Novel Insights into Hb Shaare Zedek Associated with β0-Thalassemia: Molecular Characteristics, Genetic Origin and Diagnostic Approaches
Abstract
1. Introduction
2. Results
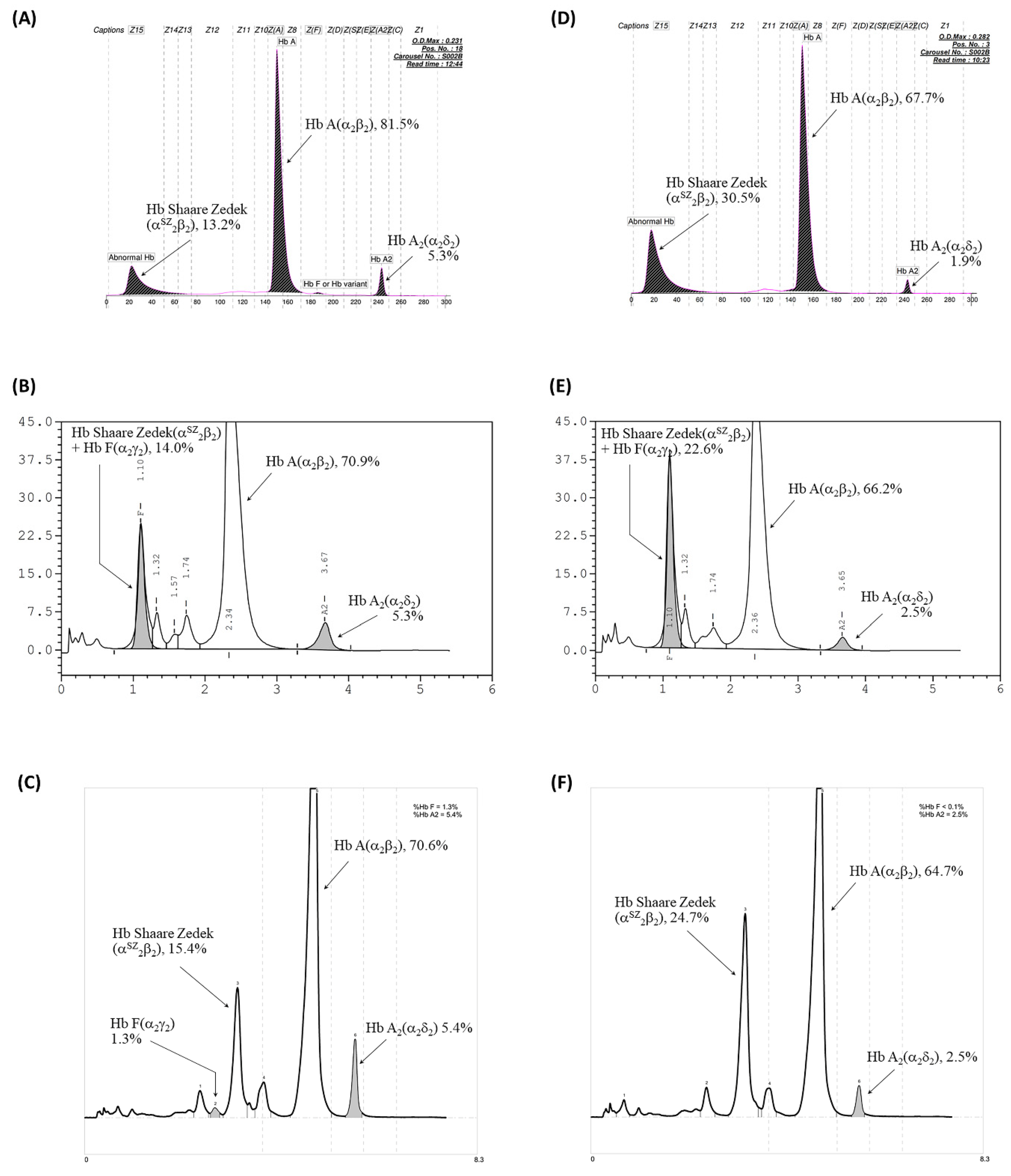
2.1. Hematological and Hb Analyses
2.2. Globin Gene Analysis
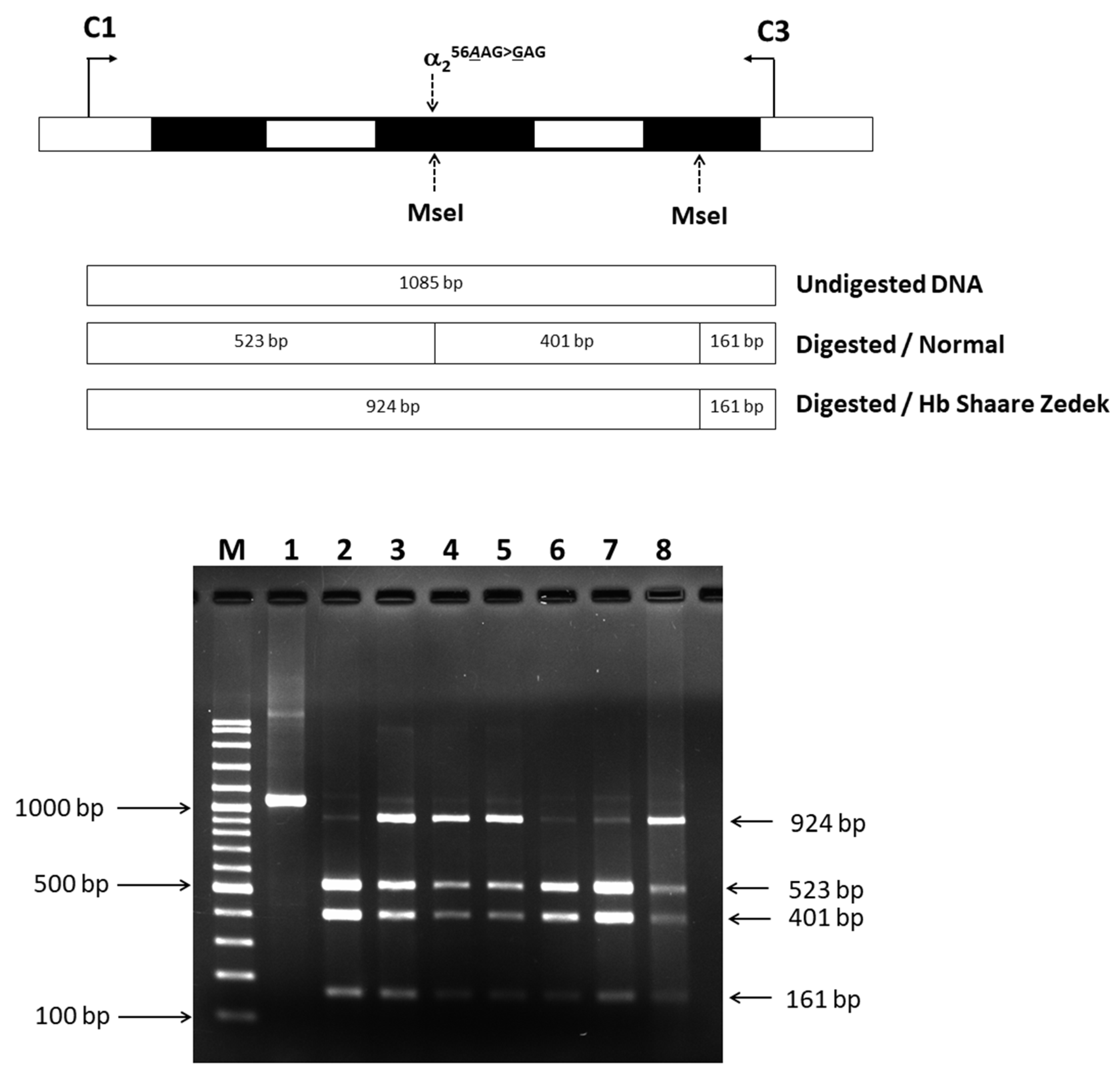
2.3. Confirmation of Mutated Hb SZ Variant by PCR-Restriction Fragment Length Polymorphism (RFLP) Analysis
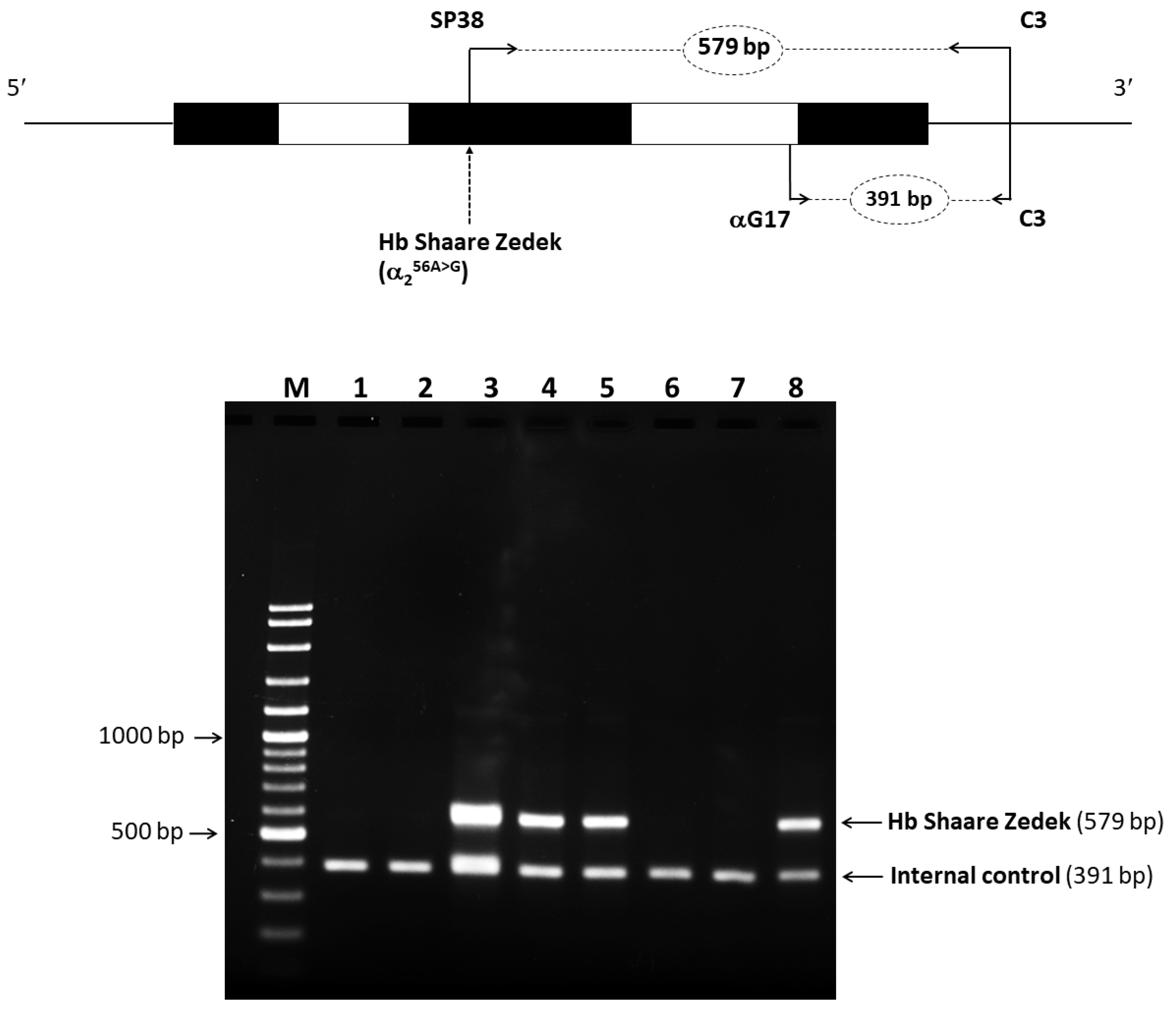
2.4. Identification of Mutated Hb Variant via Allele-Specific PCR
2.5. In Silico Analysis of Pathogenicity and Structure
2.6. α-Globin Gene Haplotype Analysis
3. Discussion
4. Materials and Methods
4.1. Study Participants and Hematological Analysis
4.2. Molecular Characterization
4.3. Identification of Hb SZ by PCR-RFLP
4.4. Allele-Specific PCR for Rapid Identification of Hb SZ
4.5. Analysis of α-Globin Gene Haplotypes
4.6. In Silico Modeling of Hb SZ
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weatherall, D.J.; Clegg, J.B. Inherited haemoglobin disorders: An increasing global health problem. Bull. World Health Organ. 2001, 79, 704–712. [Google Scholar] [PubMed] [PubMed Central]
- Weatherall, D.J. The inherited diseases of hemoglobin are an emerging global health burden. Blood 2010, 115, 4331–4336. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giardine, B.; Borg, J.; Viennas, E.; Pavlidis, C.; Moradkhani, K.; Joly, P.; Bartsakoulia, M.; Riemer, C.; Miller, W.; Tzimas, G.; et al. Updates of the HbVar database of human hemoglobin variants and thalassemia mutations. Nucleic Acids Res. 2014, 42, D1063–D1069. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kountouris, P.; Lederer, C.W.; Fanis, P.; Feleki, X.; Old, J.; Kleanthous, M. IthaGenes: An interactive database for haemoglobin variations and epidemiology. PLoS ONE 2014, 9, e103020. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Thom, C.S.; Dickson, C.F.; Gell, D.A.; Weiss, M.J. Hemoglobin variants: Biochemical properties and clinical correlates. Cold Spring Harb. Perspect. Med. 2013, 3, a011858. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Srivorakun, H.; Singha, K.; Fucharoen, G.; Sanchaisuriya, K.; Fucharoen, S. A large cohort of hemoglobin variants in Thailand: Molecular epidemiological study and diagnostic consideration. PLoS ONE 2014, 9, e108365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Panyasai, S.; Fucharoen, G.; Fucharoen, S. Hemoglobin Variants in Northern Thailand: Prevalence, Heterogeneity and Molecular Characteristics. Genet. Test. Mol. Biomark. 2016, 20, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Tepakhan, W.; Kanjanaopas, S.; Sreworadechpisal, K.; Penglong, T.; Sripornsawan, P.; Wangchauy, C.; Nokkong, C.; Kongkan, C.; Buathong, S. Molecular epidemiology and hematological profiles of hemoglobin variants in southern Thailand. Sci. Rep. 2024, 14, 9255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fucharoen, S.; Winichagoon, P. Hemoglobinopathies in Southeast Asia. Hemoglobin 1987, 11, 65–88, Erratum in: Hemoglobin 1988, 12, 99. [Google Scholar] [CrossRef] [PubMed]
- Abramov, A.; Lehmann, H.; Robb, L. Hb Shaare Zedek (alpha 56 E5 Lys leads to Glu). FEBS Lett. 1980, 113, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Eliakim, R.; Rachmilewitz, E.A. Hemoglobinopathies in Israel. Hemoglobin 1983, 7, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tian, M.; Qin, T.; Wan, L. Capillary