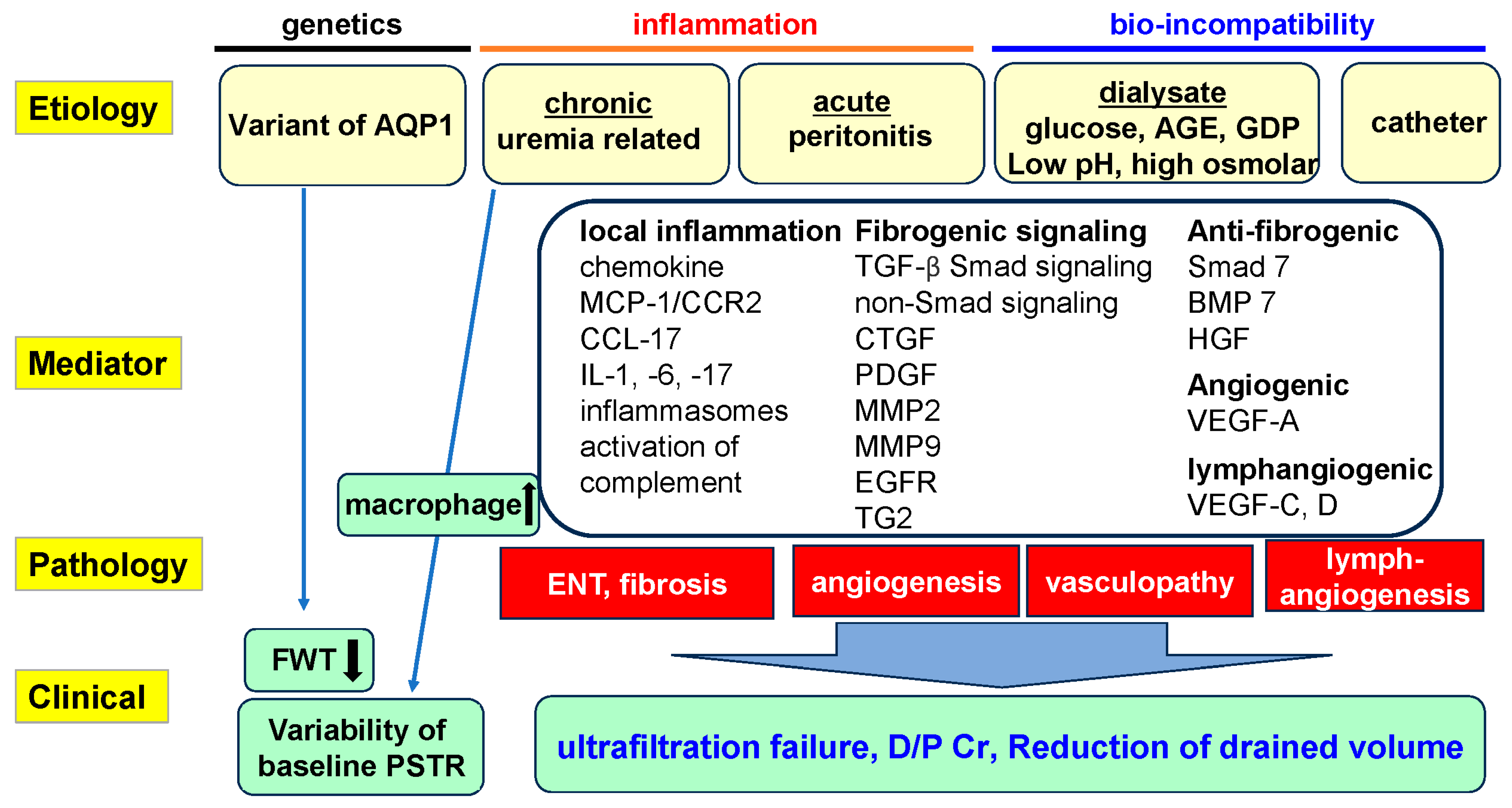
Pathophysiological Mechanisms of Peritoneal Fibrosis and Peritoneal Membrane Dysfunction in Peritoneal Dialysis
Abstract
1. Introduction
2. History and Characteristics of Human Peritoneal Membrane Alterations in PD
3. Peritoneal Membrane Dysfunction and Pathological Changes in PD: Roles of Fibrosis, Angiogenesis, and Lymphangiogenesis
3.1. Angiogenesis, Peritoneal Fibrosis, and Vasculopathy
3.1.1. Peritoneal Fibrosis and Angiogenesis
3.1.2. Relationship between Peritoneal Fibrosis and Angiogenesis
3.1.3. Vasculopathy
3.2. Major Factors Inducing Peritoneal Fibrosis and Injury
3.2.1. Peritonitis
3.2.2. AGEs as Bioincompatible Factors in PDF
3.3. Relationship between Peritoneal Fibrosis and Lymphangiogenesis
3.4. Changes to Peritoneal Membrane Transport in PD
4. Roles of Cytokines and Growth Factors in Peritoneal Inflammation and Fibrosis in PD
4.1. Inflammation
4.2. Macrophage Infiltration
4.3. Inflammatory Cytokines
4.4. Inflammasomes
4.5. Growth Factors for Development of Peritoneal Fibrosis (Table 1)
4.5.1. TGF-β
| Target | Intervention | Model | Results | References |
|---|---|---|---|---|
| TGF-β | Gene induction | Rat | fibrosis↑, angiogenesis↑, peritoneal function↓ | [89] |
| Receptor inhibitor | Mouse, PD solution | fibrosis↓, angiogenesis↓, peritoneal function↑ | [90] | |
| Receptor inhibitor | Rat, CG | fibrosis↓, VEGF-A/C↓, angiogenesis/lymphangiogenesis↓ | [40,53] | |
| CTGF | Monoclonal antibody | Mouse, CG | fibrosis↓, VEGF-A↓, angiogenesis↓ | [92] |
| Genetic deletion | Mouse, CG | fibrosis↓, inflammation↓, angiogenesis/lymphangiogenesis↓, peritoneal function↑ | [34,93] | |
| EGFR | Receptor inhibitor | Rat, CG | fibrosis↓, angiogenesis↓, TGF-β signaling↓ | [94] |
| PDGF-B | Gene induction | Rat | VEGF-A↑, angiogenesis↑ | [95] |
| Core fucosylation | shRNA | Rat, PD solution | fibrosis↓, TGF-β/PDGF/EGFR signaling↓ | [96,97] |
| MMP9 | Genetic deletion | Mouse, TGF-β1 gene transfer | VEGF-A↓, angiogenesis↓ | [98] |
| MMP10 | Genetic deletion | Mouse, CG | fibrosis↓, inflammation↓, VEGF-A↓, angiogenesis↓, peritoneal function↑ | [99] |
| TG2 | Genetic deletion | Mouse, CG | fibrosis↓, angiogenesis↓, EMT↓ | [60] |
| Smad7 | Gene induction | Rat, uremia, PD solution | fibrosis↓, peritoneal function↑, Smad2/3 activation↓ | [100,101] |
| BMP7 | Recombinant protein | Rat, uremia, CG | fibrosis↓, inflammation↓, angiogenesis↓, Smad3 pathway↓ | [102] |
| HGF | Recombinant protein | Rat, PD solution | fibrosis↓, AGEs↓, TGF-β1↓, VEGF-A↓, angiogenesis↓ | [103] |
4.5.2. CTGF
4.5.3. Other Fibrogenic Factors
4.6. Anti-Fibrogenic Factors
5. Roles of Complement Activation in Peritoneal Inflammation and Fibrosis in PD
5.1. Complement System in the Process of Fibrosis and the Fibrinolytic System
| Molecule | Molecular Weight | Regulatory Point | Distribution in Peritoneum | References |
|---|---|---|---|---|
| Fluid phase | ||||
| C1 inhibitor (C1-INH, C1 inactivator) | ~80 kDa, | CP * | N/A ***** | N/A |
| ~100 kDa | ||||
| factor I (CFI) | α chain 50 kDa, | C3 convertase | N/A | N/A |
| β chain 38 kDa | ||||
| C4-binding protein (C4BP) | ~ 500 kDa | CP | N/A | N/A |
| factor H (CFH) | ~155 kDa | AP **, C3 convertase | N/A | N/A |
| vitronectin (S-protein, epibolin, plasminogen activator inhibitor (PAI)-1-binding protein) | ~75 kDa | TP *** | N/A | N/A |
| clusterin | 80 kDa | TP | N/A | N/A |
| carboxypeptidase N | 50 kDa, 85 kDa | anaphylatoxin | N/A | N/A |
| carboxypeptidase R **** (carboxypeptidase B) | 60 kDa | anaphylatoxin | N/A | N/A |
| Solid phase | ||||
| membrane cofactor protein (MCP, CD46) | ~65 kDa, ~55 kDa | C3 convertase | Mesothelial cells | [132,133] |
| Endothelial cells | ||||
| decay accelerating factor (DAF, CD55) | ~70 kDa | C3 convertase | Mesothelial cells | [132,133] |
| Endothelial cells | ||||
| complement receptor 1 (CD35) | 160-over 250 kDa | C3 convertase | N/A | N/A |
| CD59 | ~20 kDa | TP | Mesothelial cells | [132,133] |
| Endothelial cells | ||||
5.2. Peritoneal Fibrosis Associated with Complement Activation
6. Peritoneal Pathological Findings in EPS and Effects of PD Solutions on Pathologies of the Peritoneal Membrane
6.1. Peritoneal Pathological Changes of EPS (Table 3A)
| (A) | ||||||
| Number of Patients | PD Duration | Peritoneal Thickness | Vasculopathy | Vessel Density | Year | |
| Garosi et al. [160] | Non-EPS (N = 180) EPS (N = 39) | on PD for 6 months to 12 years | 75 (10–70) μm 750 (250–4000) μm (p < 0.01) | Prevalence of vasculopathy 11% 100% (p < 0.01) | 2005 | |
| Alscher et al. [161] | Non-EP: (N = 26) EPS (N = 9) | 19 ± 28 months 93 ± 27 months | Peritoneal thickness was higher in EPS group. (p < 0.001) | No significant difference. | No significant difference. | 2007 |
| Sherif et al. [162] | Non-EPS (N = 13) EPS (N = 12) | 106.3 ± 20.9 months 116.3 ± 38.2 months | 527.2 ± 457.4 μm 552.3 ± 331.9 μm (p = 0.5) | No significant differences. | 36.6 ± 27.1 /mm2 37.2 ± 21.9 /mm2 (p = 0.6) | 2008 |
| Braun et al. [163] | Non-EPS (N = 27) EPS (N = 31) | 37.6 ± 38.0 months 77.5 ± 41.2 months | 602.9 μm 1132.5 μm (p = 0.0031) | Prevalence of vasculopathy 26% 35% (p = 0.40) | 41% 52% (p = 0.61) | 2012 |
| Morelle et al. [26] | Non-EPS (N = 28) EPS (N = 7) | 57.8 ± 7.6 months 55.9 ± 4.2 months | Submethotelial thickness was higher in EPS group (p < 0.01). | Vasculopathy grade was higher in EPS group (p < 0.05). | Higher in EPS group (p < 0.01). | 2015 |
| Tawada et al. [164] | EPS-conventional solution (N = 28) EPS-pH neutral solution (N = 7) | 132.9 (105.4–157.2) months 63.1 (29.8–83.1) months | 528.4 (359.3–798.4) μm 348.1 (314.6–812.0) μm (p = 0.4). | L/V ratio 0.00 (0.00–0.51) 0.67 (0.64–0.78) (<0.001). | 12.5 (5.7–23.7)/mm 29.0 (12.3–56.0)/mm (p < 0.05). | 2021 |
| (B) | ||||||
| Number of Patients | PD Duration | Peritoneal Thickness | Vasculopathy | Vessel Density | Year | |
| Kawanishi et al. [165] | Conventional solution (N = 12) pH-neutral solution (N = 12) | 57.0 ± 5.97 months 51.9 ± 5.9 months | 482.5 ± 24.3 μm 281.4 ± 34.4 μm (p < 0.05) | L/V 0.50 ± 0.03 0.86 ± 0.03 (p < 0.05) | Vessel density 30.6 ± 3.5 /mm2 90.4 ± 3.3 /mm2 (p < 0.05) | 2013 |
| Hamada et al. [166] | Conventional solution (N = 80) pH-neutral solution (N = 61) | 62.5 ± 43.3 months 33.6 ± 23.1 months | No significant difference for at least 60 months. | Patency in neutral group was significantly higher compared to that in acidic group. | 2015 | |
| del Peso et al. [167] | Conventional solution (N = 23) pH-neutral solution (N = 23) | 24.2 ± 18 months 22.7 ± 16 months | Prevalence of fibrosis 69.6% 47.8% (p = 0.13) | Prevalence of vasculopathy 30.4% 4.3% (p = 0.02) | 2016 | |
| Tawada et al. [45] | Conventional solution (N = 54) pH-neutral solution (N = 73) | 102 (75.0–132.0) months 44 (19.0–72.0) months | 375.0 (274.06–602.00) μm 244.0 (154.68–390.25) μm (p < 0.001) | L/V 0.50 ± 0.17 0.76 ± 0.06 (p < 0.001) | CD31-vessels (number/mm) 14.0 (10.28–17.07) 12.4 (7.68–17.18) (p = 0.354) | 2019 |
| Sugiyama et al. [168] | Conventional solution (N = 11) pH-neutral solution (N = 11) | 111.9 ± 22.1 month 98.6 ± 18.2 months | 395.3 (351.1–516.4) μm 281.4 (150.1–419.3) μm (not significant) | L/V 0.43 ± 0.18 0.73 ± 0.08 (p < 0.05) | Vessels (number/mm) 16.3 ± 6.1 11.5 ± 3.0 (p < 0.05) | 2022 |
6.2. Effects of PD Solution in the Peritoneal Membrane (Table 3B)
7. Prevention and Summary
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AGEs | advanced glycation end products |
| AQP1 | aquaporin-1 |
