Antioxidant Activity and Preclinical Safety of Semen persicae Extract
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Characterization
2.2. DPPH Free Radical Scavenging Activity
2.3. Hydroxyl Radical Scavenging Activity
2.4. ABTS+ Scavenging Activity
2.5. Fe2+-Chelating Assay
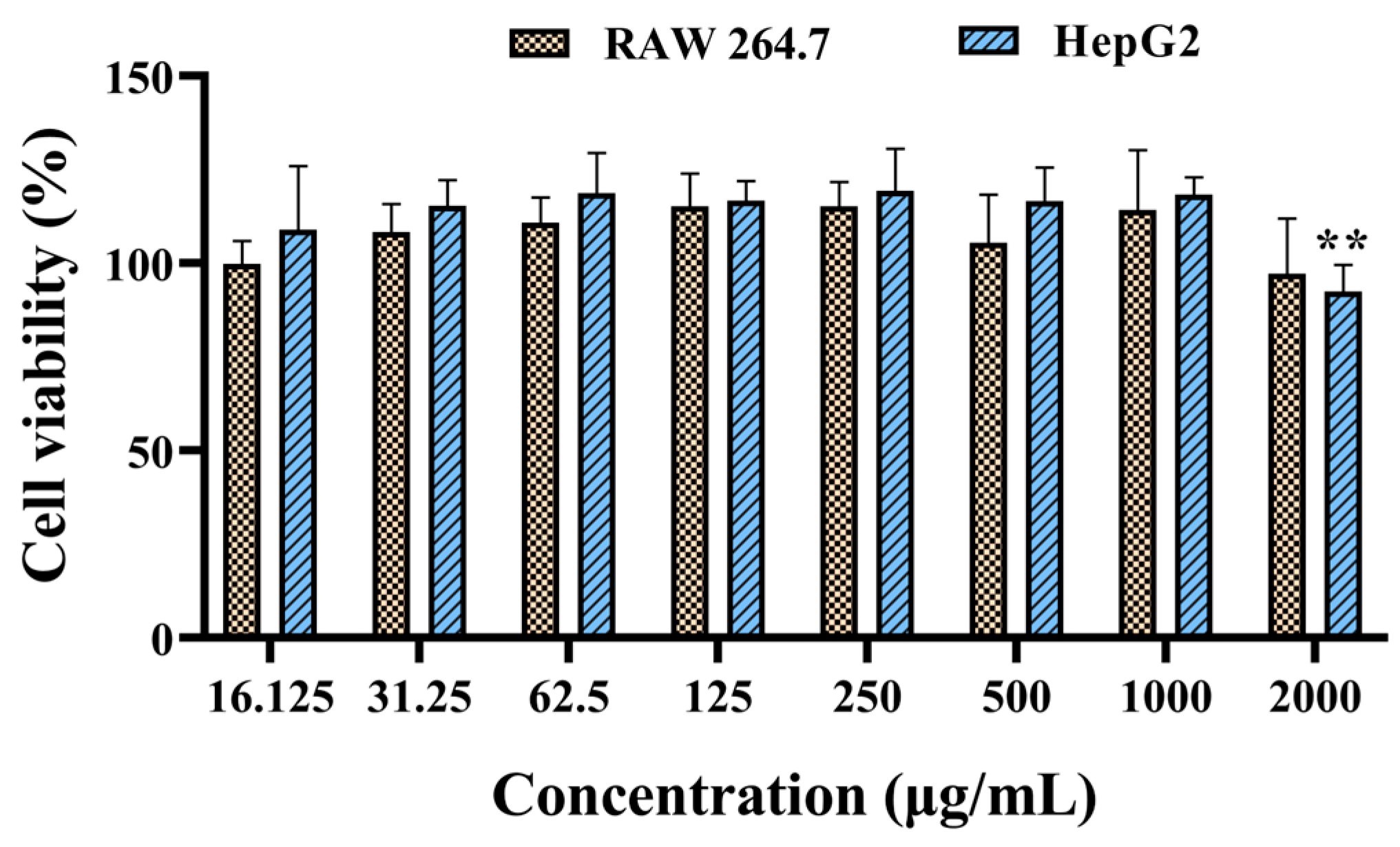
2.6. Cytotoxicity Evaluation
2.7. Acute Oral Toxicity
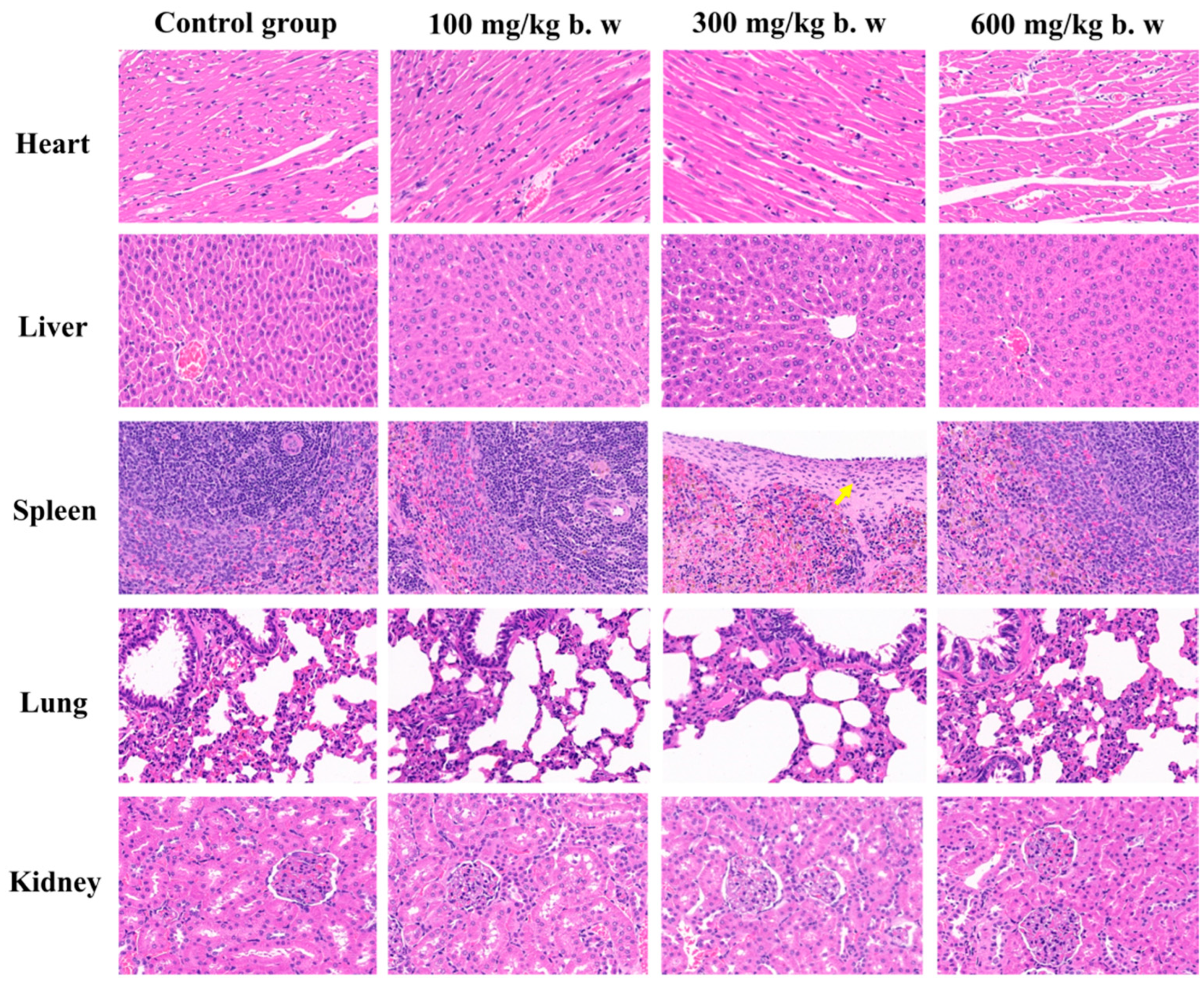
2.8. Subchronic Toxicity Study
3. Materials and Methods
3.1. Material and Reagents
3.2. Ethanol Extraction from Semen Persicae
3.3. Determination of Amygdalin in SPE
3.4. Animals
3.5. DPPH Free Radical Scavenging Assay
3.6. Hydroxyl Radical Scavenging Assay
3.7. ABTS Radical Cation Decolorization Assay
3.8. Fe2+ Chelating Activity
3.9. Cytotoxicity Assay
3.10. Acute Oral Toxicity Study
3.11. Repeated Dose 28-Day Oral Toxicity
3.12. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rauf, A.; Khalil, A.A.; Awadallah, S.; Khan, S.A.; Abu-Izneid, T.; Kamran, M.; Hemeg, H.A.; Mubarak, M.S.; Khalid, A.; Wilairatana, P. Reactive oxygen species in biological systems: Pathways, associated diseases, and potential inhibitors—A review. Food Sci. Nutr. 2024, 12, 675–3693. [Google Scholar] [CrossRef] [PubMed]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef]
- Weng, M.; Xie, X.; Liu, C.; Lim, K.L.; Zhang, C.W.; Li, L. The sources of reactive oxygen species and its possible role in the pathogenesis of Parkinson’s disease. Park. Dis. US 2018, 2018, 9163040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lv, G.; He, W.; Shi, L.; Pan, H.; Fan, L. Effects of extraction methods on the antioxidant activities of polysaccharides obtained from Flammulina velutipes. Carbohyd. Polym. 2013, 98, 1524–1531. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, B.; Sen, D.J.; Mahanti, B.; Nayak, A.K. Antioxidant potential of herbal polysaccharides: An overview on recent researches. Sens. Int. 2022, 3, 100158. [Google Scholar] [CrossRef]
- Mohanta, B.; Sen, D.J.; Mahanti, B.; Nayak, A.K. Extraction, characterization, haematocompatibility and antioxidant activity of linseed polysaccharide. Carbohydr. Polym. Tech. 2023, 5, 100321. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.H.; Chang, H.; Si, N. Network pharmacology and bioinformatics study on the treatment of renal fibrosis with persicae semen-carthami flos drug pair. Medicine 2023, 102, e32946. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.Y.; Liu, L.; Tao, W.W.; Duan, J.A.; Liu, X.H.; Huang, S.P. Antithrombotic lipids from Semen Persicae. Nat. Prod. Res. 2011, 25, 1650–11656. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.M. Research progress on chemical components and pharmacological effects of Semen persicae. J. Snake 2007, 19, 130–132. [Google Scholar]
