In Vivo Luciferin–Luciferase Reaction in Micro-Mini Pigs Using Xenogeneic Rat Bone Marrow Transplantation
Abstract
1. Introduction
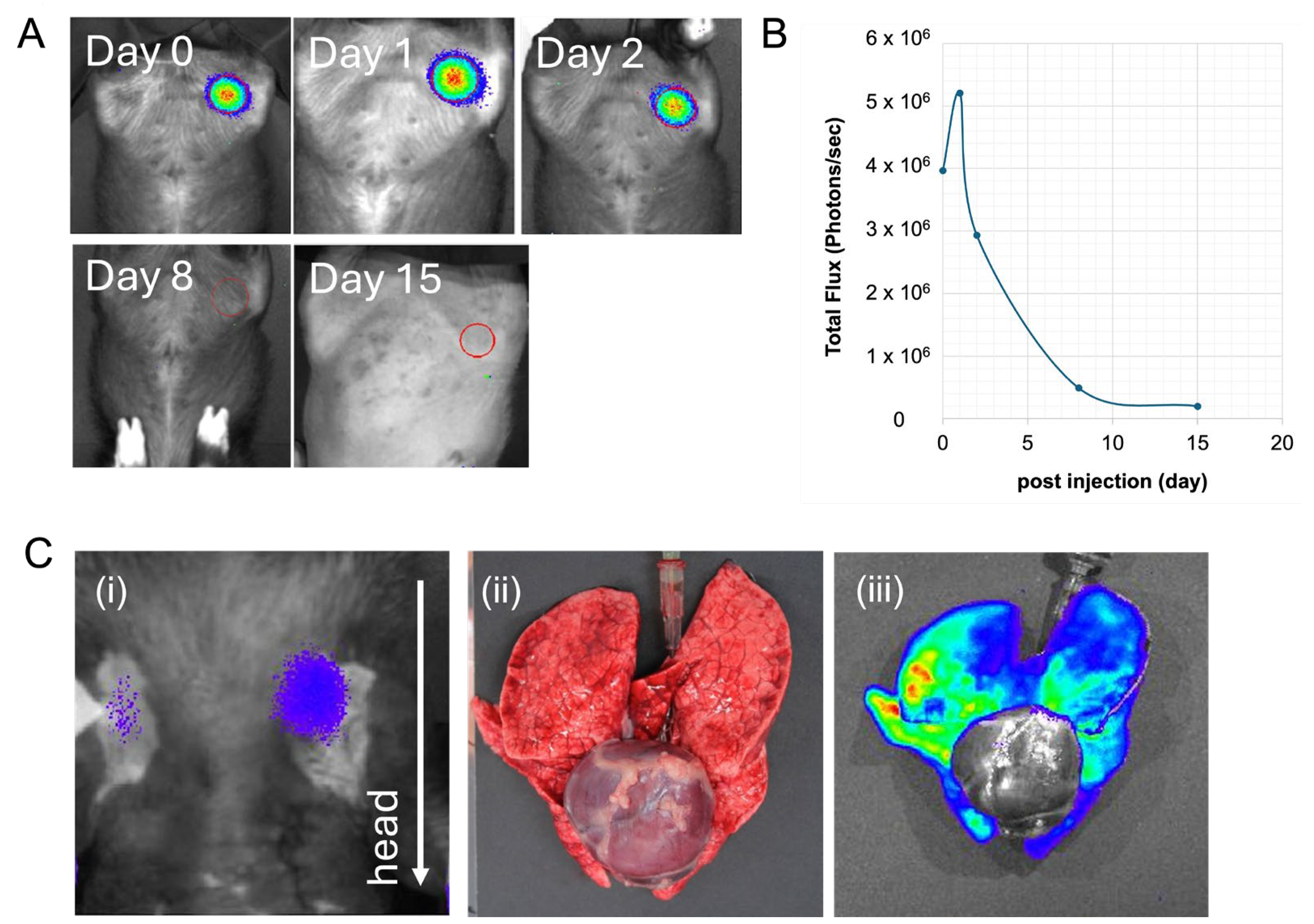
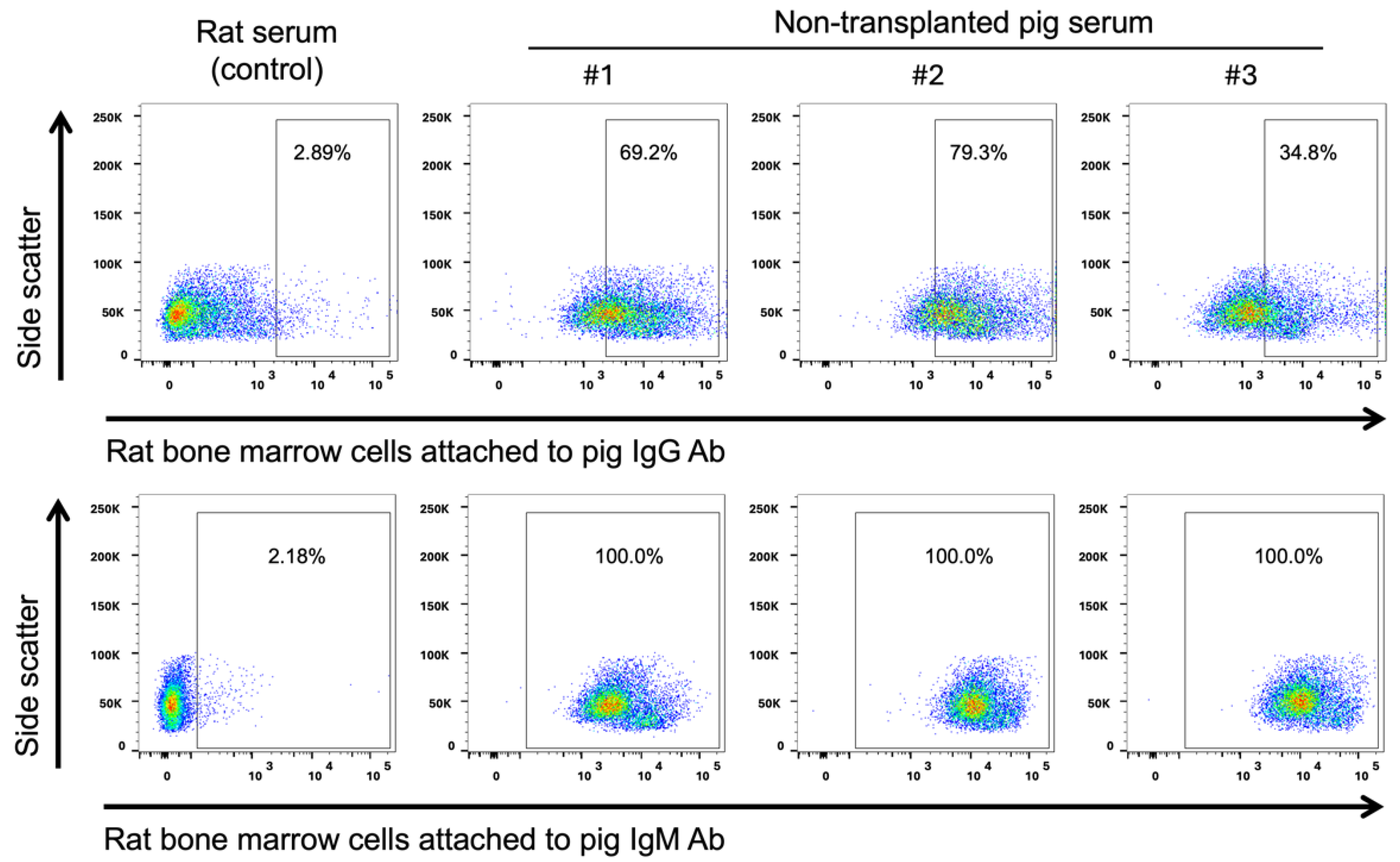
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, Y.; Sun, Y.; Wang, X.; Li, H.; Qiu, J.; Lu, W. Application of photoacoustic computed tomography in biomedical imaging: A literature review. Bioeng. Transl. Med. 2023, 8, e10419. [Google Scholar] [CrossRef] [PubMed]
- Hirai, M.; Sakurada, T.; Ikeda, T.; Monden, Y.; Shimoizumi, H.; Yamagata, T. Developmental changes of the neural mechanisms underlying Level 2 visual perspective-taking: A functional near-infrared spectroscopy study. Dev. Psychobiol. 2022, 64, e22229. [Google Scholar] [CrossRef] [PubMed]
- Noel, S.J.; Jørgensen, H.J.H.; Knudsen, K.E.B. The use of near-infrared spectroscopy (NIRS) to determine the energy value of individual feedstuffs and mixed diets for pigs. Anim. Feed Sci. Technol. 2022, 283, 115156. [Google Scholar] [CrossRef]
- James, M.L.; Gambhir, S.S. A molecular imaging primer: Modalities, imaging agents, and applications. Physiol. Rev. 2012, 92, 897–965. [Google Scholar] [CrossRef] [PubMed]
- Saito-Moriya, R.; Obata, R.; Maki, S.A. Near-Infrared luciferin Analogs for in vivo optical Imaging. In Bioluminescence—Technology and Biology; Suzuki, H., Ogoh, K., Eds.; IntechOpen: London, UK, 2021; ISBN 978-1-83962-385-1. [Google Scholar]
- Gyöngyösi, M.; Hemetsberger, R.; Wolbank, S.; Pichler, V.; Kaun, C.; Posa, A.; Petrasi, Z.; Petnehazy, Ö.; Hofer-Warbinek, R.; De Martin, R.; et al. Delayed recovery of myocardial blood flow after intracoronary stem cell administration. Stem Cell Rev. Rep. 2011, 7, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Rodenberg, E.J.; Patel, D.S.; Shirley, B.; Young, B.W.; Taylor, A.F.; Steidinger, H.R.; Fisher, S.J.; Patel, A.N. Catheter-based retrograde coronary sinus infusion is a practical delivery technique for introducing biological molecules into the cardiac system. Catheter. Cardiovasc. Interv. 