| CCL2 | C-C motif chemokine ligand 2 |
| CTGF/CCN2 | connective tissue growth factor/cellular communication network factor 2 |
| D/P Cr | dialysate-to-plasma ratio of creatinine |
| ECM | extracellular matrix |
| EMT | epithelial–mesenchymal transition |
| EPS | encapsulating peritoneal sclerosis |
| GDPs | glucose degradation products |
| HPMCs | human peritoneal mesothelial cells |
| IL | interleukin |
| L/V ratio | ratio of luminal diameter (L) to vessel diameter (V) |
| MAC | membrane attack complex |
| MMPs | matrix metalloproteinases |
| PCV | post-capillary venule |
| PD | peritoneal dialysis |
| peritoneal dialysis fluid | |
| pH-neutral solution | low-GDP pH-neutral solution |
| Smad | Suppressor of mothers against decapentaplegic |
| STAT3 | signal transducers and activators of transcription 3 |
| TGF-β | transforming growth factor-β |
| TNF-α | tumor necrosis factor α |
| UFF | ultrafiltration failure |
| VEGF | vascular endothelial growth factor |
References
- Li, P.K.; Chow, K.M.; Van de Luijtgaarden, M.W.; Johnson, D.W.; Jager, K.J.; Mehrotra, R.; Naicker, S.; Pecoits-Filho, R.; Yu, X.Q.; Lameire, N. Changes in the worldwide epidemiology of peritoneal dialysis. Nat. Rev. Nephrol. 2017, 13, 90–103. [Google Scholar] [CrossRef]
- Lambie, M.; Zhao, J.; McCullough, K.; Davies, S.J.; Kawanishi, H.; Johnson, D.W.; Sloand, J.A.; Sanabria, M.; Kanjanabuch, T.; Kim, Y.L.; et al. Variation in Peritoneal Dialysis Time on Therapy by Country: Results from the Peritoneal Dialysis Outcomes and Practice Patterns Study. Clin. J. Am. Soc. Nephrol. 2022, 17, 861–871. [Google Scholar] [CrossRef]
- IRODaT, International Registry in Organ Donation and Transplantation. Available online: https://www.irodat.org/?p=database)%20(% (accessed on 30 March 2024).
- Mizuno, M.; Ito, Y.; Tanaka, A.; Suzuki, Y.; Hiramatsu, H.; Watanabe, M.; Tsuruta, Y.; Matsuoka, T.; Ito, I.; Tamai, H.; et al. Peritonitis is still an important factor for withdrawal from peritoneal dialysis therapy in the Tokai area of Japan. Clin. Exp. Nephrol. 2011, 15, 727–737. [Google Scholar] [CrossRef]
- Mizuno, M.; Ito, Y.; Suzuki, Y.; Sakata, F.; Saka, Y.; Hiramatsu, T.; Tamai, H.; Mizutani, M.; Naruse, T.; Ohashi, N.; et al. Recent analysis of status and outcomes of peritoneal dialysis in the Tokai area of Japan: The second report of the Tokai peritoneal dialysis registry. Clin. Exp. Nephrol. 2016, 20, 960–971. [Google Scholar] [CrossRef]
- Ateş, K.; Nergizoğlu, G.; Keven, K.; Sen, A.; Kutlay, S.; Ertürk, S.; Duman, N.; Karatan, O.; Ertuğ, A.E. Effect of fluid and sodium removal on mortality in peritoneal dialysis patients. Kidney Int. 2001, 60, 767–776. [Google Scholar] [CrossRef]
- Van Biesen, W.; Verger, C.; Heaf, J.; Vrtovsnik, F.; Britto, Z.M.L.; Do, J.Y.; Prieto-Velasco, M.; Martínez, J.P.; Crepaldi, C.; De Los Ríos, T.; et al. Evolution Over Time of Volume Status and PD-Related Practice Patterns in an Incident Peritoneal Dialysis Cohort. Clin. J. Am. Soc. Nephrol. 2019, 14, 882–893. [Google Scholar] [CrossRef]
- Tabinor, M.; Elphick, E.; Dudson, M.; Kwok, C.S.; Lambie, M.; Davies, S.J. Bioimpedance-defined overhydration predicts survival in end stage kidney failure (ESKF): Systematic review and subgroup meta-analysis. Sci. Rep. 2018, 8, 4441. [Google Scholar] [CrossRef]
- Devuyst, O.; Margetts, P.J.; Topley, N. The pathophysiology of the peritoneal membrane. J. Am. Soc. Nephrol. 2010, 21, 1077–1085. [Google Scholar] [CrossRef]
- Honda, K.; Hamada, C.; Nakayama, M.; Miyazaki, M.; Sherif, A.M.; Harada, T.; Hirano, H. Impact of uremia, diabetes, and peritoneal dialysis itself on the pathogenesis of peritoneal sclerosis: A quantitative study of peritoneal membrane morphology. Clin. J. Am. Soc. Nephrol. 2008, 3, 720–728. [Google Scholar] [CrossRef]
- Terri, M.; Trionfetti, F.; Montaldo, C.; Cordani, M.; Tripodi, M.; Lopez-Cabrera, M.; Strippoli, R. Mechanisms of Peritoneal Fibrosis: Focus on Immune Cells-Peritoneal Stroma Interactions. Front. Immunol. 2021, 12, 607204. [Google Scholar] [CrossRef]
- Schaefer, B.; Bartosova, M.; Macher-Goeppinger, S.; Ujszaszi, A.; Wallwiener, M.; Nyarangi-Dix, J.; Sallay, P.; Burkhardt, D.; Querfeld, U.; Pfeifle, V.; et al. Quantitative Histomorphometry of the Healthy Peritoneum. Sci. Rep. 2016, 6, 21344. [Google Scholar] [CrossRef]
- Dobbie, J.W.; Zaki, M.; Wilson, L. Ultrastructural studies on the peritoneum with special reference to chronic ambulatory peritoneal dialysis. Scott Med. J. 1981, 26, 213–223. [Google Scholar] [CrossRef]
- Pollock, C.A.; Ibels, L.S.; Eckstein, R.P.; Graham, J.C.; Caterson, R.J.; Mahony, J.F.; Sheil, A.G. Peritoneal morphology on maintenance dialysis. Am. J. Nephrol. 1989, 9, 198–204. [Google Scholar] [CrossRef]
- Di Paolo, N.; Sacchi, G. Peritoneal vascular changes in continuous ambulatory peritoneal dialysis (CAPD): An in vivo model for the study of diabetic microangiopathy. Perit. Dial. Int. 1989, 9, 41–45. [Google Scholar] [CrossRef]
- Honda, K.; Nitta, K.; Horita, S.; Yumura, W.; Nihei, H. Morphological changes in the peritoneal vasculature of patients on CAPD with ultrafiltration failure. Nephron 1996, 72, 171–176. [Google Scholar] [CrossRef]
- Plum, J.; Hermann, S.; Fusshöller, A.; Schoenicke, G.; Donner, A.; Röhrborn, A.; Grabensee, B. Peritoneal sclerosis in peritoneal dialysis patients related to dialysis settings and peritoneal transport properties. Kidney Int. Suppl. 2001, 78, S42–S47. [Google Scholar] [CrossRef]
- Williams, J.D.; Craig, K.J.; Topley, N.; Von Ruhland, C.; Fallon, M.; Newman, G.R.; Mackenzie, R.K.; Williams, G.T. Morphologic changes in the peritoneal membrane of patients with renal disease. J. Am. Soc. Nephrol. 2002, 13, 470–479. [Google Scholar] [CrossRef]
- Williams, J.D.; Craig, K.J.; Topley, N.; Williams, G.T. Peritoneal dialysis: Changes to the structure of the peritoneal membrane and potential for biocompatible solutions. Kidney Int. Suppl. 2003, 63, S158–S161. [Google Scholar] [CrossRef]
- Tăranu, T.; Florea, L.; Păduraru, D.; Georgescu, S.O.; Frâncu, L.L.; Stan, C.I. Morphological changes of the peritoneal membrane in patients with long-term dialysis. Rom. J. Morphol. Embryol. 2014, 55, 927–932. [Google Scholar] [PubMed]
- Sherif, A.M.; Nakayama, M.; Maruyama, Y.; Yoshida, H.; Yamamoto, H.; Yokoyama, K.; Kawakami, M. Quantitative assessment of the peritoneal vessel density and vasculopathy in CAPD patients. Nephrol. Dial. Transpl. 2006, 21, 1675–1681. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shimaoka, T.; Hamada, C.; Kaneko, K.; Io, H.; Sekiguchi, Y.; Aruga, S.; Inuma, J.; Inami, Y.; Hotta, Y.; Horikoshi, S.; et al. Quantitative evaluation and assessment of peritoneal morphologic changes in peritoneal dialysis patients. Nephrol. Dial. Transpl. 2010, 25, 3379–3385. [Google Scholar] [CrossRef] [PubMed]
- Numata, M.; Nakayama, M.; Nimura, S.; Kawakami, M.; Lindholm, B.; Kawaguchi, Y. Association between an increased surface area of peritoneal microvessels and a high peritoneal solute transport rate. Perit. Dial. Int. 2003, 23, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Morelle, J.; Marechal, C.; Yu, Z.; Debaix, H.; Corre, T.; Lambie, M.; Verduijn, M.; Dekker, F.; Bovy, P.; Evenepoel, P.; et al. AQP1 Promoter Variant, Water Transport, and Outcomes in Peritoneal Dialysis. N. Engl. J. Med. 2021, 385, 1570–1580. [Google Scholar] [CrossRef]
