Association of Inflammation and Immune Cell Infiltration with Estrogen Receptor Alpha in an Estrogen and Ionizing Radiation-Induced Breast Cancer Model
Abstract
1. Introduction
2. Results
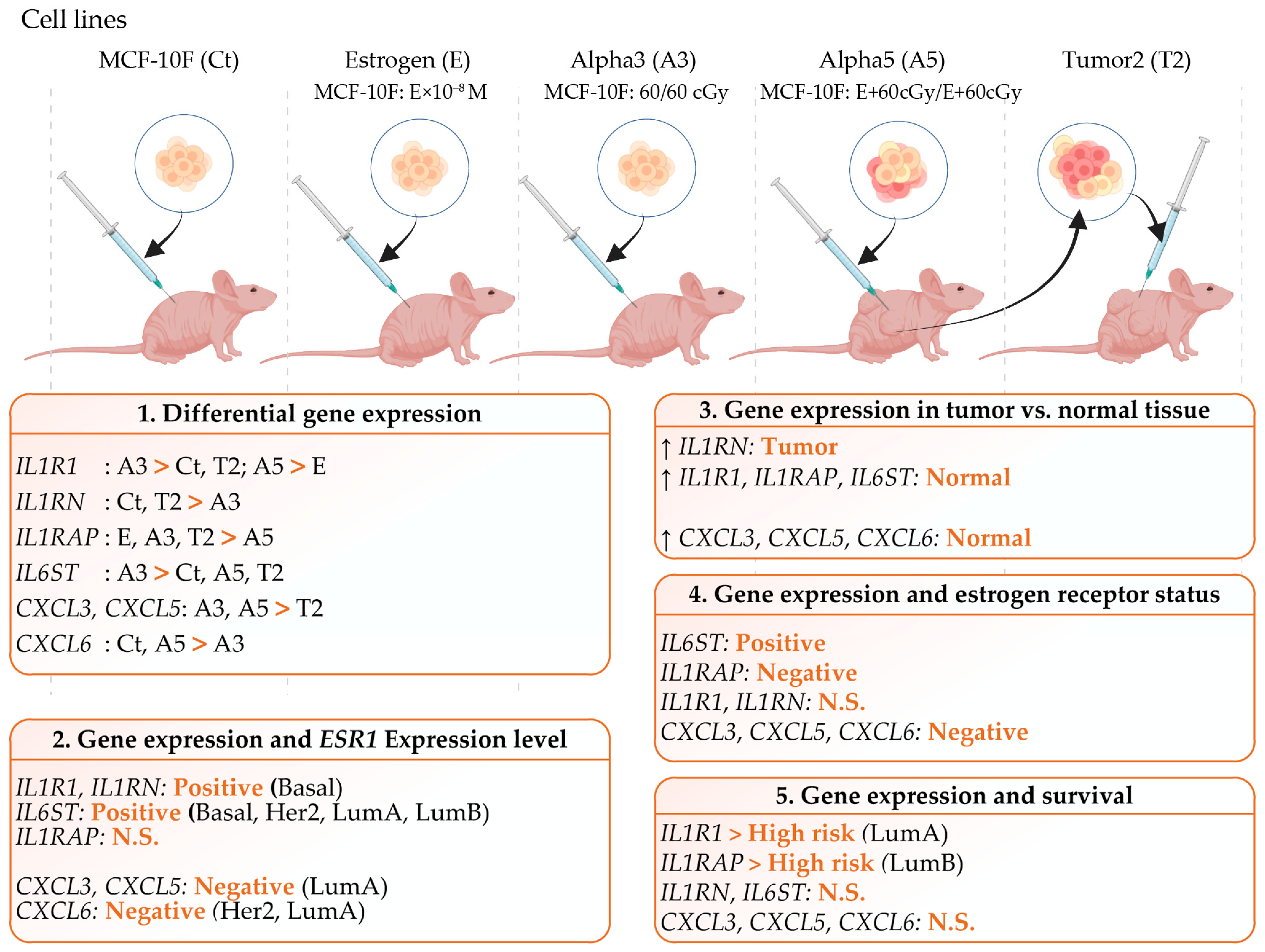
2.1. Differential Gene Expression in a Radiation and Estrogen Experimental Breast Cancer Model
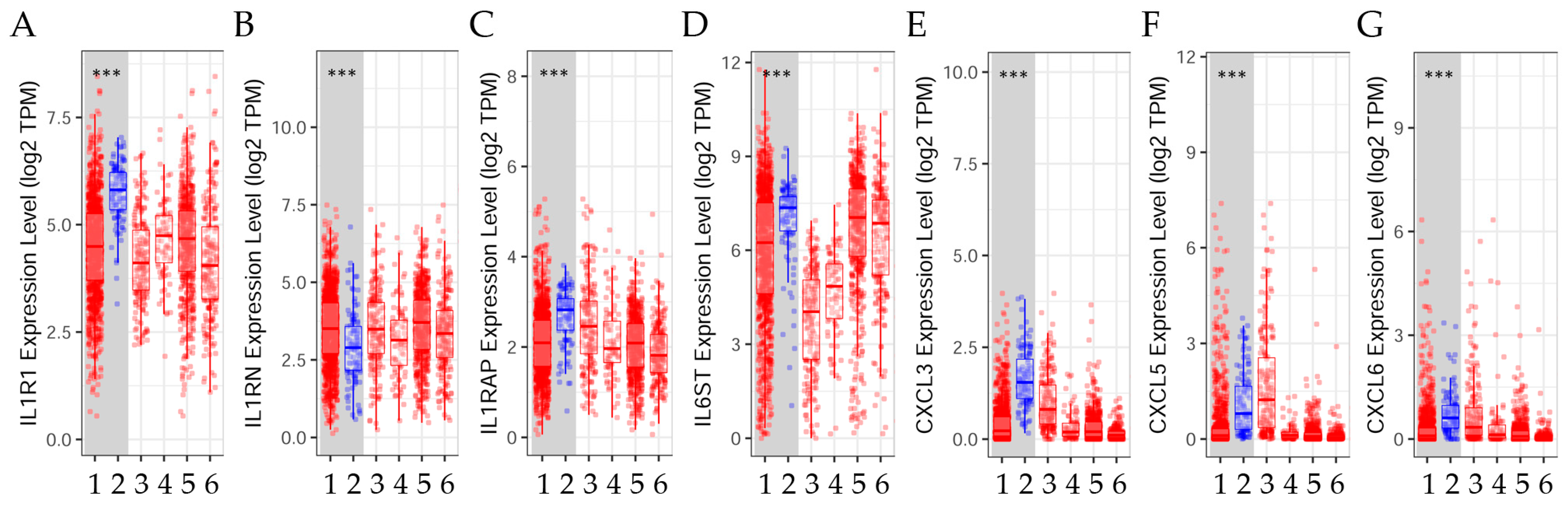
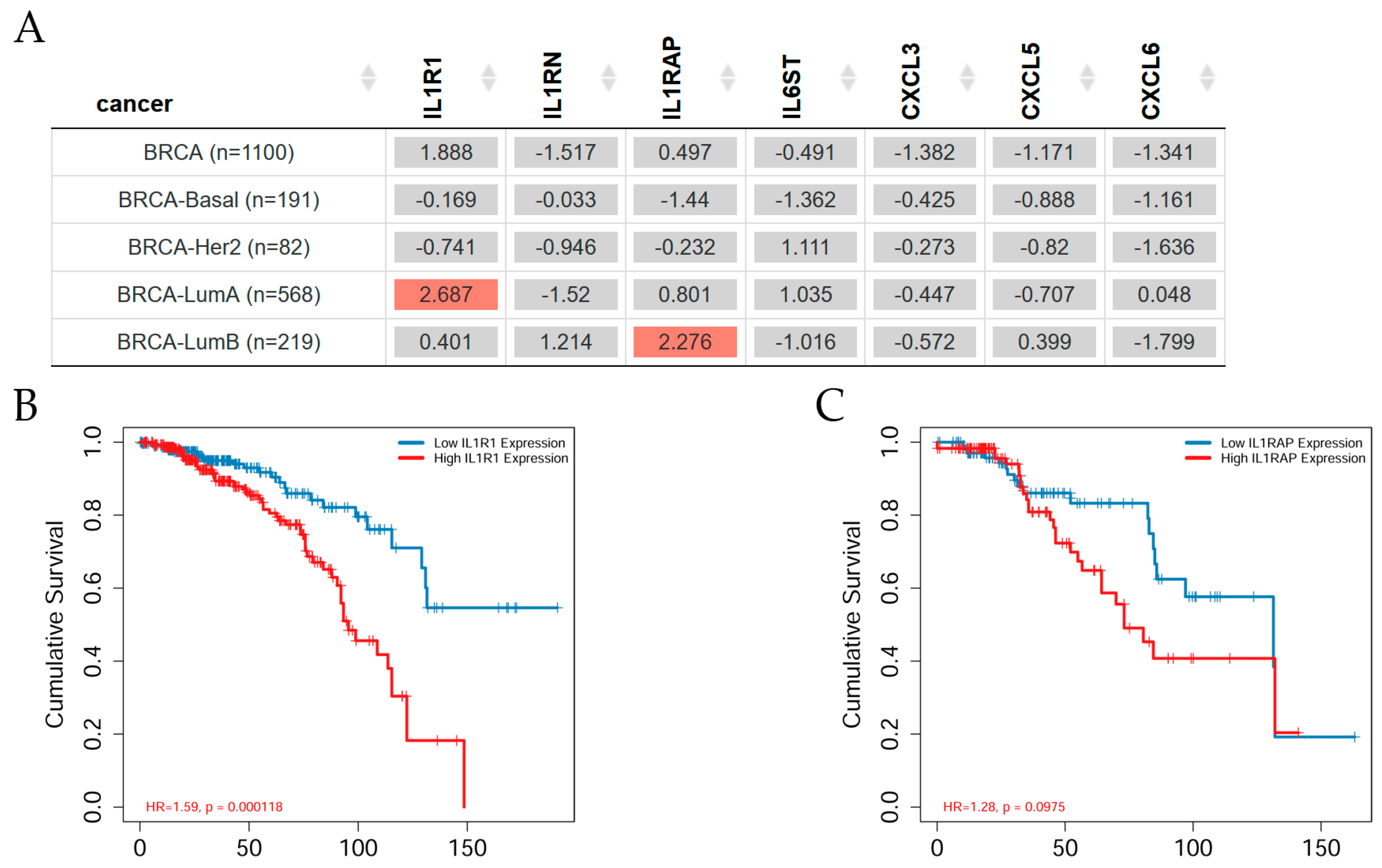
2.2. Gene Expression Levels in Tumor Versus Normal Tissues across Breast Cancer Subtypes
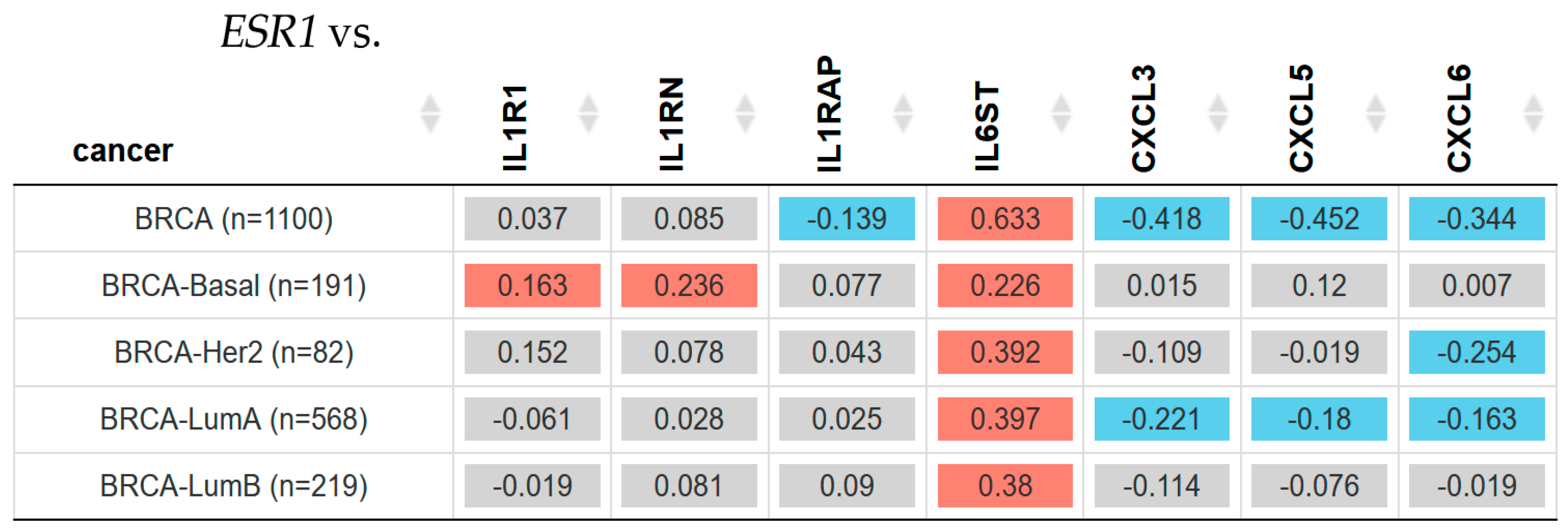
2.3. Gene Expression Associated with Inflammation and ESR1 Gene Expression in Breast Cancer Subtypes
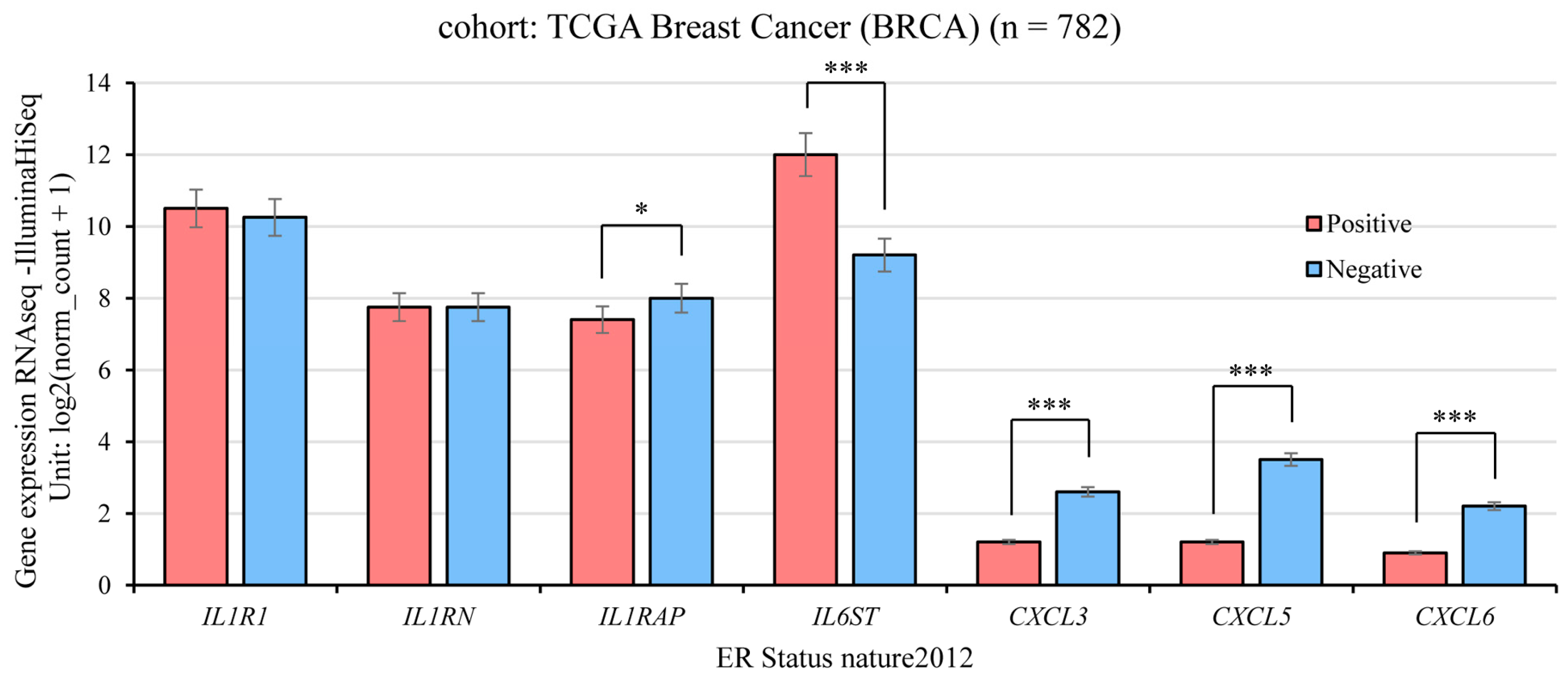
2.4. Gene Expression and ER Status in TCGA Breast Cancer
2.5. Gene Expression and Clinical Survival in Breast Cancer Subtypes
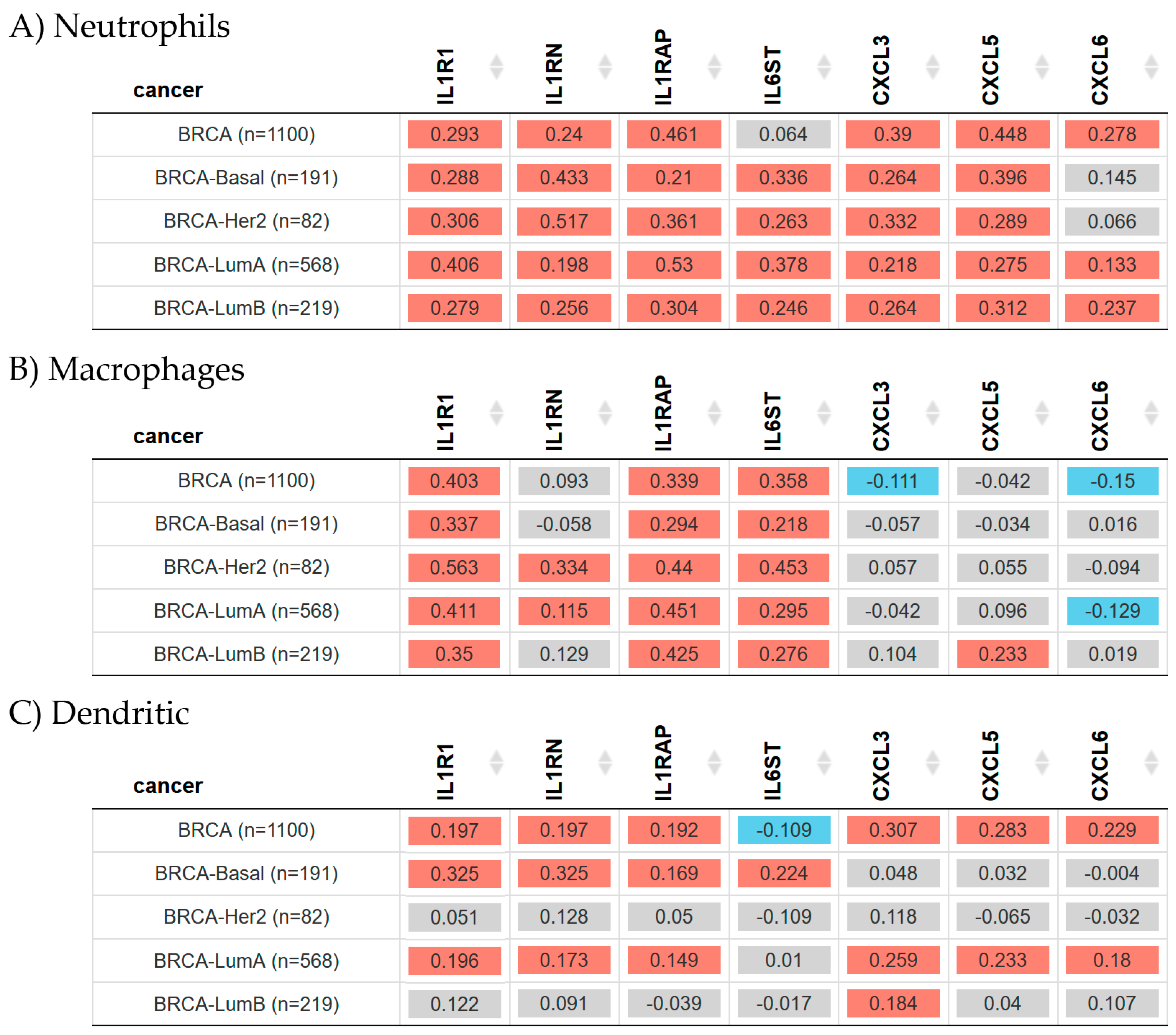
2.6. Gene Expression and Immune Infiltration in Breast Cancer
3. Discussion
4. Materials and Methods
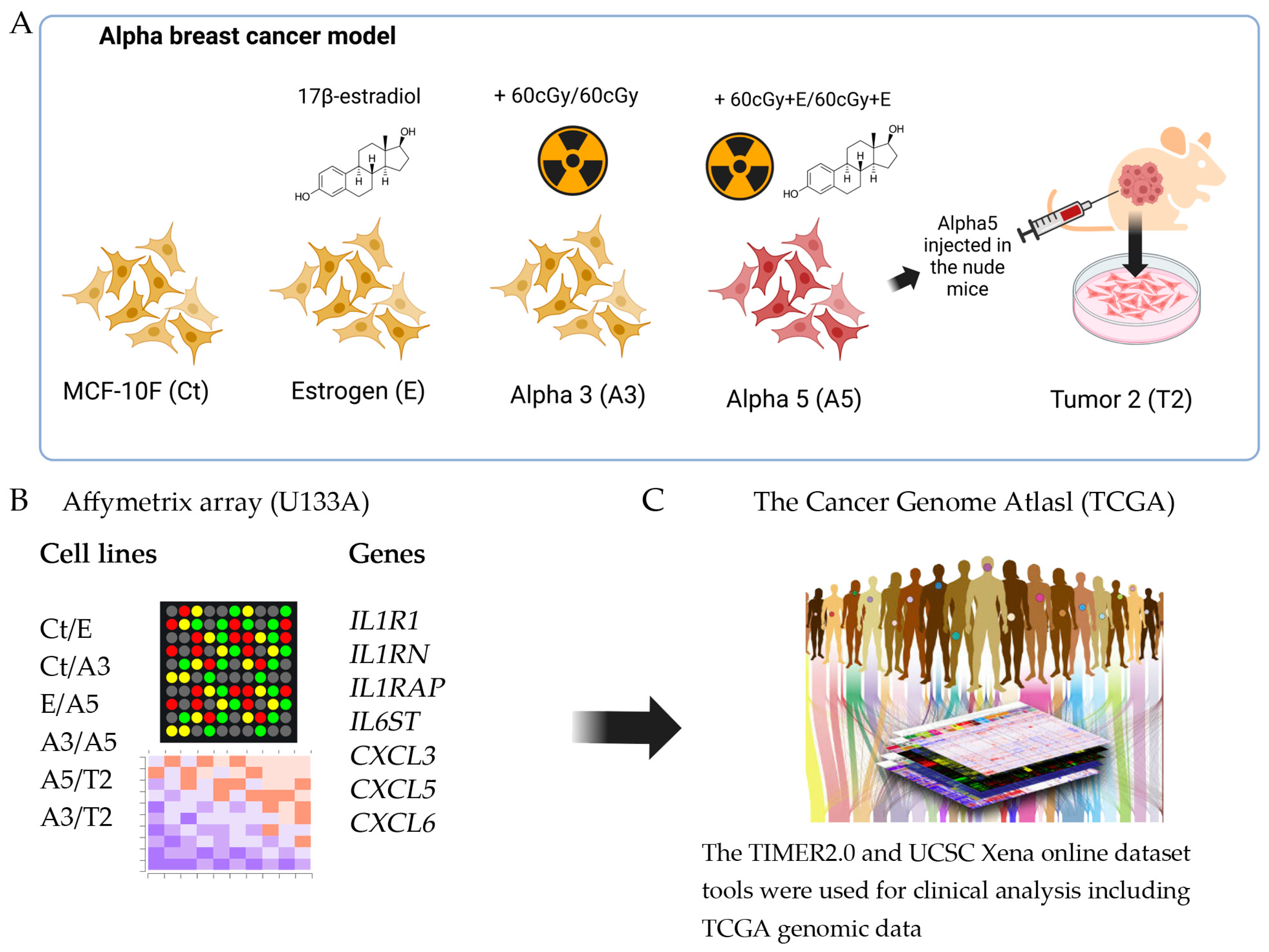
4.1. An Experimental Radiation- and Estrogen-Induced Breast Cancer Model, Named Alpha Model
4.2. Cell Lines
4.3. Affymetrix (U133A) Oligonucleotide Microarray
4.4. Bioinformatics
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IARC. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020; p. 596. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Polyak, K. Breast cancer: Origins and evolution. J. Clin. Investig. 2007, 117, 3155–3163. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sorlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Sorlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar] [CrossRef] [PubMed]
- Sorlie, T.; Wang, Y.; Xiao, C.; Johnsen, H.; Naume, B.; Samaha, R.R.; Borresen-Dale, A.L. Distinct molecular mechanisms underlying clinically relevant subtypes of breast cancer: Gene expression analyses across three different platforms. BMC Genom. 2006, 7, 127. [Google Scholar] [CrossRef]
- Calaf, G.M.; Crispin, L.A.; Munoz, J.P.; Aguayo, F.; Roy, D.; Narayan, G. Ionizing Radiation and Estrogen Affecting Growth Factor Genes in an Experimental Breast Cancer Model. Int. J. Mol. Sci. 2022, 23, 14284. [Google Scholar] [CrossRef] [PubMed]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schutz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar] [CrossRef]
- Xia, S.; Lin, Q. Estrogen Receptor Bio-Activities Determine Clinical Endocrine Treatment Options in Estrogen Receptor-Positive Breast Cancer. Technol. Cancer Res. Treat. 2022, 21, 15330338221090351. [Google Scholar] [CrossRef]
