Microbial Immobilized Enzyme Biocatalysts for Multipollutant Mitigation: Harnessing Nature’s Toolkit for Environmental Sustainability
Abstract
:1. Introduction
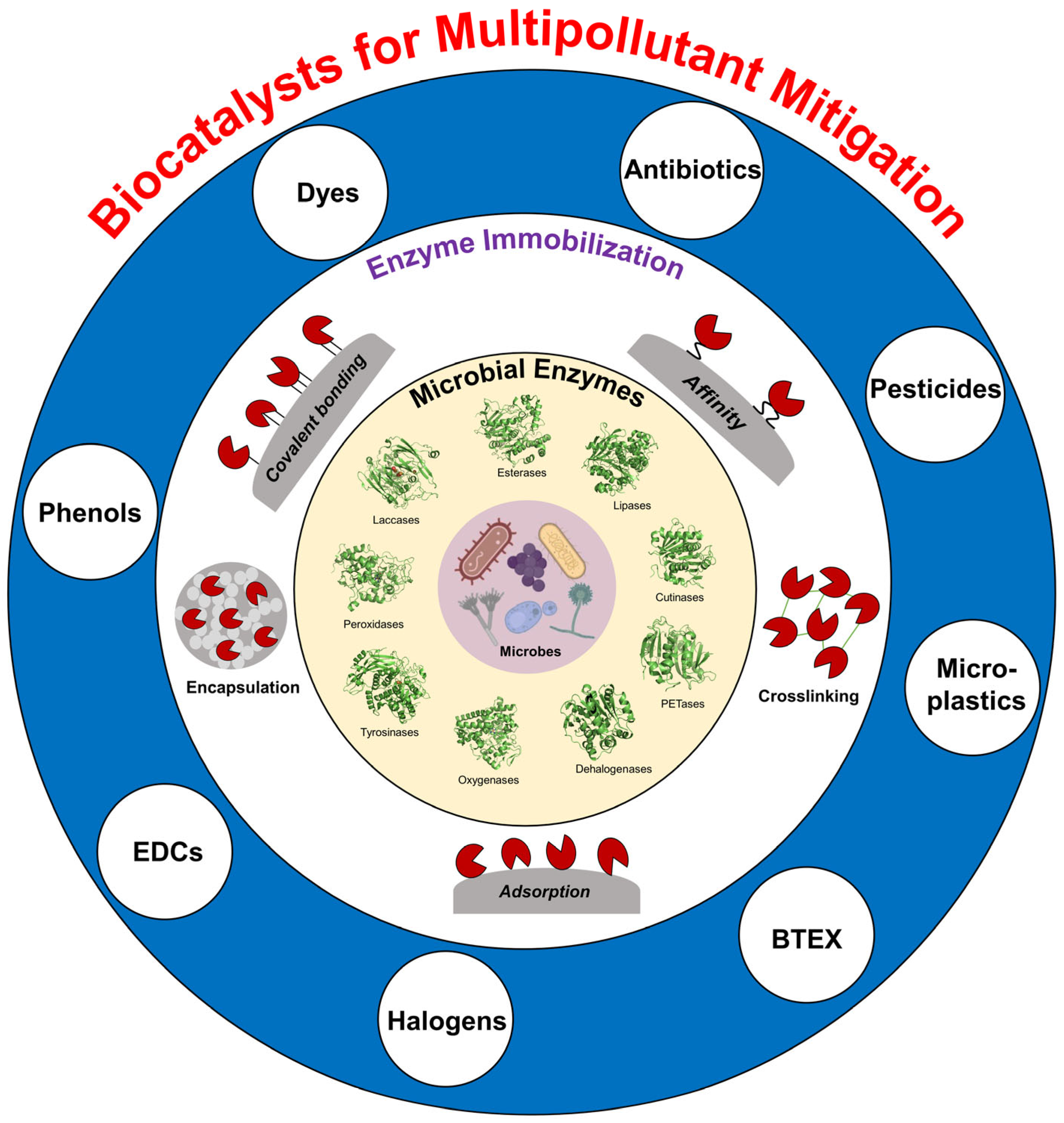
2. Microbial Enzymes for Multipollutant Mitigation
2.1. Oxidoreductases: Powerful Tools for Pollutant Oxidation
2.1.1. Laccases
2.1.2. Peroxidases
2.1.3. Tyrosinases
2.1.4. Oxygenases
2.2. Hydrolases
2.2.1. Esterases
2.2.2. Lipases
2.2.3. Cutinases
2.2.4. PETase and MHETase
2.2.5. Dehalogenases
3. Microbial Enzyme Immobilization
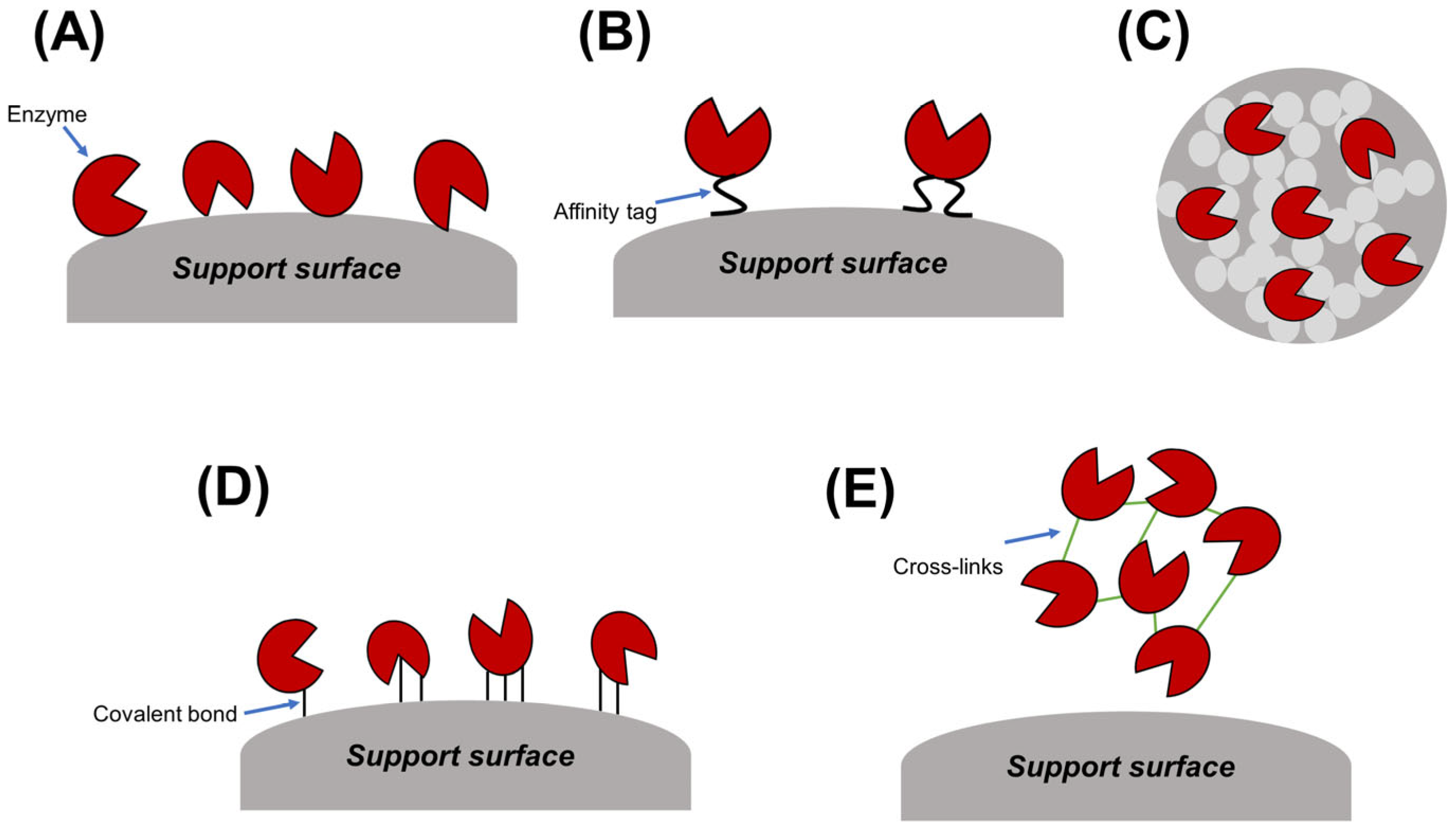
3.1. Immobilization Approaches for Enhancing Enzyme Performance
3.1.1. Adsorption Immobilization
3.1.2. Encapsulation
3.1.3. Covalent Immobilization
3.1.4. Cross-Linking
3.1.5. Affinity-Based Immobilization
3.2. Techno-Economic Evaluation of Enzyme Immobilization for Bioremediation
4. Applications of Immobilized Microbial Enzymes for Multipollutant Mitigation
4.1. Immobilized Microbial Enzymes for Mitigating Pharmaceutical Pollutants
4.2. Dyes and Pigments as Environmental Micropollutants
4.3. Pesticides as Environmental Micropollutants
4.4. Degradation of Microplastics with Immobilized Enzymes
4.5. Degradation of Industrial Chemical Pollutants with Immobilized Enzymes
5. Conclusions and Future Outlooks
Author Contributions
Funding
Conflicts of Interest
References
- Liang, W.; Yang, M. Urbanization, economic growth and environmental pollution: Evidence from China. Sustain. Comput. Inform. Syst. 2019, 21, 1–9. [Google Scholar]