- Schoenicke, G.; Diamant, R.; Donner, A.; Roehrborn, A.; Grabensee, B.; Plum, J. Histochemical distribution and expression of aquaporin 1 in the peritoneum of patients undergoing peritoneal dialysis: Relation to peritoneal transport. Am. J. Kidney Dis. 2004, 44, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Morelle, J.; Sow, A.; Hautem, N.; Bouzin, C.; Crott, R.; Devuyst, O.; Goffin, E. Interstitial Fibrosis Restricts Osmotic Water Transport in Encapsulating Peritoneal Sclerosis. J. Am. Soc. Nephrol. 2015, 26, 2521–2533. [Google Scholar] [CrossRef]
- Nakayama, M.; Kawaguchi, Y.; Yamada, K.; Hasegawa, T.; Takazoe, K.; Katoh, N.; Hayakawa, H.; Osaka, N.; Yamamoto, H.; Ogawa, A.; et al. Immunohistochemical detection of advanced glycosylation end-products in the peritoneum and its possible pathophysiological role in CAPD. Kidney Int. 1997, 51, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Nitta, K.; Horita, S.; Yumura, W.; Nihei, H.; Nagai, R.; Ikeda, K.; Horiuchi, S. Accumulation of advanced glycation end products in the peritoneal vasculature of continuous ambulatory peritoneal dialysis patients with low ultra-filtration. Nephrol. Dial. Transpl. 1999, 14, 1541–1549. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Mizumasa, T.; Kuroki, Y.; Eriguchi, M.; Yoshida, H.; Taniguchi, M.; Masutani, K.; Tsuruya, K.; Kitazono, T. Advanced glycation end products are associated with immature angiogenesis and peritoneal dysfunction in patients on peritoneal dialysis. Perit. Dial. Int. 2020, 40, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, H.; Hamada, C.; Shimaoka, T.; Sekiguchi, Y.; Io, H.; Kaneko, K.; Horikoshi, S.; Tomino, Y. Accumulation of advanced glycation end products and beta 2-microglobulin in fibrotic thickening of the peritoneum in long-term peritoneal dialysis patients. J. Artif. Organs 2014, 17, 60–68. [Google Scholar] [CrossRef]
- Mizumasa, T.; Honda, K.; Aoki, S.; Hamada, C.; Miyazaki, M.; Ito, Y.; Tanno, Y.; Nakano, T.; Nakayama, M.; Peritoneal Biopsy Study Group of the Japanese Society for Peritoneal Dialysis. Proposal of peritoneal biopsy procedures for patients undergoing peritoneal dialysis. Ren. Replace. Ther. 2020, 6, 8. [Google Scholar] [CrossRef]
- Tawada, M.; Ito, Y.; Hamada, C.; Honda, K.; Mizuno, M.; Suzuki, Y.; Sakata, F.; Terabayashi, T.; Matsukawa, Y.; Maruyama, S.; et al. Vascular Endothelial Cell Injury Is an Important Factor in the Development of Encapsulating Peritoneal Sclerosis in Long-Term Peritoneal Dialysis Patients. PLoS ONE 2016, 11, e0154644. [Google Scholar] [CrossRef]
- Sawai, A.; Ito, Y.; Mizuno, M.; Suzuki, Y.; Toda, S.; Ito, I.; Hattori, R.; Matsukawa, Y.; Gotoh, M.; Takei, Y.; et al. Peritoneal macrophage infiltration is correlated with baseline peritoneal solute transport rate in peritoneal dialysis patients. Nephrol. Dial. Transpl. 2011, 26, 2322–2332. [Google Scholar] [CrossRef]
- Kinashi, H.; Toda, N.; Sun, T.; Nguyen, T.Q.; Suzuki, Y.; Katsuno, T.; Yokoi, H.; Aten, J.; Mizuno, M.; Maruyama, S.; et al. Connective tissue growth factor is correlated with peritoneal lymphangiogenesis. Sci. Rep. 2019, 9, 12175. [Google Scholar] [CrossRef]
- Terabayashi, T.; Ito, Y.; Mizuno, M.; Suzuki, Y.; Kinashi, H.; Sakata, F.; Tomita, T.; Iguchi, D.; Tawada, M.; Nishio, R.; et al. Vascular endothelial growth factor receptor-3 is a novel target to improve net ultrafiltration in methylglyoxal-induced peritoneal injury. Lab. Investig. 2015, 95, 1029–1043. [Google Scholar] [CrossRef]
- Fabbrini, P.; Schilte, M.N.; Zareie, M.; ter Wee, P.M.; Keuning, E.D.; Beelen, R.H.; van den Born, J. Celecoxib treatment reduces peritoneal fibrosis and angiogenesis and prevents ultrafiltration failure in experimental peritoneal dialysis. Nephrol. Dial. Transpl. 2009, 24, 3669–3676. [Google Scholar] [CrossRef]
- Kinashi, H.; Ito, Y.; Sun, T.; Katsuno, T.; Takei, Y. Roles of the TGF-β–VEGF-C Pathway in Fibrosis-Related Lymphangiogenesis. Int. J. Mol. Sci. 2018, 19, 2487. [Google Scholar] [CrossRef]
- Krediet, R.T.; Zweers, M.M.; van der Wal, A.C.; Struijk, D.G. Neoangiogenesis in the peritoneal membrane. Perit. Dial. Int 2000, 20, S19–S25. [Google Scholar] [CrossRef]
- Masola, V.; Bonomini, M.; Borrelli, S.; Di Liberato, L.; Vecchi, L.; Onisto, M.; Gambaro, G.; Palumbo, R.; Arduini, A. Fibrosis of Peritoneal Membrane as Target of New Therapies in Peritoneal Dialysis. Int. J. Mol. Sci. 2022, 23, 4831. [Google Scholar] [CrossRef]
- Kariya, T.; Nishimura, H.; Mizuno, M.; Suzuki, Y.; Matsukawa, Y.; Sakata, F.; Maruyama, S.; Takei, Y.; Ito, Y. TGF-β1–VEGF-A pathway induces neoangiogenesis with peritoneal fibrosis in patients undergoing peritoneal dialysis. Am. J. Physiol. Ren. Physiol. 2018, 314, F167–F180. [Google Scholar] [CrossRef]
- Jeon, S.H.; Chae, B.C.; Kim, H.A.; Seo, G.Y.; Seo, D.W.; Chun, G.T.; Kim, N.S.; Yie, S.W.; Byeon, W.H.; Eom, S.H.; et al. Mechanisms underlying TGF-beta1-induced expression of VEGF and Flk-1 in mouse macrophages and their implications for angiogenesis. J. Leukoc. Biol. 2007, 81, 557–566. [Google Scholar] [CrossRef]
- Carmi, Y.; Voronov, E.; Dotan, S.; Lahat, N.; Rahat, M.A.; Fogel, M.; Huszar, M.; White, M.R.; Dinarello, C.A.; Apte, R.N. The role of macrophage-derived IL-1 in induction and maintenance of angiogenesis. J. Immunol. 2009, 183, 4705–4714. [Google Scholar] [CrossRef]
- Darby, I.A.; Hewitson, T.D. Hypoxia in tissue repair and fibrosis. Cell Tissue Res. 2016, 365, 553–562. [Google Scholar] [CrossRef]
- Krediet, R.T.; Parikova, A. Relative Contributions of Pseudohypoxia and Inflammation to Peritoneal Alterations with Long-Term Peritoneal Dialysis Patients. Clin. J. Am. Soc. Nephrol. 2022, 17, 1259–1266. [Google Scholar] [CrossRef]
- Tawada, M.; Hamada, C.; Suzuki, Y.; Sakata, F.; Sun, T.; Kinashi, H.; Katsuno, T.; Takei, Y.; Maruyama, S.; Honda, K.; et al. Effects of long-term treatment with low-GDP, pH-neutral solutions on peritoneal membranes in peritoneal dialysis patients. Clin. Exp. Nephrol. 2019, 23, 689–699. [Google Scholar] [CrossRef]
- Ito, Y.; Kinashi, H.; Katsuno, T.; Suzuki, Y.; Mizuno, M. Peritonitis-induced peritoneal injury models for research in peritoneal dialysis review of infectious and non-infectious models. Ren. Replace Ther. 2017, 3, 16. [Google Scholar] [CrossRef]
- Cueto-Manzano, A.M.; González-Espinoza, L.; Martin del Campo, F.; Fortes, P.C.; Pecoits-Filho, R. Inflammation in peritoneal dialysis: A Latin-American perspective. Perit Dial. Int. 2007, 27, 347–352. [Google Scholar] [CrossRef]
- Ito, Y.; Sun, T.; Tanaka, H.; Yamaguchi, M.; Kinashi, H.; Sakata, F.; Kunoki, S.; Sakai, Y.; Ishimoto, T. Tissue Sodium Accumulation Induces Organ Inflammation and Injury in Chronic Kidney Disease. Int. J. Mol. Sci. 2023, 24, 8329. [Google Scholar] [CrossRef]
- Witowski, J.; Wisniewska, J.; Korybalska, K.; Bender, T.O.; Breborowicz, A.; Gahl, G.M.; Frei, U.; Passlick-Deetjen, J.; Jörres, A. Prolonged exposure to glucose degradation products impairs viability and function of human peritoneal mesothelial cells. J. Am. Soc. Nephrol. 2001, 12, 2434–2441. [Google Scholar] [CrossRef]
- Liu, S.H.; Sheu, W.H.; Lee, M.R.; Lee, W.J.; Yi, Y.C.; Yang, T.J.; Jen, J.F.; Pan, H.C.; Shen, C.C.; Chen, W.B.; et al. Advanced glycation end product Nε-carboxymethyllysine induces endothelial cell injury: The involvement of SHP-1-regulated VEGFR-2 dephosphorylation. J. Pathol. 2013, 230, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Y.; Li, X.L.; Gao, H.Q.; Zhang, J.H.; Cai, Q.; Cheng, M.; Lu, M. Grape seed procyanidin B2 inhibits advanced glycation end product-induced endothelial cell apoptosis through regulating GSK3β phosphorylation. Cell Biol. Int. 2011, 35, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, L.; Shu, B.; Tang, J.; Zhang, L.; Xie, J.; Liu, X.; Xu, Y.; Qi, S. Angiopoietin-1 protects the endothelial cells against advanced glycation end product injury by strengthening cell junctions and inhibiting cell apoptosis. J. Cell Physiol. 2015, 230, 1895–1905. [Google Scholar] [CrossRef]