2019, 94, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K.; Jablonska, A.; Chu, C.; Gregg, L.; Bulte, J.W.M.; Koehler, R.C.; Walczak, P.; Janowski, M. Biodistribution of glial progenitors in a three dimensional-printed model of the piglet cerebral ventricular system. Stem Cells Dev. 2019, 28, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Watano, R.; Ohmori, T.; Hishikawa, S.; Sakata, A.; Mizukami, H. Utility of microminipigs for evaluating liver-mediated gene expression in the presence of neutralizing antibody against vector capsid. Gene Ther. 2020, 27, 427–434. [Google Scholar] [CrossRef]
- Kremen, T.J.; Stefanovic, T.; Tawackoli, W.; Salehi, K.; Avalos, P.; Reichel, D.; Perez, M.J.; Glaeser, J.D.; Sheyn, D. A translational porcine model for human cell-based therapies in the treatment of posttraumatic osteoarthritis after anterior cruciate ligament injury. Am. J. Sports Med. 2020, 48, 3002–3012. [Google Scholar] [CrossRef]
- Hakamata, Y.; Murakami, T.; Kobayashi, E. “Firefly rats” as an organ/cellular source for long-term in vivo bioluminescent imaging. Transplantation 2006, 81, 1179–1184. [Google Scholar] [CrossRef]
- Horie, M.; Sekiya, I.; Muneta, T.; Ichinose, S.; Matsumoto, K.; Saito, H.; Murakami, T.; Kobayashi, E. Intra-articular injected synovial stem cells differentiate into meniscal cells directly and promote meniscal regeneration without mobilization to distant organs in rat massive meniscal defect. Stem Cells 2009, 27, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Kainuma, S.; Miyagawa, S.; Fukushima, S.; Pearson, J.; Chen, Y.C.; Saito, A.; Harada, A.; Shiozaki, M.; Iseoka, H.; Watabe, T.; et al. Cell-sheet therapy with omentopexy promotes arteriogenesis and improves coronary circulation physiology in failing heart. Mol. Ther. 2015, 23, 374–386. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, S.; Soma, Y.; Nakajima, K.; Kanazawa, H.; Tohyama, S.; Tabei, R.; Hirano, A.; Handa, N.; Yamada, Y.; Okuda, S.; et al. Intramyocardial transplantation of human iPS cell–derived cardiac spheroids improves cardiac function in heart failure animals. JACC Basic Transl. Sci. 2021, 6, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, Y.; Nakayama, K.; Taguchi, T.; Enosawa, S.; Tamura, T.; Yoshimaru, K.; Matsuura, T.; Hayashida, M.; Kohashi, K.; Oda, Y.; et al. In vivo and ex vivo methods of growing a liver bud through tissue connection. Sci. Rep. 2017, 7, 14085. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, S.; Kobayashi, E.; Fujii, M.; Ohta, Y.; Arai, K.; Matano, M.; Ishikawa, K.; Miyamoto, K.; Toshimitsu, K.; Takahashi, S.; et al. An organoid-based organ-repurposing approach to treat short bowel syndrome. Nature 2021, 592, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Griffith, B.P.; Goerlich, C.E.; Singh, A.K.; Rothblatt, M.; Lau, C.L.; Shah, A.; Lorber, M.; Grazioli, A.; Saharia, K.K.; Hong, S.N.; et al. Genetically modified porcine-to-human cardiac xenotransplantation. N. Engl. J. Med. 2022, 387, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, R.A.; Stern, J.M.; Lonze, B.E.; Tatapudi, V.S.; Mangiola, M.; Wu, M.; Weldon, E.; Lawson, N.; Deterville, C.; Dieter, R.A.; et al. Results of two cases of pig-to-human kidney xenotransplantation. N. Engl. J. Med. 2022, 386, 1889–1898. [Google Scholar] [CrossRef] [PubMed]
- Platt, J.L.; Cascalho, M.; Piedrahita, J.A. Xenotransplantation: Progress along paths uncertain from models to application. ILAR J. 2018, 59, 286–308. [Google Scholar] [CrossRef] [PubMed]
- McGregor, C.G.A.; Davies, W.R.; Oi, K.; Teotia, S.S.; Schirmer, J.M.; Risdahl, J.M.; Tazelaar, H.D.; Kremers, W.K.; Walker, R.C.; Byrne, G.W.; et al. Cardiac xenotransplantation: Recent preclinical progress with 3-month median survival. J. Thorac. Cardiovasc. Surg. 2005, 130, 844–851. [Google Scholar] [CrossRef]
- Abicht, J.-M.; Mayr, T.; Reichart, B.; Buchholz, S.; Werner, F.; Lutzmann, I.; Schmoeckel, M.; Bauer, A.; Thormann, M.; Langenmayer, M.; et al. Pre-clinical heterotopic intrathoracic heart xenotransplantation: A possibly useful clinical technique. Xenotransplantation 2015, 22, 427–442. [Google Scholar] [CrossRef]
- Loupy, A.; Goutaudier, V.; Giarraputo, A.; Mezine, F.; Morgand, E.; Robin, B.; Khalil, K.; Mehta, S.; Keating, B.; Dandro, A.; et al. Immune response after pig-to-human kidney xenotransplantation: A multimodal phenotyping study. Lancet 2023, 402, 1158–1169. [Google Scholar] [CrossRef]
