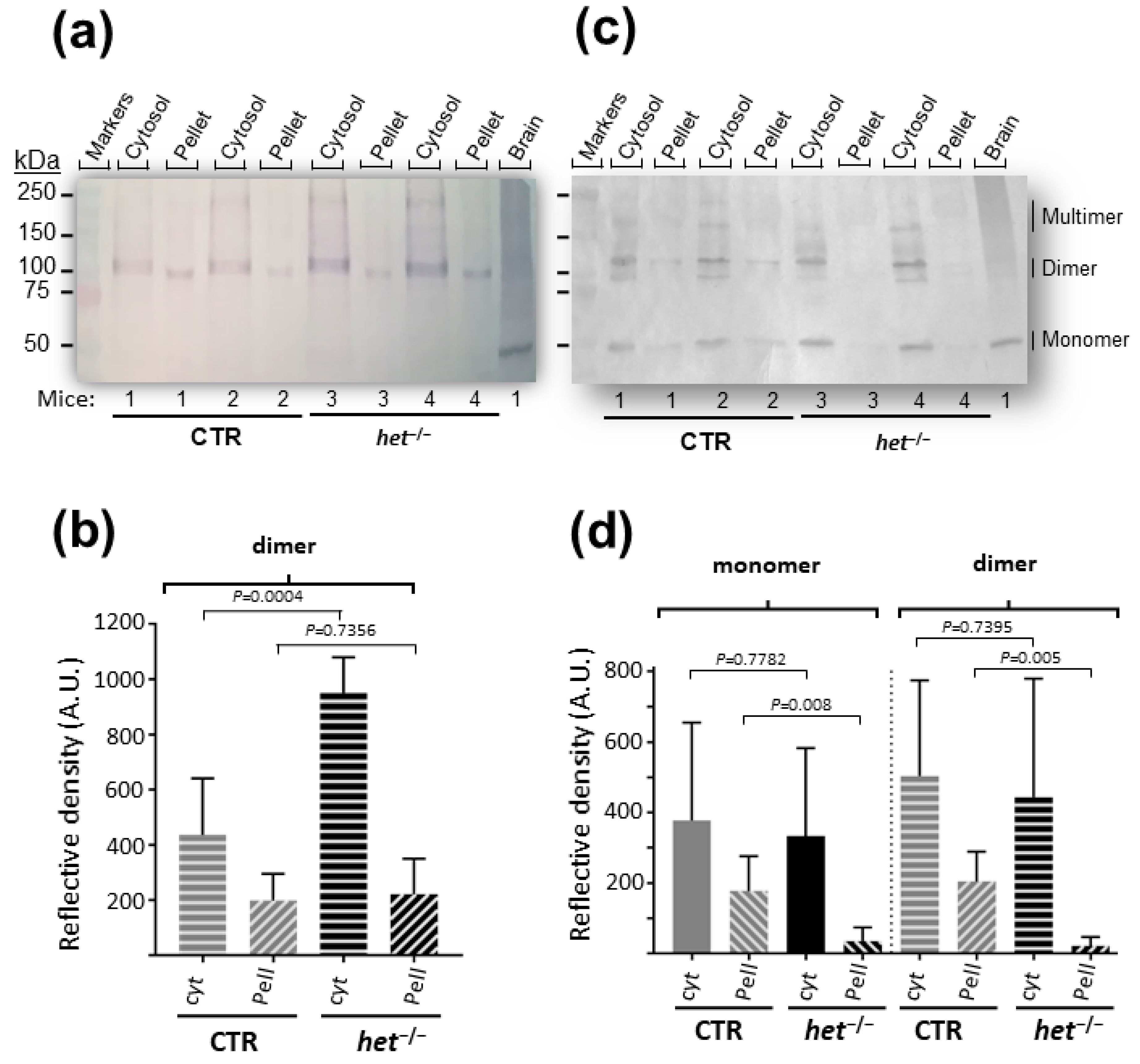
2.1. The het−/− Mouse Is Characterized by Increased Homer Dimer in the Cytosolic Fraction of SOL Muscle and Loss of Both Homer Monomer and Dimer in the Insoluble/Pellet Fraction of GAS Muscle
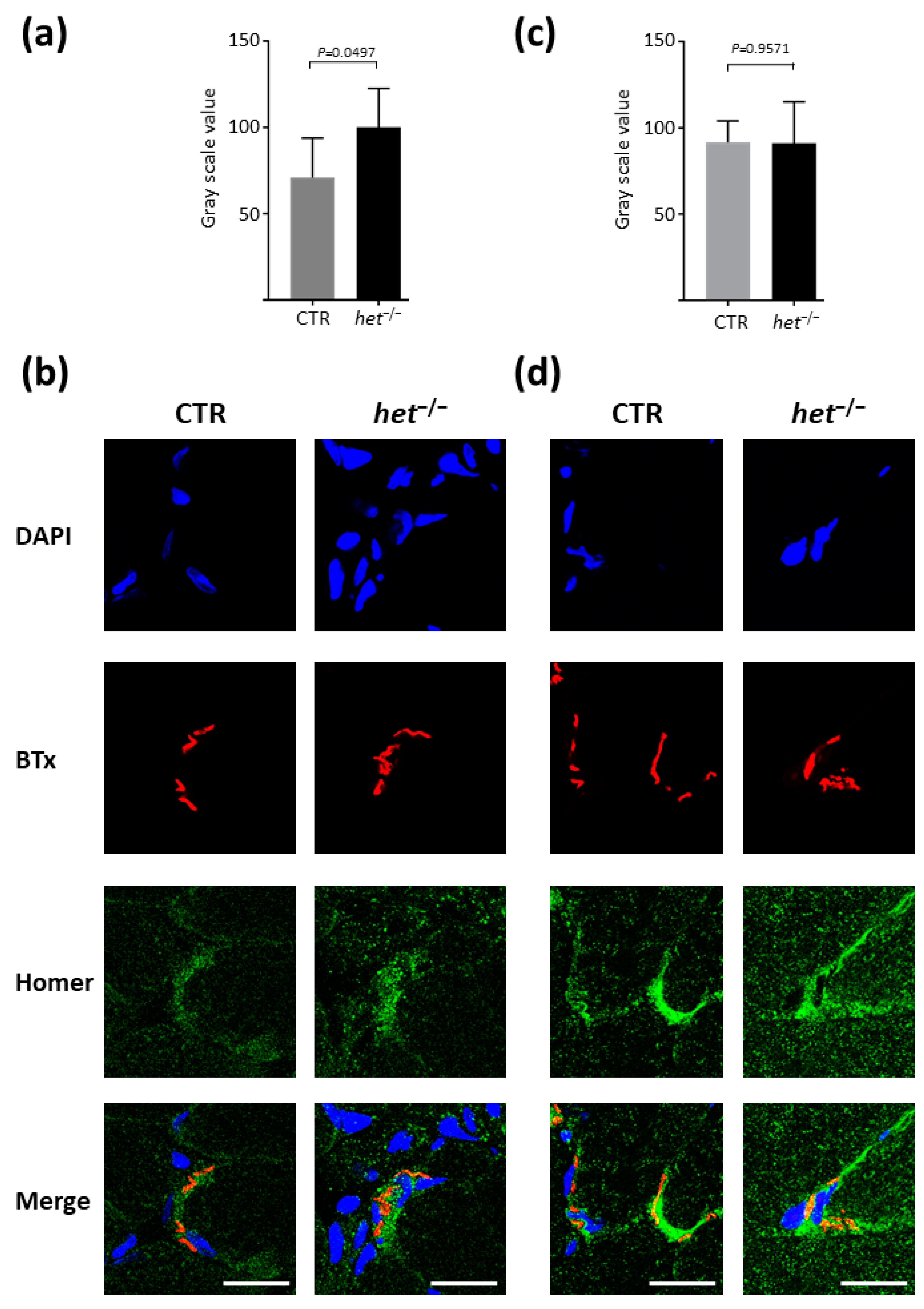
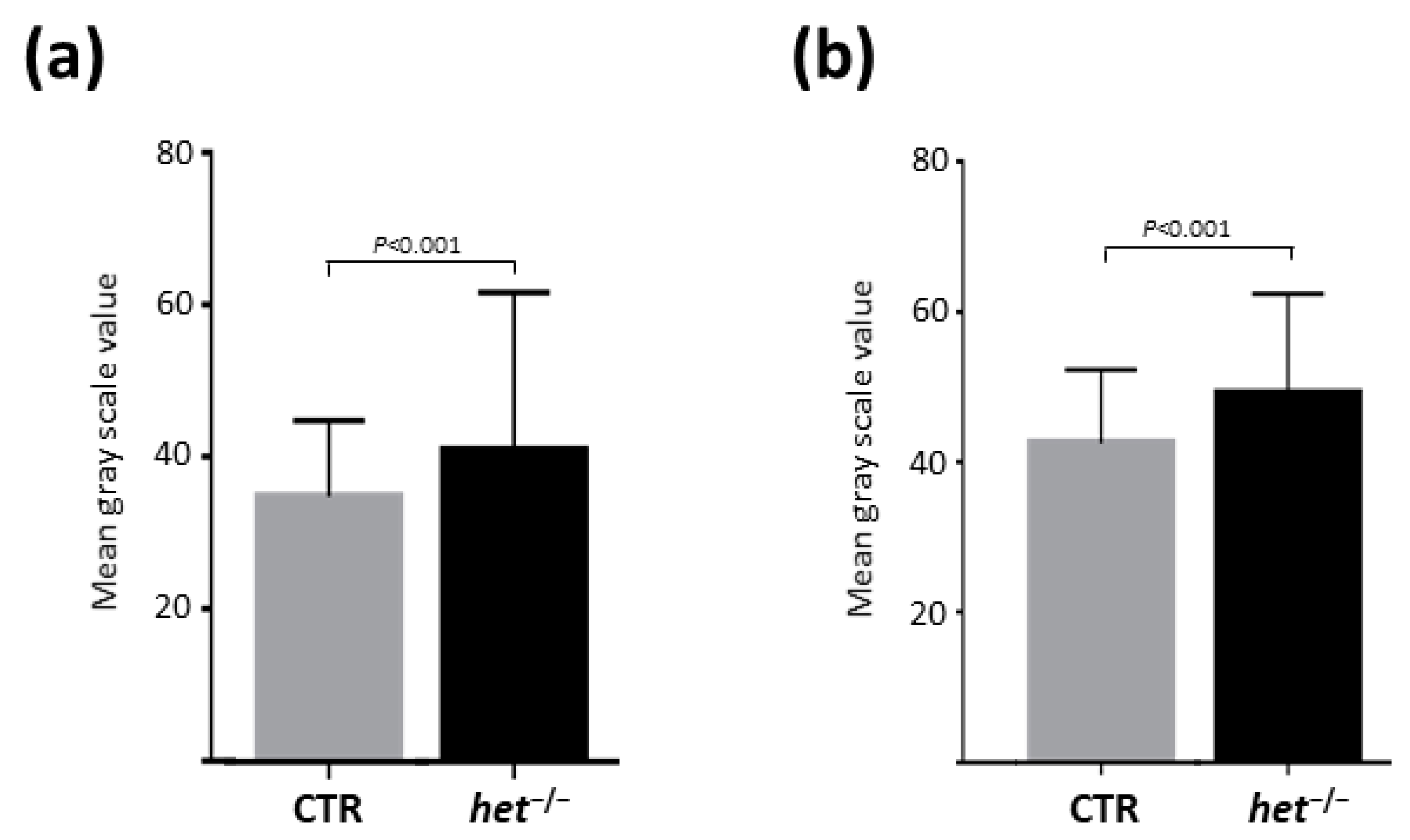
2.2. Increased Homer and Nicotinic Acetylcholine Receptor (nAChR) Protein Expression at the NMJ Postsynaptic Microdomain of het−/− Mouse SOL
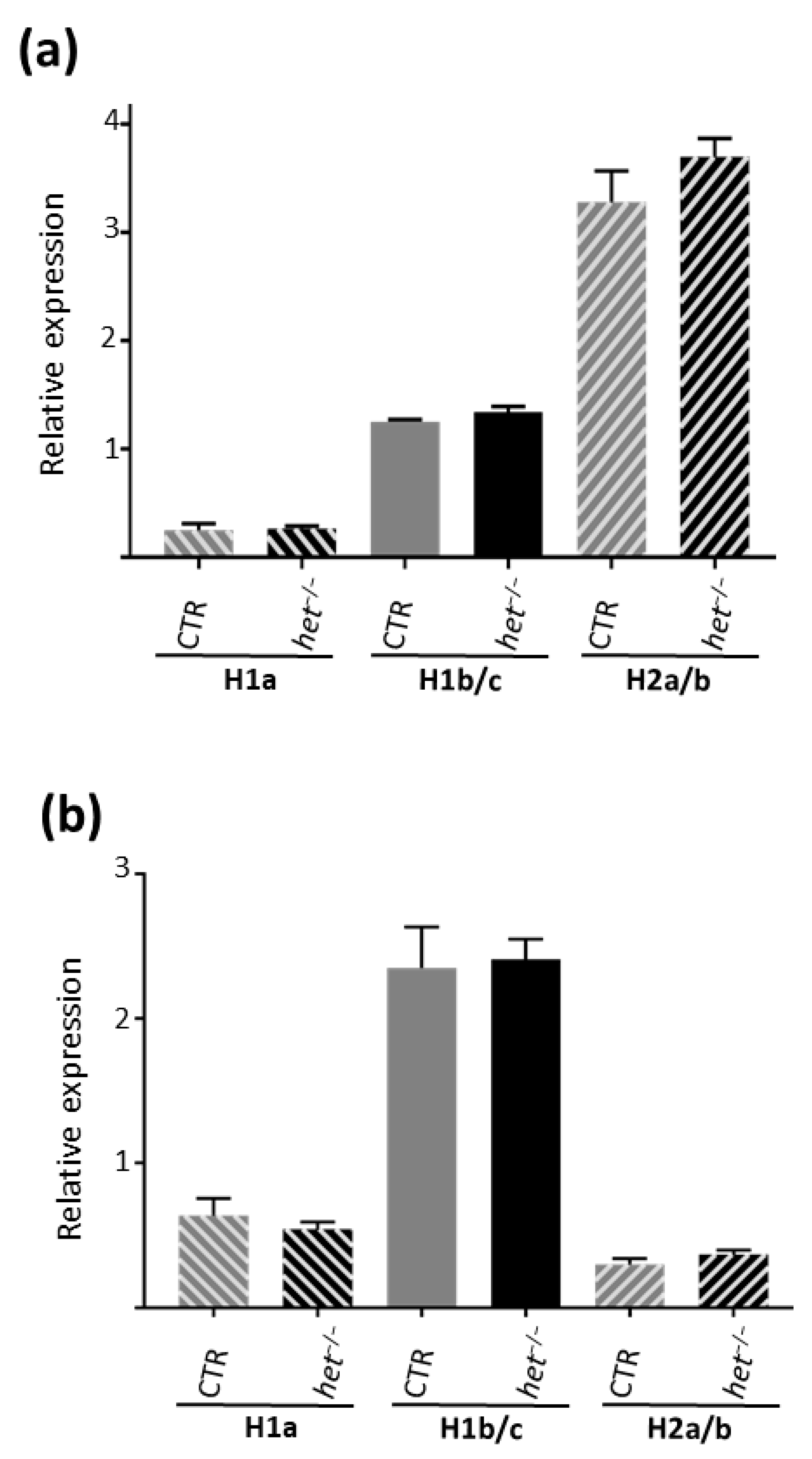
2.3. Homer 1b/c in the GAS and H2a/b in the SOL Muscles Are the Dominating Expressed Homer Isoforms
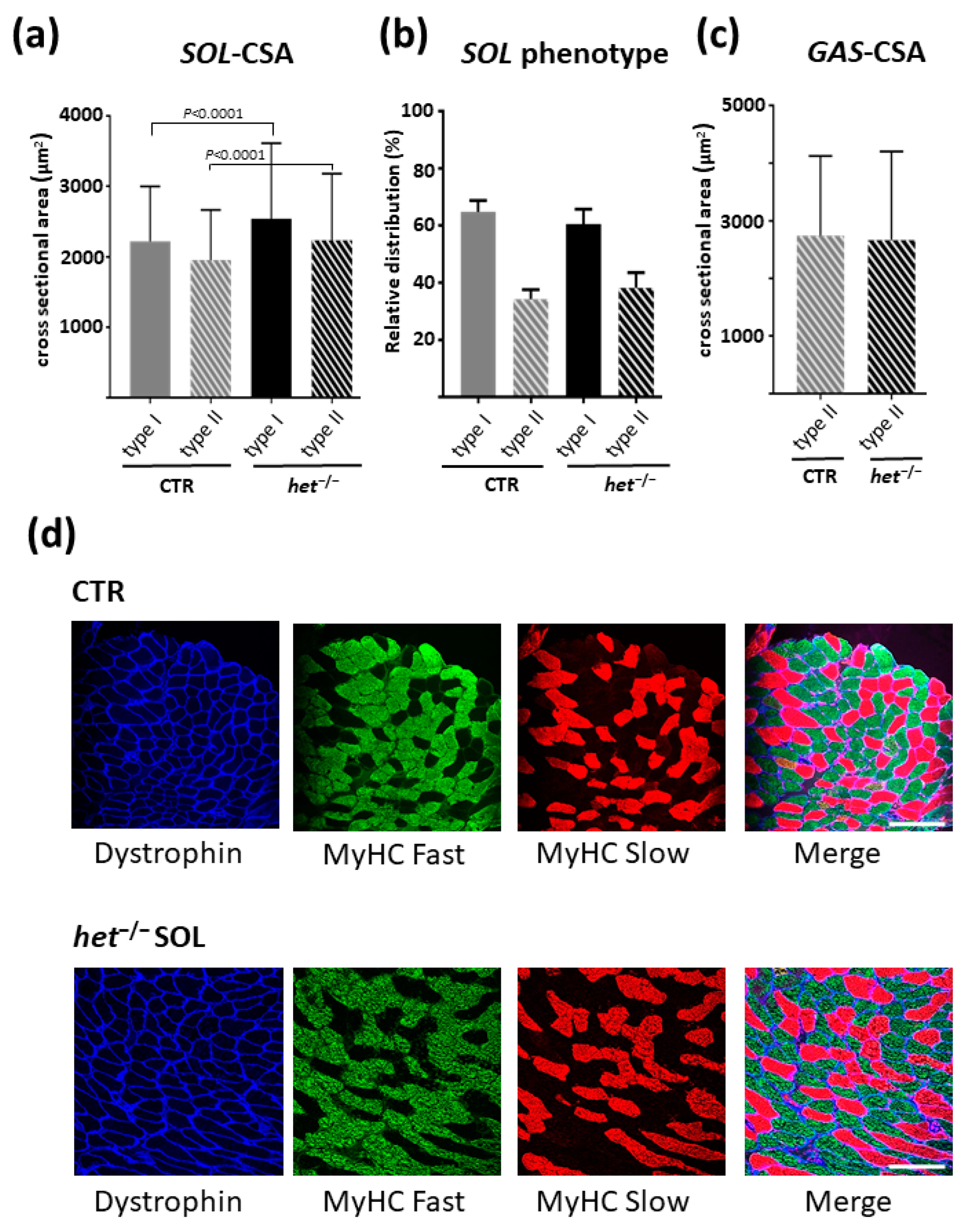
2.4. The het−/− Mouse Is Characterized by a Higher Myofiber Cross-Sectional Area in the Postural SOL Muscle
3. Discussion
4. Conclusions
5. Study Limitations
6. Materials and Methods
6.1. het−/− Mice
6.2. RNA Extraction and Quantitative Polymerase Chain Reaction (qPCR) Analysis
6.3. Myofiber Cross-Sectional Area and Phenotype Composition Analysis
6.4. Tissue Fractionation and Western Blot Analysis
6.5. Immunohistochemistry
6.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Angelaki, D.E.; Cullen, K.E. Vestibular system: The many facets of a multimodal sense. Annu. Rev. Neurosci. 2008, 31, 125–150. [Google Scholar] [CrossRef] [PubMed]
- Brosel, S.; Strupp, M. The Vestibular System and Ageing. Subcell. Biochem. 2019, 91, 195–225. [Google Scholar]
- Grillner, S.; Hongo, T.; Lund, S. The vestibulospinal tract. Effects on alpha-motoneurones in the lumbosacral spinal cord in the cat. Exp. Brain Res. 1970, 10, 94–120. [Google Scholar] [CrossRef]