- Kinashi, H.; Ito, Y.; Mizuno, M.; Suzuki, Y.; Terabayashi, T.; Nagura, F.; Hattori, R.; Matsukawa, Y.; Mizuno, T.; Noda, Y.; et al. TGF-β1 promotes lymphangiogenesis during peritoneal fibrosis. J. Am. Soc. Nephrol. 2013, 24, 1627–1642. [Google Scholar] [CrossRef] [PubMed]
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell 2010, 140, 460–476. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, J.; Wu, F.; Wei, F.; Han, W.; Xu, X.; Zhang, Y. Current Status of Lymphangiogenesis: Molecular Mechanism, Immune Tolerance, and Application Prospect. Cancers 2023, 15, 1169. [Google Scholar] [CrossRef]
- Krediet, R.T. The effective lymphatic absorption rate is an accurate and useful concept in the physiology of peritoneal dialysis. Perit. Dial. Int. 2004, 24, 309–313. [Google Scholar] [CrossRef]
- Flessner, M. Effective lymphatic absorption rate is not a useful or accurate term to use in the physiology of peritoneal dialysis. Perit. Dial. Int. 2004, 24, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Tsai, T.J.; Shih, C.L.; Huang, J.W.; Chuang, H.F.; Chen, M.H.; Fang, C.C. Intraperitoneal vascular endothelial growth factor C level is related to peritoneal dialysis ultrafiltration. Blood Purif. 2009, 28, 69–74. [Google Scholar] [CrossRef]
- Krediet, R.T. Ultrafiltration Failure Is a Reflection of Peritoneal Alterations in Patients Treated With Peritoneal Dialysis. Front. Physiol. 2018, 9, 1815. [Google Scholar] [CrossRef] [PubMed]
- Kunoki, S.; Tatsukawa, H.; Sakai, Y.; Kinashi, H.; Kariya, T.; Suzuki, Y.; Mizuno, M.; Yamaguchi, M.; Sasakura, H.; Ikeno, M.; et al. Inhibition of Transglutaminase 2 Reduces Peritoneal Injury in a Chlorhexidine-Induced Peritoneal Fibrosis Model. Lab. Investig. 2023, 103, 100050. [Google Scholar] [CrossRef]
- Morelle, J.; Stachowska-Pietka, J.; Öberg, C.; Gadola, L.; La Milia, V.; Yu, Z.; Lambie, M.; Mehrotra, R.; de Arteaga, J.; Davies, S. ISPD recommendations for the evaluation of peritoneal membrane dysfunction in adults: Classification, measurement, interpretation and rationale for intervention. Perit. Dial. Int. 2021, 41, 352–372. [Google Scholar] [CrossRef]
- Trionfetti, F.; Marchant, V.; González-Mateo, G.T.; Kawka, E.; Márquez-Expósito, L.; Ortiz, A.; López-Cabrera, M.; Ruiz-Ortega, M.; Strippoli, R. Novel Aspects of the Immune Response Involved in the Peritoneal Damage in Chronic Kidney Disease Patients under Dialysis. Int. J. Mol. Sci. 2023, 24, 5763. [Google Scholar] [CrossRef] [PubMed]
- Witowski, J.; Kamhieh-Milz, J.; Kawka, E.; Catar, R.; Jörres, A. IL-17 in Peritoneal Dialysis-Associated Inflammation and Angiogenesis: Conclusions and Perspectives. Front. Physiol. 2018, 9, 1694. [Google Scholar] [CrossRef] [PubMed]
- Sakata, F.; Ito, Y.; Mizuno, M.; Sawai, A.; Suzuki, Y.; Tomita, T.; Tawada, M.; Tanaka, A.; Hirayama, A.; Sagara, A.; et al. Sodium chloride promotes tissue inflammation via osmotic stimuli in subtotal-nephrectomized mice. Lab. Investig. 2017, 97, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Sakata, F.; Ishii, T.; Tawada, M.; Suzuki, Y.; Kinashi, H.; Katsuno, T.; Takei, Y.; Maruyama, S.; Mizuno, M.; et al. Excessive salt intake increases peritoneal solute transport rate via local tonicity-responsive enhancer binding protein in subtotal nephrectomized mice. Nephrol. Dial. Transpl. 2019, 34, 2031–2042. [Google Scholar] [CrossRef] [PubMed]
- Seeger, H.; Kitterer, D.; Latus, J.; Alscher, M.D.; Braun, N.; Segerer, S. The potential role of NFAT5 and osmolarity in peritoneal injury. Biomed. Res. Int. 2015, 2015, 578453. [Google Scholar] [CrossRef] [PubMed]
- Sahinoz, M.; Tintara, S.; Deger, S.M.; Alsouqi, A.; Crescenzi, R.L.; Mambungu, C.; Vincz, A.; Mason, O.; Prigmore, H.L.; Guide, A.; et al. Tissue sodium stores in peritoneal dialysis and hemodialysis patients determined by 23-sodium magnetic resonance imaging. Nephrol. Dial. Transpl. 2020, 36, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Flessner, M.F.; Credit, K.; Henderson, K.; Vanpelt, H.M.; Potter, R.; He, Z.; Henegar, J.; Robert, B. Peritoneal changes after exposure to sterile solutions by catheter. J. Am. Soc. Nephrol. 2007, 18, 2294–2302. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Qin, X.Y.; Furutani, Y.; Yu, W.; Kojima, S. Imaging of the ex vivo transglutaminase activity in liver macrophages of sepsis mice. Anal. Biochem. 2020, 597, 113654. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Hsu, H.; Lin, C.C.; Pan, S.Y.; Liu, S.Y.; Wu, C.F.; Tsai, P.Z.; Liao, C.T.; Cheng, H.T.; Chiang, W.C.; et al. Inflammatory macrophages switch to CCL17-expressing phenotype and promote peritoneal fibrosis. J. Pathol. 2020, 250, 55–66. [Google Scholar] [CrossRef]
- Lee, S.H.; Kang, H.Y.; Kim, K.S.; Nam, B.Y.; Paeng, J.; Kim, S.; Li, J.J.; Park, J.T.; Kim, D.K.; Han, S.H.; et al. The monocyte chemoattractant protein-1 (MCP-1)/CCR2 system is involved in peritoneal dialysis-related epithelial-mesenchymal transition of peritoneal mesothelial cells. Lab. Investig. 2012, 92, 1698–1711. [Google Scholar] [CrossRef]
- Li, Q.; Zheng, M.; Liu, Y.; Sun, W.; Shi, J.; Ni, J.; Wang, Q. A pathogenetic role for M1 macrophages in peritoneal dialysis-associated fibrosis. Mol. Immunol. 2018, 94, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, T.E.; Shaw, T.N.; Lennon, R.; Herrick, S.E.; Rückerl, D. Ongoing Exposure to Peritoneal Dialysis Fluid Alters Resident Peritoneal Macrophage Phenotype and Activation Propensity. Front. Immunol. 2021, 12, 715209. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Li, L.; Li, L.; Zhou, X.; Wan, M.; Lou, P.; Zhao, M.; Lv, K.; Yuan, Y.; et al. Peritoneal M2 macrophage-derived extracellular vesicles as natural multitarget nanotherapeutics to attenuate cytokine storms after severe infections. J. Control. Release 2022, 349, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Lambie, M.R.; Chess, J.; Summers, A.M.; Williams, P.F.; Topley, N.; Davies, S.J. Peritoneal inflammation precedes encapsulating peritoneal sclerosis: Results from the GLOBAL Fluid Study. Nephrol. Dial. Transpl. 2016, 31, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Gong, Y.; Chen, Y.; Yu, D.; Wang, X.; Zhang, X.; Dou, Y.; Liu, D.; Cheng, G.; Lu, S.; et al. IL-6 promotes epithelial-to-mesenchymal transition of human peritoneal mesothelial cells possibly through the JAK2/STAT3 signaling pathway. Am. J. Physiol. Ren. Physiol. 2017, 313, F310–F318. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Sun, T.; Kinashi, H.; Kamiya, K.; Yamaguchi, M.; Nobata, H.; Sakata, F.; Kim, H.; Mizuno, M.; Kunoki, S.; et al. Interleukin-6 blockade reduces salt-induced cardiac inflammation and fibrosis in subtotal nephrectomized mice. Am. J. Physiol. Ren. Physiol. 2022, 323, F654–F665. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Bao, M.; Fang, Y.; Yu, X.; Ji, J.; Ding, X. STAT3/HIF-1α signaling activation mediates peritoneal fibrosis induced by high glucose. J. Transl. Med. 2021, 19, 283. [Google Scholar] [CrossRef]
- Schnell, A.; Littman, D.R.; Kuchroo, V.K. T(H)17 cell heterogeneity and its role in tissue inflammation. Nat. Immunol. 2023, 24, 19–29. [Google Scholar] [CrossRef]