- Little, J.B. Radiation carcinogenesis. Carcinogenesis 2000, 21, 397–404. [Google Scholar] [CrossRef]
- Reisz, J.A.; Bansal, N.; Qian, J.; Zhao, W.; Furdui, C.M. Effects of ionizing radiation on biological molecules--mechanisms of damage and emerging methods of detection. Antioxid. Redox Signal. 2014, 21, 260–292. [Google Scholar] [CrossRef]
- Citrin, D.E.; Mitchell, J.B. Mechanisms of Normal Tissue Injury From Irradiation. Semin. Radiat. Oncol. 2017, 27, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Riches, A.C.; Herceg, Z.; Bryant, P.E.; Wynford-Thomas, D. Radiation-induced transformation of SV40-immortalized human thyroid epithelial cells by single and fractionated exposure to gamma-irradiation in vitro. Int. J. Radiat. Biol. 1994, 66, 757–765. [Google Scholar] [PubMed]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Dinarello, C.A.; Molgora, M.; Garlanda, C. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity 2019, 50, 778–795. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.E.; Smith, D.E. The IL-1 family: Regulators of immunity. Nat. Rev. Immunol. 2010, 10, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Xiaoping, X.; Cai, H.; Li, D.; Wang, J.; Fu, X.; Yu, F.; Sun, M.; Lv, Z. Cytokines as prognostic tool in breast carcinoma. Front. Biosci. 2011, 16, 2515–2526. [Google Scholar] [CrossRef] [PubMed]
- Fujiwaki, R.; Iida, K.; Nakayama, K.; Kanasaki, H.; Hata, K.; Katabuchi, H.; Okamura, H.; Miyazaki, K. Clinical significance of interleukin-1 receptor antagonist in patients with cervical carcinoma. Gynecol. Oncol. 2003, 89, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Barak, V.; Nisman, B.; Polliack, A.; Vannier, E.; Dinarello, C.A. Correlation of serum levels of interleukin-1 family members with disease activity and response to treatment in hairy cell leukemia. Eur. Cytokine Netw. 1998, 9, 33–39. [Google Scholar] [PubMed]
- Mustea, A.; Pirvulescu, C.; Konsgen, D.; Braicu, E.I.; Yuan, S.; Sun, P.; Lichtenegger, W.; Sehouli, J. Decreased IL-1 RA concentration in ascites is associated with a significant improvement in overall survival in ovarian cancer. Cytokine 2008, 42, 77–84. [Google Scholar] [CrossRef]
- Kaminska, J.; Kowalska, M.M.; Nowacki, M.P.; Chwalinski, M.G.; Rysinska, A.; Fuksiewicz, M. CRP, TNF-alpha, IL-1ra, IL-6, IL-8 and IL-10 in blood serum of colorectal cancer patients. Pathol. Oncol. Res. 2000, 6, 38–41. [Google Scholar] [CrossRef]