- Curthoys, I.S.; Grant, J.W.; Burgess, A.M.; Pastras, C.J.; Brown, D.J.; Manzari, L. Otolithic Receptor Mechanisms for Vestibular-Evoked Myogenic Potentials: A Review. Front. Neurol. 2018, 9, 366. [Google Scholar] [CrossRef]
- Kasri, M.; Picquet, F.; Falempin, M. Effects of unilateral and bilateral labyrinthectomy on rat postural muscle properties: The soleus. Exp. Neurol. 2004, 185, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Luxa, N.; Salanova, M.; Schiffl, G.; Gutsmann, M.; Besnard, S.; Denise, P.; Clarke, A.; Blottner, D. Increased myofiber remodelling and NFATc1-myonuclear translocation in rat postural skeletal muscle after experimental vestibular deafferentation. J. Vestib. Res. 2013, 23, 187–193. [Google Scholar] [CrossRef]
- Kawao, N.; Morita, H.; Obata, K.; Tamura, Y.; Okumoto, K.; Kaji, H. The vestibular system is critical for the changes in muscle and bone induced by hypergravity in mice. Physiol. Rep. 2016, 4, e12979. [Google Scholar] [CrossRef] [PubMed]
- Iles, J.F.; Ali, A.S.; Savic, G. Vestibular-evoked muscle responses in patients with spinal cord injury. Brain 2004, 127, 1584–1592. [Google Scholar] [CrossRef]
- Tartaglia, G.M.; Barozzi, S.; Marin, F.; Cesarani, A.; Ferrario, V.F. Electromyographic Activity of Sternocleidomastoid and Masticatory Muscles in Patients with Vestibular Lesions. J. Appl. Oral. Sci. 2008, 16, 391–396. [Google Scholar] [CrossRef]