- Liu, F.; Yu, C.; Qin, H.; Zhang, S.; Fang, L.; Wang, Y.; Wang, J.; Cui, B.; Hu, S.; Liu, N.; et al. Nintedanib attenuates peritoneal fibrosis by inhibiting mesothelial-to-mesenchymal transition, inflammation and angiogenesis. J. Cell Mol. Med. 2021, 25, 6103–6114. [Google Scholar] [CrossRef]
- Kimura, A.; Kishimoto, T. IL-6: Regulator of Treg/Th17 balance. Eur. J. Immunol. 2010, 40, 1830–1835. [Google Scholar] [CrossRef]
- Martín, P.; Sánchez-Madrid, F. CD69: An unexpected regulator of TH17 cell-driven inflammatory responses. Sci. Signal 2011, 4, pe14. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Hishida, E.; Ito, H.; Komada, T.; Karasawa, T.; Kimura, H.; Watanabe, S.; Kamata, R.; Aizawa, E.; Kasahara, T.; Morishita, Y.; et al. Crucial Role of NLRP3 Inflammasome in the Development of Peritoneal Dialysis-related Peritoneal Fibrosis. Sci. Rep. 2019, 9, 10363. [Google Scholar] [CrossRef] [PubMed]
- Kadoya, H.; Hirano, A.; Umeno, R.; Kajimoto, E.; Iwakura, T.; Kondo, M.; Wada, Y.; Kidokoro, K.; Kishi, S.; Nagasu, H.; et al. Activation of the inflammasome drives peritoneal deterioration in a mouse model of peritoneal fibrosis. FASEB J. 2023, 37, e23129. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.H.; Hong, Y.S.; Lim, H.J.; Choi, J.H.; Han, D.S.; Yoon, K.I. High glucose solution and spent dialysate stimulate the synthesis of transforming growth factor-beta1 of human peritoneal mesothelial cells: Effect of cytokine costimulation. Perit. Dial. Int. 1999, 19, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.N.; Lai, K.B.; Szeto, C.C.; Lam, C.W.; Leung, J.C. Growth factors in continuous ambulatory peritoneal dialysis effluent. Their relation with peritoneal transport of small solutes. Am. J. Nephrol. 1999, 19, 416–422. [Google Scholar] [CrossRef]
- Margetts, P.J.; Kolb, M.; Galt, T.; Hoff, C.M.; Shockley, T.R.; Gauldie, J. Gene transfer of transforming growth factor-beta1 to the rat peritoneum: Effects on membrane function. J. Am. Soc. Nephrol. 2001, 12, 2029–2039. [Google Scholar] [CrossRef]
- Loureiro, J.; Aguilera, A.; Selgas, R.; Sandoval, P.; Albar-Vizcaíno, P.; Pérez-Lozano, M.L.; Ruiz-Carpio, V.; Majano, P.L.; Lamas, S.; Rodríguez-Pascual, F.; et al. Blocking TGF-β1 protects the peritoneal membrane from dialysate-induced damage. J. Am. Soc. Nephrol. 2011, 22, 1682–1695. [Google Scholar] [CrossRef]
- Lho, Y.; Do, J.Y.; Heo, J.Y.; Kim, A.Y.; Kim, S.W.; Kang, S.H. Effects of TGF-β1 Receptor Inhibitor GW788388 on the Epithelial to Mesenchymal Transition of Peritoneal Mesothelial Cells. Int. J. Mol. Sci. 2021, 22, 4739. [Google Scholar] [CrossRef]
- Sakai, N.; Nakamura, M.; Lipson, K.E.; Miyake, T.; Kamikawa, Y.; Sagara, A.; Shinozaki, Y.; Kitajima, S.; Toyama, T.; Hara, A.; et al. Inhibition of CTGF ameliorates peritoneal fibrosis through suppression of fibroblast and myofibroblast accumulation and angiogenesis. Sci. Rep. 2017, 7, 5392. [Google Scholar] [CrossRef] [PubMed]
- Toda, N.; Mori, K.; Kasahara, M.; Koga, K.; Ishii, A.; Mori, K.P.; Osaki, K.; Mukoyama, M.; Yanagita, M.; Yokoi, H. Deletion of connective tissue growth factor ameliorates peritoneal fibrosis by inhibiting angiogenesis and inflammation. Nephrol. Dial. Transpl. 2018, 33, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, N.; Xiong, C.; Xu, L.; Shi, Y.; Qiu, A.; Zang, X.; Mao, H.; Zhuang, S. Inhibition of EGF Receptor Blocks the Development and Progression of Peritoneal Fibrosis. J. Am. Soc. Nephrol. 2016, 27, 2631–2644. [Google Scholar] [CrossRef] [PubMed]
- Cina, D.; Patel, P.; Bethune, J.C.; Thoma, J.; Rodriguez-Lecompte, J.C.; Hoff, C.M.; Liu, L.; Margetts, P.J. Peritoneal morphological and functional changes associated with platelet-derived growth factor B. Nephrol. Dial. Transpl. 2009, 24, 448–457. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, L.; Shen, N.; Wang, N.; Wang, W.; Tang, Q.; Du, X.; Carrero, J.J.; Wang, K.; Deng, Y.; Li, Z.; et al. Inhibiting core fucosylation attenuates glucose-induced peritoneal fibrosis in rats. Kidney Int. 2018, 93, 1384–1396. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Yang, N.; Wang, W.; Du, X.; Tang, Q.; Lin, H.; Li, L. Blocking core fucosylation of epidermal growth factor (EGF) receptor prevents peritoneal fibrosis progression. Ren. Fail. 2021, 43, 869–877. [Google Scholar] [CrossRef]
- Padwal, M.; Siddique, I.; Wu, L.; Tang, K.; Boivin, F.; Liu, L.; Robertson, J.; Bridgewater, D.; West-Mays, J.; Gangji, A.; et al. Matrix metalloproteinase 9 is associated with peritoneal membrane solute transport and induces angiogenesis through β-catenin signaling. Nephrol. Dial. Transpl. 2017, 32, 50–61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ishimura, T.; Ishii, A.; Yamada, H.; Osaki, K.; Toda, N.; Mori, K.P.; Ohno, S.; Kato, Y.; Handa, T.; Sugioka, S.; et al. Matrix metalloproteinase-10 deficiency has protective effects against peritoneal inflammation and fibrosis via transcription factor NFκΒ pathway inhibition. Kidney Int. 2023, 104, 929–942. [Google Scholar] [CrossRef]
- Guo, H.; Leung, J.C.; Lam, M.F.; Chan, L.Y.; Tsang, A.W.; Lan, H.Y.; Lai, K.N. Smad7 transgene attenuates peritoneal fibrosis in uremic rats treated with peritoneal dialysis. J. Am. Soc. Nephrol. 2007, 18, 2689–2703. [Google Scholar] [CrossRef]
- Nie, J.; Dou, X.; Hao, W.; Wang, X.; Peng, W.; Jia, Z.; Chen, W.; Li, X.; Luo, N.; Lan, H.Y.; et al. Smad7 gene transfer inhibits peritoneal fibrosis. Kidney Int. 2007, 72, 1336–1344. [Google Scholar] [CrossRef]
- Silva, F.M.O.; Costalonga, E.C.; Silva, C.; Carreira, A.C.O.; Gomes, S.A.; Sogayar, M.C.; Fanelli, C.; Noronha, I.L. Tamoxifen and bone morphogenic protein-7 modulate fibrosis and inflammation in the peritoneal fibrosis model developed in uremic rats. Mol. Med. 2019, 25, 41. [Google Scholar] [CrossRef]
- Nakamura, S.; Niwa, T. Pyridoxal phosphate and hepatocyte growth factor prevent dialysate-induced peritoneal damage. J. Am. Soc. Nephrol. 2005, 16, 144–150. [Google Scholar] [CrossRef]
- Abreu, J.G.; Ketpura, N.I.; Reversade, B.; De Robertis, E.M. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat. Cell Biol. 2002, 4, 599–604. [Google Scholar] [CrossRef]
- Leung, J.C.; Chan, L.Y.; Tam, K.Y.; Tang, S.C.; Lam, M.F.; Cheng, A.S.; Chu, K.M.; Lai, K.N. Regulation of CCN2/CTGF and related cytokines in cultured peritoneal cells under conditions simulating peritoneal dialysis. Nephrol. Dial. Transpl. 2009, 24, 458–469. [Google Scholar] [CrossRef]
- Mizutani, M.; Ito, Y.; Mizuno, M.; Nishimura, H.; Suzuki, Y.; Hattori, R.; Matsukawa, Y.; Imai, M.; Oliver, N.; Goldschmeding, R.; et al. Connective tissue growth factor (CTGF/CCN2) is increased in peritoneal dialysis patients with high peritoneal solute transport rate. Am. J. Physiol. Ren. Physiol. 2010, 298, F721–F733. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; West-Mays, J.; Kolb, M.; Rodrigues, J.C.; Hoff, C.M.; Margetts, P.J. Platelet derived growth factor B and epithelial mesenchymal transition of peritoneal mesothelial cells. Matrix Biol. 2010, 29, 97–106. [Google Scholar] [CrossRef]
- Venkatachalam, M.A.; Weinberg, J.M. New wrinkles in old receptors: Core fucosylation is yet another target to inhibit TGF-β signaling. Kidney Int. 2013, 84, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Catania, J.M.; Chen, G.; Parrish, A.R. Role of matrix metalloproteinases in renal pathophysiologies. Am. J. Physiol. Ren. Physiol. 2007, 292, F905–F911. [Google Scholar] [CrossRef] [PubMed]