- Ebrahimi, B.; Tucker, S.L.; Li, D.; Abbruzzese, J.L.; Kurzrock, R. Cytokines in pancreatic carcinoma: Correlation with phenotypic characteristics and prognosis. Cancer 2004, 101, 2727–2736. [Google Scholar] [CrossRef] [PubMed]
- Ricote, M.; Garcia-Tunon, I.; Bethencourt, F.R.; Fraile, B.; Paniagua, R.; Royuela, M. Interleukin-1 (IL-1alpha and IL-1beta) and its receptors (IL-1RI, IL-1RII, and IL-1Ra) in prostate carcinoma. Cancer 2004, 100, 1388–1396. [Google Scholar] [CrossRef]
- Iwagaki, H.; Hizuta, A.; Tanaka, N. Interleukin-1 receptor antagonists and other markers in colorectal cancer patients. Scand. J. Gastroenterol. 1997, 32, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Miki, C. Profile of circulating levels of interleukin-1 receptor antagonist and interleukin-6 in colorectal cancer patients. Scand. J. Gastroenterol. 1999, 34, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Parekh, D.J.; Ankerst, D.P.; Baillargeon, J.; Higgins, B.; Platz, E.A.; Troyer, D.; Hernandez, J.; Leach, R.J.; Lokshin, A.; Thompson, I.M. Assessment of 54 biomarkers for biopsy-detectable prostate cancer. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1966–1972. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, S.; Stepien, T.; Kuzdak, K.; Stepien, H.; Krupinski, R.; Seehofer, D.; Rayes, N.; Ulrich, F. Serum levels of interleukin-1 receptor antagonist (IL-1ra) in thyroid cancer patients. Langenbecks Arch. Surg. 2008, 393, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, R.K.; Belec, L.; Soubrier, M.; Malapert, D.; Zuber, M.; Viard, J.P.; Intrator, L.; Degos, J.D.; Authier, F.J. Overproduction of proinflammatory cytokines imbalanced by their antagonists in POEMS syndrome. Blood 1996, 87, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Poch, B.; Lotspeich, E.; Ramadani, M.; Gansauge, S.; Beger, H.G.; Gansauge, F. Systemic immune dysfunction in pancreatic cancer patients. Langenbecks Arch. Surg. 2007, 392, 353–358. [Google Scholar] [CrossRef]
- Rose-John, S. Interleukin-6 Family Cytokines. Cold Spring Harb. Perspect. Biol. 2018, 10, a028415. [Google Scholar] [CrossRef]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Muller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef]
- Bravo, J.; Heath, J.K. Receptor recognition by gp130 cytokines. EMBO J. 2000, 19, 2399–2411. [Google Scholar] [CrossRef]