- Joziasse, D.H.; Oriol, R. Xenotransplantation: The importance of the Galα1,3Gal epitope in hyperacute vascular rejection. Biochim. Biophys. Acta 1999, 1455, 403–418. [Google Scholar] [CrossRef]
- Cooper, D.K.C. A brief history of cross-species organ transplantation. Bayl. Univ. Med. Cent. Proc. 2012, 25, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Huai, G.; Du, J.; Zhang, Z.; Gonelle-Gispert, C.; Zhang, X.; Dou, K.; Deng, S.; Pan, D.; Buhler, L.H. Gene-modified pigs as donors for liver xenotransplantation: How many modifications are needed? EJT 2023, 1, 234–245. [Google Scholar] [CrossRef]
- Kolber-Simonds, D.; Lai, L.; Watt, S.R.; Denaro, M.; Arn, S.; Augenstein, M.L.; Betthauser, J.; Carter, D.B.; Greenstein, J.L.; Hao, Y.; et al. Production of α-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc. Natl. Acad. Sci. USA 2004, 101, 7335–7340. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Yazawa, K.; Shimizu, A.; Iwanaga, T.; Hisashi, Y.; Nuhn, M.; O’Malley, P.; Nobori, S.; Vagefi, P.A.; Patience, C.; et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of A1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat. Med. 2005, 11, 32–34. [Google Scholar] [CrossRef]
- Pan, W.; Zhang, W.; Zheng, B.; Camellato, B.R.; Stern, J.; Lin, Z.; Khodadadi-Jamayran, A.; Kim, J.; Sommer, P.; Khalil, K.; et al. Cellular Dynamics in Pig-to-Human Kidney Xenotransplantation. Med 2024, S2666-6340(24)00207-1. [Google Scholar] [CrossRef] [PubMed]
- Fujisaki, J.; Wu, J.; Carlson, A.L.; Silberstein, L.; Putheti, P.; Larocca, R.; Gao, W.; Saito, T.I.; Lo Celso, C.L.; Tsuyuzaki, H.; et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature 2011, 474, 216–219. [Google Scholar] [CrossRef]
- Zou, L.; Barnett, B.; Safah, H.; Larussa, V.F.; Evdemon-Hogan, M.; Mottram, P.; Wei, S.; David, O.; Curiel, T.J.; Zou, W. Bone Marrow Is a Reservoir for CD4+CD25+ Regulatory T Cells That Traffic through CXCL12/CXCR4 Signals. Cancer Res 2004, 64, 8451–8455. [Google Scholar] [CrossRef]
- Niederkorn, J.Y. See no evil, hear no evil, do no evil: The lessons of immune privilege. Nat. Immunol. 2006, 7, 354–359. [Google Scholar] [CrossRef]