- Vignaux, G.; Besnard, S.; Denise, P.; Elefteriou, F. The Vestibular System: A Newly Identified Regulator of Bone Homeostasis Acting Through the Sympathetic Nervous System. Curr. Osteoporos. Rep. 2015, 13, 198–205. [Google Scholar] [CrossRef]
- Ronca, A.E.; Fritzsch, B.; Alberts, J.R.; Bruce, L.L. Effects of microgravity on vestibular development and function in rats: Genetics and environment. Korean J. Biol. Sci. 2000, 4, 215–221. [Google Scholar] [CrossRef]
- Mulavara, A.P.; Ruttley, T.; Cohen, H.S.; Peters, B.T.; Miller, C.; Brady, R.; Merkle, L.; Bloomberg, J.J. Vestibular-somatosensory convergence in head movement control during locomotion after long-duration space flight. J. Vestib. Res. 2012, 22, 153–166. [Google Scholar] [CrossRef]
- Dilda, V.; Morris, T.R.; Yungher, D.A.; MacDougall, H.G.; Moore, S.T. Central adaptation to repeated galvanic vestibular stimulation: Implications for pre-flight astronaut training. PLoS ONE 2014, 9, e112131. [Google Scholar] [CrossRef] [PubMed]
- Arntz, A.I.; van der Putte, D.A.M.; Jonker, Z.D.; Hauwert, C.M.; Frens, M.A.; Forbes, P.A. The Vestibular Drive for Balance Control Is Dependent on Multiple Sensory Cues of Gravity. Front. Physiol. 2019, 10, 476. [Google Scholar] [CrossRef]
- Lai, K.O.; Ip, N.Y. Postsynaptic signaling of new players at the neuromuscular junction. J. Neurocytol. 2003, 32, 727–741. [Google Scholar] [CrossRef]
- Good, M.C.; Zalatan, J.G.; Lim, W.A. Scaffold proteins: Hubs for controlling the flow of cellular information. Science 2011, 332, 680–686. [Google Scholar] [CrossRef] [PubMed]