- Hirahara, I.; Inoue, M.; Umino, T.; Saito, O.; Muto, S.; Kusano, E. Matrix metalloproteinase levels in the drained dialysate reflect the peritoneal solute transport rate: A multicentre study in Japan. Nephrol. Dial. Transpl. 2011, 26, 1695–1701. [Google Scholar] [CrossRef][Green Version]
- Soltani, F.; Kaartinen, M.T. Transglutaminases in fibrosis-overview and recent advances. Am. J. Physiol. Cell Physiol. 2023, 325, C885–C894. [Google Scholar] [CrossRef]
- Nakao, A.; Afrakhte, M.; Morén, A.; Nakayama, T.; Christian, J.L.; Heuchel, R.; Itoh, S.; Kawabata, M.; Heldin, N.E.; Heldin, C.H.; et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature 1997, 389, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Balzer, M.S. Molecular pathways in peritoneal fibrosis. Cell Signal. 2020, 75, 109778. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; LeBleu, V.S.; Bosukonda, D.; Keck, P.; Taduri, G.; Bechtel, W.; Okada, H.; Carlson, W., Jr.; Bey, P.; Rusckowski, M.; et al. Activin-like kinase 3 is important for kidney regeneration and reversal of fibrosis. Nat. Med. 2012, 18, 396–404. [Google Scholar] [CrossRef]
- Yu, M.A.; Shin, K.S.; Kim, J.H.; Kim, Y.I.; Chung, S.S.; Park, S.H.; Kim, Y.L.; Kang, D.H. HGF and BMP-7 ameliorate high glucose-induced epithelial-to-mesenchymal transition of peritoneal mesothelium. J. Am. Soc. Nephrol. 2009, 20, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Ogawa, K.; Kawaguchi, M.; Fumoto, S.; Mukai, H.; Kawakami, S. Suppression of Peritoneal Fibrosis by Sonoporation of Hepatocyte Growth Factor Gene-Encoding Plasmid DNA in Mice. Pharmaceutics 2021, 13, 115. [Google Scholar] [CrossRef]
- Obata, Y.; Abe, K.; Miyazaki, M.; Koji, T.; Tabata, Y.; Nishino, T. The Transfer of the Hepatocyte Growth Factor Gene by Macrophages Ameliorates the Progression of Peritoneal Fibrosis in Mice. Int. J. Mol. Sci. 2023, 24, 6951. [Google Scholar] [CrossRef] [PubMed]
- Yoshimine, H.; Tanoue, S.; Ibi, Y.; Minami, M.; Nakahara, M.; Tokunaga, K.; Kanmura, S.; Ido, A. Hepatocyte growth factor ameliorates methylglyoxal-induced peritoneal inflammation and fibrosis in mouse model. Clin. Exp. Nephrol. 2021, 25, 935–943. [Google Scholar] [CrossRef]
- Mizuno, M.; Morgan, B.P. The possibilities and pitfalls for anti-complement therapies in inflammatory diseases. Curr. Drug Targets Inflamm. Allergy 2004, 3, 87–96. [Google Scholar] [CrossRef]
- Mizuno, M.; Suzuki, Y.; Ito, Y. Complement regulation and kidney diseases: Recent knowledge of the double-edged roles of complement activation in nephrology. Clin. Exp. Nephrol. 2018, 22, 3–14. [Google Scholar] [CrossRef]
- Pandya, P.H.; Wilkes, D.S. Complement system in lung disease. Am. J. Respir. Cell Mol. Biol. 2014, 51, 467–473. [Google Scholar] [CrossRef]
- Gangadharan, B.; Antrobus, R.; Dwek, R.A.; Zitzmann, N. Novel serum biomarker candidates for liver fibrosis in hepatitis C patients. Clin. Chem. 2007, 53, 1792–1799. [Google Scholar] [CrossRef] [PubMed]
- Vasel, M.; Rutz, R.; Bersch, C.; Feick, P.; Singer, M.V.; Kirschfink, M.; Nakchbandi, I.A. Complement activation correlates with liver necrosis and fibrosis in chronic hepatitis C. Clin. Immunol. 2014, 150, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Li, X.; Liu, J.; Lee, H.; Salciccioli, L.; Lazar, J.; Zhang, M. The role of complement C3 in the outcome of regional myocardial infarction. Biochem. Biophys. Rep. 2023, 33, 101434. [Google Scholar]
- Keir, L.S.; Firth, R.; Aponik, L.; Feitelberg, D.; Sakimoto, S.; Aguilar, E.; Welsh, G.I.; Richards, A.; Usui, Y.; Satchell, S.C.; et al. VEGF regulates local inhibitory complement proteins in the eye and kidney. J. Clin. Investig. 2017, 127, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tan, S.; Liu, H.; Jiang, J.; Wang, X.; Li, L.; Wu, B. Hepatocyte-derived MASP1-enriched small extracellular vesicles activate HSCs to promote liver fibrosis. Hepatology 2023, 77, 1181–1197. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Roderfeld, M.; Sabrane, K.; Zhang, P.; Tian, Y.; Mertens, J.C.; Frei, P.; Stieger, B.; Weber, A.; Müllhaupt, B.; et al. Complement factor C5 deficiency significantly delays the progression of biliary fibrosis in bile duct-ligated mice. Biochem. Biophys. Res. Commun. 2012, 418, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Bohlson, S.S.; O’Conner, S.D.; Hulsebus, H.J.; Ho, M.M.; Fraser, D.A. Complement, c1q, and c1q-related molecules regulate macrophage polarization. Front. Immunol. 2014, 5, 402. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wu, X.; Song, Y.; Chen, Y.; Wan, J. Complement C3 exacerbates renal interstitial fibrosis by facilitating the M1 macrophage phenotype in a mouse model of unilateral ureteral obstruction. Am. J. Physiol. Ren. Physiol. 2019, 317, F1171–F1182. [Google Scholar] [CrossRef]
- Nesheim, M.; Wang, W.; Boffa, M.; Nagashima, M.; Morser, J.; Bajzar, L. Thrombin, thrombomodulin and TAFI in the molecular link between coagulation and fibrinolysis. Thromb. Haemost. 1997, 78, 386–391. [Google Scholar] [CrossRef]
- Fujimoto, H.; Gabazza, E.C.; Taguchi, O.; Nishii, Y.; Nakahara, H.; Bruno, N.E.; D’Alessandro-Gabazza, C.N.; Kasper, M.; Yano, Y.; Nagashima, M.; et al. Thrombin-activatable fibrinolysis inhibitor deficiency attenuates bleomycin-induced lung fibrosis. Am. J. Pathol. 2006, 168, 1086–1096. [Google Scholar] [CrossRef]
- Sei, Y.; Mizuno, M.; Suzuki, Y.; Imai, M.; Higashide, K.; Harris, C.L.; Sakata, F.; Iguchi, D.; Fujiwara, M.; Kodera, Y.; et al. Expression of membrane complement regulators, CD46, CD55 and CD59, in mesothelial cells of patients on peritoneal dialysis therapy. Mol. Immunol. 2015, 65, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Kitterer, D.; Biegger, D.; Segerer, S.; Braun, N.; Alscher, M.D.; Latus, J. Alteration of membrane complement regulators is associated with transporter status in patients on peritoneal dialysis. PLoS ONE 2017, 12, e0177487. [Google Scholar] [CrossRef]
- Schvartz, I.; Seger, D.; Shaltiel, S. Vitronectin. Int. J. Biochem. Cell Biol. 1999, 31, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Vittal, R.; Fisher, A.J.; Thompson, E.L.; Cipolla, E.M.; Gu, H.; Mickler, E.A.; Varre, A.; Agarwal, M.; Kim, K.K.; Vasko, M.R.; et al. Overexpression of Decay Accelerating Factor Mitigates Fibrotic Responses to Lung Injury. Am. J. Respir. Cell Mol. Biol. 2022, 67, 459–470. [Google Scholar] [CrossRef]
- Blumenkrantz, M.J.; Gahl, G.M.; Kopple, J.D.; Kamdar, A.V.; Jones, M.R.; Kessel, M.; Coburn, J.W. Protein losses during peritoneal dialysis. Kidney Int. 1981, 19, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Young, G.A.; Kendall, S.; Brownjohn, A.M. Complement activation during CAPD. Nephrol. Dial. Transpl. 1993, 8, 1372–1375. [Google Scholar]
- Barbano, G.; Cappa, F.; Prigione, I.; Tedesco, F.; Pausa, M.; Gugliemino, R.; Pistoia, V.; Gusmano, R.; Perfumo, F. Peritoneal mesothelial cells produce complement factors and express CD59 that inhibits C5b-9-mediated cell lysis. Adv. Perit. Dial. 1999, 15, 253–257. [Google Scholar] [PubMed]
- Mizuno, T.; Mizuno, M.; Imai, M.; Suzuki, Y.; Kushida, M.; Noda, Y.; Maruyama, S.; Okada, H.; Okada, N.; Matsuo, S.; et al. Anti-C5a complementary peptide ameliorates acute peritoneal injury induced by neutralization of Crry and CD59. Am. J. Physiol. Ren. Physiol. 2013, 305, F1603–F1616. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.; Hettinga, J.; Flückiger, R.; Krumrei, N.; Goldfine, A.; Angarita, L.; Halperin, J. Molecular basis for a link between complement and the vascular complications of diabetes. Proc. Natl. Acad. Sci. USA 2000, 97, 5450–5455. [Google Scholar] [CrossRef]