Electrophoresis Resolves Inconclusive HPLC Analysis for Hemoglobin Variants: A Study of Two Cases. Clin. Lab. 2018, 64, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Sack, J.S.; Andrews, L.C.; Magnus, K.A.; Hanson, J.C.; Rubin, J.; Love, W.E. Location of amino acid residues in human deoxy hemoglobin. Hemoglobin 1978, 2, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Singha, K.; Taweenan, W.; Fucharoen, G.; Fucharoen, S. Erythrocyte indices in a large cohort of β-thalassemia carrier: Implication for population screening in an area with high prevalence and heterogeneity of thalassemia. Int. J. Lab. Hematol. 2019, 41, 513–518. [Google Scholar] [CrossRef]
- Pootrakul, S.; Boonyarat, D.; Kematorn, B.; Suanpan, S.; Wasi, P. Hemoglobin Thailand [alpha 56 (E 5) Lys leads to Thr]: A new abnormal human hemoglobin. Hemoglobin 1977, 1, 781–798. [Google Scholar] [CrossRef] [PubMed]
- Zwerdling, T.; Williams, S.; Nasr, S.A.; Rucknagel, D.L. Hb Port Huron [alpha 56 (E5)Lys----ARG]: A new alpha chain variant. Hemoglobin 1991, 15, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Wajcman, H.; Gombaud-Saintonge, G.; Galacteros, F.; Martha, M.; Vertongen, F. Hb Belliard [alpha 56(E5)Lys----Asn] a new fast-moving alpha chain variant found in a subject of Spanish origin. Hemoglobin 1989, 13, 157–162. [Google Scholar] [CrossRef]
- Borbely, N.; Phelan, L.; Szydlo, R.; Bain, B. Capillary zone electrophoresis for haemoglobinopathy diagnosis. J. Clin. Pathol. 2013, 66, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Fujita, S.; Ohta, Y.; Kobayashi, Y. Hemoglobin I (alpha 16(A14) Lys replaced by Glu) and hemoglobin J Iran (beta 77(EF1) His replaced by Asp) discovered in Japanese. Hemoglobin 1982, 6, 537–541. [Google Scholar] [CrossRef]