- Soloviev, M.M.; Ciruela, F.; Chan, W.Y.; McIlhinney, R.A. Mouse brain and muscle tissues constitutively express high levels of Homer proteins. Eur. J. Biochem. 2000, 267, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Volpe, P.; Sandri, C.; Bortoloso, E.; Valle, G.; Nori, A. Topology of Homer 1c and Homer 1a in C2C12 myotubes and transgenic skeletal muscle fibers. Biochem. Biophys. Res. Commun. 2004, 316, 884–892. [Google Scholar] [CrossRef]
- Shiraishi-Yamaguchi, Y.; Furuichi, T. The Homer family proteins. Genome Biol. 2007, 8, 206. [Google Scholar] [CrossRef]
- Tu, J.C.; Xiao, B.; Yuan, J.P.; Lanahan, A.A.; Leoffert, K.; Li, M.; Linden, D.J.; Worley, P.F. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron 1998, 21, 717–726. [Google Scholar] [CrossRef]
- Tanaka, M.; Duncan, R.S.; McClung, N.; Yannazzo, J.A.S.; Hwang, S.Y.; Marunouchi, T.; Inokuchi, K.; Koulen, P. Homer proteins control neuronal differentiation through IP3 receptor signaling. Febs Lett. 2006, 580, 6145–6150. [Google Scholar] [CrossRef]
- Feng, W.; Tu, J.; Yang, T.; Vernon, P.S.; Allen, P.D.; Worley, P.F.; Pessah, I.N. Homer regulates gain of ryanodine receptor type 1 channel complex. J. Biol. Chem. 2002, 277, 44722–44730. [Google Scholar] [CrossRef]
- Yuan, J.P.; Kiselyov, K.; Shin, D.M.; Chen, J.; Shcheynikov, N.; Kang, S.H.; Dehoff, M.H.; Schwarz, M.K.; Seeburg, P.H.; Muallem, S.; et al. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell 2003, 114, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Ward, C.W.; Feng, W.; Tu, J.; Pessah, I.N.; Worley, P.K.; Schneider, M.F. Homer protein increases activation of Ca2+ sparks in permeabilized skeletal muscle. J. Biol. Chem. 2004, 279, 5781–5787. [Google Scholar] [CrossRef]