- Mizuno, M.; Ito, Y.; Hepburn, N.; Mizuno, T.; Noda, Y.; Yuzawa, Y.; Harris, C.L.; Morgan, B.P.; Matsuo, S. Zymosan, but not lipopolysaccharide, triggers severe and progressive peritoneal injury accompanied by complement activation in a rat peritonitis model. J. Immunol. 2009, 183, 1403–1412. [Google Scholar] [CrossRef]
- Iguchi, D.; Mizuno, M.; Suzuki, Y.; Sakata, F.; Maruyama, S.; Okada, A.; Okada, H.; Ito, Y. Anti-C5a complementary peptide mitigates zymosan-induced severe peritonitis with fibrotic encapsulation in rats pretreated with methylglyoxal. Am. J. Physiol. Ren. Physiol. 2018, 315, F1732–F1746. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, T.; Mizuno, M.; Iguchi, D.; Kojima, H.; Kim, H.; Suzuki, Y.; Kinashi, H.; Ishimoto, T.; Maruyama, S.; Ito, Y. C1 inhibitor mitigates peritoneal injury in zymosan-induced peritonitis. Am. J. Physiol. Ren. Physiol. 2021, 320, F1123–F1132. [Google Scholar] [CrossRef] [PubMed]
- Fukui, S.; Mizuno, M.; Tawada, M.; Suzuki, Y.; Kojima, H.; Matsukawa, Y.; Imai, M.; Kim, H.; Kinashi, H.; Mizutani, M.; et al. Peritoneal Expression of Membrane Complement Regulators Is Decreased in Peritoneal Dialysis Patients with Infected Peritonitis. Int. J. Mol. Sci. 2023, 24, 9146. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, T.; Mizuno, M.; Morgan, B.P.; Noda, Y.; Yamada, K.; Okada, N.; Yuzawa, Y.; Matsuo, S.; Ito, Y. Specific collaboration between rat membrane complement regulators Crry and CD59 protects peritoneum from damage by autologous complement activation. Nephrol. Dial. Transpl. 2011, 26, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Leung, J.C.; Chan, L.Y.; Tsang, A.W.; Chen, C.X.; Zhou, W.; Lai, K.N.; Sacks, S.H. Regulation of complement C3 and C4 synthesis in human peritoneal mesothelial cells by peritoneal dialysis fluid. Clin. Exp. Immunol. 2004, 136, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Bartosova, M.; Schaefer, B.; Bermejo, J.L.; Tarantino, S.; Lasitschka, F.; Macher-Goeppinger, S.; Sinn, P.; Warady, B.A.; Zaloszyc, A.; Parapatics, K.; et al. Complement Activation in Peritoneal Dialysis-Induced Arteriolopathy. J. Am. Soc. Nephrol. 2018, 29, 268–282. [Google Scholar] [CrossRef]
- Zelek, W.M.; Xie, L.; Morgan, B.P.; Harris, C.L. Compendium of current complement therapeutics. Mol. Immunol. 2019, 114, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Anliker-Ort, M.; Dingemanse, J.; van den Anker, J.; Kaufmann, P. Treatment of Rare Inflammatory Kidney Diseases: Drugs Targeting the Terminal Complement Pathway. Front. Immunol. 2020, 11, 599417. [Google Scholar] [CrossRef] [PubMed]
- Kamegai, N.; Kim, H.; Suzuki, Y.; Fukui, S.; Kojima, H.; Maruyama, S.; Morgan, B.P.; Zelek, W.M.; Mizuno, M. Complement terminal pathway inhibition reduces peritoneal injuries in a rat peritonitis model. Clin. Exp. Immunol. 2023, 214, 209–218. [Google Scholar] [CrossRef]
- Johnson, D.W.; Cho, Y.; Livingston, B.E.; Hawley, C.M.; McDonald, S.P.; Brown, F.G.; Rosman, J.B.; Bannister, K.M.; Wiggins, K.J. Encapsulating peritoneal sclerosis: Incidence, predictors, and outcomes. Kidney Int. 2010, 77, 904–912. [Google Scholar] [CrossRef]
- Brown, E.A.; Bargman, J.; van Biesen, W.; Chang, M.Y.; Finkelstein, F.O.; Hurst, H.; Johnson, D.W.; Kawanishi, H.; Lambie, M.; de Moraes, T.P.; et al. Length of Time on Peritoneal Dialysis and Encapsulating Peritoneal Sclerosis—Position Paper for ISPD: 2017 Update. Perit. Dial. Int. 2017, 37, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.A.; Van Biesen, W.; Finkelstein, F.O.; Hurst, H.; Johnson, D.W.; Kawanishi, H.; Pecoits-Filho, R.; Woodrow, G. Length of time on peritoneal dialysis and encapsulating peritoneal sclerosis: Position paper for ISPD. Perit. Dial. Int. 2009, 29, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, H.; Kawaguchi, Y.; Fukui, H.; Hara, S.; Imada, A.; Kubo, H.; Kin, M.; Nakamoto, M.; Ohira, S.; Shoji, T. Encapsulating peritoneal sclerosis in Japan: A prospective, controlled, multicenter study. Am. J. Kidney Dis. 2004, 44, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Korte, M.R.; Sampimon, D.E.; Lingsma, H.F.; Fieren, M.W.; Looman, C.W.; Zietse, R.; Weimar, W.; Betjes, M.G. Risk factors associated with encapsulating peritoneal sclerosis in Dutch EPS study. Perit. Dial. Int. 2011, 31, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Rigby, R.J.; Hawley, C.M. Sclerosing peritonitis: The experience in Australia. Nephrol. Dial. Transpl. 1998, 13, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Korte, M.R.; Yo, M.; Betjes, M.G.; Fieren, M.W.; van Saase, J.C.; Boer, W.H.; Weimar, W.; Zietse, R. Increasing incidence of severe encapsulating peritoneal sclerosis after kidney transplantation. Nephrol. Dial. Transpl. 2007, 22, 2412–2414. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Balasubramaniam, G.; Brown, E.A.; Davenport, A.; Cairns, H.; Cooper, B.; Fan, S.L.; Farrington, K.; Gallagher, H.; Harnett, P.; Krausze, S.; et al. The Pan-Thames EPS study: Treatment and outcomes of encapsulating peritoneal sclerosis. Nephrol. Dial. Transpl. 2009, 24, 3209–3215. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Oda, H. Pathology of encapsulating peritoneal sclerosis. Perit. Dial. Int. 2005, 25 (Suppl. S4), S19–S29. [Google Scholar] [CrossRef]
- Garosi, G.; Di Paolo, N.; Sacchi, G.; Gaggiotti, E. Sclerosing peritonitis: A nosological entity. Perit. Dial. Int. 2005, 25 (Suppl. S3), S110–S112. [Google Scholar] [CrossRef]
- Alscher, D.M.; Braun, N.; Biegger, D.; Fritz, P. Peritoneal mast cells in peritoneal dialysis patients, particularly in encapsulating peritoneal sclerosis patients. Am. J. Kidney Dis. 2007, 49, 452–461. [Google Scholar] [CrossRef]
- Sherif, A.M.; Yoshida, H.; Maruyama, Y.; Yamamoto, H.; Yokoyama, K.; Hosoya, T.; Kawakami, M.; Nakayama, M. Comparison between the pathology of encapsulating sclerosis and simple sclerosis of the peritoneal membrane in chronic peritoneal dialysis. Ther. Apher. Dial. 2008, 12, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Braun, N.; Fritz, P.; Ulmer, C.; Latus, J.; Kimmel, M.; Biegger, D.; Ott, G.; Reimold, F.; Thon, K.P.; Dippon, J.; et al. Histological criteria for encapsulating peritoneal sclerosis—A standardized approach. PLoS ONE 2012, 7, e48647. [Google Scholar] [CrossRef] [PubMed]
- Tawada, M.; Ito, Y.; Banshodani, M.; Yamashita, M.; Shintaku, S.; Sun, T.; Suzuki, Y.; Kinashi, H.; Kubo, Y.; Ando, M.; et al. Vasculopathy plays an important role during the development and relapse of encapsulating peritoneal sclerosis with conventional peritoneal dialysis solutions. Nephrol. Dial. Transpl. 2021, 36, 1519–1526. [Google Scholar] [CrossRef]
- Kawanishi, K.; Honda, K.; Tsukada, M.; Oda, H.; Nitta, K. Neutral solution low in glucose degradation products is associated with less peritoneal fibrosis and vascular sclerosis in patients receiving peritoneal dialysis. Perit. Dial. Int. 2013, 33, 242–251. [Google Scholar] [CrossRef]
- Hamada, C.; Honda, K.; Kawanishi, K.; Nakamoto, H.; Ito, Y.; Sakurada, T.; Tanno, Y.; Mizumasa, T.; Miyazaki, M.; Moriishi, M.; et al. Morphological characteristics in peritoneum in patients with neutral peritoneal dialysis solution. J. Artif. Organs 2015, 18, 243–250. [Google Scholar] [CrossRef]