- Wang, N.; Dai, L.; Chen, Z.; Li, T.; Wu, J.; Wu, H.; Wu, H.; Xiang, W. Extraction optimization, physicochemical characterization, and antioxidant activity of polysaccharides from Rhodosorus sp. SCSIO-45730. J. Appl. Phycol. 2022, 34, 285–299. [Google Scholar] [CrossRef]
- Jun, J.Y.; Kim, J.H.; Kim, M.; Hong, S.Y.; Kim, M.; Ryu, G.H.; Park, J.H.; Jung, H.S.; Sohn, Y. Persicae semen promotes bone union in rat fractures by stimulating osteoblastogenesis through BMP-2 and Wnt signaling. Int. J. Mol. Sci. 2023, 24, 7388. [Google Scholar] [CrossRef]
- Rong, J.; Tilton, R.; Shen, J.; Ng, K.M.; Liu, C.; Tam, P.K.; Lau, A.S.; Cheng, Y.C. Genome-wide biological response fingerprinting (BioReF) of the Chinese botanical formulation ISF-1 enables the selection of multiple marker genes as a potential metric for quality control. J. Ethnopharmacol. 2007, 113, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Duan, J.A.; Tang, Y.; Guo, J.M.; Yang, N.Y.; Ma, H.Y.; Shi, X.Q. Taoren-Honghua herb pair and its main components promoting blood circulation through influencing on hemorheology, plasma coagulation and platelet aggregation. J. Ethnopharmacol. 2012, 139, 381–387. [Google Scholar] [CrossRef]
- Fukuda, T.; Ito, H.; Mukainaka, T.; Tokuda, H.; Nishino, H.; Yoshida, T. Anti-tumor promoting effect of glycosides from Prunus persica seeds. Biol. Pharm. Bull. 2003, 26, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R. Amygdalin content of seeds, kernels and food products commercially-available in the UK. Food Chem. 2014, 152, 133–139. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Liu, J.; Gong, Y.; Lu, W.; Sha, X.; Cao, C.; Li, Y.; Wang, J. Amygdalin ameliorates alopecia areata on C3H/HeJ mice by inhibiting inflammation through JAK2/STAT3 pathway. J. Ethnopharmacol. 2024, 331, 118317. [Google Scholar] [CrossRef]
- Yang, H.Y.; Chang, H.K.; Lee, J.W.; Kim, Y.S.; Kim, H.; Lee, M.H.; Shin, M.S.; Ham, D.H.; Park, H.K.; Lee, H. Amygdalin suppresses lipopolysaccharide-induced expressions of cyclooxygenase-2 and inducible nitric oxide synthase in mouse BV2 microglial cells. Neurol. Res. 2007, 29, S59–S64. [Google Scholar] [CrossRef]
- Tanaka, R.; Nitta, A.; Nagatsu, A. Application of a quantitative 1H-NMR method for the determination of amygdalin in Persicae semen, Armeniacae semen, and Mume fructus. J. Nat. Med. 2014, 68, 225–230. [Google Scholar] [CrossRef]
- Markowitsch, S.D.; Binali, S.; Rutz, J.; Chun, F.K.; Haferkamp, A.; Tsaur, I.; Juengel, E.; Fischer, N.D.; Thomas, A.; Blaheta, R.A. Survey of physicians and healers using amygdalin to treat cancer patients. Nutrients 2024, 16, 2068. [Google Scholar] [CrossRef]