- Worley, P.F.; Zeng, W.; Huang, G.; Kim, J.Y.; Shin, D.M.; Kim, M.S.; Yuan, J.P.; Kiselyov, K.; Muallem, S. Homer proteins in Ca2+ signaling by excitable and non-excitable cells. Cell Calcium 2007, 42, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Soloviev, M.M.; Ciruela, F.; Chan, W.Y.; McIlhinney, R.A. Molecular characterisation of two structurally distinct groups of human homers, generated by extensive alternative splicing. J. Mol. Biol. 2000, 295, 1185–1200. [Google Scholar] [CrossRef] [PubMed]
- Okabe, S.; Urushido, T.; Konno, D.; Okado, H.; Sobue, K. Rapid redistribution of the postsynaptic density protein PSD-Zip45 (Homer 1c) and its differential regulation by NMDA receptors and calcium channels. J. Neurosci. 2001, 21, 9561–9571. [Google Scholar] [CrossRef]
- Hayashi, M.K.; Ames, H.M.; Hayashi, Y. Tetrameric hub structure of postsynaptic scaffolding protein homer. J. Neurosci. 2006, 26, 8492–8501. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Pu, S.; Jiang, W.; Worley, P.F.; Xu, T. Deficiency of SHANK3 isoforms impairs thermal hyperalgesia and dysregulates the expression of postsynaptic proteins in the spinal cord. Neurosci. Res. 2021, 163, 26–33. [Google Scholar] [CrossRef]
- Bockaert, J.; Perroy, J.; Ango, F. The Complex Formed by Group I Metabotropic Glutamate Receptor (mGluR) and Homer1a Plays a Central Role in Metaplasticity and Homeostatic Synaptic Scaling. J. Neurosci. 2021, 41, 5567–5578. [Google Scholar] [CrossRef]
