10-Eicosanol Alleviates Patulin-Induced Cell Cycle Arrest and Apoptosis by Activating AKT (Protein Kinase B) in Porcine Intestinal Epithelial Cells
Abstract
1. Introduction
2. Results
2.1. Patulin Reduced Viability in IPEC-J2 Cell
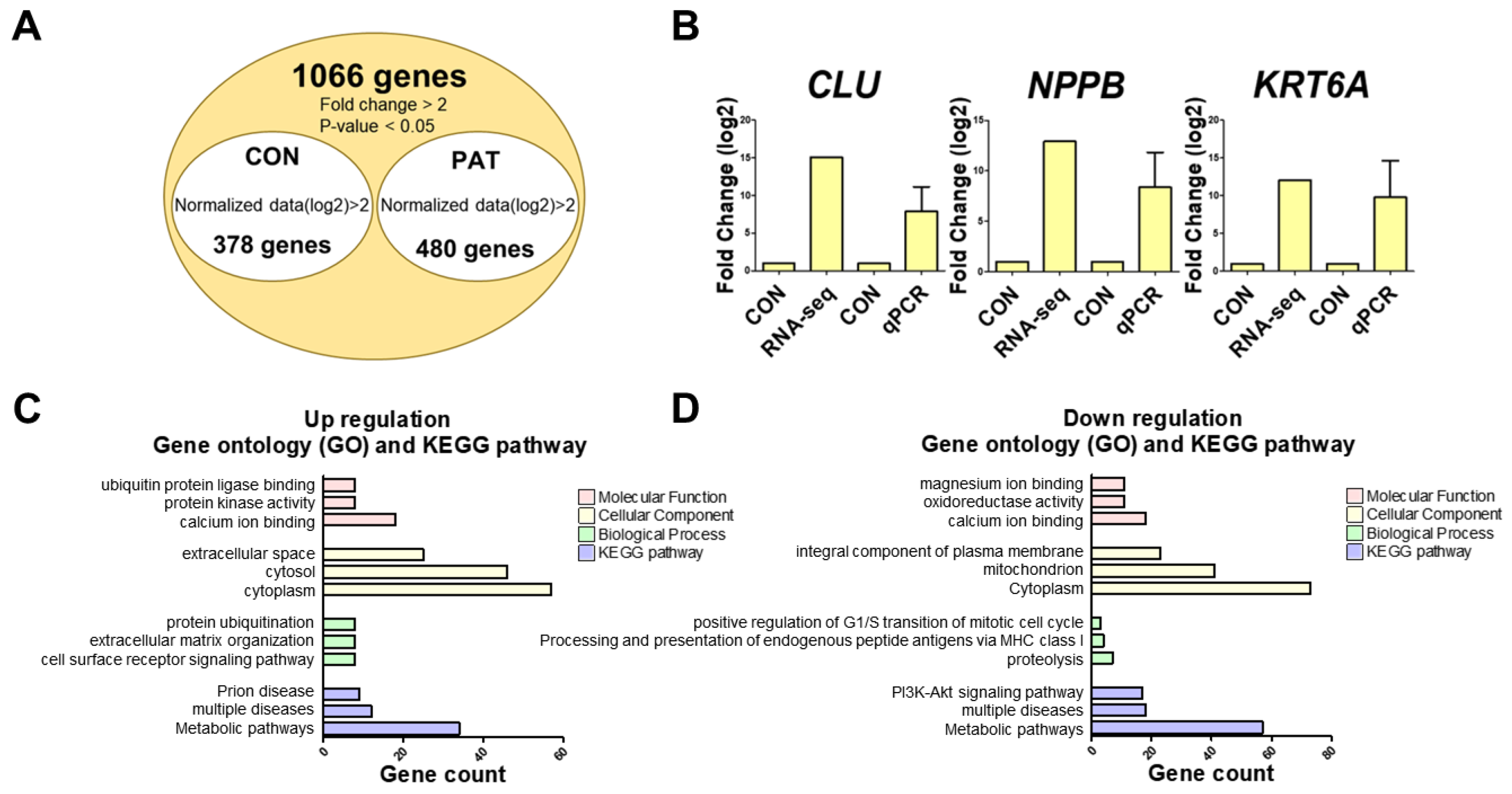
2.2. Identification and Validation of Differentially Expressed Genes
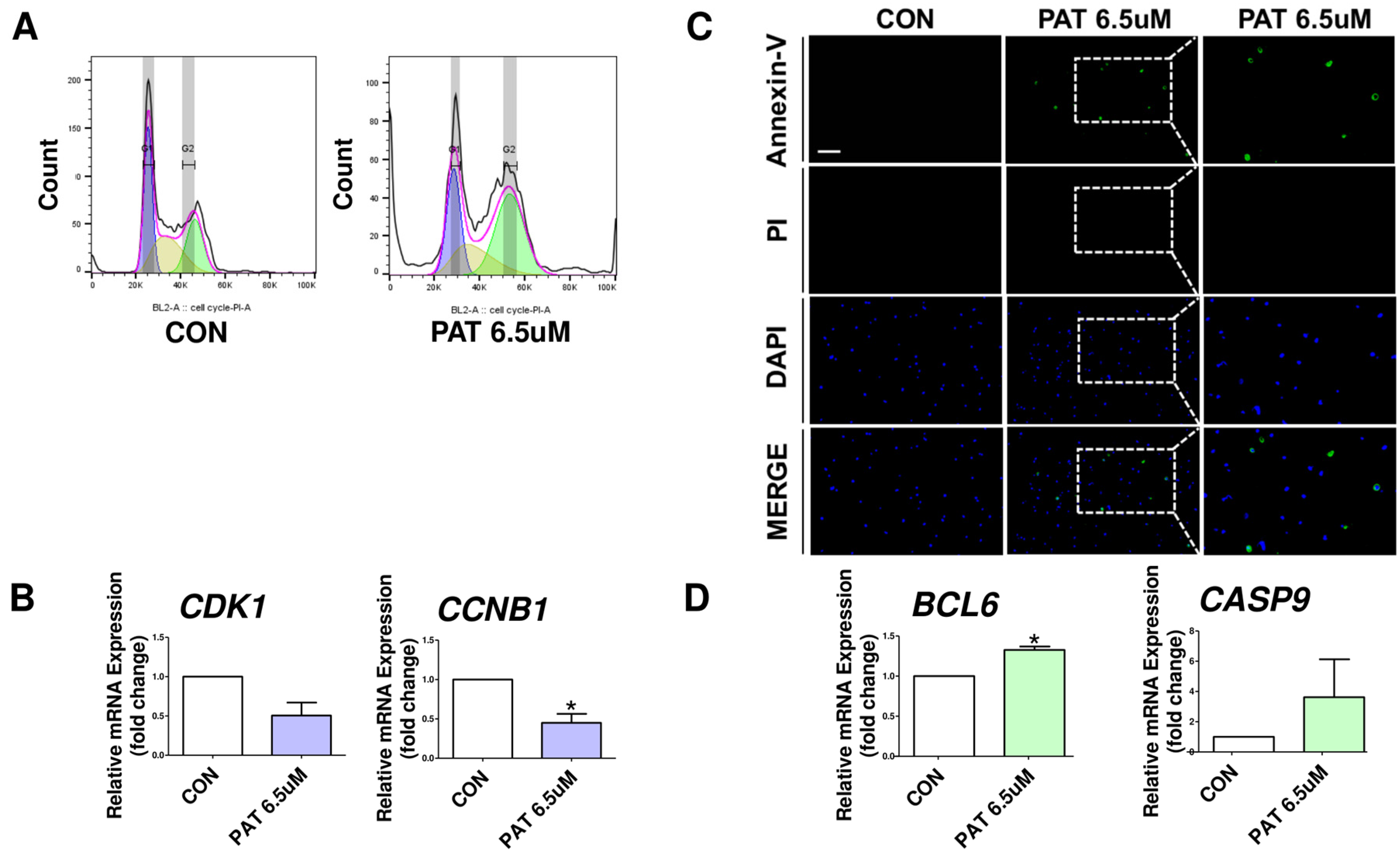
2.3. Patulin Causes Cell Cycle Arrest and Apoptosis
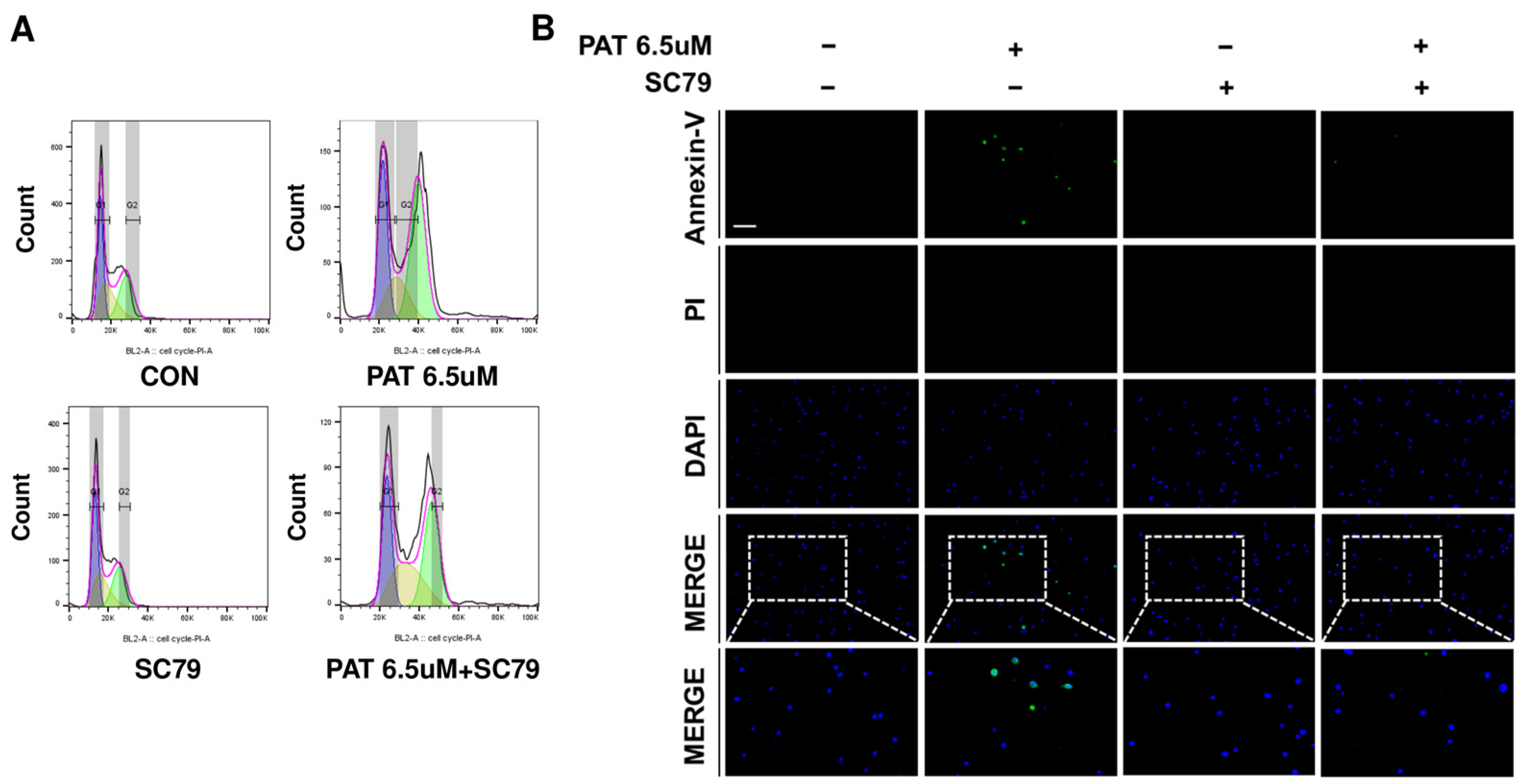
2.4. Activation of AKT Restores the Cell Cycle and Alleviates Apoptosis
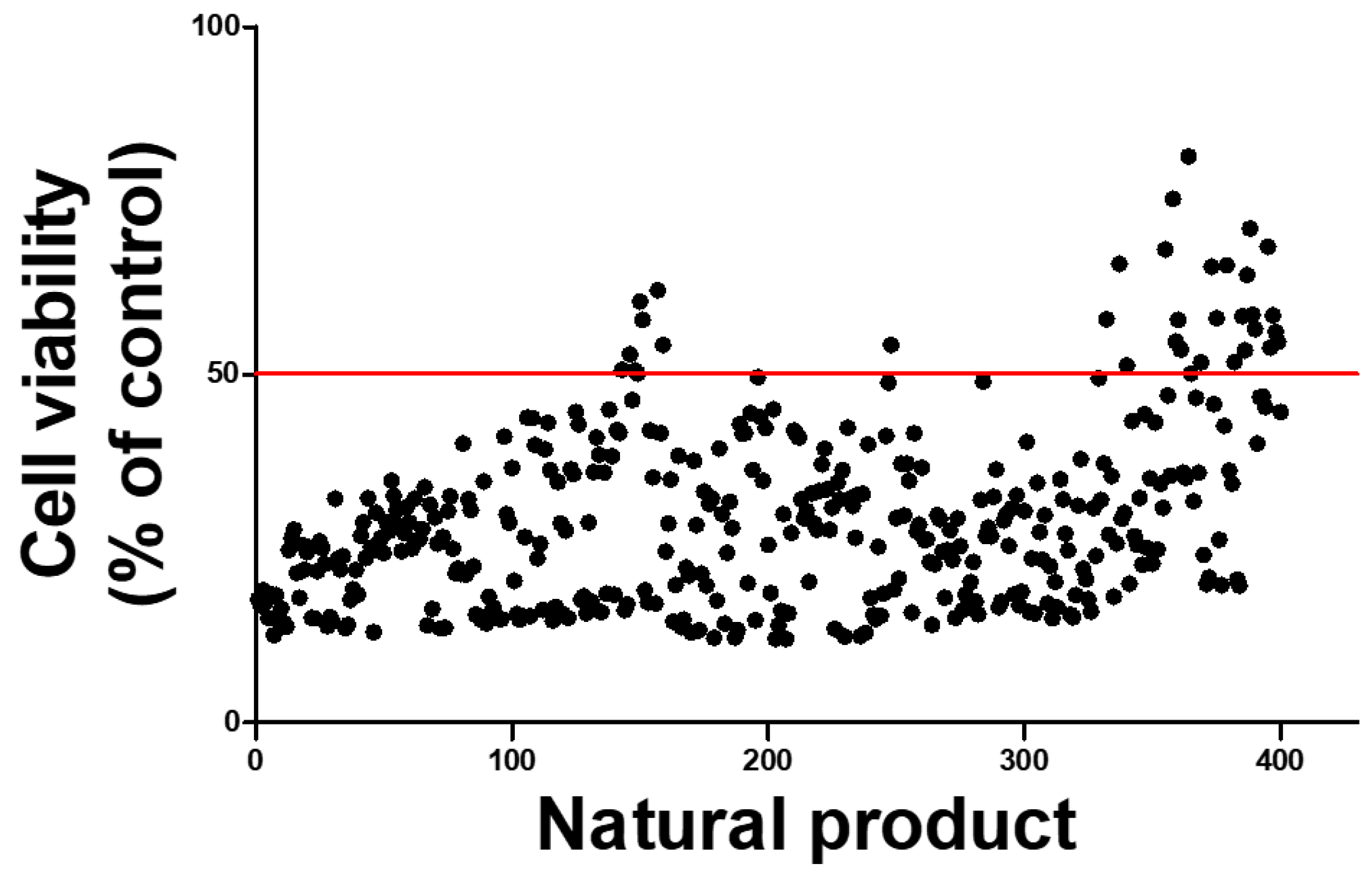
2.5. High-Throughput Screening to Discover Natural Products That Mitigate Patulin Toxicity in IPEC-J2 Cells
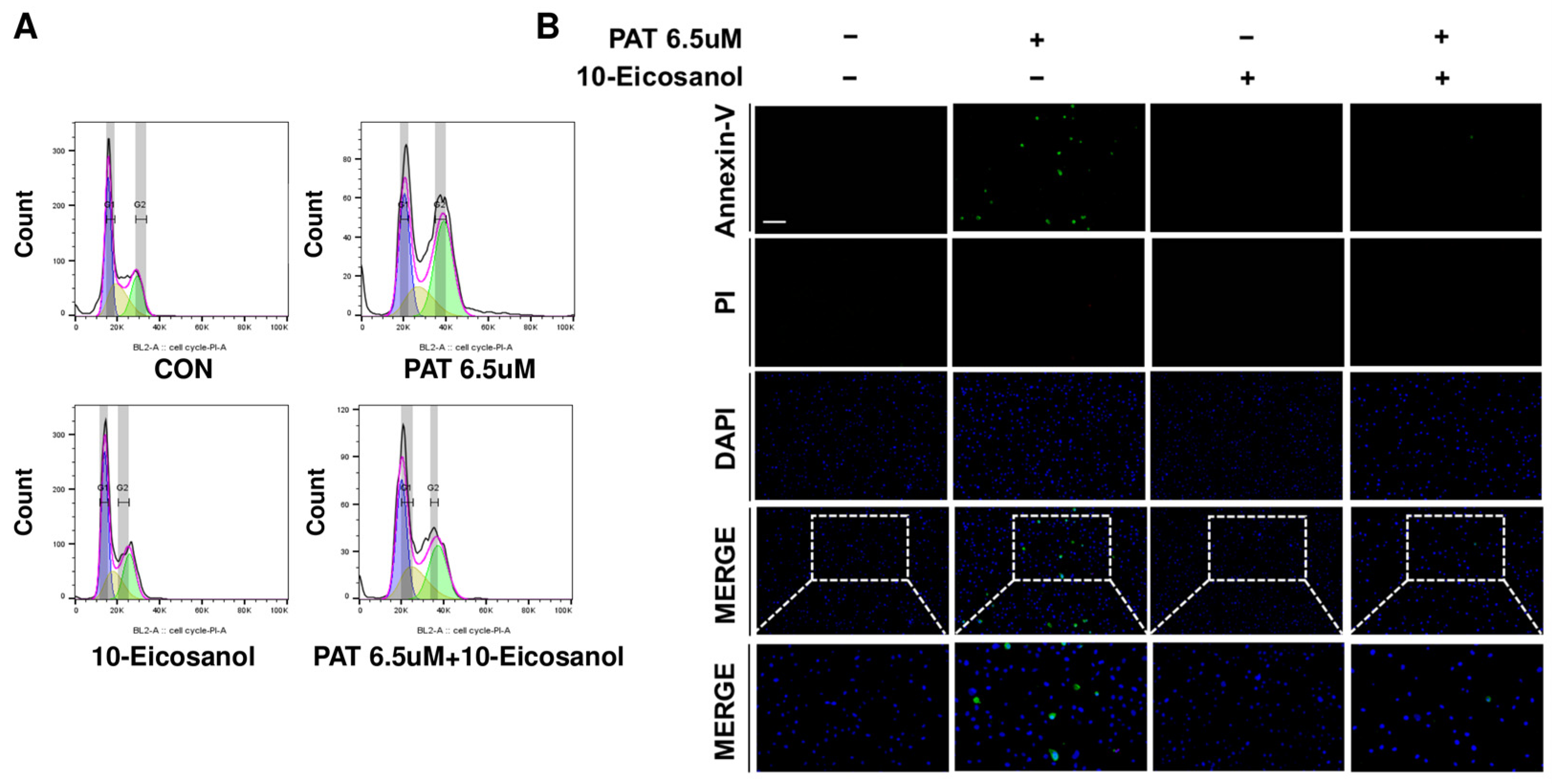
2.6. 10-Eicosanol Mitigates G2/M Phase Arrest and Apoptosis in IPEC-J2 Cells
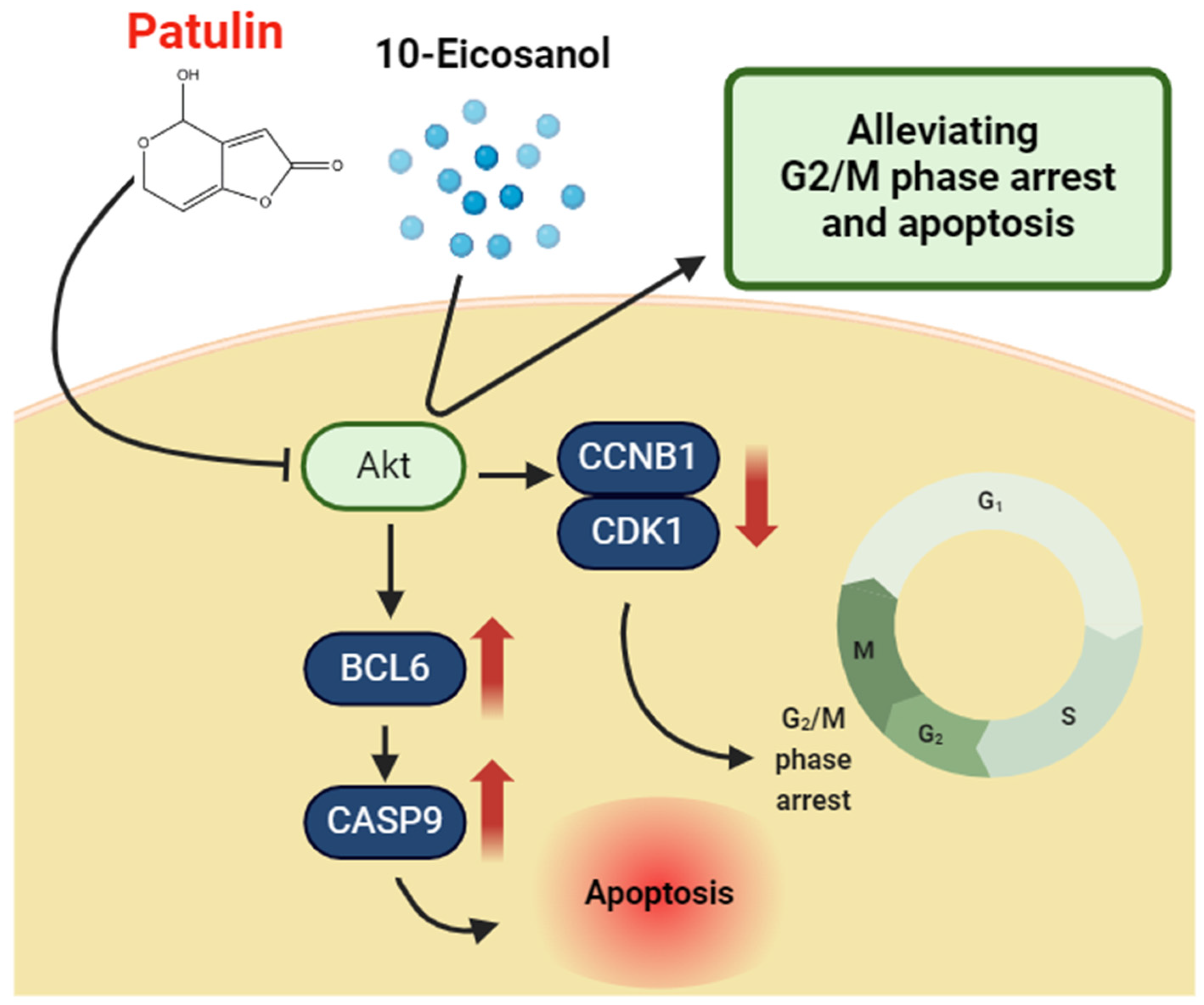
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. Cell Viability Analysis
4.3. Gene-Expression Profiling
4.4. Cell Cycle Analysis
4.5. Annexin-V and Propidium Iodide Staining
4.6. Quantitative Real-Time Polymerase Chain Reaction
4.7. High-Throughput Screening Assay
4.8. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Awuchi, C.G.; Ondari, E.N.; Ogbonna, C.U.; Upadhyay, A.K.; Baran, K.; Okpala, C.O.R.; Korzeniowska, M.; Guiné, R.P.F. Mycotoxins Affecting Animals, Foods, Humans, and Plants: Types, Occurrence, Toxicities, Action Mechanisms, Prevention, and Detoxification Strategies—A Revisit. Foods 2021, 10, 1279. [Google Scholar] [CrossRef] [PubMed]
- Cunha, S.C.; Faria, M.A.; Pereira, V.L.; Oliveira, T.M.; Lima, A.C.; Pinto, E. Patulin assessment and fungi identification in organic and conventional fruits and derived products. Food Control 2014, 44, 185–190. [Google Scholar] [CrossRef]