- Molchanova, T.P.; Pobedimskaya, D.D.; Huisman, T.H. The differences in quantities of alpha 2- and alpha 1-globin gene variants in heterozygotes. Br. J. Haematol. 1994, 88, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Mosca, A.; Paleari, R.; Ivaldi, G.; Galanello, R.; Giordano, P.C. The role of haemoglobin A(2) testing in the diagnosis of thalassaemias and related haemoglobinopathies. J. Clin. Pathol. 2009, 62, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Khongthai, K.; Ruengdit, C.; Panyasai, S.; Pornprasert, S. Analysis of Deletional Hb H Diseases in Samples with Hb A2-Hb H and Hb A2-Hb Bart's on Capillary Electrophoresis. Hemoglobin 2019, 43, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Laosombat, V.; Viprakasit, V.; Chotsampancharoen, T.; Wongchanchailert, M.; Khodchawan, S.; Chinchang, W.; Sattayasevana, B. Clinical features and molecular analysis in Thai patients with Hb H disease. Ann. Hematol. 2009, 88, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Bain, B.J.; Bates, I.; Laffan, M.A.; Lewis, S.M. Dacie and Lewis Practical Haematology: Expert Consult, 11th ed.; Elsevier: Amsterdam, The Netherlands, 2011; p. 329. [Google Scholar]
- Dodé, C.; Rochette, J.; Krishnamoorthy, R. Locus assignment of human alpha globin mutations by selective amplification and direct sequencing. Br. J. Haematol. 1990, 76, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Sae-ung, N.; Fucharoen, G.; Sanchaisuriya, K.; Fucharoen, S. Alpha 0-Thalassemia and related disorder in Northeast Thailand: A molecular and hematological characterization. Acta Haematol. 