Control of Dopamine Signal in High-Order Receptor Complex on Striatal Astrocytes
Abstract
1. Introduction
2. Results
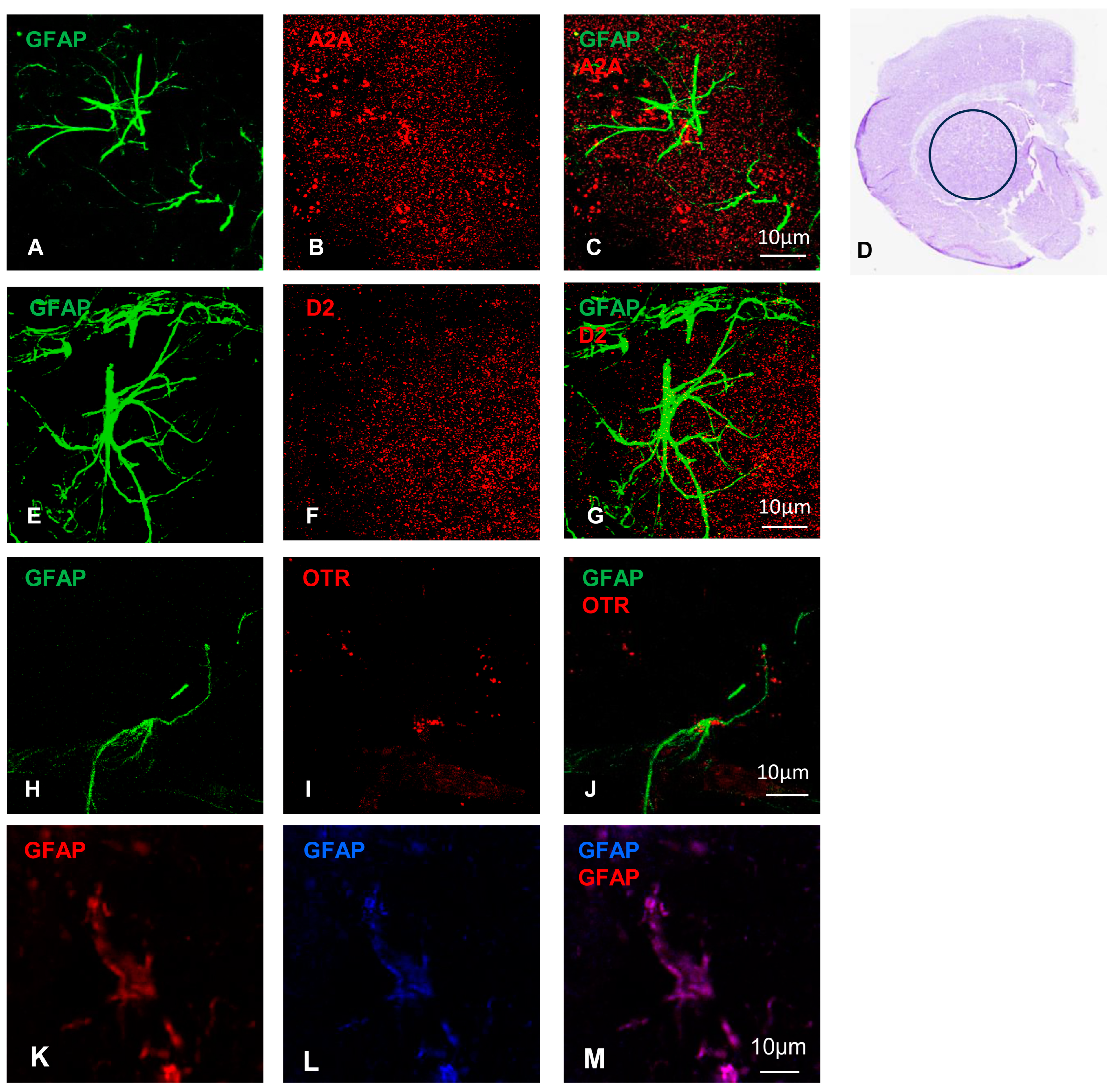
2.1. On Striatal Astrocytes the OTR, A2A, and D2 Receptors Are Co-Localized on GFAP–Ezrin Positive Processes
2.2. On Striatal Astrocytic Processes the A2A, D2 and OT Receptors Are Co-Localized
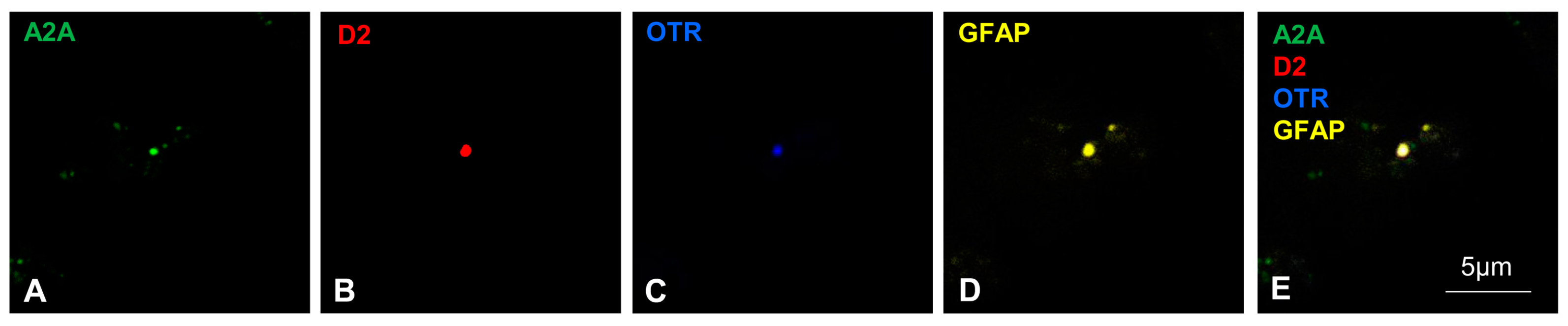
2.3. On the Membrane of Striatal Astrocytic Processes, the A2A, OTR and D2 Receptors Physically Interact
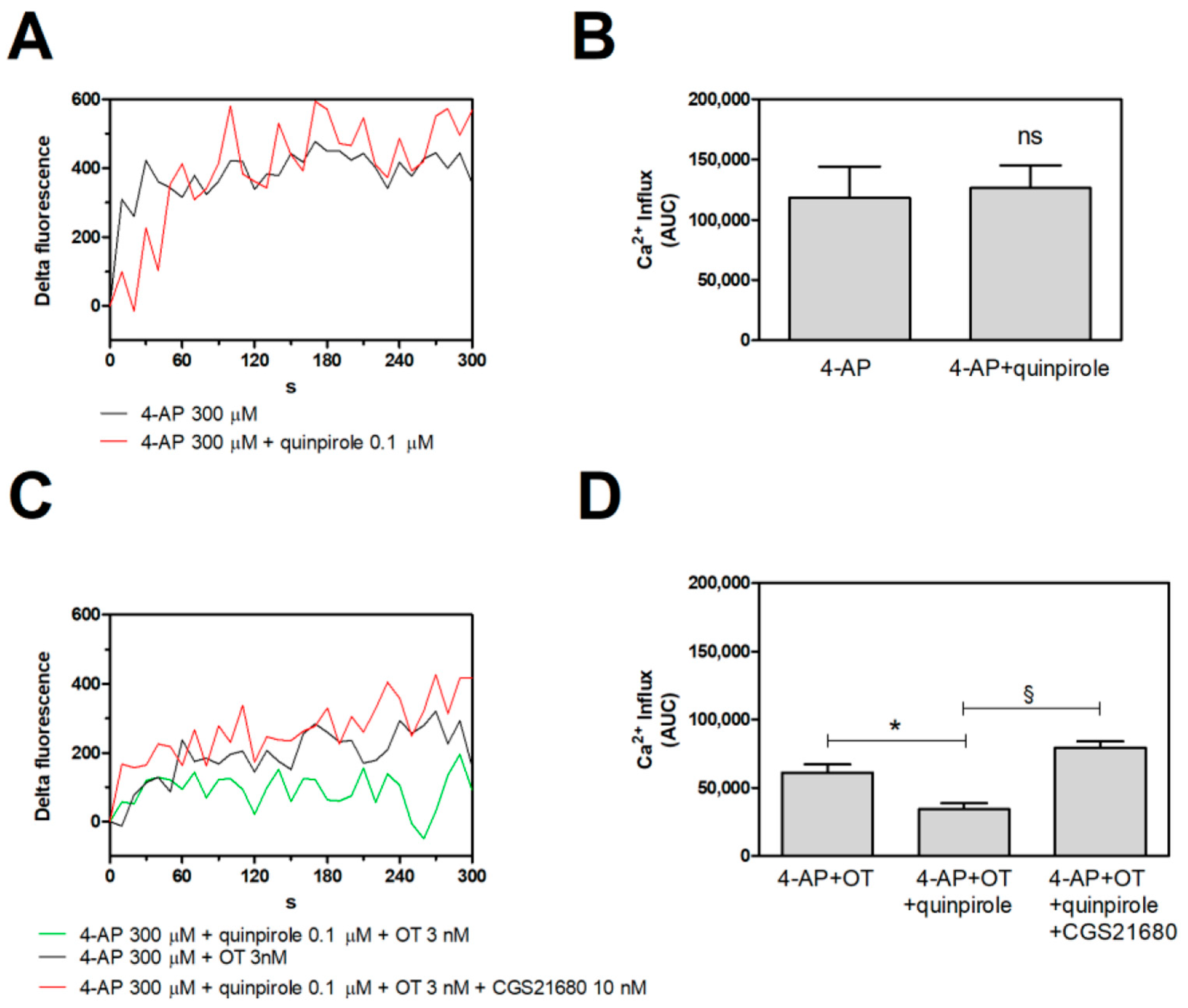
2.4. Functional Evidence for a Putative A2A-D2-OTR Mosaic: D2-OTR Heteromer-Mediated Inhibition of the 4-AP-Evoked Calcium Signals Is Nullified by A2A Receptor Activation
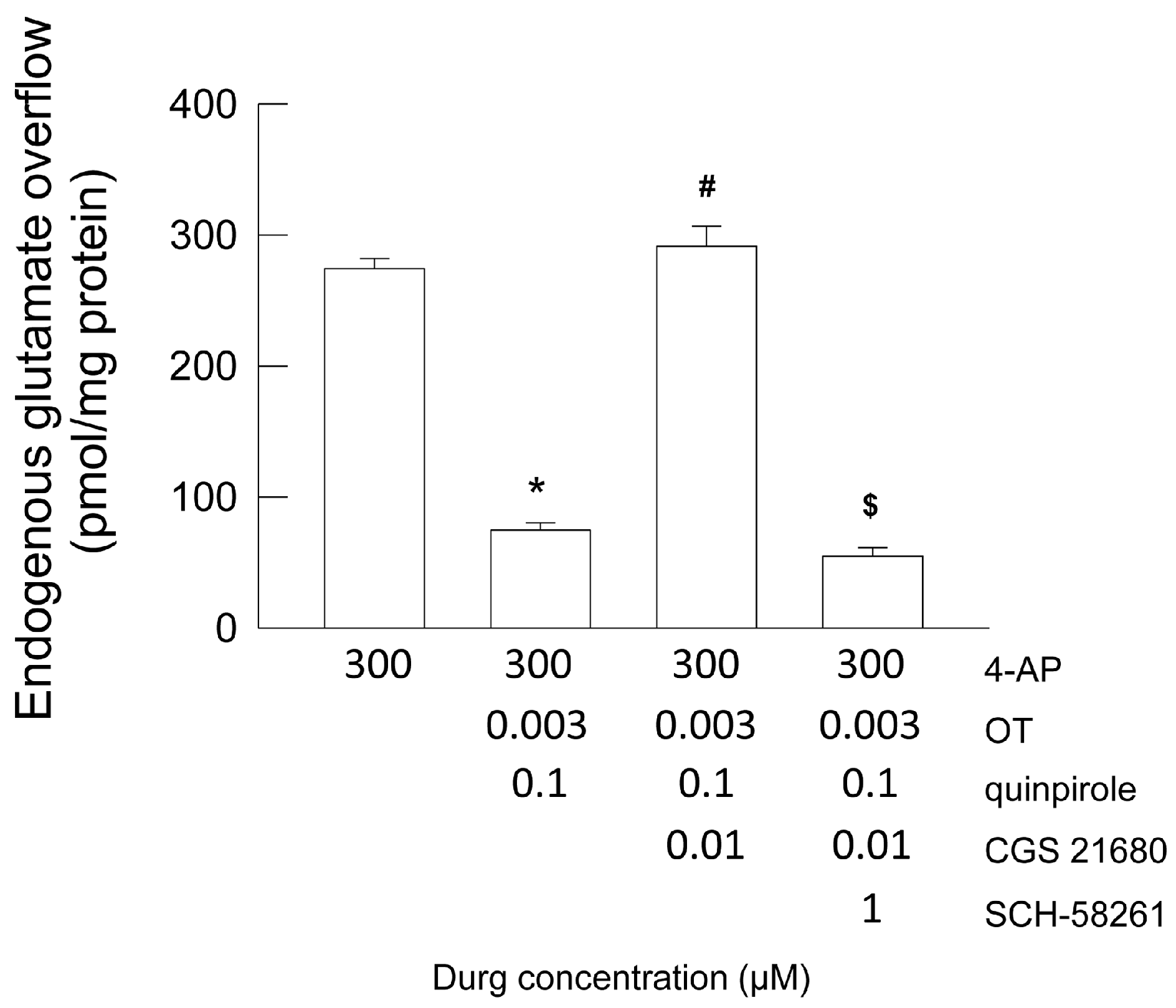
2.5. Functional Evidence for a Putative A2A-D2-OTR Mosaic: D2-OTR Heteromer-Mediated Inhibition of the 4-AP-Evoked Glutamate Release Is Nullified by A2A Receptor Activation
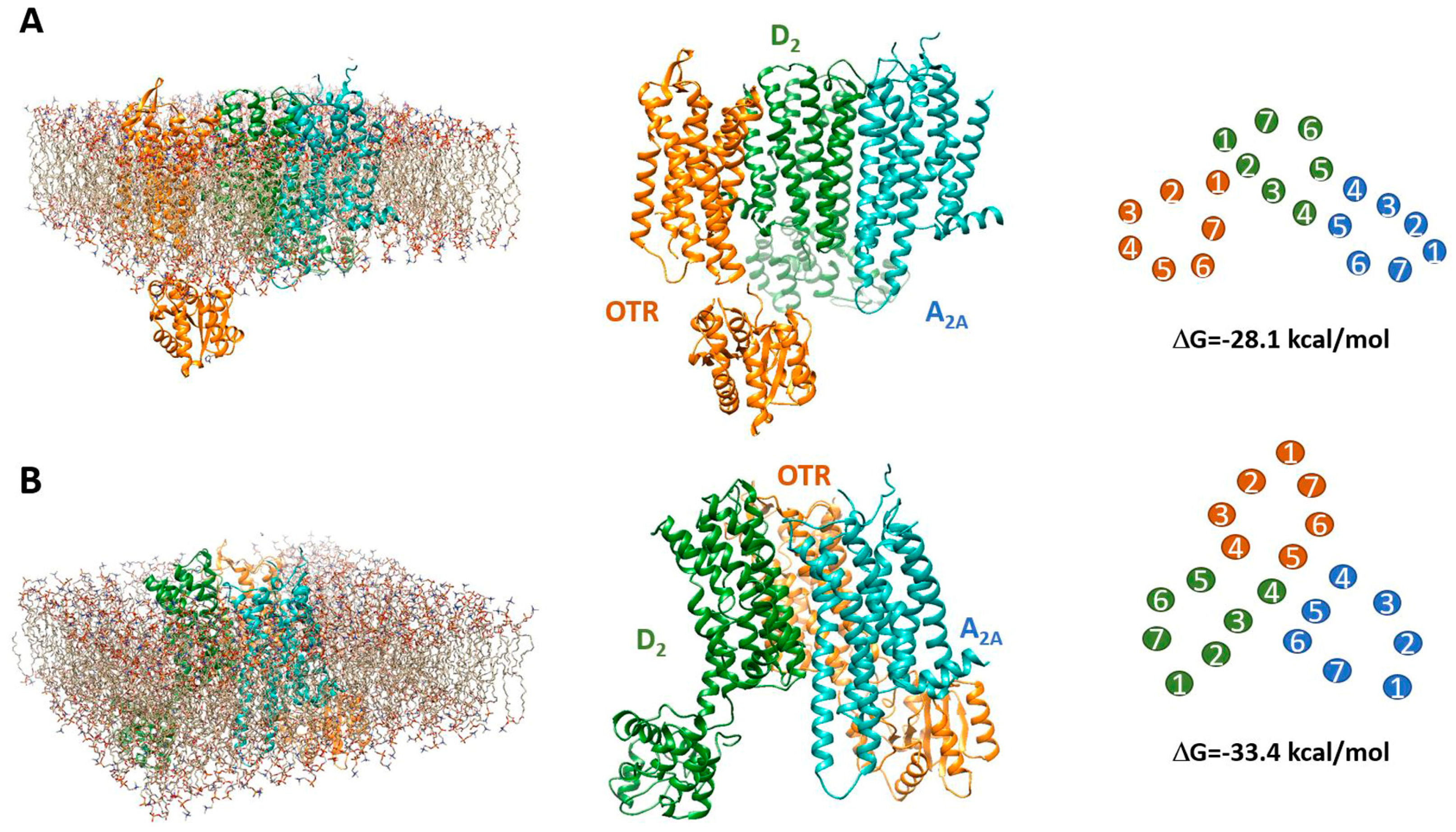
2.6. Estimated Model of a Putative A2A-D2-OTR Mosaic
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Slices Preparation and Cresyl Violet Staining
4.3. Proximity Ligation Assay (PLA) and Immunofluorescent Confocal Microscopy on Slices
4.4. Preparation of Purified Striatal Astrocytic Processes
4.5. Immunofluorescent Labeling in Gliosomes and Confocal Microscopy
4.6. Immunoprecipitation and Immunoblotting Experiments
4.7. Endogenous Glutamate Release
4.8. Intracellular [Ca2+] Assay
4.9. Modeling of the A2A-D2-OTR Mosaic
4.10. Calculations and Statistical Analysis
4.11. Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marshall, F.H.; Jones, K.A.; Kaupmann, K.; Bettler, B. GABAB receptors: The first 7TM heterodimers. Trends Pharmacol. Sci. 1999, 20, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, M. Oligomerization of G-protein-coupled transmitter receptors. Nat. Rev. Neurosci. 2001, 2, 274–286. [Google Scholar] [CrossRef]
- Agnati, L.F.; Fuxe, K.; Zini, I.; Lenzi, P.; Hökfelt, T. Aspects on receptor regulation and isoreceptor identification. Med. Biol. 1980, 58, 182–187. [Google Scholar] [PubMed]
- Fuxe, K.; Agnati, L.F.; Benfenati, F.; Celani, M.; Zini, I.; Zoli, M.; Zini, I.; Zoli, M.; Mutt, V. Evidence for the existence of receptor-receptor interactions in the central nervous system. Studies on the regulation of monoamine receptors by neuropeptides. J. Neural Transm. 1983, 18, 165–179. [Google Scholar]
- Agnati, L.F.; Fuxe, K.; Giardino, L.; Calzà, L.; Zoli, M.; Battistini, N.; Benfenati, F.; Vanderhaeghen, J.J.; Guidolin, D.; Ruggeri, M. Evidence for cholecystokinin-dopamine receptor interactions in the central nervous system of the adult and old rat. Studies on their functional meaning. Ann. N. Y. Acad. Sci. 1985, 448, 315–333. [Google Scholar] [CrossRef] [PubMed]
- El-Asmar, L.; Springael, J.Y.; Ballet, S.; Andrieu, E.U.; Vassart, G.; Parmentier, M. Evi-dence for negative binding cooperativity within CCR5-CCR2b heterodimers. Mol. Pharmacol. 2005, 67, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, K.; Canals, M.; Torvinen, M.; Marcellino, D.; Terasmaa, A.; Genedani, S.; Leo, G.; Guidolin, D.; Diaz-Cabiale, Z.; Rivera, A.; et al. Intramembrane receptor-receptor interactions: A novel principle in molecular medicine. J. Neural Transm. 2007, 114, 49–75. [Google Scholar] [CrossRef]
- Rashid, A.J.; So, C.H.; Kong, M.M.C.; Furtak, T.; El-Ghundi, M.; Cheng, R.; O’Dowd, B.F.; George, S.R. D1–D2 dopa-mine receptor heterooligomers with unique pharmacology are coupled to rapid activa-tion of Gq/11 in the striatum. Proc. Natl. Acad. Sci. USA 2007, 104, 654–659. [Google Scholar] [CrossRef]
- Franco, R.; Casadó, V.; Cortés, A.; Mallol, J.; Ciruela, F.; Ferré, S.; Lluis, C.; Canela, E.I. G-protein-coupled receptor heteromers: Function and ligand phar-macology. Br. J. Pharmacol. 2008, 153, S90–S98. [Google Scholar] [CrossRef]
- Fuxe, K.; Borroto-Escuela, D.O.; Marcellino, D.; Romero-Fernández, W.; Frankowska, M.; Guidolin, D.; Filip, M.; Ferraro, L.; Woods, A.S.; Tarakanov, A.; et al. GPCR Heteromers and their Allosteric Receptor-Receptor Interactions. Curr. Med. Chem. 2012, 19, 356–363. [Google Scholar] [CrossRef]
- Brugarolas, M.; Navarro, G.; Martínez-Pinilla, E.; Angelats, E.; Casadó, V.; Lanciego, J.L.; Franco, R. G-protein-coupled receptor heteromers as key players in the molecular architecture of the central nervous system. CNS Neurosci. Ther. 2014, 20, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Zoli, M.; Agnati, L.F.; Hedlund, P.B.; Li, X.M.; Ferré, S.; Fuxe, K. Receptor–receptor interactions as an integrative mechanism in nerve cells. Mol. Neurobiol. 1993, 7, 293–334. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, D.; Tortorella, C.; Marcoli, M.; Cervetto, C.; De Caro, R.; Maura, G.; Agnati, L.F. Modulation of Neuron and Astrocyte Dopamine Receptors via Receptor–Receptor Interactions. Pharmaceuticals 2023, 16, 1427. [Google Scholar] [CrossRef] [PubMed]