- Hussain, S.; Asi, M.R.; Iqbal, M.; Akhtar, M.; Imran, M.; Ariño, A. Surveillance of Patulin in Apple, Grapes, Juices and Value-Added Products for Sale in Pakistan. Foods 2020, 9, 1744. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-H.; Wu, T.-S.; Yu, F.-Y.; Su, C.-C. Induction of Oxidative Stress Response by the Mycotoxin Patulin in Mammalian Cells. Toxicol. Sci. 2006, 95, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Chen, X.; Chen, Z.; Zeng, X.; Yue, T.; Yuan, Y. Effects of Selenium Nanoparticles on Preventing Patulin-Induced Liver, Kidney and Gastrointestinal Damage. Foods 2022, 11, 749. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Shan, S.; Zhou, S.; Chen, Z.; Wu, Y.; Liao, W.; Zhao, C.; Chu, Q. Saccharomyces cerevisiae: A patulin degradation candidate both in vitro and in vivo. Food Funct. 2023, 14, 3083–3091. [Google Scholar] [CrossRef] [PubMed]
- Boussabbeh, M.; Ben Salem, I.; Prola, A.; Guilbert, A.; Bacha, H.; Abid-Essefi, S.; Lemaire, C. Patulin Induces Apoptosis through ROS-Mediated Endoplasmic Reticulum Stress Pathway. Toxicol. Sci. 2015, 144, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; Bahuguna, A.; Kim, M. The effects of mycotoxin patulin on cells and cellular components. Trends Food Sci. Technol. 2019, 83, 99–113. [Google Scholar] [CrossRef]
- Ali, A.; Tan, H.; Kaiko, G.E. Role of the Intestinal Epithelium and Its Interaction with the Microbiota in Food Allergy. Front. Immunol. 2020, 11, 604054. [Google Scholar] [CrossRef]
- Oda, M.; Hatano, Y.; Sato, T. Intestinal epithelial organoids: Regeneration and maintenance of the intestinal epithelium. Curr. Opin. Genet. Dev. 2022, 76, 101977. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Goto, Y.; Ivanov, I.I. Intestinal epithelial cells as mediators of the commensal-host immune crosstalk. Immunol. Cell Biol. 2013, 91, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Patankar, J.V.; Becker, C. Cell death in the gut epithelium and implications for chronic inflammation. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Speijers, G.J.A.; Franken, M.A.M.; van Leeuwen, F.X.R. Subacute toxicity study of patulin in the rat: Effects on the kidney and the gastro-intestinal tract. Food Chem. Toxicol. 1988, 26, 23–30. [Google Scholar] [CrossRef] [PubMed]
- McKinley, E.R.; Carlton, W.W. Patulin mycotoxicosis in Swiss ICR mice. Food Cosmet. Toxicol. 1980, 18, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Galani Yamdeu, J.H.; Gong, Y.Y.; Orfila, C. A review of postharvest approaches to reduce fungal and mycotoxin contamination of foods. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1521–1560. [Google Scholar] [CrossRef] [PubMed]
- Kihal, A.; Rodríguez-Prado, M.; Calsamiglia, S. The efficacy of mycotoxin binders to control mycotoxins in feeds and the potential risk of interactions with nutrient: A review. J. Anim. Sci. 2022, 100, skac328. [Google Scholar] [CrossRef]
- Koo, B.; Adeshakin, O.; Nyachoti, C.M. Energy content of intact and heat-treated dry extruded-expelled soybean meal fed to growing pigs. J. Anim. Sci. 2021, 99, skab131. [Google Scholar] [CrossRef]
- Yu, Y.; Shi, J.; Xie, B.; He, Y.; Qin, Y.; Wang, D.; Shi, H.; Ke, Y.; Sun, Q. Detoxification of aflatoxin B(1) in corn by chlorine dioxide gas. Food Chem. 2020, 328, 127121. [Google Scholar] [CrossRef]
- Tran, T.M.; Atanasova, V.; Tardif, C.; Richard-Forget, F. Stilbenoids as Promising Natural Product-Based Solutions in a Race against Mycotoxigenic Fungi: A Comprehensive Review. J. Agric. Food Chem. 2023, 71, 5075–5092. [Google Scholar] [CrossRef]
- Carter, A.C.; King, J.B.; Mattes, A.O.; Cai, S.; Singh, N.; Cichewicz, R.H. Natural-Product-Inspired Compounds as Countermeasures against the Liver Carcinogen Aflatoxin B(1). J. Nat. Prod. 2019, 82, 1694–1703. [Google Scholar] [CrossRef]
- Jing, S.; Liu, C.; Zheng, J.; Dong, Z.; Guo, N. Toxicity of zearalenone and its nutritional intervention by natural products. Food Funct. 2022, 13, 10374–10400. [Google Scholar] [CrossRef]
- Cai, J.; Yan, R.; Shi, J.; Chen, J.; Long, M.; Wu, W.; Kuca, K. Antifungal and mycotoxin detoxification ability of essential oils: A review. Phytother. Res. 2022, 36, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Ofori-Attah, E.; Hashimoto, M.; Oki, M.; Kadowaki, D. Therapeutic Effect of Natural Products and Dietary Supplements on Aflatoxin-Induced Nephropathy. Int. J. Mol. Sci. 2024, 25, 2849. [Google Scholar] [CrossRef]
- Agnihotri, V.K.; ElSohly, H.N.; Khan, S.I.; Jacob, M.R.; Joshi, V.C.; Smillie, T.; Khan, I.A.; Walker, L.A. Constituents of Nelumbo nucifera leaves and their antimalarial and antifungal activity. Phytochem. Lett. 2008, 1, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Shinbori, C.; Saito, M.; Kinoshita, Y.; Satoh, I.; Kono, T.; Hanada, T.; Nanba, E.; Adachi, K.; Suzuki, H.; Yamada, M.; et al. Cyclohexenonic long-chain fatty alcohol has therapeutic effects on diabetes-induced angiopathy in the rat aorta. Eur. J. Pharmacol. 2007, 567, 139–144. [Google Scholar] [CrossRef]
- Montserrat-de la Paz, S.; García-Giménez, M.D.; Ángel-Martín, M.; Pérez-Camino, M.C.; Fernández Arche, A. Long-chain fatty alcohols from evening primrose oil inhibit the inflammatory response in murine peritoneal macrophages. J. Ethnopharmacol. 2014, 151, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Saito, M.; Kinoshita, Y.; Satoh, I.; Kawaba, Y.; Hayashi, A.; Oite, T.; Satoh, K.; Kanzaki, S. Effects of cyclohexenonic long-chain fatty alcohol in type 2 diabetic rat nephropathy. Biomed. Res. 2010, 31, 219–230. [Google Scholar] [CrossRef]
- Saleh, I.; Goktepe, I. The characteristics, occurrence, and toxicological effects of patulin. Food Chem. Toxicol. 2019, 129, 301–311. [Google Scholar] [CrossRef]
- Mohan, H.M.; Collins, D.; Maher, S.; Walsh, E.G.; Winter, D.C.; O’Brien, P.J.; Brayden, D.J.; Baird, A.W. The mycotoxin patulin increases colonic epithelial permeability in vitro. Food Chem. Toxicol. 2012, 50, 4097–4102. [Google Scholar] [CrossRef]
- Robert, H.; Payros, D.; Pinton, P.; Théodorou, V.; Mercier-Bonin, M.; Oswald, I.P. Impact of mycotoxins on the intestine: Are mucus and microbiota new targets? J. Toxicol. Environ. Health Part B 2017, 20, 249–275. [Google Scholar] [CrossRef]
- Xie, Y.; Shi, X.; Sheng, K.; Han, G.; Li, W.; Zhao, Q.; Jiang, B.; Feng, J.; Li, J.; Gu, Y. PI3K/Akt signaling transduction pathway, erythropoiesis and glycolysis in hypoxia (Review). Mol. Med. Rep. 2019, 19, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Hoxhaj, G.; Manning, B.D. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat. Rev. Cancer 2020, 20, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cen, L.; Zhang, X.; Tang, C.; Chen, Y.; Zhang, Y.; Yu, M.; Lu, C.; Li, M.; Li, S.; et al. MPST deficiency promotes intestinal epithelial cell apoptosis and aggravates inflammatory bowel disease via AKT. Redox Biol. 2022, 56, 102469. [Google Scholar] [CrossRef] [PubMed]
- Maharati, A.; Moghbeli, M. PI3K/AKT signaling pathway as a critical regulator of epithelial-mesenchymal transition in colorectal tumor cells. Cell Commun. Signal. 2023, 21, 201. [Google Scholar] [CrossRef]
- Karimian, A.; Ahmadi, Y.; Yousefi, B. Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair. 2016, 42, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.B.R.; Kim, G.C. Induction apoptosis and G2/M phase cell cycle arrest by non-thermal plasma in human osteosarcoma MG-63 cell line. Oral. Biol. Res. 2017, 41, 229–235. [Google Scholar] [CrossRef]
- Chen, H.; Du, G.; Yan, X.; Ye, H.; Guo, Q.; Wang, Z.; Yuan, Y.; Yue, T. Selenium-Enriched Pediococcus acidilactici MRS-7 Alleviates Patulin-Induced Jejunum Injuries in Mice and Its Possible Mechanisms. J. Agric. Food Chem. 2022, 70, 4755–4764. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Simpson, E.R.; Brown, K.A. p53: Protection against Tumor Growth beyond Effects on Cell Cycle and Apoptosis. Cancer Res. 2015, 75, 5001–5007. [Google Scholar] [CrossRef]
- Roos, W.P.; Kaina, B. DNA damage-induced cell death by apoptosis. Trends Mol. Med. 2006, 12, 440–450. [Google Scholar] [CrossRef]
- Saxena, N.; Ansari, K.M.; Kumar, R.; Dhawan, A.; Dwivedi, P.D.; Das, M. Patulin causes DNA damage leading to cell cycle arrest and apoptosis through modulation of Bax, p53 and p21/WAF1 proteins in skin of mice. Toxicol. Appl. Pharmacol. 2009, 234, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Pucci, B.; Kasten, M.; Giordano, A. Cell cycle and apoptosis. Neoplasia 2000, 2, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Fedarko, N.S. The biology of aging and frailty. Clin. Geriatr. Med. 2011, 27, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lai, Y.; Hua, Z.C. Apoptosis and apoptotic body: Disease message and therapeutic target potentials. Biosci. Rep. 2019, 39, BSR20180992. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.; Lee, J.T.; Navolanic, P.M.; Steelman, L.S.; Shelton, J.G.; Blalock, W.L.; Franklin, R.A.; McCubrey, J.A. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: A target for cancer chemotherapy. Leukemia 2003, 17, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Pillay, Y.; Nagiah, S.; Phulukdaree, A.; Krishnan, A.; Chuturgoon, A.A. Patulin suppresses α(1)-adrenergic receptor expression in HEK293 cells. Sci. Rep. 2020, 10, 20115. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.O.; Na, S.I.; Lee, M.Y.; Heo, J.S.; Han, H.J. Epinephrine increases DNA synthesis via ERK1/2s through cAMP, Ca(2+)/PKC, and PI3K/Akt signaling pathways in mouse embryonic stem cells. J. Cell. Biochem. 2008, 104, 1407–1420. [Google Scholar] [CrossRef] [PubMed]
- Ballou, L.M.; Tian, P.Y.; Lin, H.Y.; Jiang, Y.P.; Lin, R.Z. Dual regulation of glycogen synthase kinase-3beta by the alpha1A-adrenergic receptor. J. Biol. Chem. 2001, 276, 40910–40916. [Google Scholar] [CrossRef]
- Franke, T.F.; Hornik, C.P.; Segev, L.; Shostak, G.A.; Sugimoto, C. PI3K/Akt and apoptosis: Size matters. Oncogene 2003, 22, 8983–8998. [Google Scholar] [CrossRef]
- Fesik, S.W. Promoting apoptosis as a strategy for cancer drug discovery. Nat. Rev. Cancer 2005, 5, 876–885. [Google Scholar] [CrossRef]
- Qiang, Z.; Truong, M.; Meynen, K.; Murphy, P.A.; Hendrich, S. Efficacy of a mycotoxin binder against dietary fumonisin, deoxynivalenol, and zearalenone in rats. J. Agric. Food Chem. 2011, 59, 7527–7533. [Google Scholar] [CrossRef] [PubMed]
- Feizollahi, E.; Roopesh, M.S. Mechanisms of deoxynivalenol (DON) degradation during different treatments: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 5903–5924. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, L.; Sun, J.; Wang, L.; Guo, H.; Ye, Y.; Sun, X. Microbial detoxification of mycotoxins in food and feed. Crit. Rev. Food Sci. Nutr. 2022, 62, 4951–4969. [Google Scholar] [CrossRef] [PubMed]
- Diaz, D.E.; Hagler, W.M.; Blackwelder, J.T.; Eve, J.A.; Hopkins, B.A.; Anderson, K.L.; Jones, F.T.; Whitlow, L.W. Aflatoxin Binders II: Reduction of aflatoxin M1 in milk by sequestering agents of cows consuming aflatoxin in feed. Mycopathologia 2004, 157, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Mu, P.; Deng, Y. Mycotoxins: Cytotoxicity and biotransformation in animal cells. Toxicol. Res. 2016, 5, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Xiao, Z.-H.; Liu, M.; Zhang, N.-Y.; Khalil, M.M.; Gu, C.-Q.; Qi, D.-S.; Sun, L.-H. Dietary Silymarin Supplementation Alleviates Zearalenone-Induced Hepatotoxicity and Reproductive Toxicity in Rats. J. Nutr. 2018, 148, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.; Chen, X.; Ma, H.; Zhang, C.; Luo, Y.; Jiang, J.; Yin, Y. Limonene formulation exhibited potential application in the control of mycelial growth and deoxynivalenol production in Fusarium graminearum. Front. Microbiol. 2023, 14, 1161244. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Qi, W.; Xiong, D.; Long, M. Quercetin: Its Antioxidant Mechanism, Antibacterial Properties and Potential Application in Prevention and Control of Toxipathy. Molecules 2022, 27, 6545. [Google Scholar] [CrossRef]
- Fernández-Blanco, C.; Elmo, L.; Waldner, T.; Ruiz, M.-J. Cytotoxic effects induced by patulin, deoxynivalenol and toxin T2 individually and in combination in hepatic cells (HepG2). Food Chem. Toxicol. 2018, 120, 12–23. [Google Scholar] [CrossRef]
- Vergauwen, H. The IPEC-J2 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; pp. 125–134. [Google Scholar]

| Gene Name | Sequence (5′-3′) | Accession No. |
|---|---|---|
| GAPDH | Forward: ACACCGAGCATCTCCTGACTReverse: GACGAGGCAGGTCTCCCTAA | NM_001206359 |
| CLU | Forward: ATGGATGCCCACCTTCATAGReverse: GCACTTCTCACACTGGTCCTT | NM_213971 |
| KRT6A | Forward: CCAGGAGTGGATTTGGTTTTReverse: TCAGAGGGGTGAGGAGATTC | XM_021091688 |
| NPPB | Forward: CCGCAGTAGCATCTTCCAAReverse: TGTCAGCCAGGACTTCTCAG | NM_213846 |
| CDK1 | Forward: TAATAAGCTGGGATCTACCACATCReverse: CGAATGGCAGTACTAGGAACAC | NM_001159304 |
| CCNB1 | Forward: AGCTAGTGGTGGCTTCAAGGReverse: GCGCCATGACTTCCTCTGTA | NM_001170768 |
| BCL6 | Forward: GTGTCCTACGGTGCCTTTTTReverse: TGACGCAGAATGTGATGAGA | XM_005657112 |
| CASP9 | Forward: TACCCTGCCTTACCTTCCACReverse: CTGGTCTTCGGTCATCTGG | XM_013998997 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.H.; Shin, S.; Lee, S.I. 10-Eicosanol Alleviates Patulin-Induced Cell Cycle Arrest and Apoptosis by Activating AKT (Protein Kinase B) in Porcine Intestinal Epithelial Cells. Int. J. Mol. Sci. 2024, 25, 8597. https://doi.org/10.3390/ijms25168597
Lee CH, Shin S, Lee SI. 10-Eicosanol Alleviates Patulin-Induced Cell Cycle Arrest and Apoptosis by Activating AKT (Protein Kinase B) in Porcine Intestinal Epithelial Cells. International Journal of Molecular Sciences. 2024; 25(16):8597. https://doi.org/10.3390/ijms25168597
Chicago/Turabian StyleLee, Chae Hyun, Sangsu Shin, and Sang In Lee. 2024. "10-Eicosanol Alleviates Patulin-Induced Cell Cycle Arrest and Apoptosis by Activating AKT (Protein Kinase B) in Porcine Intestinal Epithelial Cells" International Journal of Molecular Sciences 25, no. 16: 8597. https://doi.org/10.3390/ijms25168597
APA StyleLee, C. H., Shin, S., & Lee, S. I. (2024). 10-Eicosanol Alleviates Patulin-Induced Cell Cycle Arrest and Apoptosis by Activating AKT (Protein Kinase B) in Porcine Intestinal Epithelial Cells. International Journal of Molecular Sciences, 25(16), 8597. https://doi.org/10.3390/ijms25168597