2007, 117, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Fucharoen, S.; Fucharoen, G.; Sanchaisuriya, K.; Pengjam, Y. Molecular analysis of a thai beta-thalassaemia heterozygote with normal haemoglobin A2 level: Implication for population screening. Ann. Clin. Biochem. 2002, 39, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Fucharoen, S.; Sanchaisuriya, K.; Fucharoen, G.; Panyasai, S.; Devenish, R.; Luy, L. Interaction of hemoglobin E and several forms of alpha-thalassemia in Cambodian families. Haematologica 2003, 88, 1092–1098. [Google Scholar]
- Miles, K.L.; Norwich, J.T.; Martinson, J.J.; Clegg, J.B. Polymerase chain reaction protocols for alpha globin haplotype polymorphisms. Br. J. Haematol. 2001, 113, 694–698. [Google Scholar] [CrossRef]



| Parameters | Grandfather | Grandmother | Paternal Aunt-1 | Paternal Aunt-2 | Father | Mother | Proband |
|---|---|---|---|---|---|---|---|
| Age (year) | 65 | 59 | 34 | 31 | 36 | 37 | 5 |
| RBC (×1012/L) | 4.77 | 4.60 | 4.14 | 4.71 | 5.52 | 5.59 | 5.75 |
| Hb (g/dL) | 14.4 | 13.6 | 11.5 | 13.7 | 15.4 | 11.9 | 10.3 |
| Hct (%) | 44.3 | 43.2 | 36.3 | 42.5 | 48.3 | 34.7 | 31.2 |
| MCV (fL) | 92.8 | 94.1 | 87.5 | 90.2 | 87.5 | 62.1 | 54.3 |
| MCH (pg) | 30.3 | 29.6 | 27.8 | 29.1 | 27.9 | 21.3 | 17.9 |
| MCHC (g/dL) | 32.6 | 31.5 | 31.7 | 32.3 | 31.9 | 34.3 | 33 |
| RDW-CV (%) | 13.4 | 12.7 | 13.0 | 13.5 | 14.0 | 16.6 | 18.9 |
| CE-Hb profile a | A2AH | A2A | A2AH | A2A | A2AH | A2A | A2AH |
| Hb A (%) | 67.5 | 67.5 | 66.5 | 97.1 | 67.6 | 93.8 | 81.5 |
| Hb A2 (%) | 1.9 | 2.9 | 1.8 | 2.9 | 1.9 | 5.8 | 5.3 |
| Hb F (%) | 0 | 0 | 0 | 0 | 0 | 0.4 | 0 |
| Hb SZ (%) | 30.6 | 0 | 31.7 | 0 | 30.5 | 0 | 13.2 |