- Salanova, M.; Bortoloso, E.; Schiffl, G.; Gutsmann, M.; Belavy, D.L.; Felsenberg, D.; Furlan, S.; Volpe, P.; Blottner, D. Expression and regulation of Homer in human skeletal muscle during neuromuscular junction adaptation to disuse and exercise. FASEB J. 2011, 25, 4312–4325. [Google Scholar] [CrossRef] [PubMed]
- Blottner, D.; Trautmann, G.; Furlan, S.; Gambara, G.; Block, K.; Gutsmann, M.; Sun, L.W.; Worley, P.F.; Gorza, L.; Scano, M.; et al. Reciprocal Homer1a and Homer2 Isoform Expression Is a Key Mechanism for Muscle Soleus Atrophy in Spaceflown Mice. Int. J. Mol. Sci. 2021, 23, 75. [Google Scholar] [CrossRef]
- Bergstrom, R.A.; You, Y.; Erway, L.C.; Lyon, M.F.; Schimenti, J.C. Deletion mapping of the head tilt (het) gene in mice: A vestibular mutation causing specific absence of otoliths. Genetics 1998, 150, 815–822. [Google Scholar] [CrossRef]
- Paffenholz, R.; Bergstrom, R.A.; Pasutto, F.; Wabnitz, P.; Munroe, R.J.; Jagla, W.; Heinzmann, U.; Marquardt, A.; Bareiss, A.; Laufs, J.; et al. Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes. Dev. 2004, 18, 486–491. [Google Scholar] [CrossRef]
- Bortoloso, E.; Megighian, A.; Furlan, S.; Gorza, L.; Volpe, P. Homer 2 antagonizes protein degradation in slow-twitch skeletal muscles. Am. J. Physiol. Cell Physiol. 2013, 304, C68–C77. [Google Scholar] [CrossRef]
- Hallgren, E.; Migeotte, P.F.; Kornilova, L.; Deliere, Q.; Fransen, E.; Glukhikh, D.; Moore, S.T.; Clement, G.; Diedrich, A.; MacDougall, H.; et al. Dysfunctional vestibular system causes a blood pressure drop in astronauts returning from space. Sci. Rep. 2015, 5, 17627. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.L.; Kroichvili, N.; Freret, T.; Philoxene, B.; Lelong-Boulouard, V.; Denise, P.; Besnard, S. Spatial and non-spatial performance in mutant mice devoid of otoliths. Neurosci. Lett. 2012, 522, 57–61. [Google Scholar] [CrossRef]
- Proske, U.; Gandevia, S.C. The Proprioceptive Senses: Their Roles in Signaling Body Shape, Body Position and Movement, and Muscle Force. Physiol. Rev. 2012, 92, 1651–1697. [Google Scholar] [CrossRef]
- Mahoney, J.R.; Holtzer, R.; Verghese, J. Visual-somatosensory integration and balance: Evidence for psychophysical integrative differences in aging. Multisens. Res. 2014, 27, 17–42. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, C.; Pette, D.; Ohlendieck, K. Increased expression of the nicotinic acetylcholine receptor in stimulated muscle. Biochem. Biophys. Res. Commun. 2003, 300, 585–591. [Google Scholar] [CrossRef]
- Murphy, S. Subcellular Fractionation for DIGE-Based Proteomics. Methods Mol. Biol. 2023, 2596, 351–362. [Google Scholar] [PubMed]
- Rai, M.; Curley, M.; Coleman, Z.; Nityanandam, A.; Jiao, J.; Graca, F.A.; Hunt, L.C.; Demontis, F. Analysis of proteostasis during aging with western blot of detergent-soluble and insoluble protein fractions. STAR Protoc. 2021, 2, 100628. [Google Scholar] [CrossRef]