- del Peso, G.; Jiménez-Heffernan, J.A.; Selgas, R.; Remón, C.; Ossorio, M.; Fernández-Perpén, A.; Sánchez-Tomero, J.A.; Cirugeda, A.; de Sousa, E.; Sandoval, P.; et al. Biocompatible Dialysis Solutions Preserve Peritoneal Mesothelial Cell and Vessel Wall Integrity. A Case-Control Study on Human Biopsies. Perit. Dial. Int. 2016, 36, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, N.; Tawada, M.; Sun, T.; Suzuki, Y.; Kinashi, H.; Yamaguchi, M.; Katsuno, T.; Aten, J.; Vlahu, C.A.; van Kuppevelt, T.H.; et al. Low-GDP, pH-neutral solutions preserve peritoneal endothelial glycocalyx during long-term peritoneal dialysis. Clin. Exp. Nephrol. 2021, 25, 1035–1046. [Google Scholar] [CrossRef]
- Garosi, G.; Di Paolo, N. Peritoneal sclerosis: One or two nosological entities? Semin. Dial. 2000, 13, 297–308. [Google Scholar] [CrossRef]
- Honda, K.; Nitta, K.; Horita, S.; Tsukada, M.; Itabashi, M.; Nihei, H.; Akiba, T.; Oda, H. Histologic criteria for diagnosing encapsulating peritoneal sclerosis in continuous ambulatory peritoneal dialysis patients. Adv. Perit. Dial. 2003, 19, 169–175. [Google Scholar]
- Braun, N.; Alscher, D.M.; Fritz, P.; Edenhofer, I.; Kimmel, M.; Gaspert, A.; Reimold, F.; Bode-Lesniewska, B.; Ziegler, U.; Biegger, D.; et al. Podoplanin-positive cells are a hallmark of encapsulating peritoneal sclerosis. Nephrol. Dial. Transpl. 2011, 26, 1033–1041. [Google Scholar] [CrossRef]
- Braun, N.; Alscher, M.D.; Fritz, P.; Latus, J.; Edenhofer, I.; Reimold, F.; Alper, S.L.; Kimmel, M.; Biegger, D.; Lindenmeyer, M.; et al. The spectrum of podoplanin expression in encapsulating peritoneal sclerosis. PLoS ONE 2012, 7, e53382. [Google Scholar] [CrossRef]
- Honda, K.; Hamada, C.; Kawanishi, K.; Nakayama, M.; Miyazaki, M.; Ito, Y. Significance of new membrane formation in peritoneal biopsies of peritoneal dialysis patients: A case–control study. Ren. Replace. Ther. 2017, 3, 33. [Google Scholar] [CrossRef]
- Nakamoto, H. Encapsulating peritoneal sclerosis—A clinician’s approach to diagnosis and medical treatment. Perit. Dial. Int. 2005, 25 (Suppl. S4), S30–S38. [Google Scholar] [CrossRef]
- Nakayama, M. The plasma leak-to-response hypothesis: A working hypothesis on the pathogenesis of encapsulating peritoneal sclerosis after long-term peritoneal dialysis treatment. Perit. Dial. Int. 2005, 25 (Suppl. S4), S71–S76. [Google Scholar] [CrossRef] [PubMed]
- Ayuzawa, N.; Ishibashi, Y.; Takazawa, Y.; Kume, H.; Fujita, T. Peritoneal morphology after long-term peritoneal dialysis with biocompatible fluid: Recent clinical practice in Japan. Perit. Dial. Int. 2012, 32, 159–167. [Google Scholar] [CrossRef]
- Nakayama, M.; Miyazaki, M.; Honda, K.; Kasai, K.; Tomo, T.; Nakamoto, H.; Kawanishi, H. Encapsulating peritoneal sclerosis in the era of a multi-disciplinary approach based on biocompatible solutions: The NEXT-PD study. Perit. Dial. Int. 2014, 34, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, B.; Bartosova, M.; Macher-Goeppinger, S.; Sallay, P.; Vörös, P.; Ranchin, B.; Vondrak, K.; Ariceta, G.; Zaloszyc, A.; Bayazit, A.K.; et al. Neutral pH and low-glucose degradation product dialysis fluids induce major early alterations of the peritoneal membrane in children on peritoneal dialysis. Kidney Int. 2018, 94, 419–429. [Google Scholar] [CrossRef]
- Kim, Y.L.; Cho, J.H.; Choi, J.Y.; Kim, C.D.; Park, S.H. Systemic and local impact of glucose and glucose degradation products in peritoneal dialysis solution. J. Ren. Nutr. 2013, 23, 218–222. [Google Scholar] [CrossRef]
- Szeto, C.C.; Johnson, D.W. Low GDP Solution and Glucose-Sparing Strategies for Peritoneal Dialysis. Semin. Nephrol. 2017, 37, 30–42. [Google Scholar] [CrossRef]
- Krediet, R.T.; Struijk, D.G. Peritoneal changes in patients on long-term peritoneal dialysis. Nat. Rev. Nephrol. 2013, 9, 419–429. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Liu, J. A review of research progress on mechanisms of peritoneal fibrosis related to peritoneal dialysis. Front. Physiol. 2023, 14, 1220450. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Anton, M.; Bowen, T.; Jenkins, R.H. microRNA regulation of peritoneal cavity homeostasis in peritoneal dialysis. Biomed. Res. Int. 2015, 2015, 929806. [Google Scholar] [CrossRef] [PubMed]
- Liakopoulos, V.; Georgianos, P.I.; Vaios, V.; Roumeliotis, S.; Karligkiotis, A.; Zebekakis, P.E. 10-year-long survival in a PD patient with severe calcifying encapsulating peritoneal sclerosis treated with tamoxifen: A case-report. BMC Nephrol. 2020, 21, 110. [Google Scholar] [CrossRef] [PubMed]
- Fenglin, X.; Shengyuan, W.; Zhiyong, Z.; Hai, Y.; Mingxu, L. Pirfenidone ameliorated AGE-induced EMT and attenuated peritoneal fibrosis in peritoneal mesothelial cells. Mol. Cell. Toxicol. 2021, 17, 315–323. [Google Scholar] [CrossRef]
- Rahmanian-Devin, P.; Rakhshandeh, H.; Baradaran Rahimi, V.; Sanei-Far, Z.; Hasanpour, M.; Memarzia, A.; Iranshahi, M.; Askari, V.R. Intraperitoneal Lavage with Crocus sativus Prevents Postoperative-Induced Peritoneal Adhesion in a Rat Model: Evidence from Animal and Cellular Studies. Oxidative Med. Cell. Longev. 2021, 2021, 5945101. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wu, K.; Jiang, S.; Li, Y.; Wang, Y.; Li, H.; Li, G.; Liu, Q.; Zhou, Y.; Chen, W.; et al. Therapeutic mechanism of baicalein in peritoneal dialysis-associated peritoneal fibrosis based on network pharmacology and experimental validation. Front. Pharmacol. 2023, 14, 1153503. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chu, C.; Mai, Y.; Zhao, Y.; Cao, L.; Ji, S.; Zhu, B.; Shen, Q. Treatment of peritoneal fibrosis: Therapeutic prospects of bioactive Agents from Astragalus membranaceus. Front. Pharmacol. 2024, 15, 1347234. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Wang, L.; TingYang; Wang, L.; Ge, W. Silymarin ameliorates peritoneal fibrosis by inhibiting the TGF-β/Smad signaling pathway. Naunyn Schmiedebergs Arch. Pharmacol. 2023, 396, 2379–2391. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.; Park, M.; Seo, A.; Kim, K.H.; Kim, S.; Kang, M.; Kang, E.; Yoo, K.D.; Lee, S.; et al. Inflammatory chemokine (C–C motif) ligand 8 inhibition ameliorates peritoneal fibrosis. FASEB J. 2023, 37, e22632. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ito, Y.; Sun, T.; Tawada, M.; Kinashi, H.; Yamaguchi, M.; Katsuno, T.; Kim, H.; Mizuno, M.; Ishimoto, T. Pathophysiological Mechanisms of Peritoneal Fibrosis and Peritoneal Membrane Dysfunction in Peritoneal Dialysis. Int. J. Mol. Sci. 2024, 25, 8607. https://doi.org/10.3390/ijms25168607
Ito Y, Sun T, Tawada M, Kinashi H, Yamaguchi M, Katsuno T, Kim H, Mizuno M, Ishimoto T. Pathophysiological Mechanisms of Peritoneal Fibrosis and Peritoneal Membrane Dysfunction in Peritoneal Dialysis. International Journal of Molecular Sciences. 2024; 25(16):8607. https://doi.org/10.3390/ijms25168607
Chicago/Turabian StyleIto, Yasuhiko, Ting Sun, Mitsuhiro Tawada, Hiroshi Kinashi, Makoto Yamaguchi, Takayuki Katsuno, Hangsoo Kim, Masashi Mizuno, and Takuji Ishimoto. 2024. "Pathophysiological Mechanisms of Peritoneal Fibrosis and Peritoneal Membrane Dysfunction in Peritoneal Dialysis" International Journal of Molecular Sciences 25, no. 16: 8607. https://doi.org/10.3390/ijms25168607
APA StyleIto, Y., Sun, T., Tawada, M., Kinashi, H., Yamaguchi, M., Katsuno, T., Kim, H., Mizuno, M., & Ishimoto, T. (2024). Pathophysiological Mechanisms of Peritoneal Fibrosis and Peritoneal Membrane Dysfunction in Peritoneal Dialysis. International Journal of Molecular Sciences, 25(16), 8607. https://doi.org/10.3390/ijms25168607