- Gul, B.; Naseem, M.K.; Malik, W.-N.; Gurmani, A.R.; Mehmood, A.; Rafique, M. Environmental micropollutants and their impact on human health with special focus on agriculture. In Hazardous Environmental Micro-Pollutants, Health Impacts and Allied Treatment Technologies; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1–19. [Google Scholar]
- Goutte, A.; Alliot, F.; Budzinski, H.; Simonnet-Laprade, C.; Santos, R.; Lachaux, V.; Maciejewski, K.; Le Menach, K.; Labadie, P. Trophic transfer of micropollutants and their metabolites in an urban riverine food web. Environ. Sci. Technol. 2020, 54, 8043–8050. [Google Scholar] [CrossRef]
- Desiante, W.L.; Minas, N.S.; Fenner, K. Micropollutant biotransformation and bioaccumulation in natural stream biofilms. Water Res. 2021, 193, 116846. [Google Scholar] [CrossRef] [PubMed]
- Pinto, I.; Simões, M.; Gomes, I.B. An overview of the impact of pharmaceuticals on aquatic microbial communities. Antibiotics 2022, 11, 1700. [Google Scholar] [CrossRef] [PubMed]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, X.; Jiang, J.; Han, J.; Li, W.; Li, X.; Yee Leung, K.M.; Snyder, S.A.; Alvarez, P.J. Which micropollutants in water environments deserve more attention globally? Environ. Sci. Technol. 2021, 56, 13–29. [Google Scholar] [CrossRef]
- Anku, W.W.; Mamo, M.A.; Govender, P.P. Phenolic compounds in water: Sources, reactivity, toxicity and treatment methods. In Phenolic Compounds-Natural Sources, Importance and Applications; InTechOpen: London, UK, 2017; pp. 419–443. [Google Scholar]