- Khan, M.M.; Lustrino, D.; Silveira, W.A.; Wild, F.; Straka, T.; Issop, Y.; O’Connor, E.; Cox, D.; Reischl, M.; Marquardt, T.; et al. Sympathetic innervation controls homeostasis of neuromuscular junctions in health and disease. Proc. Natl. Acad. Sci. USA 2016, 113, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Lorenzon, P.; Furlan, S.; Ravara, B.; Bosutti, A.; Massaria, G.; Bernareggi, A.; Sciancalepore, M.; Trautmann, G.; Block, K.; Blottner, D.; et al. Preliminary Observations on Skeletal Muscle Adaptation and Plasticity in Homer 2−/− Mice. Metabolites 2021, 11, 642. [Google Scholar] [CrossRef]
- Smith, L.R.; Barton, E.R. SMASH—Semi-automatic muscle analysis using segmentation of histology: A MATLAB application. Skelet. Muscle 2014, 4, 21. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trautmann, G.; Block, K.; Gutsmann, M.; Besnard, S.; Furlan, S.; Denise, P.; Volpe, P.; Blottner, D.; Salanova, M. Increased Homer Activity and NMJ Localization in the Vestibular Lesion het−/− Mouse soleus Muscle. Int. J. Mol. Sci. 2024, 25, 8577. https://doi.org/10.3390/ijms25168577
Trautmann G, Block K, Gutsmann M, Besnard S, Furlan S, Denise P, Volpe P, Blottner D, Salanova M. Increased Homer Activity and NMJ Localization in the Vestibular Lesion het−/− Mouse soleus Muscle. International Journal of Molecular Sciences. 2024; 25(16):8577. https://doi.org/10.3390/ijms25168577
Chicago/Turabian StyleTrautmann, Gabor, Katharina Block, Martina Gutsmann, Stéphane Besnard, Sandra Furlan, Pierre Denise, Pompeo Volpe, Dieter Blottner, and Michele Salanova. 2024. "Increased Homer Activity and NMJ Localization in the Vestibular Lesion het−/− Mouse soleus Muscle" International Journal of Molecular Sciences 25, no. 16: 8577. https://doi.org/10.3390/ijms25168577