| Premier HPLC-Hb Profile b | A2A with Hb SZ | A2A | A2A with Hb SZ | A2A | A2A with Hb SZ | A2A | A2A with Hb SZ |
| Hb A (%) | 66.2 | 86.9 | 66.7 | 88.6 | 64.7 | 86.8 | 70.6 |
| Hb A2 (%) | 2.3 | 3.2 | 2.3 | 3.2 | 2.5 | 5.9 | 5.4 |
| Hb F (%) | 0 | 0 | 0 | 0 | 0 | 1.1 | 1.3 |
| Hb SZ (%) | 23.5 | 0 | 25.0 | 0 | 24.7 | 0 | 15.4 |
| VARIANT II HPLC-Hb Profile c | A2FA | ND | A2FA | ND | A2FA | ND | A2FA |
| Hb A (%) | 64.4 | ND | 64.4 | ND | 66.2 | ND | 70.9 |
| Hb A2 (%) | 2.3 | ND | 2.3 | ND | 2.5 | ND | 5.3 |
| Hb F + Hb SZ | 18.3 | ND | 19.5 | ND | 22.6 | ND | 14.0 |
| α-globin genotype | α56A>Gα/αα | αα/αα | α56A>Gα/αα | αα/αα | α56A>Gα/αα | αα/αα | α56A>Gα/αα |
| β-globin genotype | βA/βA | βA/βA | βA/βA | βA/βA | βA/βA | βA/β0 | βA/β0 |
| α-Globin Haplotype (5′>3′) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subjects | α-Genotype | 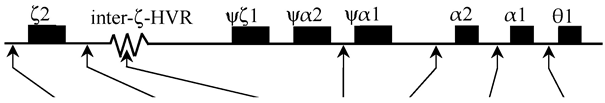 | Hb SZ (α SZ) Linked α-Haplotype | |||||||||||||
| Xba I | Sac I | [S/M/L] | Acc I | Rsa I | αPst I | θPst I | ||||||||||
| Grandfather | αα SZ/αα | [+/−] | [+/−] | [M/M] | [+/−] | [−/−] | [+/−] | [−/−] | αα SZ | [- | - | M | - | - | - | -] |
| αα | [+ | + | M | + | - | + | -] | |||||||||
| Grandmother | αα/αα | [+/−] | [+/−] | [L/S] | [+/+] | [+/−] | [−/−] | [+/−] | αα | [+ | - | S | + | + | - | +] |
| αα | [- | + | L | + | - | - | -] | |||||||||
| Paternal Aunt-1 | αα SZ/αα | [+/−] | [−/−] | [M/S] | [+/−] | [+/−] | [−/−] | [+/−] | αα SZ | [- | - | M | - | - | - | -] |
| αα | [+ | - | S | + | + | - | +] | |||||||||
| Paternal Aunt-2 | αα/αα | [+/−] | [+/+] | [L/M] | [+/+] | [−/−] | [+/−] | [−/−] | αα | [- | + | L | + | - | - | -] |
| αα | [+ | + | M | + | - | + | -] | |||||||||