- Omokehinde, T.; Johnson, R.W. GP130 Cytokines in Breast Cancer and Bone. Cancers 2020, 12, 326. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Jenkins, B.J. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat. Rev. Immunol. 2018, 18, 773–789. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Bansal, Y.; Kumar, R.; Bansal, G. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorg. Med. Chem. 2020, 28, 115327. [Google Scholar] [CrossRef] [PubMed]
- Rossi, D.; Zlotnik, A. The biology of chemokines and their receptors. Annu. Rev. Immunol. 2000, 18, 217–242. [Google Scholar] [CrossRef] [PubMed]
- Freund, A.; Chauveau, C.; Brouillet, J.P.; Lucas, A.; Lacroix, M.; Licznar, A.; Vignon, F.; Lazennec, G. IL-8 expression and its possible relationship with estrogen-receptor-negative status of breast cancer cells. Oncogene 2003, 22, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F. Cancer and the chemokine network. Nat. Rev. Cancer 2004, 4, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Walser, T.C.; Fulton, A.M. The role of chemokines in the biology and therapy of breast cancer. Breast Dis. 2004, 20, 137–143. [Google Scholar] [CrossRef]
- Zhu, Y.M.; Woll, P.J. Mitogenic effects of interleukin-8/CXCL8 on cancer cells. Future Oncol. 2005, 1, 699–704. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Khusnurrokhman, G.; Wati, F.F. Tumor-promoting inflammation in lung cancer: A literature review. Ann. Med. Surg. 2022, 79, 104022. [Google Scholar] [CrossRef] [PubMed]
- Calaf, G.M.; Roy, D.; Jara, L.; Aguayo, F.; Crispin, L.A. Gene Signature Associated with Nervous System in an Experimental Radiation- and Estrogen-Induced Breast Cancer Model. Biomedicines 2023, 11, 3111. [Google Scholar] [CrossRef] [PubMed]
- Calaf, G.M.; Crispin, L.A.; Munoz, J.P.; Aguayo, F.; Narayan, G.; Roy, D. Cell Adhesion Molecules Affected by Ionizing Radiation and Estrogen in an Experimental Breast Cancer Model. Int. J. Mol. Sci. 2022, 23, 12674. [Google Scholar] [CrossRef] [PubMed]
- Calaf, G.M.; Hei, T.K. Establishment of a radiation- and estrogen-induced breast cancer model. Carcinogenesis 2000, 21, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.J.; Craft, B.; Hastie, M.; Repecka, K.; McDade, F.; Kamath, A.; Baberjee, A.; Luo, Y.; Rogers, D.; Brooks, A.N.; et al. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020, 38, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Apte, R.N.; Dotan, S.; Elkabets, M.; White, M.R.; Reich, E.; Carmi, Y.; Song, X.; Dvozkin, T.; Krelin, Y.; Voronov, E. The involvement of IL-1 in tumorigenesis, tumor invasiveness, metastasis, and tumor-host interactions. Cancer Metastasis Rev. 2006, 25, 387–408. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Voronov, E.; Dvorkin, T.; Fima, E.; Cagnano, E.; Benharroch, D.; Shendler, Y.; Bjorkdahl, O.; Segal, S.; Dinarello, C.A.; et al. Differential effects of IL-1 alpha and IL-1 beta on tumorigenicity patterns and invasiveness. J. Immunol. 2003, 171, 6448–6456. [Google Scholar] [CrossRef]
- Douvdevani, A.; Huleihel, M.; Zoller, M.; Segal, S.; Apte, R.N. Reduced tumorigenicity of fibrosarcomas which constitutively generate IL-1 alpha either spontaneously or following IL-1 alpha gene transfer. Int. J. Cancer 1992, 51, 822–830. [Google Scholar] [CrossRef]
- Apte, R.N.; Douvdevani, A.; Zoller, M.; White, R.M.; Dvorkin, T.; Shimoni, N.; Fima, E.; Hacham, M.; Huleihel, M.; Benharroch, D.; et al. Cytokine-induced tumor immunogenicity: Endogenous interleukin-1 alpha expressed by fibrosarcoma cells confers reduced tumorigenicity. Immunol. Lett. 1993, 39, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Voronov, E.; Weinstein, Y.; Benharroch, D.; Cagnano, E.; Ofir, R.; Dobkin, M.; White, R.M.; Zoller, M.; Barak, V.; Segal, S.; et al. Antitumor and immunotherapeutic effects of activated invasive T lymphoma cells that display short-term interleukin 1alpha expression. Cancer Res. 1999, 59, 1029–1035. [Google Scholar]
- Dvorkin, T.; Song, X.; Argov, S.; White, R.M.; Zoller, M.; Segal, S.; Dinarello, C.A.; Voronov, E.; Apte, R.N. Immune phenomena involved in the in vivo regression of fibrosarcoma cells expressing cell-associated IL-1alpha. J. Leukoc. Biol. 2006, 80, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Why not treat human cancer with interleukin-1 blockade? Cancer Metastasis Rev. 2010, 29, 317–329. [Google Scholar] [CrossRef]
- Voronov, E.; Shouval, D.S.; Krelin, Y.; Cagnano, E.; Benharroch, D.; Iwakura, Y.; Dinarello, C.A.; Apte, R.N. IL-1 is required for tumor invasiveness and angiogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 2645–2650. [Google Scholar] [CrossRef]
- Hong, J.H.; Chiang, C.S.; Tsao, C.Y.; Lin, P.Y.; McBride, W.H.; Wu, C.J. Rapid induction of cytokine gene expression in the lung after single and fractionated doses of radiation. Int. J. Radiat. Biol. 1999, 75, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Garlanda, C.; Dinarello, C.A.; Mantovani, A. The interleukin-1 family: Back to the future. Immunity 2013, 39, 1003–1018. [Google Scholar] [CrossRef]
- Muhlberg, T.; Joba, W.; Spitzweg, C.; Schworm, H.D.; Heberling, H.J.; Heufelder, A.E. Interleukin-1 receptor antagonist ribonucleic acid and protein expression by cultured Graves’ and normal orbital fibroblasts is differentially modulated by dexamethasone and irradiation. J. Clin. Endocrinol. Metab. 2000, 85, 734–742. [Google Scholar] [CrossRef][Green Version]
- Iwakawa, M.; Hamada, N.; Imadome, K.; Funayama, T.; Sakashita, T.; Kobayashi, Y.; Imai, T. Expression profiles are different in carbon ion-irradiated normal human fibroblasts and their bystander cells. Mutat. Res. 2008, 642, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Raskova, M.; Lacina, L.; Kejik, Z.; Venhauerova, A.; Skalickova, M.; Kolar, M.; Jakubek, M.; Rosel, D.; Smetana, K., Jr.; Brabek, J. The Role of IL-6 in Cancer Cell Invasiveness and Metastasis-Overview and Therapeutic Opportunities. Cells 2022, 11, 3698. [Google Scholar] [CrossRef]
- Dawes, J.M.; Antunes-Martins, A.; Perkins, J.R.; Paterson, K.J.; Sisignano, M.; Schmid, R.; Rust, W.; Hildebrandt, T.; Geisslinger, G.; Orengo, C.; et al. Genome-wide transcriptional profiling of skin and dorsal root ganglia after ultraviolet-B-induced inflammation. PLoS ONE 2014, 9, e93338. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Adachi, A.; Honda, T.; Egawa, G.; Kanameishi, S.; Takimoto, R.; Miyake, T.; Hossain, M.R.; Komine, M.; Ohtsuki, M.; Gunzer, M.; et al. Estradiol suppresses psoriatic inflammation in mice by regulating neutrophil and macrophage functions. J. Allergy Clin. Immunol. 2022, 150, 909–919 e908. [Google Scholar] [CrossRef]
- Mosly, D.; MacLeod, K.; Moir, N.; Turnbull, A.; Sims, A.H.; Langdon, S.P. Variation in IL6ST cytokine family function and the potential of IL6 trans-signalling in ERalpha positive breast cancer cells. Cell. Signal. 2023, 103, 110563. [Google Scholar] [CrossRef]
- Kovats, S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell. Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Feldman, I.; Feldman, G.M.; Mobarak, C.; Dunkelberg, J.C.; Leslie, K.K. Identification of proteins within the nuclear factor-kappa B transcriptional complex including estrogen receptor-alpha. Am. J. Obstet. Gynecol. 2007, 196, 394.e1–394.e13. [Google Scholar] [CrossRef]
- Kalaitzidis, D.; Gilmore, T.D. Transcription factor cross-talk: The estrogen receptor and NF-kappaB. Trends Endocrinol. Metab. 2005, 16, 46–52. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef]
- Ehling, J.; Tacke, F. Role of chemokine pathways in hepatobiliary cancer. Cancer Lett. 2016, 379, 173–183. [Google Scholar] [CrossRef]
- Allavena, P.; Germano, G.; Marchesi, F.; Mantovani, A. Chemokines in cancer related inflammation. Exp. Cell Res. 2011, 317, 664–673. [Google Scholar] [CrossRef]
- Bates, J.P.; Derakhshandeh, R.; Jones, L.; Webb, T.J. Mechanisms of immune evasion in breast cancer. BMC Cancer 2018, 18, 556. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; An, R.; Yu, S.; He, J.; Zhang, H. A novel immune subtype classification of ER-positive, PR-negative, and HER2-negative breast cancer based on the genomic and transcriptomic landscape. J. Transl. Med. 2021, 19, 398. [Google Scholar] [CrossRef] [PubMed]
- Soule, H.D.; Maloney, T.M.; Wolman, S.R.; Peterson, W.D., Jr.; Brenz, R.; McGrath, C.M.; Russo, J.; Pauley, R.J.; Jones, R.F.; Brooks, S.C. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res. 1990, 50, 6075–6086. [Google Scholar] [PubMed]
- Calaf, G.; Russo, J. Transformation of human breast epithelial cells by chemical carcinogens. Carcinogenesis 1993, 14, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Calaf, G.M.; Roy, D. Gene and protein expressions induced by 17beta-estradiol and parathion in cultured breast epithelial cells. Mol. Med. 2007, 13, 255–265. [Google Scholar] [CrossRef]
- Calaf, G.M.; Roy, D. Cell adhesion proteins altered by 17beta estradiol and parathion in breast epithelial cells. Oncol. Rep. 2008, 19, 165–169. [Google Scholar]
- Hei, T.K.; Zhao, Y.L.; Roy, D.; Piao, C.Q.; Calaf, G.; Hall, E.J. Molecular alterations in tumorigenic human bronchial and breast epithelial cells induced by high LET radiation. Adv. Space Res. 2001, 27, 411–419. [Google Scholar] [CrossRef]
- Calaf, G.; Hei, T.K. Oncoprotein expression in human breast epithelial cells transformed by high-LET radiation. Int. J. Radiat. Biol. 2001, 77, 31–40. [Google Scholar]
- Calaf, G.M.; Roy, D.; Hei, T.K. Immunochemical analysis of protein expression in breast epithelial cells transformed by estrogens and high linear energy transfer (LET) radiation. Histochem. Cell Biol. 2005, 124, 261–274. [Google Scholar] [CrossRef]
- Califano, A. SPLASH: Structural pattern localization analysis by sequential histograms. Bioinformatics 2000, 16, 341–357. [Google Scholar] [CrossRef][Green Version]
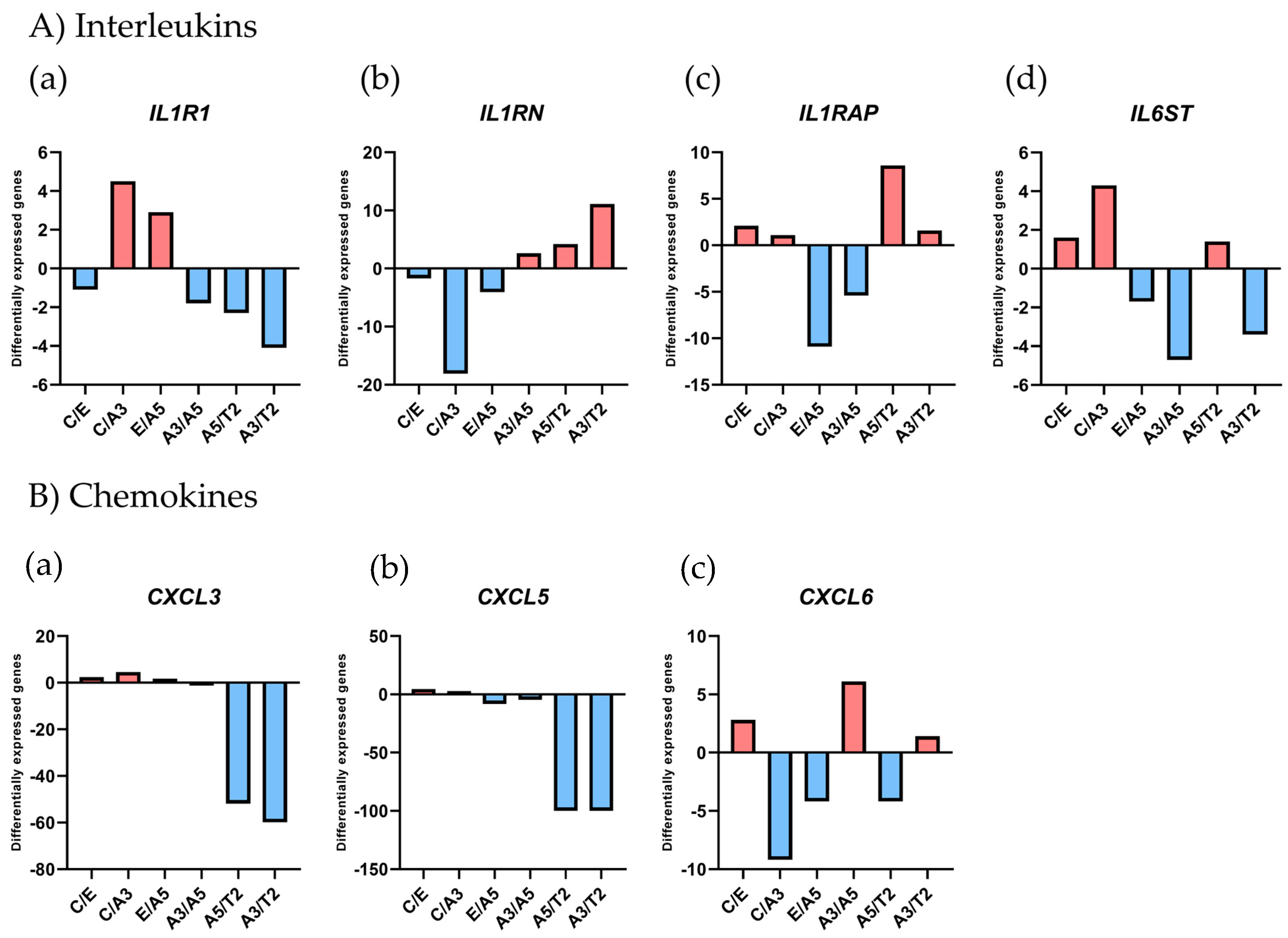
| GeneBank | Gene Symbol | Ct/E | Ct/A3 | E/A5 | A3/A5 | A5/T2 | A3/T2 |
|---|---|---|---|---|---|---|---|
| NM_000877 | IL1R1 | −1.1 | 4.5 | 2.9 | −1.8 | −2.3 | −4.1 |
| NM_002182 | IL1RAP | 2.1 | 1.1 | −10.9 | −5.4 | 8.6 | 1.6 |
| AW0833557 | IL1RN | −1.7 | −18.1 | −4.1 | 2.6 | 4.2 | 11.1 |
| NM_002184 | IL6ST | 1.6 | 4.3 | −1.7 | −4.7 | 1.4 | −3.4 |
| NM_002090 | CXCL3 | 2.4 | 4.6 | 1.7 | −1.2 | −51.9 | −59.9 |
| BG166705 | CXCL5 | 4.6 | 2.7 | −8.3 | −4.8 | −100 | −100 |
| NM_002993 | CXCL6 | 2.8 | −9.2 | −4.2 | 6.1 | −4.2 | 1.4 |
| Cell lines | Agar a | Invasion b | Tumorigenicity c | Immunocytochemistry d | Classification e | ||
|---|---|---|---|---|---|---|---|
| ER | PgR | ErbB2 | |||||
| MCF-10F | − | − | − | − | − | − | normal |
| MCF-10F + E | − | − | − | − | − | − | normal |
| A3 | + | + | − | + | + | + | malignant |
| A5 | + | + | + | + | + | + | tumorigenic |
| T2 | + | + | + | + | + | + | tumorigenic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koning, T.; Calaf, G.M. Association of Inflammation and Immune Cell Infiltration with Estrogen Receptor Alpha in an Estrogen and Ionizing Radiation-Induced Breast Cancer Model. Int. J. Mol. Sci. 2024, 25, 8604. https://doi.org/10.3390/ijms25168604
Koning T, Calaf GM. Association of Inflammation and Immune Cell Infiltration with Estrogen Receptor Alpha in an Estrogen and Ionizing Radiation-Induced Breast Cancer Model. International Journal of Molecular Sciences. 2024; 25(16):8604. https://doi.org/10.3390/ijms25168604
Chicago/Turabian StyleKoning, Tania, and Gloria M. Calaf. 2024. "Association of Inflammation and Immune Cell Infiltration with Estrogen Receptor Alpha in an Estrogen and Ionizing Radiation-Induced Breast Cancer Model" International Journal of Molecular Sciences 25, no. 16: 8604. https://doi.org/10.3390/ijms25168604
APA StyleKoning, T., & Calaf, G. M. (2024). Association of Inflammation and Immune Cell Infiltration with Estrogen Receptor Alpha in an Estrogen and Ionizing Radiation-Induced Breast Cancer Model. International Journal of Molecular Sciences, 25(16), 8604. https://doi.org/10.3390/ijms25168604