- Love, A.C.; Prescher, J.A. Seeing (and using) the light: Recent developments in bioluminescence technology. Cell Chem. Biol. 2020, 27, 904–920. [Google Scholar] [CrossRef] [PubMed]
- Saito-Moriya, R.; Nakayama, J.; Kamiya, G.; Kitada, N.; Obata, R.; Maki, S.A.; Aoyama, H. How to select firefly luciferin analogues for in vivo imaging. Int. J. Mol. Sci. 2021, 22, 1848. [Google Scholar] [CrossRef] [PubMed]
- Iwano, S.; Sugiyama, M.; Hama, H.; Watakabe, A.; Hasegawa, N.; Kuchimaru, T.; Tanaka, K.Z.; Takahashi, M.; Ishida, Y.; Hata, J.; et al. Single-cell bioluminescence imaging of deep tissue in freely moving animals. Science 2018, 359, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-B.; Paulmurugan, R. Bioluminescent imaging systems for assay developments. Anal. Sci. 2021, 37, 233–247. [Google Scholar] [CrossRef]
- Abe, T.; Kono, S.; Ohnuki, T.; Hishikawa, S.; Kunita, S.; Hanazono, Y. A Swine model of acute thrombocytopenia with prolonged bleeding time produced by busulfan. Exp. Anim. 2016, 65, 345–351. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abe, T.; Endo, K.; Hanazono, Y.; Kobayashi, E. In Vivo Luciferin–Luciferase Reaction in Micro-Mini Pigs Using Xenogeneic Rat Bone Marrow Transplantation. Int. J. Mol. Sci. 2024, 25, 8609. https://doi.org/10.3390/ijms25168609
Abe T, Endo K, Hanazono Y, Kobayashi E. In Vivo Luciferin–Luciferase Reaction in Micro-Mini Pigs Using Xenogeneic Rat Bone Marrow Transplantation. International Journal of Molecular Sciences. 2024; 25(16):8609. https://doi.org/10.3390/ijms25168609
Chicago/Turabian StyleAbe, Tomoyuki, Kazuhiro Endo, Yutaka Hanazono, and Eiji Kobayashi. 2024. "In Vivo Luciferin–Luciferase Reaction in Micro-Mini Pigs Using Xenogeneic Rat Bone Marrow Transplantation" International Journal of Molecular Sciences 25, no. 16: 8609. https://doi.org/10.3390/ijms25168609
APA StyleAbe, T., Endo, K., Hanazono, Y., & Kobayashi, E. (2024). In Vivo Luciferin–Luciferase Reaction in Micro-Mini Pigs Using Xenogeneic Rat Bone Marrow Transplantation. International Journal of Molecular Sciences, 25(16), 8609. https://doi.org/10.3390/ijms25168609