- Ferré, S.; Ciruela, F.; Canals, M.; Marcellino, D.; Burgueno, J.; Casadó, V.; Hillion, J.; Torvinen, M.; Fanelli, F.; de Benedetti, P.; et al. Adenosine A2A-dopamine D2 receptor–receptor heteromers. Targets for neuro-psychiatric disorders. Park. Relat. Disord. 2004, 10, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Agnati, L.F.; Guidolin, D.; Vilardaga, J.P.; Ciruela, F.; Fuxe, K. On the expanding terminology in the GPCR field: The meaning of receptor mosaics and receptor heteromers. J. Recept. Signal Transduct. Res. 2010, 30, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, D.; Marcoli, M.; Tortorella, C.; Maura, G.; Agnati, L.F. Receptor-receptor interactions as a widespread phenomenon: Novel targets for drug development? Front. Endocrinol. 2019, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Agnati, L.F.; Leo, G.; Genedani, S.; Andreoli, N.; Marcellino, D.; Woods, A.; Piron, L.; Guidolin, D.; Fuxe, K. Structural plasticity in G-protein coupled receptors as demonstrated by the allosteric actions of homocysteine and computer-assisted analysis of disordered domains. Brain Res. Rev. 2008, 58, 459–474. [Google Scholar] [CrossRef]
- Agnati, L.F.; Guidolin, D.; Leo, G.; Carone, C.; Genedani, S.; Fuxe, K. Receptor-receptor interactions: A novel concept in brain integration. Prog. Neurobiol. 2010, 90, 157–175. [Google Scholar] [CrossRef]
- Farran, B. An update on the physiological and therapeutic relevance of GPCR oligomers. Pharmacol. Res. 2017, 117, 303–327. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, D.; Marcoli, M.; Tortorella, C.; Maura, G.; Agnati, L.F. G protein-coupled receptor-receptor interactions give integrative dynamics to intercellular communication. Rev. Neurosci. 2018, 29, 703–726. [Google Scholar] [CrossRef]
- Agnati, L.F.; Marcoli, M.; Maura, G.; Woods, A.S.; Guidolin, D. The brain as a “hyper-network”: The key role of neural networks as main producers of the integrated brain actions especially via the “broadcasted” neuroconnectomics. J. Neural Transm. 2018, 125, 883–897. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, D.; Tortorella, C.; Marcoli, M.; Maura, G.; Agnati, L.F. Intercellular Communication in the Central Nervous System as Deduced by Chemical Neuroanatomy and Quantitative Analysis of Images: Impact on Neuropharmacology. Int. J. Mol. Sci. 2022, 23, 5805. [Google Scholar] [CrossRef] [PubMed]
- Marcoli, M.; Agnati, L.F.; Franco, R.; Cortelli, P.; Anderlini, D.; Guidolin, D.; Cervetto, C.; Maura, G. Modulating brain integrative actions as a new perspective on pharmacological approaches to neuropsychiatric diseases. Front. Endocrinol. 2023, 13, 1038874. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, D.; Tortorella, C.; Marcoli, M.; Cervetto, C.; Maura, G.; Agnati, L.F. Receptor-receptor interactions and glial cells functions with a special focus on G protein-coupled receptors. Int. J. Mol. Sci. 2021, 22, 8656. [Google Scholar] [CrossRef] [PubMed]
- Di Menna, L.; Joffe, M.E.; Iacovelli, L.; Orlando, R.; Lindsley, C.W.; Mairesse, J.; Gress`ens, P.; Cannella, M.; Caraci, F.; Copani, A.; et al. Functional partnership between mGlu3 and mGlu5 metabotropic glutamate receptors in the central nervous system. Neuropharmacology 2017, 128, 301–313. [Google Scholar] [CrossRef]
- Mariotti, L.; Losi, G.; Lia, A.; Melone, M.; Chiavegato, A.; Gomez-Gonzalo, M.; Sessolo, M.; Bovetti, S.; Forli, A.; Zonta, M.; et al. Interneuron-specific signaling evokes distinctive somatostatin-mediated responses in adult cortical astrocytes. Nat. Commun. 2018, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Kolasa, M.; Solich, J.; Faron-Goreka, A.; Zurawek, D.; Pabian, P.; Lukasiewicz, S.; Kuśmider, M.; Szafran-Pilch, K.; Szlachta, M.; Dziedzicka-Wasylewska, M. Paroxetine and low-dose risperidone induce serotonin 5-HT1A and Dopamine D2 receptor heteromerization in the mouse prefrontal cortex. Neuroscience 2018, 377, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Cervetto, C.; Venturini, A.; Passalacqua, M.; Guidolin, D.; Genedani, S.; Fuxe, K.; Borroto-Esquela, D.O.; Cortelli, P.; Woods, A.S.; Maura, G.; et al. A2A-D2 receptor–receptor interaction modulates gliotransmitter release from striatal astrocyte processes. J. Neurochem. 2017, 140, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Cervetto, C.; Venturini, A.; Guidolin, D.; Maura, G.; Passalacqua, M.; Tacchetti, C.; Cortelli, P.; Genedani, S.; Candiani, S.; Ramoino, P.; et al. Homocysteine and A2A-D2 receptor-receptor interaction at striatal astrocyte processes. J. Mol. Neurosci. 2018, 65, 456–466. [Google Scholar] [CrossRef]
- Pelassa, S.; Guidolin, D.; Venturini, A.; Averna, M.; Frumento, G.; Campanini, L.; Bernardi, R.; Cortelli, P.; Buonaura, G.C.; Maura, G.; et al. A2A-D2 heteromers on striatal astrocytes: Biochemical and biophysical evidence. Int. J. Mol. Sci. 2019, 20, 2457. [Google Scholar] [CrossRef]
- Amato, S.; Averna, M.; Guidolin, D.; Pedrazzi, M.; Pelassa, S.; Capraro, M.; Passalacqua, M.; Bozzo, M.; Gatta, E.; Anderlini, D.; et al. Heterodimer of A2A and Oxytocin Receptors Regulating Glutamate Release in Adult Striatal Astrocytes. Int. J. Mol. Sci. 2022, 23, 2326. [Google Scholar] [CrossRef]
- Amato, S.; Averna, M.; Guidolin, D.; Ceccoli, C.; Gatta, E.; Candiani, S.; Pedrazzi, M.; Capraro, M.; Maura, G.; Agnati, L.F.; et al. Heteromerization of Dopamine D2 and Oxytocin Receptor in Adult Striatal Astrocytes. Int. J. Mol. Sci. 2023, 24, 4677. [Google Scholar] [CrossRef]
- Trifilieff, P.; Rives, M.L.; Urizar, E.; Piskorowski, R.A.; Vishwasrao, H.D.; Castrillon, J.; Schmauss, C.; Slättman, M.; Gullberg, M.; Javitch, J.A. Detection of antigen interactions ex vivo by proximity ligation assay: Endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. Biotechniques 2011, 51, 111–118. [Google Scholar] [CrossRef]
- Lavialle, M.; Aumann, G.; Anlauf, E.; Pröls, F.; Arpin, M.; Derouiche, A. Structural plasticity of perisynaptic astrocyte processes involves ezrin and metabotropic glutamate receptors. Proc. Natl. Acad. Sci. USA 2011, 108, 12915–12919. [Google Scholar] [CrossRef] [PubMed]
- Halassa, M.M.; Haydon, P.G. Integrated brain circuits: Astrocytic networks modulate neuronal activity and behavior. Annu. Rev. Physiol. 2010, 72, 335–355. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Butt, A.; Li, B.; Illes, P.; Zorec, R.; Semyanov, A.; Tang, Y.; Sofroniew, M.V. Astrocytes in human central nervous system diseases: A frontier for new therapies. Singal Transduct. Target. Ther. 2023, 8, 396. [Google Scholar] [CrossRef]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Chounlamountry, K.; Kessler, J.P. The ultrastructure of perisynaptic glia in the nucleus tractus solitarii of the adult rat: Comparison between single synapses and multisynaptic arrangements. Glia 2011, 59, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Lachamp, P.; Crest, M.; Kessler, J.P. Vesicular glutamate transporters type 1 and 2 expression in axon terminals of the rat nucleus of the solitary tract. Neuroscience 2006, 137, 73–81. [Google Scholar] [CrossRef]
- Newman, E.A.; Zahs, K.R. Modulation of neuronal activity by glial cells in the retina. J. Neurosci. 1998, 18, 4022–4028. [Google Scholar] [CrossRef]
- Papouin, T.; Dunphy, J.; Tolman, M.; Foley, J.C.; Haydon, P.G. Astrocytic control of synaptic function. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160154. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.T.; McCarthy, K.D. Astrocytic neurotransmitter receptors in situ and in vivo. Prog. Neurobiol. 1997, 51, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Perea, G.; Navarrete, M.; Araque, A. Tripartite synapses: Astrocytes process and control synaptic information. Trends Neurosci. 2009, 32, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, M.; Verkhratsky, A. Artifact versus reality-how astrocytes contribute to synaptic events. Glia 2012, 60, 1013–1023. [Google Scholar] [CrossRef] [PubMed]
- Haydon, P.G.; Carmignoto, G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 2006, 86, 1009–1031. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.M.; Swanson, R.A. Astrocyte glutamate transport: Review of properties, regulation, and physiological functions. Glia 2000, 32, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Matott, M.P.; Kline, D.D.; Hasser, E.M. Glial EAAT2 regulation of extracellular nTS glutamate critically controls neuronal activity and cardiorespiratory reflexes. J. Physiol. 2017, 595, 6045–6063. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.; Rönnbäck, L.; Hansson, E. Astrocyte–endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Giaume, C.; Tabernero, A.; Medina, J.M. Metabolic trafficking through astrocytic gap junctions. Glia 1997, 21, 114–123. [Google Scholar] [CrossRef]
- Simard, M.; Arcuino, G.; Takano, T.; Liu, Q.S.; Nedergaard, M. Signaling at the gliovascular interface. J. Neurosci. 2003, 23, 9254–9262. [Google Scholar] [CrossRef]
- Xie, L.; Kang, H.; Xu, Q.; Chen, M.J.; Liao, Y. Sleep drives metabolite clearance from the adult brain. Science 2013, 342, 373–377. [Google Scholar] [CrossRef]
- Montana, V.; Malarkey, E.B.; Verderio, C.; Matteoli, M.; Parpura, V. Vesicular transmitter release from astrocytes. Glia 2006, 54, 700–715. [Google Scholar] [CrossRef]
- Bergersen, L.H.; Gundersen, V. Morphological evidence for vesicular glutamate release from astrocytes. Neuroscience 2009, 158, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Santello, M.; Volterra, A. Synaptic modulation by astrocytes Ca2+ -dependent glutamate release. Neuroscience 2009, 158, 253–259. [Google Scholar] [CrossRef]
- Parpura, V.; Verkhratsky, A. The astrocyte excitability brief: From receptors to gliotransmission. Neurochem. Int. 2012, 61, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Martın, R.; Bajo-Grañeras, R.; Moratalla, R.; Perea, G.; Araque, A. Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 2015, 349, 730–734. [Google Scholar] [CrossRef]
- Di Benedetto, B.; Rupprecht, R. Targeting glia cells: Novel perspectives for the treatment of neuro-psychiatric diseases. Curr. Neuropharmacol. 2013, 11, 171–185. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 2015, 16, 249–263. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Parpura, V. Astrogliopathology in neurological, neurodevelopmental and psychiatric disorders. Neurobiol. Dis. 2016, 85, 254–261. [Google Scholar] [CrossRef]
- Mitterauer, B.J. Pathophysiology of schizophrenia based on impaired glial-neuronal interactions. Open J. Med. Psychol. 2014, 3, 42110. [Google Scholar] [CrossRef]
- Xia, M.; Abazyan, S.; Jouroukhin, Y.; Pletnikov, M. Behavioral sequelae of astrocyte dysfunction: Focus on animal models of schizophrenia. Schizophr. Res. 2014, 176, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, H.G.; Steiner, J.; Guest, P.C.; Dobrowolny, H.; Bogerts, B. Glial cells as key players in schizophrenia pathology: Recent insights and concepts of therapy. Schizophr. Res. 2015, 161, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.F.; Sardinha, V.M.; Guerra-Gomes, S.; Araque, A.; Sousa, N. Do stars govern our actions? Astrocyte involvement in rodent behavior. Trends Neurosci. 2015, 38, 535–549. [Google Scholar] [CrossRef]
- Kruyer, A.; Kalivas, P.W. Astrocytes as cellular mediators of cue reactivity in addiction. Curr. Opin. Pharmacol. 2021, 56, 1–6. [Google Scholar] [CrossRef]
- Villalba, R.M.; Smith, Y. Striatal spine plasticity in Parkison’s disease. Front. Neuroanat. 2010, 4, 133. [Google Scholar] [CrossRef] [PubMed]
- Villalba, R.M.; Smith, Y. Differential structural plasticity of corticostriatal and thalamostriatal axo-spinous synapses in MPTP-treated parkinsonian monkeys. J. Comp. Neurol. 2011, 519, 989–1005. [Google Scholar] [CrossRef] [PubMed]
- Villalba, R.M.; Mathai, A.; Smith, Y. Morphological changes of glutamatergic synapses in animal models of Parkinson’s disease. Front. Neuroanat. 2015, 9, 117. [Google Scholar] [CrossRef]
- Chassain, C.; Melon, C.; Salin, P.; Vitale, F.; Couraud, S.; Durif, F.; Kerkerian-Le Goff, L.; Gubellini, P. Metabolic, synaptic and behavioral im- pact of 5-week chronic deep brain stimulation in hemiparkinsonian rats. J. Neurochem. 2016, 136, 1004–1016. [Google Scholar] [CrossRef] [PubMed]
- Booth, H.D.E.; Hirst, W.D.; Wade-Martins, R. The role of astrocyte dysfunction in Parkinson’s disease pathogenesis. Trends Neurosci. 2017, 40, 358–370. [Google Scholar] [CrossRef]
- Ingham, C.A.; Hood, S.H.; Arbuthnott, G.W. Spine density on neostriatal neurones changes with 6-hydroxydopamine lesions and with age. Brain Res. 1989, 503, 334–338. [Google Scholar] [CrossRef]
- Ingham, C.A.; Hood, S.H.; Taggart, P.; Arbuthnott, G.W. Plasticity of synapses in the rat neostriatum after unilateral lesion of the nigrostriatal dopaminergic pathway. J. Neurosci. 1998, 18, 4732–4743. [Google Scholar] [CrossRef] [PubMed]
- Stephens, B.; Mueller, A.J.; Shering, A.F.; Hood, S.H.; Taggart, P.; Arbuthnott, G.W.; Bell, J.E.; Kilford, L.; Kingsbury, A.E.; Daniel, S.E.; et al. Evidence of a breakdown of corticostriatal connections in Parkinson’s disease. Neuroscience 2005, 132, 741–754. [Google Scholar] [CrossRef] [PubMed]
- Day, M.; Wang, Z.; Ding, J.; An, X.; Ingham, C.A.; Shering, A.F.; Wokosin, D.; Ilijic, E.; Sun, Z.; Sampson, A.R.; et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat. Neurosci. 2006, 9, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Deutch, A.Y.; Colbran, R.J.; Winder, D.J. Striatal plasticity and medium spiny neuron dendritic remodeling in parkinsonism. Park. Relat. Disord. 2007, 13 (Suppl. 3), S251–S258. [Google Scholar] [CrossRef] [PubMed]
- Neely, M.D.; Schmidt, D.E.; Deutch, A.Y. Cortical regulation of dopamine depletion-induced dendritic spine loss in striatal medium spiny neurons. Neuroscience 2007, 149, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Scholz, B.; Svensson, M.; Alm, H.; Skold, K.; Falth, M.; Kultima, K.; Guigoni, C.; Doudnikoff, E.; Li, Q.; Crossman, A.R.; et al. Striatal proteomic analysis suggests that first L-dopa dose equates to chronic exposure. PLoS ONE 2008, 3, e1589. [Google Scholar] [CrossRef]
- Schuster, S.; Doudnikoff, E.; Rylander, D.; Berthet, A.; Aubert, I.; Ittrich, C.; Bloch, B.; Cenci, M.A.; Surmeier, D.J.; Hengerer, B.; et al. Antagonizing L-type Ca2+ channel reduces development of abnormal involuntary movement in the rat model of L-3,4-dihydroxyphenylalanine-induced dyskinesia. Biol. Psychiatry 2009, 65, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Smith, Y.; Villalba, R.M.; Raju, D.V. Striatal spine plasticity in Parkinson’s disease: Pathological or not? Park. Relat. Disord. 2009, 15 (Suppl. 3), S156–S161. [Google Scholar] [CrossRef]
- Villalba, R.M.; Lee, H.; Smith, Y. Dopaminergic denervation and spine loss in the striatum of MPTP-treated monkeys. Exp. Neurol. 2009, 215, 220–227. [Google Scholar] [CrossRef]
- Garcia, B.G.; Neely, M.D.; Deutch, A.Y. Cortical regulation of striatal medium spiny neuron dendritic remodeling in parkinsonism: Modulation of glutamate release reverses dopamine depletion-induced dendritic spine loss. Cereb. Cortex 2010, 20, 2423–2432. [Google Scholar] [CrossRef]
- Soderstrom, K.E.; O’Malley, J.A.; Levine, N.D.; Sortwell, C.E.; Collier, T.J.; Steece-Collier, K. Impact of dendritic spine preservation in medium spiny neurons on dopamine graft efficacy and the expression of dyskinesias in parkinsonian rats. Eur. J. Neurosci. 2010, 31, 478–490. [Google Scholar] [CrossRef] [PubMed]
- Galarraga, E.; Bargas, J.; Martinez-Fong, D.; Aceves, J. Spontaneous synaptic potentials in dopamine-denervated neostriatal neurons. Neurosci. Lett. 1987, 81, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, P.; Pisani, A.; Mercuri, N.B.; Bernardi, G. The corticostriatal projection: From synaptic plasticity to dysfunctions of the basal ganglia. Trends Neurosci. 1996, 19, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Marti, M.; Sbrenna, S.; Fuxe, K.; Bianchi, C.; Beani, L.; Morari, M. In vitro evidence for increased facilitation of striatal acetylcholine release via pre- and postsynaptic NMDA receptors in hemiparkinsonian rats. J. Neurochem. 1999, 72, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Raju, D.V.; Ahern, T.H.; Shah, D.J.; Wright, T.M.; Standaert, D.G.; Hall, R.A.; Smith, Y. Differential synaptic plasticity of the corticostriatal and thalamostriatal systems in an MPTP-treated monkey model of parkinsonism. Eur. J. Neurosci. 2008, 27, 1647–1658. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.; Betancur, C.; Giros, B.; Hirsch, E.; El Mestikawy, S. Altered expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in Parkinson disease. Neurobiol. Aging 2007, 28, 568–578. [Google Scholar] [CrossRef] [PubMed]
- Meshul, C.K.; Emre, N.; Nakamura, C.M.; Allen, C.; Donohue, M.K.; Buckman, J.F. Time-dependent changes in striatal glutamate synapses following a 6-hydroxydopamine lesion. Neuroscience 1999, 88, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Meshul, C.K.; Cogen, J.P.; Cheng, H.W.; Moore, C.; Krentz, L.; McNeill, T.H. Alterations in rat striatal glutamate synapses following a lesion of the cortico- and/or nigrostriatal pathway. Exp. Neurol. 2000, 165, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Bandopadhyay, R.; Kingsbury, A.E.; Cookson, M.R.; Reid, A.R.; Evans, I.M.; Hope, A.D.; Pittman, A.M.; Lashley, T.; Canet-Aviles, R.; Miller, D.W.; et al. The expression of DJ-1 (PARK7) in normal human CNS and idiopathic Parkinson’s disease. Brain 2004, 127, 420–430. [Google Scholar] [CrossRef]
- Neumann, M.; Müller, V.; Görner, K.; Kretzschmar, H.A.; Haass, C.; Kahle, P.J. Pathological properties of the Parkinson’s disease-associated protein DJ-1 in alpha-synucleinopathies and tauopathies: Relevance for multiple system atrophy and Pick’s disease. Acta Neuropathol. 2004, 107, 489–496. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, J.S.; Park, J.Y.; Suh, Y.H.; Jou, I.; Joe, E.H.; Park, S.M. DJ-1 associates with lipid rafts by palmitoylation and regulates lipid rafts-dependent endocytosis in astrocytes. Hum. Mol. Genet. 2013, 22, 4805–4817. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Cha, S.H.; Choi, Y.R.; Jou, I.; Joe, E.H.; Park, S.M. DJ-1 deficiency impairs glutamate uptake into astrocytes via the regulation of flotillin-1 and caveolin-1 expression. Sci. Rep. 2016, 6, 28823. [Google Scholar] [CrossRef] [PubMed]
- Butchbach, M.E.; Tian, G.; Guo, H.; Lin, C.L. Association of excitatory amino acid transporters, especially EAAT2, with cholesterol-rich lipid raft microdomains: Importance for excitatory amino acid transporter localization and function. J. Biol. Chem. 2004, 279, 34388–34396. [Google Scholar] [CrossRef] [PubMed]
- Fuxe, K.; Borroto-Escuela, D.O.; Romero-Fernandez, W.; Ciruela, F.; Manger, P.; Leo, G.; Diaz-Cabiale, Z.; Agnati, L.F. On the role of volume transmission and receptor-receptor interactions in social behaviour: Focus on central catecholamine and oxytocin neurons. Brain Res. 2012, 1476, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.A.; Brunton, P.J. Oxytocin: Control of Secretion by the Brain and Central Roles. In Reference Module in Neuroscience an Biobehavioral Psychology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–14. [Google Scholar]
- Ghazy, A.A.; Soliman, O.A.; Elbahnasi, A.I.; Alawy, A.Y.; Mansour, A.M.; Gowayed, M.A. Role of Oxytocin in Different Neuropsychiatric, Neurodegenerative, and Neurodevelopmental Disorders. In Reviews of Physiology, Biochemistry and Pharmacology; Pedersen, S.H.F., Ed.; Springer: Cham, Switzerland, 2022; p. 186. [Google Scholar]
- Erbaş, O.; Oltulu, F.; Taşkiran, D. Amelioration of rotenone-induced dopaminergic cell death in the striatum by oxytocin treatment. Peptides 2012, 38, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Erbaş, O.; Oltulu, F.; Taşkiran, D. Suppression of exaggerated neuronal oscillations by oxytocin in a rat model of Parkinson’s disease. Gen. Physiol. Biophys. 2013, 32, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Almansoub, H.A.M.M.; Tang, H.; Wu, Y.; Wang, D.Q.; Mahaman, Y.A.R.; Salissou, M.T.M.; Lu, Y.; Hu, F.; Zhou, L.T.; Almansob, Y.A.M.; et al. Oxytocin Alleviates MPTP-Induced Neurotoxicity in Mice by Targeting MicroRNA-26a/Death-Associated Protein Kinase 1 Pathway. J. Alzheimer’s Dis. 2020, 74, 883–901. [Google Scholar] [CrossRef] [PubMed]
- Petersson, M.; Lagumdzija, A.; Stark, A.; Bucht, E. Oxytocin stimulates proliferation of human osteoblast-like cells. Peptides 2002, 23, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Rashed, L.A.; Hashem, R.M.; Soliman, H.M. Oxytocin inhibits NADPH oxidase and P38 MAPK in cisplatin-induced nephrotoxicity. Biomed. Pharmacother. 2011, 65, 474–480. [Google Scholar] [CrossRef]
- Jankowski, M.; Broderick, T.L.; Gutkowska, J. The Role of Oxytocin in Cardiovascular Protection. Front. Psychol. 2020, 11, 2139. [Google Scholar] [CrossRef]
- Erbaş, O.; Altuntaş, I. Oxytocin and Neuroprotective Effects. In Oxytocin and Health; Wu, W., Kostoglou-Athanassiouand, I., Eds.; IntechOpen: London, UK, 2021. [Google Scholar]
- Gamal-Eltrabily, M.; Manzano-García, A. Role of central oxytocin and dopamine systems in nociception and their possible interactions: Suggested hypotheses. Rev. Neurosci. 2018, 29, 377–386. [Google Scholar] [CrossRef] [PubMed]
- de la Mora, M.P.; Pérez-Carrera, D.; Crespo-Ramírez, M.; Tarakanov, A.; Fuxe, K.; Borroto-Escuela, D.O. Signaling in dopamine D2 receptor-oxytocin receptor heterocomplexes and its relevance for the anxiolytic effects of dopamine and oxytocin interactions in the amygdala of the rat. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1862, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Romero-Fernandez, W.; Borroto-Escuela, D.O.; Agnati, L.F.; Fuxe, K. Evidence for the existence of dopamine D2-oxytocin receptor heteromers in the ventral and dorsal striatum with facilitatory receptor-receptor interactions. Mol. Psychiatry 2013, 18, 849–850. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Zhang, S.Z.; Tang, M.; Zhang, X.H.; Zhou, Z.; Yin, Y.Q.; Zhou, Q.B.; Huang, Y.Y.; Liu, Y.J.; Wawrousek, E.; et al. Suppression of neuroinflammation by astrocytic dopamine D2 receptors via αB-crystallin. Nature 2012, 494, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Minchev, D.; Kazakova, M.; Sarafian, V. Neuroinflammation and Autophagy in Parkinson’s Disease-Novel Perspectives. Int. J. Mol. Sci. 2022, 23, 14997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, Y.; Wu, J.; Manaenko, A.; Yang, P.; Tang, J.; Fu, W.; Zhang, J.H. Activation of dopamine D2 receptor suppresses neuroinflammation through AB-crystalline by inhibition of NF-ΚB nuclear translocation in experimental ICH mice model. Stroke 2015, 46, 2637–2646. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.I.; Jo, M.G.; Park, T.J.; Ullah, R.; Ahmad, S.; Rehman, S.U.; Kim, M.O. Quinpirole-mediated regulation of dopamine D2 receptors inhibits glial cell-induced neuroinflammation in cortex and striatum after brain injury. Biomedicines 2021, 9, 47. [Google Scholar] [CrossRef]
- Cervetto, C.; Maura, G.; Guidolin, D.; Amato, S.; Ceccoli, C.; Agnati, L.F.; Marcoli, M. Striatal astrocytic A2A-D2 receptor-receptor interactions and their role in neuropsychiatric disorders. Neuropharmacology 2023, 237, 109636. [Google Scholar] [CrossRef]
- Augusto, E.; Matos, M.; Sevigny, J.; El-Tayeb, A.; Bynoe, M.S.; Müller, C.E.; Cunha, R.A.; Chen, J.F. Ecto-5′-nucleotidase (CD73)-mediated formation of adenosine is critical for the striatal adenosine A2A receptor functions. J. Neurosci. 2013, 33, 11390–11399. [Google Scholar] [CrossRef]
- Sperlágh, B.; Vizi, E.S. The role of extracellular adenosine in chemical neurotransmission in the ippocampus and basal ganglia: Phamacological and clinical aspects. Curr. Top. Med. Chem. 2011, 11, 1034–1046. [Google Scholar] [CrossRef]
- Villar-Menéndez, I.; Porta, S.; Buira, S.P.; Pereira-Veiga, T.; Díaz-Sánchez, S.; Albasanz, J.L.; Ferrer, I.; Martín, M.; Barrachina, M. Increased striatal adenosine A2A receptor levels is an early event in Parkinson’s disease-related pathology and it is potentially regulated by miR-34b. Neurobiol. Dis. 2014, 69, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Maeda, T.; Kimura, T.; Sugiyama, K.; Yamada, K.; Hiraiwa, R.; Nishi, M.; Hattori, N.; Abe, T.; Deguchi, K.; Fujimoto, K.; et al. Randomized controlled trial of KW-6356 monotherapy in patients with early untreated Parkinson’s disease. Park. Relat. Disord. 2023, 117, 105907. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; de Vries, E.F.J.; van der Meiden, E.; Moraga-Amaro, R.; Vazquez-Matias, D.A.; Barazzuol, L.; Dierckx, R.A.J.O.; van Waarde, A. Effects of the adenosine A2A receptor antagonist KW6002 on the dopaminergic system, motor performance, and neuroinflammation in a rat model of Parkinson’s disease. Neuropharmacology 2024, 247, 109862. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Barawkar, D.A.; Thorat, S.; Shejul, Y.D.; Patel, M.; Naykodi, M.; Jain, V.; Salve, Y.; Prasad, V.; Chaudhary, S.; et al. Design, Synthesis of Novel, Potent, Selective, Orally Bioavailable Adenosine A2A Receptor Antagonists and Their Biological Evaluation. J. Med. Chem. 2017, 60, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Shen, H.Y.; Coelho, J.E.; Araújo, I.M.; Huang, Q.Y.; Day, Y.J.; Rebola, N.; Canas, P.M.; Rapp, E.K.; Ferrara, J.; et al. Adenosine A2A receptor antagonists exert motor and neuroprotective effects by distinct cellular mechanisms. Ann. Neurol. 2008, 63, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Dungo, R.; Deeks, E.D. Istradefylline: First global approval. Drugs 2013, 73, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.F.; Cunha, R.A. The belated US FDA approval of the adenosine A2A receptor antagonist istradefylline for treatment of Parkinson’s disease. Purinergic Signal. 2020, 16, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Jenner, P. Istradefylline, a novel adenosine A2A receptor antagonist, for the treatment of Parkinson’s disease. Expert Opin. Investig. Drugs 2005, 14, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Fahn, S.; Oakes, D.; Shoulson, I.; Kieburtz, K.; Rudolph, A.; Marek, K.; Seibyl, J.; Lang, A.; Olanow, C.W.; Tanner, C.; et al. Levodopa and the progression of Parkinson’s disease. N. Engl. J. Med. 2004, 351, 2498–2508. [Google Scholar]
- Muzerengi, S.; Clarke, C.E. Initial drug treatment in Parkinson’s disease. BMJ 2015, 351, h4669. [Google Scholar] [CrossRef]
- Ahlskog, J.E.; Muenter, M.D. Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov. Disord. 2001, 16, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.A.; Winnick, A.; Welschmeyer, A.; Kaneb, A.; Berardino, K.; Cornett, E.M.; Kaye, A.D.; Viswanath, O.; Urits, I. Istradefylline to Treat Patients with Parkinson’s Disease Experiencing “Off” Episodes: A Comprehensive Review. Neurol. Int. 2020, 12, 109–129. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Cabiale, Z.; Vivó, M.; Del Arco, A.; O’Connor, W.T.; Harte, M.K.; Müller, C.E.; Martínez, E.; Popoli, P.; Fuxe, K.; Ferré, S. Metabotropic glutamate mGlu5 receptor-mediated modulation of the ventral striopallidal GABA pathway in rats. Interactions with adenosine A(2A) and dopamine D(2) receptors. Neurosci. Lett. 2002, 324, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Cabello, N.; Gandia, J.; Bertarelli, D.C.; Watanabe, M.; Lluis, C.; Franco, R.; Ferre, S.; Lujan, R.; Ciruela, F. Metabotropic glutamate type 5, dopamine d2 and adenosine a2a receptors form higher-order oligomers in living cells. J. Neurochem. 2009, 109, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Romero-Fernandez, W.; Taura, J.J.; Crans, R.A.J.; Lopez-Cano, M.; Fores-Pons, R.; Narváez, M.; Carlsson, J.; Ciruela, F.; Fuxe, K.; Borroto-Escuela, D.O. The mGlu5 Receptor Protomer-Mediated Dopamine D2 Receptor Trans-Inhibition Is Dependent on the Adenosine A2A Receptor Protomer: Implications for Parkinson’s Disease. Mol. Neurobiol. 2022, 59, 5955–5969. [Google Scholar] [CrossRef] [PubMed]
- Borroto-Escuela, D.O.; Narvaez, M.; Wydra, K.; Pintsuk, J.; Pinton, L.; Jimenez-Beristain, A.; Di Palma, M.; Jastrzebska, J.; Filip, M.; Fuxe, K. Cocaine self-administration specifically increases A2Ar-D2r and D2r-sigma1r heteroreceptor complexes in the rat nucleus accumbens shell. Relevance for cocaine use disorder. Pharmacol. Biochem. Behav. 2017, 155, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Ferré, S.; Quiroz, C.; Woods, A.S.; Cunha, R.; Popoli, P.; Ciruela, F.; Lluis, C.; Franco, R.; Azdad, K.; Schiffmann, S.N. An update on adenosine A2A-dopamine D2 receptor interactions: Implications for the function of G protein-coupled receptors. Curr. Pharm. Des. 2008, 14, 1468–1474. [Google Scholar] [CrossRef] [PubMed]
- Popoli, P.; Pezzola, A.; Torvinen, M.; Reggio, R.; Pintor, A.; Scarchilli, L.; Fuxe, K.; Ferré, S. The selective mglu(5) receptor agonist chpg inhibits quinpirole-induced turning in 6-hydroxydopamine-lesioned rats and modulates the binding characteristics of dopamine d(2) receptors in the rat striatum: Interactions with adenosine a(2a) receptors. Neuropsychopharmacology 2001, 25, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Coccurello, R.; Breysse, N.; Amalric, M. Simultaneous blockade of adenosine A2A and metabotropic glutamate mGlu5 receptors increase their efficacy in reversing Parkinsonian deficits in rats. Neuropsychopharmacology 2004, 29, 1451–1461. [Google Scholar] [CrossRef]
- Black, Y.D.; Xiao, D.; Pellegrino, D.; Kachroo, A.; Brownell, A.L.; Schwarzschild, M.A. Protective effect of metabotropic glutamate mGluR5 receptor elimination in a 6-hydroxydopamine model of Parkinson’s disease. Neurosci. Lett. 2010, 486, 161–165. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Wydra, K.; Filip, M.; Fuxe, K. A2AR-D2R heteroreceptor complexes in cocaine reward and addiction. Trends Pharmacol. Sci. 2018, 39, 1008–1020. [Google Scholar] [CrossRef] [PubMed]
- Romieu, P.; Phan, V.L.; Martin-Fardon, R.; Maurice, T. Involvement of the sigma(1) receptor in cocaine-induced conditioned place preference: Possible dependence on dopamine uptake blockade. Neuropsychopharmacology 2002, 26, 444–455. [Google Scholar] [CrossRef]
- Borroto-Escuela, D.O.; Lopez-Salas, A.; Wydra, K.; Bartolini, M.; Zhou, Z.; Frankowska, M.; Suder, A.; Benitez-Porres, J.; Romero-Fernandez, W.; Filip, M.; et al. Combined treatment with Sigma1R and A2AR agonists fails to inhibit cocaine self-administration despite causing strong antagonistic accumbal A2AR-D2R complex interactions: The potential role of astrocytes. Front. Mol. Neurosci. 2023, 16, 1106765. [Google Scholar] [CrossRef]
- Kaczor, A.A.; Wróbel, T.M.; Bartuzi, D. Allosteric Modulators of Dopamine D2 Receptors for Fine-Tuning of Dopaminergic Neurotransmission in CNS Diseases: Overview, Pharmacology, Structural Aspects and Synthesis. Molecules 2023, 28, 178. [Google Scholar] [CrossRef]
- Nakamura, Y.; Iga, K.; Shibata, T.; Shudo, M.; Kataoka, K. Glial plasmalemmal vesicles: A subcellular fraction from rat hippocampal homogenate distinct from synaptosomes. Glia 1993, 9, 48–56. [Google Scholar] [CrossRef]
- Cervetto, C.; Vergani, L.; Passalacqua, M.; Ragazzoni, M.; Venturini, A.; Cecconi, F.; Berretta, N.; Mercuri, N.; D’Amelio, M.; Maura, G.; et al. Astrocyte-Dependent Vulnerability to Excitotoxicity in Spermine Oxidase-Overexpressing Mouse. Neuromol. Med. 2016, 18, 50–68. [Google Scholar] [CrossRef]
- Venturini, A.; Passalacqua, M.; Pelassa, S.; Pastorino, F.; Tedesco, M.; Cortese, K.; Gagliani, M.C.; Leo, G.; Maura, G.; Guidolin, D.; et al. Exosomes From Astrocyte Processes: Signaling to Neurons. Front. Pharmacol. 2019, 10, 1452. [Google Scholar] [CrossRef]
- Derouiche, A.; Geiger, K.D. Perspectives for ezrin and radixin in astrocytes: Kinases, functions and pathology. Int. J. Mol. Sci. 2019, 20, 3776. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Marcoli, M.; Venturini, A.; Passalacqua, M.; Agnati, L.F.; Signore, A.; Raffetto, M.; Maura, G.; Benedicenti, S.; Cervetto, C. Near-infrared laser photons induce glutamate release from cerebrocortical nerve terminals. J. Biophotonics 2018, 11, e201800102. [Google Scholar] [CrossRef]
- Cervetto, C.; Mazzotta, M.C.; Frattaroli, D.; Alloisio, S.; Nobile, M.; Maura, G.; Marcoli, M. Calmidazolium selectively inhibits exocytotic glutamate release evoked by P2X7 receptor activation. Neurochem. Int. 2012, 60, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare Mannelli, L.; Micheli, L.; Cervetto, C.; Toti, A.; Lucarini, E.; Parisio, C.; Marcoli, M.; Ghelardini, C. Neuronal alarmin IL-1α evokes astrocyte-mediated protective signals: Effectiveness in chemotherapy-induced neuropathic pain. Neurobiol. Dis. 2022, 168, 105716. [Google Scholar] [CrossRef] [PubMed]
- Cervetto, C.; Amaroli, A.; Amato, S.; Gatta, E.; Diaspro, A.; Maura, G.; Signore, A.; Benedicenti, S.; Marcoli, M. Photons Induce Vesicular Exocytotic Release of Glutamate in a Power-Dependent Way. Int. J. Mol. Sci. 2023, 24, 10977. [Google Scholar] [CrossRef] [PubMed]
- Cervetto, C.; Averna, M.; Vergani, L.; Pedrazzi, M.; Amato, S.; Pelassa, S.; Giuliani, S.; Baldini, F.; Maura, G.; Mariottini, P.; et al. Reactive Astrocytosis in a Mouse Model of Chronic Polyamine Catabolism Activation. Biomolecules 2021, 11, 1274. [Google Scholar] [CrossRef]
- RCSB PDB Protein Data Bank. Available online: https://www.rcsb.org (accessed on 10 September 2021).
- Doré, A.S.; Robertson, N.; Errey, J.C.; Ng, I.; Hollenstein, K.; Tehan, B.; Hurrell, E.; Bennett, K.; Congreve, M.; Magnani, F.; et al. Structure of the adenosine A(2A) receptor in complex with ZM241385 and the xanthines XAC and caffeine. Structure 2011, 19, 1283–1293. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Che, T.; Levit, A.; Shoichet, B.K.; Wacker, D.; Roth, B.L. Structure of the D2 dopamine receptor bound to the atypical antipsychotic drug risperidone. Nature 2018, 555, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Waltenspühl, Y.; Schöppe, J.; Ehrenmann, J.; Kummer, L.; Plückthun, A. crystal structure of the human oxytocin receptor. Sci. Adv. 2020, 6, eabb5419. [Google Scholar] [CrossRef] [PubMed]
- YASARA Energy Minimization Server. Available online: http://www.yasara.org/minimizationserver.htm (accessed on 8 March 2024).
- Krieger, E.; Joo, K.; Lee, J.; Lee, J.; Raman, S.; Thompson, J.; Tyka, M.; Baker, D.; Karplus, K. Improving physical realism, stereochemistry, and side-chain accuracy in homology modeling: Four approaches that performed well in CASP8. Proteins 2009, 77, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Mem-LZerD Protein Docking Web Server. Available online: https://lzerd.kiharalab.org/upload/ (accessed on 10 March 2024).
- Christoffer, C.; Harini, K.; Archit, G.; Kihara, D. Assembly of Protein Complexes in and on the Membrane with Predicted Spatial Arrangement Constraints. J. Mol. Biol. 2024, 436, 168486. [Google Scholar] [CrossRef]
- CHARMM-GUI Membrane Builder. Available online: http://www.charmm-gui.org/?doc=input (accessed on 12 March 2024).
- Lee, J.; Patel, D.S.; Ståhle, J.; Park, S.-J.; Kern, N.R.; Kim, S.; Lee, J.; Cheng, X.; Valvano, M.A.; Holst, O.; et al. CHARMM-GUI membrane builder for complex biological membrane simulations with glycolipids and lipoglycans. J. Chem. Theory Comput. 2019, 15, 775–786. [Google Scholar] [CrossRef]
- Krissinel, E.; Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef] [PubMed]
- Protein Data Bank in Europe (Proteins, Interfaces, Structures and Assemblies)—PDBePISA. Available online: https://www.ebi.ac.uk/pdbe/pisa/ (accessed on 15 March 2024).


| A2A | D2 | OTR | |
|---|---|---|---|
| Agonist | CGS 21680 | quinpirole | oxytocin (OT) |
| concentration | 0.01 µM | 0.1 µM | 3 nM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amato, S.; Averna, M.; Farsetti, E.; Guidolin, D.; Pedrazzi, M.; Gatta, E.; Candiani, S.; Maura, G.; Agnati, L.F.; Cervetto, C.; et al. Control of Dopamine Signal in High-Order Receptor Complex on Striatal Astrocytes. Int. J. Mol. Sci. 2024, 25, 8610. https://doi.org/10.3390/ijms25168610
Amato S, Averna M, Farsetti E, Guidolin D, Pedrazzi M, Gatta E, Candiani S, Maura G, Agnati LF, Cervetto C, et al. Control of Dopamine Signal in High-Order Receptor Complex on Striatal Astrocytes. International Journal of Molecular Sciences. 2024; 25(16):8610. https://doi.org/10.3390/ijms25168610
Chicago/Turabian StyleAmato, Sarah, Monica Averna, Elisa Farsetti, Diego Guidolin, Marco Pedrazzi, Elena Gatta, Simona Candiani, Guido Maura, Luigi Francesco Agnati, Chiara Cervetto, and et al. 2024. "Control of Dopamine Signal in High-Order Receptor Complex on Striatal Astrocytes" International Journal of Molecular Sciences 25, no. 16: 8610. https://doi.org/10.3390/ijms25168610
APA StyleAmato, S., Averna, M., Farsetti, E., Guidolin, D., Pedrazzi, M., Gatta, E., Candiani, S., Maura, G., Agnati, L. F., Cervetto, C., & Marcoli, M. (2024). Control of Dopamine Signal in High-Order Receptor Complex on Striatal Astrocytes. International Journal of Molecular Sciences, 25(16), 8610. https://doi.org/10.3390/ijms25168610