- Liu, C.; Li, X.; Yang, H.; Mao, X.; Wang, J.; Gao, W. Effect of natural β-glucosidase inhibitors in reducing toxicity of amygdalin in Persicae semen. Phytother. Res. 2017, 31, 771–777. [Google Scholar] [CrossRef]
- Xi, S.; Qian, L.; Tong, H.; Yue, L.; Zhao, H.; Wang, D.; Lu, D.; Li, P.; Wang, X. Toxicity and clinical reasonable application of Taoren (Semen persicae) based on ancient and modern literature research. J. Tradit. Chin. Med. 2013, 33, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.M. Chinese Materia Medica, 2nd ed.; China Press of Traditional Chinese Medicine: Beijing, China, 2007; pp. 324–325. [Google Scholar]
- Song, Z.; Xu, X. Advanced research on anti-tumor effects of amygdalin. J. Cancer Res. Ther. 2014, 10, 3–7. [Google Scholar]
- Jaszczak-Wilke, E.; Polkowska, Z.; Koprowski, M.; Owsianik, K.; Mitchell, A.E.; Balczewski, P. Amygdalin: Toxicity, anticancer activity and analytical procedures for its determination in plant seeds. Molecules 2021, 26, 2253. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Seo, B.I.; Cho, S.Y.; Han, C.K.; Song, C.H.; Park, S.J.; Ku, S.K. Single oral dose toxicity study of prebrewed armeniacae semen in rats. Toxicol. Res. 2013, 29, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xu, X.; Yuan, S.; Liu, H.; Liu, M.; Zhang, Y.; Zhang, H.; Gao, Y.; Lin, R.; Li, X. Identification and analysis of amygdalin, neoamygdalin and amygdalin amide in different processed bitter almonds by HPLC-ESI-MS/MS and HPLC-DAD. Molecules 2017, 22, 1425. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Venkatesan, M.; Arumugam, V.; Pugalendi, R.; Ramachandran, K.; Sengodan, K.; Vijayan, S.R.; Sundaresan, U.; Ramachandran, S.; Pugazhendhi, A. Antioxidant, anticoagulant and mosquitocidal properties of water soluble polysaccharides (WSPs) from Indian seaweeds. Process Biochem. 2019, 84, 196–204. [Google Scholar] [CrossRef]
- Chen, Y.X.; Liu, X.Y.; Xiao, Z.; Huang, Y.F.; Liu, B. Antioxidant activities of polysaccharides obtained from Chlorella pyrenoidosa via different ethanol concentrations. Int. J. Biol. Macromol. 2016, 91, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Merillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Dinis, T.C.P.; Maseira, V.M.C.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, X.J.; Xie, Z.Y.; Cao, T.Y.; Jiang, S.F.; Huang, L.N. Lipoxin A4 methyl ester attenuated ketamine-induced neurotoxicity in SH-SY5Y cells via regulating leptin pathway. Toxicol. Vitr. 2023, 89, 105581. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Huang, Y.; Hu, W.; Li, R.; Wang, J.; Han, M.; Li, Z. Evaluation of the antibacterial, anti-inflammatory, and bone-promoting capacity of UiO-66 loaded with thymol or carvacrol. ACS Appl. Mater. Interfaces 2024, 16, 36017−36029. [Google Scholar] [CrossRef] [PubMed]
- OECD. Guideline for the Testing of Chemicals. Acute Oral Toxicity Acute Toxic Class Method: Test No-423; OECD: Paris, France, 2001. [Google Scholar]
- OECD. Test No. 407: Repeated Dose 28-Day Oral Toxicity Study in Rodents, OECD Guidelines for the Testing of Chemicals, Section 4; OECD: Paris, France, 2008. [Google Scholar]
- IBM SPSS. Statistics for Windows, Version 24.0; IBM: Armonk, NY, USA, 2012. [Google Scholar]
- Jing, Y.; Hu, J.; Su, Z.; Cheng, W.; Zhang, Y.; Yang, X.; Zhang, D.; Wu, L. Structural characterisation and antioxidant activities in vitro and in vivo of a novel polysaccharide from Salvia miltiorrhiza. Nat. Prod. Res. 2023, 37, 1006–1011. [Google Scholar] [CrossRef] [PubMed]
- Lupina, K.; Kowalczyk, D.; Lis, M.; Basiura-Cembala, M. Antioxidant polysaccharide/gelatin blend films loaded with curcumin-A comparative study. Int. J. Biol. Macromol. 2023, 236, 123945. [Google Scholar] [CrossRef]
- Ge, B.C.; Wang, W.; Gao, Y.R.; Chen, X.J. Optimization of extraction of lycopene from carrot and determination of its antioxidant activity. J. Food Meas. Charact. 2018, 17, 5497–5505. [Google Scholar] [CrossRef]
- Shao, Y.; Chen, H.; Lin, H.; Feng, H.; Gong, J.; Cao, G.; Hong, W.; Yao, Y.; Zou, H.; Yan, Y. Exploration on varying patterns of morphological features and quality of armeniacae semen amarum in rancid process based on colorimeter, electronic nose, and GC/MS coupled with human panel. Front. Pharmacol. 2022, 13, 599979. [Google Scholar] [CrossRef]
- Ahmad, M.M. Recent trends in chemical modification and antioxidant activities of plants-based polysaccharides: A review. Carbohydr. Polym. Tech. Appl. 2021, 2, 100045. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, Q.; Zhang, Y.; Wei, J.; Liu, P. Taoren Honghua drug attenuates atherosclerosis and plays an anti-inflammatory role in ApoE knock-out mice and RAW264.7 cells. Front. Pharmacol. 2020, 11, 1070. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; He, M.; Gu, C.; Wei, D.; Liang, Y.; Yan, J.; Wang, C. Extraction optimization, purification, antioxidant activity, and preliminary structural characterization of crude polysaccharide from an Arctic Chlorella. Polymers 2018, 10, 292. [Google Scholar] [CrossRef]
- Li, H.B.; Wong, C.C.; Cheng, K.W.; Chen, F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT 2008, 41, 385–390. [Google Scholar] [CrossRef]
- Barakat, H.; Ljutaily, T.; Almujaydil, M.S.; Algheshairy, R.M.; Alhomaid, R.M.; Almutairi, A.S.; Alshimali, S.I.; Abdellatif, A.A.H. Amygdalin: A review on its characteristics, antioxidant potential, gastrointestinal microbiota intervention, anticancer therapeutic and mechanisms, toxicity, and encapsulation. Biomolecules 2022, 12, 1514. [Google Scholar] [CrossRef]
- Lehmane, H.; Kohonou, A.N.; Tchogou, A.P.; Ba, R.; Dah-Nouvlessounon, D.; Didagbe, O.; Sina, H.; Senou, M.; Adjanohoun, A.; Baba-Moussa, L. Antioxidant, anti-Inflammatory, and anti-cancer properties of amygdalin extracted from three cassava varieties cultivated in Benin. Molecules 2023, 28, 4548. [Google Scholar] [CrossRef]
- Nampoothiri, S.V.; Prathapan, A.; Cherian, O.L.; Raghu, K.G.; Venugopalan, V.V.; Sundaresan, A. In vitro antioxidant and inhibitory potential of Terminalia bellerica and Emblica officinalis fruits against LDL oxidation and key enzymes linked to type 2 diabetes. Food Chem. Toxicol. 2011, 49, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Go, M.R.; Kim, H.J.; Yu, J.; Choi, S.J. Toxicity and toxicokinetics of amygdalin in Maesil (Prunus mume) Syrup: Protective effect of Maesil against amygdalin toxicity. J. Agric. Food Chem. 2018, 66, 11432–11440. [Google Scholar] [CrossRef]
- Porwal, M.; Khan, N.A.; Maheshwari, K.K. Evaluation of acute and subacute oral toxicity induced by ethanolic extract of Marsdenia tenacissima leaves in experimental rats. Sci. Pharm. 2017, 85, 29. [Google Scholar] [CrossRef]
- Afolayan, A.J.; Wintola, O.A.; Fouche, G. Acute and subacute toxicological evaluation of the aerial extract of Monsonia angustifolia E. Mey. Ex. A. rich in Wistar rats. Evid. Based Complement. Altern. Med. 2016, 2016, 4952485. [Google Scholar] [CrossRef] [PubMed]
- Cossetin, J.F.; de Almeida, A.S.; Antoniazzi, C.T.D.; Kudsi, S.Q.; Engelmann, A.M.; Guex, C.G.; Oliveira, J.S.; Theisen, M.C.; Ritter, C.S.; Doleski, P.H.; et al. Hydroalcoholic extract of leaf of Arachis hypogaea L. (Fabaceae) did not induce toxic effects in the repeated-dose toxicity study in rats. Regul. Toxicol. Pharmacol. 2020, 115, 104683. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.Z.; Xu, H.D.; Kim, K.H.; Ahn, T.H.; Bae, J.S.; Lee, J.Y.; Gil, K.H.; Lee, J.Y.; Woo, S.J.; Yoo, H.J.; et al. Reference data of the main physiological parameters in control sprague-dawley rats from pre-clinical toxicity studies. Lab. Anim. Res. 2010, 26, 153–164. [Google Scholar] [CrossRef]
- Loha, M.; Mulu, A.; Abay, S.M.; Ergete, W.; Geleta, B. Acute and subacute toxicity of methanol extract of Syzygium guineense leaves on the histology of the liver and kidney and biochemical compositions of blood in rats. Evid. Based Complement. Altern. Med. 2019, 2019, e5702159. [Google Scholar] [CrossRef]
- Shakya, A.; Chaudhary, S.K.; Bhat, H.R.; Ghosh, S.K. Acute and sub-chronic toxicity studies of Benincasa hispida (Thunb.) cogniaux fruit extract in rodents. Regul. Toxicol. Pharmacol. 2020, 118, 104785. [Google Scholar] [CrossRef]
- Petterinoa, C.; Argentino-Storino, A. Clinical chemistry and haematology historical data in control Sprague-Dawley rats from pre-clinical toxicity studies. Exp. Toxicol. Pathol. 2006, 57, 213–219. [Google Scholar] [CrossRef] [PubMed]
- He, X.Y.; Wu, L.J.; Wang, W.X.; Xie, P.J.; Chen, Y.H.; Wang, F. Amygdalin—A pharmacological and toxicological review. J. Ethnopharmacol. 2020, 254, 112717. [Google Scholar] [CrossRef] [PubMed]
- Qadir, M.; Fatima, K. Review on Pharmacological activity of Amygdalin. Arch. Cancer Res. 2017, 5, 160. [Google Scholar] [CrossRef]


| Parameters | Control Group | First Round Treatment | Second Round Treatment | p1 a | p2 b |
|---|---|---|---|---|---|
| Body weights (g) | |||||
| 0 day | 193.67 ± 2.45 | 191.40 ± 1.28 | 191.33 ± 2.42 | - | - |
| 14 days | 218.93 ± 2.40 | 216.47 ± 3.16 | 215.43 ± 4.45 | - | - |
| Absolute organ weight (g) | |||||
| Liver | 9.0 ± 0.22 | 8.04 ± 0.53 | 8.82 ± 0.91 | 0.205 | 0.914 |
| Kidneys | 1.72 ± 0.11 | 1.63 ± 0.18 | 1.60 ± 0.13 | 0.744 | 0.581 |
| Spleen | 0.57 ± 0.04 | 0.53 ± 0.02 | 0.53 ± 0.05 | 0.395 | 0.558 |
| Heart | 0.76 ± 0.08 | 0.74 ± 0.05 | 0.82 ± 0.09 | 0.959 | 0.580 |
| Lungs | 1.27 ± 0.16 | 1.34 ± 0.10 | 1.62 ± 0.48 | 0.951 | 0.372 |
| Uterus + ovaries | 0.88 ± 0.06 | 0.76 ± 0.04 | 1.00 ± 0.20 | 0.509 | 0.508 |
| Relative organ weight (%) | |||||
| Liver | 4.12 ± 0.07 | 3.71 ± 0.20 | 4.09 ± 0.34 | 0.152 | 0.983 |
| Kidneys | 0.78 ± 0.04 | 0.75 ± 0.07 | 0.74 ± 0.05 | 0.785 | 0.617 |
| Spleen | 0.26 ± 0.02 | 0.24 ± 0.01 | 0.25 ± 0.02 | 0.443 | 0.726 |
| Heart | 0.35 ± 0.04 | 0.34 ± 0.03 | 0.38 ± 0.05 | 0.974 | 0.556 |
| Lungs | 0.58 ± 0.02 | 0.62 ± 0.05 | 0.76 ± 0.19 | 0.902 | 0.233 |
| Uterus + ovaries | 0.40 ± 0.03 | 0.35 ± 0.02 | 0.46 ± 0.09 | 0.562 | 0.384 |
| Parameters | Control | 100 mg/kg bw | 300 mg/kg bw | 600 mg/kg bw |
|---|---|---|---|---|
| Female | ||||
| WBC (109/L) | 5.42 ± 0.95 | 5.77 ± 1.46 | 6.03 ± 1.12 | 6.02 ± 1.09 |
| NEU (109/L) | 0.70 ± 0.13 | 0.80 ± 0.17 | 0.78 ± 0.19 | 0.64 ± 0.15 |
| NEP (%) | 12.86 ± 2.22 | 12.90 ± 1.94 | 11.98 ± 2.16 | 11.68 ± 2.34 |
| RBC (1012/L) | 8.26 ± 0.58 | 8.27 ± 0.70 | 7.79 ± 0.61 | 8.07 ± 0.71 |
| HGB (g/dL) | 15.90 ± 1.19 | 15.38 ± 1.34 | 15.68 ± 1.32 | 14.84 ± 0.97 |
| HCT (%) | 49.33 ± 4.54 | 48.19 ± 5.42 | 52.85 ± 3.86 | 50.04 ± 3.60 |
| MCV (fL) | 58.77 ± 3.27 | 60.10 ± 2.81 | 59.20 ± 4.45 | 58.46 ± 4.58 |
| MCH (pg) | 19.06 ± 1.05 | 18.98 ± 1.51 | 19.04 ± 0.87 | 18.68 ± 0.96 |
| MCHC (g/dL) | 31.22 ± 1.71 | 31.12 ± 2.21 | 30.72 ± 2.69 | 31.96 ± 2.36 |
| PLT (109/L) | 669.4 ± 70.6 | 645.1 ± 105.8 | 699.8 ± 121.2 | 724.2 ± 112.8 |
| MPV (fL) | 6.50 ± 0.16 | 6.72 ± 0.17 | 7.04 ± 0.27 * | 7.16 ± 0.30 ** |
| Male | ||||