- Rahman, M.M.; Sultan, M.B.; Alam, M. Microplastics and adsorbed micropollutants as emerging contaminants in landfill: A mini review. Curr. Opin. Environ. Sci. Health 2023, 31, 100420. [Google Scholar] [CrossRef]
- Ali, S.S.; Elsamahy, T.; Koutra, E.; Kornaros, M.; El-Sheekh, M.; Abdelkarim, E.A.; Zhu, D.; Sun, J. Degradation of conventional plastic wastes in the environment: A review on current status of knowledge and future perspectives of disposal. Sci. Total Environ. 2021, 771, 144719. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Jeevanantham, S.; Karishma, S.; Tajsabreen, B.; Yaashikaa, P.; Reshma, B. Effective water/wastewater treatment methodologies for toxic pollutants removal: Processes and applications towards sustainable development. Chemosphere 2021, 280, 130595. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Conventional and non-conventional adsorbents for wastewater treatment. Environ. Chem. Lett. 2019, 17, 195–213. [Google Scholar] [CrossRef]
- Narayanan, M.; Ali, S.S.; El-Sheekh, M. A comprehensive review on the potential of microbial enzymes in multipollutant bioremediation: Mechanisms, challenges, and future prospects. J. Environ. Manag. 2023, 334, 117532. [Google Scholar] [CrossRef] [PubMed]
- Karigar, C.S.; Rao, S.S. Role of microbial enzymes in the bioremediation of pollutants: A review. Enzym. Res. 2011, 2011, 805187. [Google Scholar] [CrossRef] [PubMed]
- Spina, F.; Gea, M.; Bicchi, C.; Cordero, C.; