Exposure to Radiofrequency Induces Synaptic Dysfunction in Cortical Neurons Causing Learning and Memory Alteration in Early Postnatal Mice
Abstract
:1. Introduction
2. Results
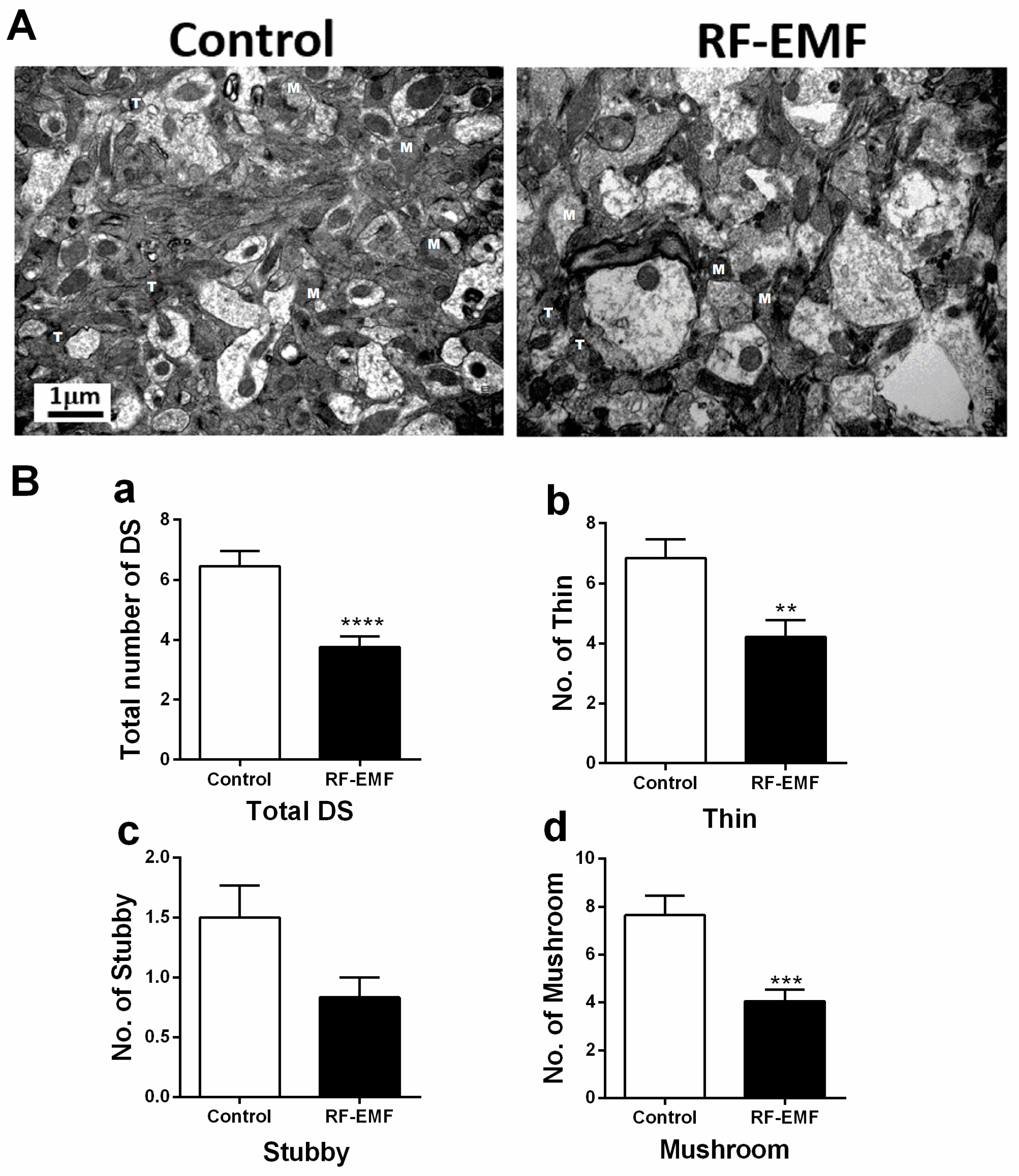
2.1. Alterations in the Number of Dendritic Spines in Cortical Neurons of Mice by RF-EMF Exposure
2.2. Effect of RF-EMF Exposure on Synaptic Maturation in Cortical Neurons of Mice
2.3. Effect of RF-EMF Exposure on Neurite Outgrowth in Developing Cortical Neurons of Mice
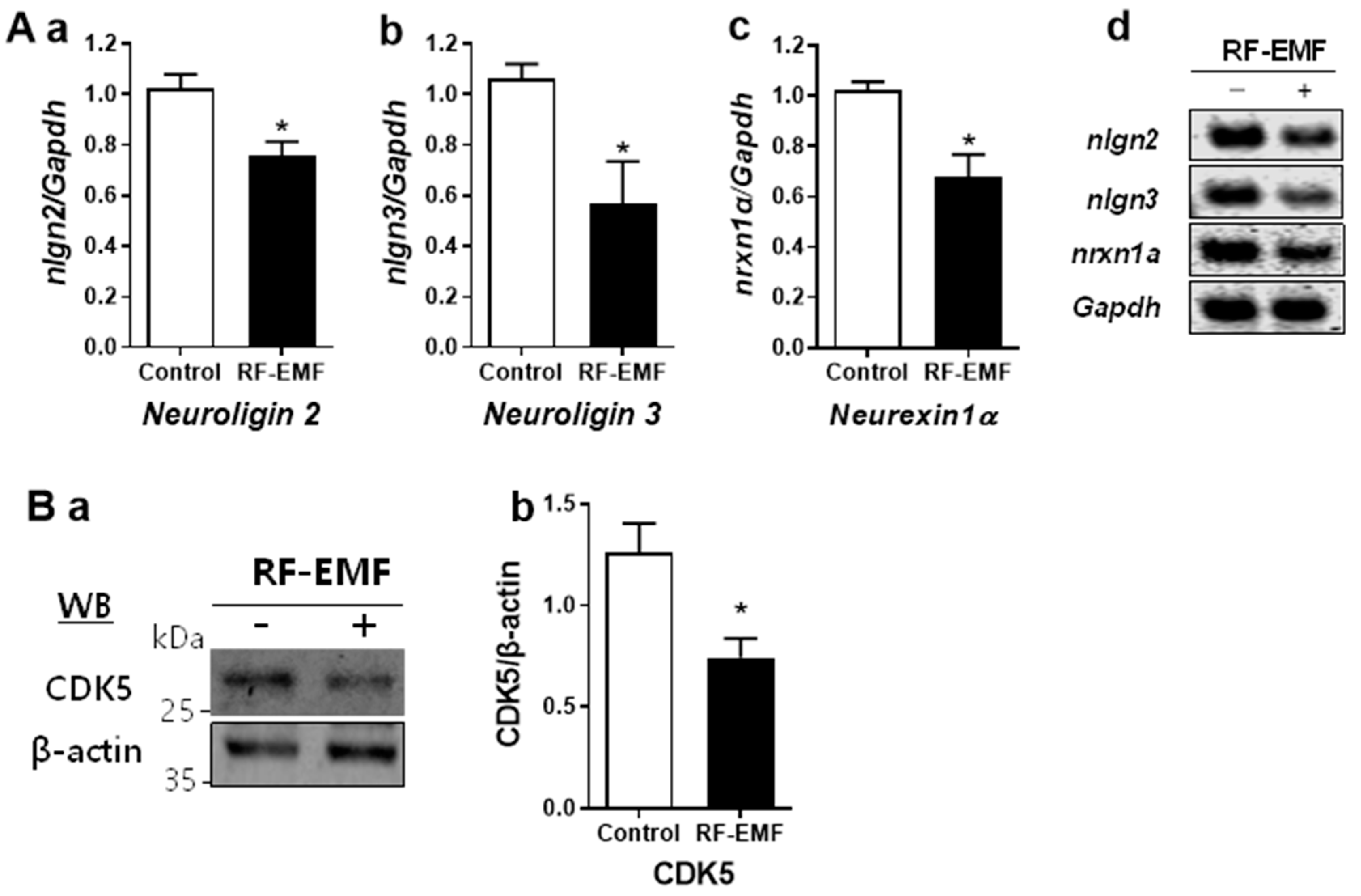
2.4. Changes in Synapse Formation-Related Genes and Proteins in the Cerebral Cortex of Mice after RF-EMF Exposure
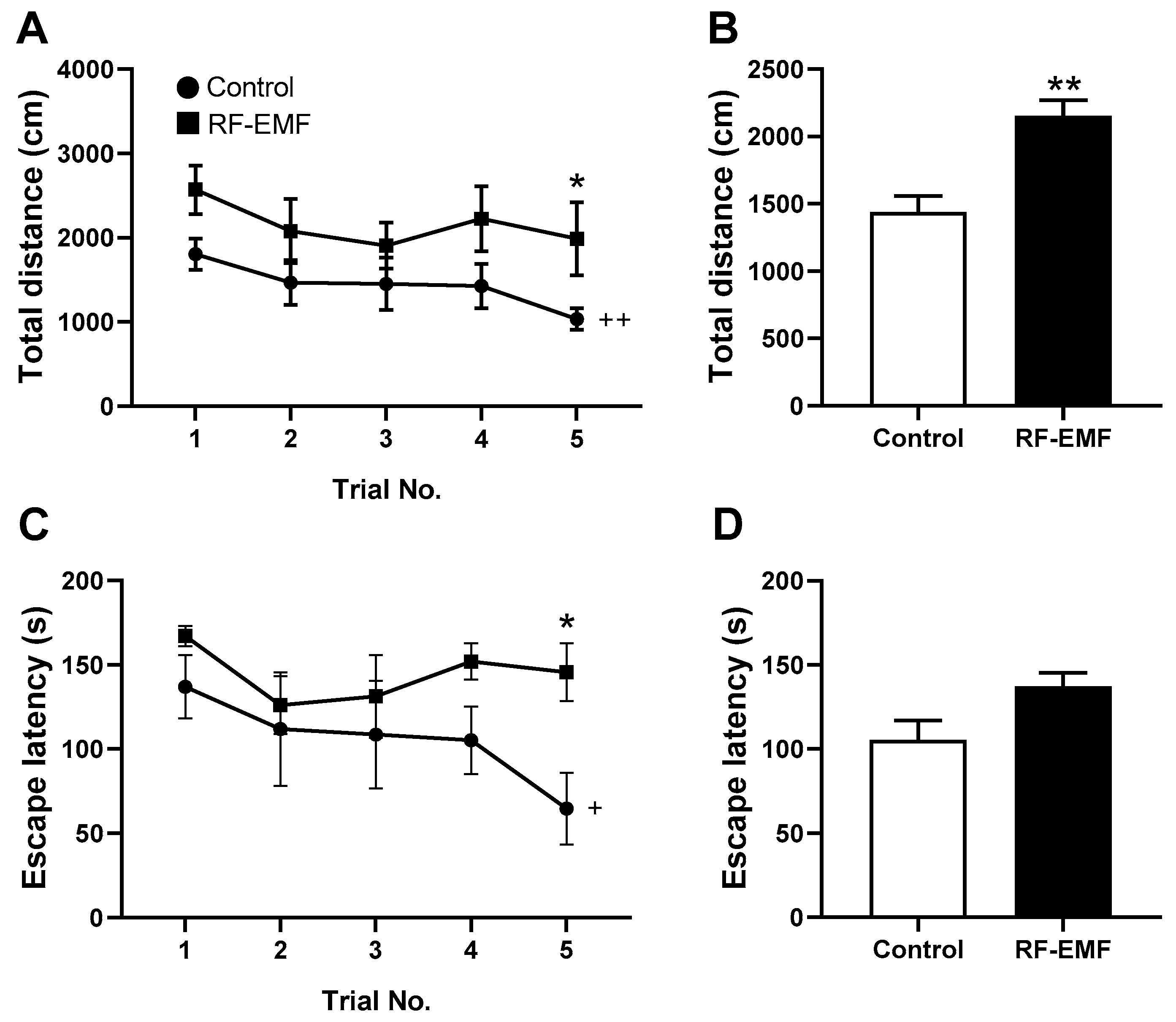
2.5. Spatial Learning and Memory Function in Young Mice Affected by RF-EMF Exposure
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. RF-EMF Exposure in Mice
4.3. Primary Cultures of Mouse Cortical Neurons
4.4. RF-EMF Exposure in Cultured Neurons
4.5. Immunocytochemistry
4.6. Confocal Imaging and Morphological Analysis
4.7. Transmission Electron Microscopy (TEM)
4.8. Quantitative RT-PCR
4.9. Western Blot
4.10. Morris Water Maze Test
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christ, A.; Gosselin, M.-C.; Christopoulou, M.; Kühn, S.; Kuster, N. Age-dependent tissue-specific exposure of cell phone users. Phys. Med. Biol. 2010, 55, 1767–1783. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yu, D.-H.; Huh, Y.H.; Lee, E.H.; Kim, H.-G.; Kim, H.R. Long-term exposure to 835 MHz RF-EMF induces hyperactivity, autophagy and demyelination in the cortical neurons of mice. Sci. Rep. 2017, 7, 41129. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Sohn, U.D.; Kim, H.-G.; Kim, H.R. Exposure to 835 MHz RF-EMF decreases the expression of calcium channels, inhibits apoptosis, but induces autophagy in the mouse hippocampus. Korean J. Physiol. Pharmacol. 2018, 22, 277. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Huh, Y.H.; Kim, H.R. Trafficking of synaptic vesicles is changed at the hypothalamus by exposure to an 835 MHz radiofrequency electromagnetic field. Gen. Physiol. Biophys. 2019, 38, 379. [Google Scholar] [CrossRef] [PubMed]
- Schüz, J. Mobile phone use and exposures in children. Bioelectromagnetics 2005, 26, S45–S50. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, T.; Yamazaki, K.; Araki, A.; Teraoka, Y.; Tamura, N.; Hikage, T.; Omiya, M.; Mizuta, M.; Kishi, R. Exposure to radiofrequency electromagnetic field in the high-frequency band and cognitive function in children and adolescents: A literature review. Int. J. Environ. Res. Public Health 2020, 17, 9179. [Google Scholar] [CrossRef]
- Gandhi, O.P.; Morgan, L.L.; De Salles, A.A.; Han, Y.-Y.; Herberman, R.B.; Davis, D.L. Exposure limits: The underestimation of absorbed cell phone radiation, especially in children. Electromagn. Biol. Med. 2012, 31, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Chung, K.H.; Hwang, Y.R.; Park, H.R.; Kim, H.J.; Kim, H.-G.; Kim, H.R. Exposure to RF-EMF alters postsynaptic structure and hinders neurite outgrowth in developing hippocampal neurons of early postnatal mice. Int. J. Mol. Sci. 2021, 22, 5340. [Google Scholar] [CrossRef] [PubMed]
- Shipp, S. Structure and function of the cerebral cortex. Curr. Biol. 2007, 17, R443–R449. [Google Scholar] [CrossRef] [PubMed]
- Fernández, V.; Llinares-Benadero, C.; Borrell, V. Cerebral cortex expansion and folding: What have we learned? EMBO J. 2016, 35, 1021–1044. [Google Scholar] [CrossRef]
- Ballmaier, M.; O’Brien, J.T.; Burton, E.J.; Thompson, P.M.; Rex, D.E.; Narr, K.L.; McKeith, I.G.; DeLuca, H.; Toga, A.W. Comparing gray matter loss profiles between dementia with Lewy bodies and Alzheimer’s disease using cortical pattern matching: Diagnosis and gender effects. Neuroimage 2004, 23, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Eckstrand, K.; Sharp, W.; Blumenthal, J.; Lerch, J.; Greenstein, D.; Clasen, L.; Evans, A.; Giedd, J.; Rapoport, J. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc. Natl. Acad. Sci. USA 2007, 104, 19649–19654. [Google Scholar] [CrossRef] [PubMed]
- Ong, L.T.; Fan, S.W.D. Morphological and Functional Changes of Cerebral Cortex in Autism Spectrum Disorder. Innov. Clin. Neurosci. 2023, 20, 40. [Google Scholar] [PubMed]
- El-Husseini, A.E.-D.; Schnell, E.; Chetkovich, D.M.; Nicoll, R.A.; Bredt, D.S. PSD-95 involvement in maturation of excitatory synapses. Science 2000, 290, 1364–1368. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, V.A.; Sabatini, B.L. Anatomical and physiological plasticity of dendritic spines. Annu. Rev. Neurosci. 2007, 30, 79–97. [Google Scholar] [CrossRef]
- Park, S.C.; Chun, H.J.; Kang, C.D.; Sul, D. Prevention and management of non-steroidal anti-inflammatory drugs-induced small intestinal injury. World J. Gastroenterol. WJG 2011, 17, 4647. [Google Scholar] [CrossRef] [PubMed]
- Okechukwu, C.E. Effects of radiofrequency electromagnetic field exposure on neurophysiology. Adv. Hum. Biol. 2020, 10, 6–10. [Google Scholar] [CrossRef]
- Christopher, B.; Khandaker, M.U.; Jojo, P. Empirical study on specific absorption rate of head tissues due to induced heating of 4G cell phone radiation. Radiat. Phys. Chem. 2021, 178, 108910. [Google Scholar] [CrossRef]
- Meral, I.; Mert, H.; Mert, N.; Deger, Y.; Yoruk, I.; Yetkin, A.; Keskin, S. Effects of 900-MHz electromagnetic field emitted from cellular phone on brain oxidative stress and some vitamin levels of guinea pigs. Brain Res. 2007, 1169, 120–124. [Google Scholar] [CrossRef]
- Consales, C.; Merla, C.; Marino, C.; Benassi, B. Electromagnetic fields, oxidative stress, and neurodegeneration. Int. J. Cell Biol. 2012, 2012, 683897. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, C.-H.; Kim, H.-G.; Kim, H.R. Decreased dopamine in striatum and difficult locomotor recovery from MPTP insult after exposure to radiofrequency electromagnetic fields. Sci. Rep. 2019, 9, 1201. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zuo, H.; Li, Y. Effects of radiofrequency electromagnetic radiation on neurotransmitters in the brain. Front. Public Health 2021, 9, 691880. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Zhang, S.; Xu, Z.; Ju, L.; Lu, D.; Yao, G. Studying gene expression profile of rat neuron exposed to 1800 MHz radiofrequency electromagnetic fields with cDNA microassay. Toxicology 2007, 235, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Lai, H. Neurological Effects of Non-Ionizing Electromagnetic Fields. The Bioinitiative Report: 2014. Available online: https://bioinitiative.org/ (accessed on 30 July 2024).
- Liu, Y.-X.; Tai, J.-L.; Li, G.-Q.; Zhang, Z.-W.; Xue, J.-H.; Liu, H.-S.; Zhu, H.; Cheng, J.-D.; Liu, Y.-L.; Li, A.-M. Exposure to 1950-MHz TD-SCDMA electromagnetic fields affects the apoptosis of astrocytes via caspase-3-dependent pathway. PLoS ONE 2012, 7, e42332. [Google Scholar] [CrossRef] [PubMed]
- Stam, R. Electromagnetic fields and the blood–brain barrier. Brain Res. Rev. 2010, 65, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, S.; Deniz, O.G.; Önger, M.E.; Türkmen, A.P.; Yurt, K.K.; Aydın, I.; Altunkaynak, B.Z.; Davis, D. Electromagnetic field and brain development. J. Chem. Neuroanat. 2016, 75, 52–61. [Google Scholar] [CrossRef]
- Hunt, C.A.; Schenker, L.J.; Kennedy, M.B. PSD-95 is associated with the postsynaptic density and not with the presynaptic membrane at forebrain synapses. J. Neurosci. 1996, 16, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Scheiffele, P.; Fan, J.; Choih, J.; Fetter, R.; Serafini, T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell 2000, 101, 657–669. [Google Scholar] [CrossRef]
- Betancur, C.; Sakurai, T.; Buxbaum, J.D. The emerging role of synaptic cell-adhesion pathways in the pathogenesis of autism spectrum disorders. Trends Neurosci. 2009, 32, 402–412. [Google Scholar] [CrossRef]
- Lawson-Yuen, A.; Saldivar, J.-S.; Sommer, S.; Picker, J. Familial deletion within NLGN4 associated with autism and Tourette syndrome. Eur. J. Hum. Genet. 2008, 16, 614–618. [Google Scholar] [CrossRef]
- Wang, H. Endocannabinoid mediates excitatory synaptic function of β-neurexins. commentary: β-neurexins control neural circuits by regulating synaptic endocannabinoid signaling. Front. Neurosci. 2016, 10, 198721. [Google Scholar] [CrossRef]
- Cao, X.; Tabuchi, K. Functions of synapse adhesion molecules neurexin/neuroligins and neurodevelopmental disorders. Neurosci. Res. 2017, 116, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Jiang, M.; Zhang, B.; Gokce, O.; Südhof, T.C. Conditional deletion of all neurexins defines diversity of essential synaptic organizer functions for neurexins. Neuron 2017, 94, 611–625.e4. [Google Scholar] [CrossRef]
- Cuttler, K.; Hassan, M.; Carr, J.; Cloete, R.; Bardien, S. Emerging evidence implicating a role for neurexins in neurodegenerative and neuropsychiatric disorders. Open Biol. 2021, 11, 210091. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hua, F.; Yang, D.; Lin, Y.; Zhang, L.; Ying, J.; Sheng, H.; Wang, X. Roles of neuroligins in central nervous system development: Focus on glial neuroligins and neuron neuroligins. J. Transl. Med. 2022, 20, 418. [Google Scholar] [CrossRef]
- Li, L.-Y.; Imai, A.; Izumi, H.; Inoue, R.; Koshidaka, Y.; Takao, K.; Mori, H.; Yoshida, T. Differential contribution of canonical and noncanonical NLGN3 pathways to early social development and memory performance. Mol. Brain 2024, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Shan, D.; Song, Y.; Zhang, Y.; Ho, C.W.; Xia, W.; Li, Z.; Ge, F.; Ou, Q.; Dai, Z.; Dai, Z. Neurexin dysfunction in neurodevelopmental and neuropsychiatric disorders: A PRIMSA-based systematic review through iPSC and animal models. Front. Behav. Neurosci. 2024, 18, 1297374. [Google Scholar] [CrossRef]
- Gilmore, E.C.; Ohshima, T.; Goffinet, A.M.; Kulkarni, A.B.; Herrup, K. Cyclin-dependent kinase 5-deficient mice demonstrate novel developmental arrest in cerebral cortex. J. Neurosci. 1998, 18, 6370–6377. [Google Scholar] [CrossRef]
- Ao, C.; Li, C.; Chen, J.; Tan, J.; Zeng, L. The role of Cdk5 in neurological disorders. Front. Cell. Neurosci. 2022, 16, 951202. [Google Scholar] [CrossRef]
- Hawasli, A.H.; Benavides, D.R.; Nguyen, C.; Kansy, J.W.; Hayashi, K.; Chambon, P.; Greengard, P.; Powell, C.M.; Cooper, D.C.; Bibb, J.A. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat. Neurosci. 2007, 10, 880–886. [Google Scholar] [CrossRef]
- Mishiba, T.; Tanaka, M.; Mita, N.; He, X.; Sasamoto, K.; Itohara, S.; Ohshima, T. Cdk5/p35 functions as a crucial regulator of spatial learning and memory. Mol. Brain 2014, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.Z.; Hassan, Z.; Has, A.T.C. Morris water maze: A versatile and pertinent tool for assessing spatial learning and memory. Exp. Anim. 2022, 71, 264–280. [Google Scholar] [CrossRef] [PubMed]
- Anckarsäter, H. Central nervous changes in social dysfunction: Autism, aggression, and psychopathy. Brain Res. Bull. 2006, 69, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Raine, A. Prefrontal structural and functional brain imaging findings in antisocial, violent, and psychopathic individuals: A meta-analysis. Psychiatry Res. Neuroimaging 2009, 174, 81–88. [Google Scholar] [CrossRef] [PubMed]
- João, R.B.; Filgueiras, R.M. Frontal lobe: Functional neuroanatomy of its circuitry and related disconnection syndromes. In Prefrontal Cortex; IntechOpen: London, UK, 2018; Volume 41. [Google Scholar]
- Badre, D.; Kayser, A.S.; D’Esposito, M. Frontal cortex and the discovery of abstract action rules. Neuron 2010, 66, 315–326. [Google Scholar] [CrossRef]
- Adams, B.; Fitch, T.; Chaney, S.; Gerlai, R. Altered performance characteristics in cognitive tasks: Comparison of the albino ICR and CD1 mouse strains. Behav. Brain Res. 2002, 133, 351–361. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Seok, J.Y.; Kim, Y.-H.; Kim, H.J.; Lee, J.-K.; Kim, H.R. Exposure to Radiofrequency Induces Synaptic Dysfunction in Cortical Neurons Causing Learning and Memory Alteration in Early Postnatal Mice. Int. J. Mol. Sci. 2024, 25, 8589. https://doi.org/10.3390/ijms25168589
Kim JH, Seok JY, Kim Y-H, Kim HJ, Lee J-K, Kim HR. Exposure to Radiofrequency Induces Synaptic Dysfunction in Cortical Neurons Causing Learning and Memory Alteration in Early Postnatal Mice. International Journal of Molecular Sciences. 2024; 25(16):8589. https://doi.org/10.3390/ijms25168589
Chicago/Turabian StyleKim, Ju Hwan, Jun Young Seok, Yun-Hee Kim, Hee Jung Kim, Jin-Koo Lee, and Hak Rim Kim. 2024. "Exposure to Radiofrequency Induces Synaptic Dysfunction in Cortical Neurons Causing Learning and Memory Alteration in Early Postnatal Mice" International Journal of Molecular Sciences 25, no. 16: 8589. https://doi.org/10.3390/ijms25168589
APA StyleKim, J. H., Seok, J. Y., Kim, Y.-H., Kim, H. J., Lee, J.-K., & Kim, H. R. (2024). Exposure to Radiofrequency Induces Synaptic Dysfunction in Cortical Neurons Causing Learning and Memory Alteration in Early Postnatal Mice. International Journal of Molecular Sciences, 25(16), 8589. https://doi.org/10.3390/ijms25168589