| WBC (109/L) | 5.86 ± 0.99 | 5.93 ± 0.95 | 5.82 ± 1.77 | 6.44 ± 1.64 |
| NEU (109/L) | 0.71 ± 0.13 | 0.75 ± 0.15 | 0.75 ± 0.13 | 0.70 ± 0.07 |
| NEP (%) | 11.78 ± 2.33 | 12.64 ± 1.83 | 12.92 ± 2.31 | 12.04 ± 2.36 |
| RBC (1012/L) | 8.42 ± 0.66 | 8.16 ± 0.90 | 8.49 ± 0.89 | 8.45 ± 0.76 |
| HGB (g/dL) | 15.92 ± 1.12 | 16.10 ± 1.10 | 16.24 ± 1.25 | 16.02 ± 0.92 |
| HCT (%) | 49.81 ± 6.24 | 51.54 ± 2.15 | 52.00 ± 3.72 | 50.82 ± 2.65 |
| MCV (fL) | 60.10 ± 3.61 | 60.77 ± 2.67 | 60.80 ± 2.48 | 59.77 ± 2.06 |
| MCH (pg) | 19.02 ± 1.14 | 18.92 ± 0.94 | 19.35 ± 1.22 | 19.37 ± 1.32 |
| MCHC (g/dL) | 30.76 ± 1.78 | 31.82 ± 2.32 | 32.04 ± 1.53 | 31.06 ± 1.58 |
| PLT (109/L) | 657.6 ± 85.1 | 593.6 ± 80.2 | 751.8 ± 176.2 | 628.2 ± 84.7 |
| MPV (fL) | 6.66 ± 0.21 | 6.76 ± 0.22 | 6.96 ± 0.34 | 7.26 ± 0.22 * |
| Female | ||||
| ALT (U/L) | 37.42 ± 5.87 | 41.34 ± 8.21 | 36.57 ± 5.85 | 37.80 ± 7.17 |
| AST (U/L) | 132.00 ± 9.31 | 129.08 ± 9.98 | 125.10 ± 13.21 | 133.54 ± 7.41 |
| TC (mmol/L) | 1.75 ± 0.25 | 1.78 ± 0.18 | 1.82 ± 0.20 | 1.73 ± 0.14 |
| Urea (mmol/L) | 9.53 ± 0.72 | 9.74 ± 0. 97 | 9.44 ± 0.53 | 9.92 ± 0.88 |
| Crea (μmol/L) | 41.48 ± 6.61 | 46.68 ± 4.53 | 54.78 ± 7.01 * | 46.80 ± 7.17 |
| ALP (U/L) | 209.30 ± 50.43 | 197.68 ± 56.56 | 185.58 ± 62.74 | 194.90 ± 67.64 |
| ALB (g/L) | 36.96 ± 2.67 | 35.48 ± 2.85 | 36.00 ± 3.97 | 35.80 ± 4.57 |
| Male | ||||
| ALT (U/L) | 43.02 ± 4.88 | 46.24 ± 7.54 | 49.06 ± 6.19 | 47.26 ± 7.31 |
| AST (U/L) | 138.34 ± 8.73 | 135.08 ± 9.48 | 140.80 ± 5.71 | 139.74 ± 9.87 |
| TC (mmol/L) | 1.71 ± 0.12 | 1.80 ± 0.09 | 1.77 ± 0.16 | 1.94 ± 0.14 |
| Urea (mmol/L) | 9.61 ± 0.93 | 9.44 ± 0.47 | 9.57 ± 0.72 | 9.57 ± 0.85 |
| Crea (μmol/L) | 41.48 ± 9.19 | 40.34 ± 7.10 | 45.76 ± 9.01 | 41.48 ± 8.85 |
| ALP (U/L) | 225.06 ± 34.37 | 195.78 ± 47.93 | 213.88 ± 53.80 | 223.66 ± 48.25 |
| ALB (g/L) | 34.32 ± 3.70 | 37.16 ± 3.62 | 34.80 ± 3.17 | 35.42 ± 3.08 |
| Parameters | Control Group | 100 mg/kg bw | 300 mg/kg bw | 600 mg/kg bw |
|---|---|---|---|---|
| Body weight (g) | ||||
| 0 days | 165.18 ± 8.32 | 159.66 ± 11.67 | 158.80 ± 7.45 | 161.81 ± 9.05 |
| 28 days | 208.84 ± 7.24 | 211.68 ± 8.33 | 207.93 ± 7.89 | 205.03 ± 9.56 |
| Absolute organ weight (g) | ||||
| Heart | 0.85 ± 0.12 | 0.83 ± 0.11 | 0.91 ± 0.06 | 0.88 ± 0.08 |
| Liver | 7.75 ± 1.07 | 7.93 ± 0.69 | 8.09 ± 1.01 | 6.96 ± 1.16 |
| Spleen | 0.49 ± 0.07 | 0.48 ± 0.06 | 0.56 ± 0.08 | 0.55 ± 0.11 |
| Lungs | 1.23 ± 0.13 | 1.14 ± 0.13 | 1.21 ± 0.10 | 1.27 ± 0.20 |
| Kidneys | 1.57 ± 0.07 | 1.65 ± 0.06 | 1.61 ± 0.09 | 1.60 ± 0.05 |
| Thymus | 0.50 ± 0.11 | 0.52 ± 0.06 | 0.49 ± 0.04 | 0.47 ± 0.05 |
| Ovaries | 0.13 ± 0.03 | 0.14 ± 0.03 | 0.15 ± 0.03 | 0.15 ± 0.02 |
| Organ-to-body-weight ratio (%) | ||||
| Heart | 0.41 ± 0.06 | 0.40 ± 0.06 | 0.44 ± 0.02 | 0.43 ± 0.02 |
| Liver | 3.71 ± 0.47 | 3.76 ± 0.43 | 3.90 ± 0.57 | 3.38 ± 0.49 |
| Spleen | 0.23 ± 0.03 | 0.23 ± 0.02 | 0.27 ± 0.03 | 0.27 ± 0.06 |
| Lungs | 0.59 ± 0.08 | 0.54 ± 0.06 | 0.58 ± 0.04 | 0.62 ± 0.09 |
| Kidneys | 0.75 ± 0.01 | 0.78 ± 0.01 | 0.77 ± 0.02 | 0.78 ± 0.04 |
| Thymus | 0.24 ± 0.05 | 0.25 ± 0.02 | 0.24 ± 0.01 | 0.23 ± 0.02 |
| Ovaries | 0.06 ± 0.01 | 0.07 ± 0.01 | 0.07 ± 0.01 | 0.08 ± 0.01 |
| Parameters | Control Group | 100 mg/kg bw | 300 mg/kg bw | 600 mg/kg bw |
|---|---|---|---|---|
| Body weight (g) | ||||
| 0 days | 192.04 ± 8.83 | 188.42 ± 13.86 | 193.10 ± 10.35 | 190.35 ± 10.62 |
| 28 days | 257.15 ± 10.82 | 254.43 ± 10.27 | 251.73 ± 7.21 | 249.70 ± 11.66 |
| Absolute organ weight (g) | ||||
| Heart | 0.94 ± 0.10 | 0.92 ± 0.12 | 0.97 ± 0.14 | 0.98 ± 0.10 |
| Liver | 9.24 ± 1.56 | 9.45 ± 1.44 | 9.19 ± 1.32 | 9.33 ± 1.17 |
| Spleen | 0.58 ± 0.07 | 0.62 ± 0.07 | 0.65 ± 0.09 | 0.62 ± 0.06 |
| Lung | 1.50 ± 0.09 | 1.33 ± 0.16 | 1.32 ± 0.08 * | 1.34 ± 0.11 |
| Kidney | 2.12 ± 0.14 | 2.04 ± 0.18 | 2.10 ± 0.23 | 1.63 ± 0.37 |
| Thymus | 0.57 ± 0.11 | 0.50 ± 0.13 | 0.56 ± 0.13 | 0.59 ± 0.15 |
| Testis | 3.06 ± 0.25 | 2.92 ± 0.25 | 2.87 ± 0.18 | 3.16 ± 0.26 |
| relative organ weight | ||||
| Heart | 0.37 ± 0.03 | 0.36 ± 0.04 | 0.39 ± 0.05 | 0.39 ± 0.06 |
| Liver | 3.58 ± 0.47 | 3.72 ± 0.61 | 3.64 ± 0.45 | 3.73 ± 0.31 |
| Spleen | 0.23 ± 0.03 | 0.25 ± 0.02 | 0.26 ± 0.03 | 0.25 ± 0.02 |
| Lungs | 0.58 ± 0.01 | 0.52 ± 0.05 | 0.52 ± 0.05 | 0.54 ± 0.04 |
| Kidneys | 0.83 ± 0.05 | 0.80 ± 0.05 | 0.83 ± 0.07 | 0.65 ± 0.15 |
| Thymus | 0.22 ± 0.05 | 0.20 ± 0.05 | 0.22 ± 0.05 | 0.24 ± 0.06 |
| Testis | 1.19 ± 0.06 | 1.15 ± 0.06 | 1.14 ± 0.08 | 1.27 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Liu, Y.; Song, Y.; Liu, Q.; Jin, L.; Shang, R. Antioxidant Activity and Preclinical Safety of Semen persicae Extract. Int. J. Mol. Sci. 2024, 25, 8580. https://doi.org/10.3390/ijms25168580
Yang J, Liu Y, Song Y, Liu Q, Jin L, Shang R. Antioxidant Activity and Preclinical Safety of Semen persicae Extract. International Journal of Molecular Sciences. 2024; 25(16):8580. https://doi.org/10.3390/ijms25168580
Chicago/Turabian StyleYang, Jing, Yu Liu, Yingying Song, Qinqin Liu, Liqiong Jin, and Ruofeng Shang. 2024. "Antioxidant Activity and Preclinical Safety of Semen persicae Extract" International Journal of Molecular Sciences 25, no. 16: 8580. https://doi.org/10.3390/ijms25168580
APA StyleYang, J., Liu, Y., Song, Y., Liu, Q., Jin, L., & Shang, R. (2024). Antioxidant Activity and Preclinical Safety of Semen persicae Extract. International Journal of Molecular Sciences, 25(16), 8580. https://doi.org/10.3390/ijms25168580