| Father | αα SZ/αα | [−/−] | [+/−] | [L/M] | [+/−] | [−/−] | [−/−] | [−/−] | αα SZ | [- | - | M | - | - | - | -] |
| αα | [- | + | L | + | - | - | -] | |||||||||
| Mother | αα/αα | [+/−] | [+/−] | [L/S] | [+/+] | [−/−] | [+/−] | [+/−] | αα | [+ | - | S | + | - | + | +] |
| αα | [- | + | L | + | - | - | -] | |||||||||
| Proband | αα SZ/αα | [+/−] | [−/−] | [M/S] | [+/−] | [−/−] | [+/−] | [+/−] | αα SZ | [- | - | M | - | - | - | -] |
| αα | [+ | - | S | + | - | + | +] | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satthakarn, S.; Srisuwan, W.; Kunyanone, N.; Panyasai, S. Novel Insights into Hb Shaare Zedek Associated with β0-Thalassemia: Molecular Characteristics, Genetic Origin and Diagnostic Approaches. Int. J. Mol. Sci. 2024, 25, 8578. https://doi.org/10.3390/ijms25168578
Satthakarn S, Srisuwan W, Kunyanone N, Panyasai S. Novel Insights into Hb Shaare Zedek Associated with β0-Thalassemia: Molecular Characteristics, Genetic Origin and Diagnostic Approaches. International Journal of Molecular Sciences. 2024; 25(16):8578. https://doi.org/10.3390/ijms25168578
Chicago/Turabian StyleSatthakarn, Surada, Wibhasiri Srisuwan, Naowarat Kunyanone, and Sitthichai Panyasai. 2024. "Novel Insights into Hb Shaare Zedek Associated with β0-Thalassemia: Molecular Characteristics, Genetic Origin and Diagnostic Approaches" International Journal of Molecular Sciences 25, no. 16: 8578. https://doi.org/10.3390/ijms25168578
APA StyleSatthakarn, S., Srisuwan, W., Kunyanone, N., & Panyasai, S. (2024). Novel Insights into Hb Shaare Zedek Associated with β0-Thalassemia: Molecular Characteristics, Genetic Origin and Diagnostic Approaches. International Journal of Molecular Sciences, 25(16), 8578. https://doi.org/10.3390/ijms25168578





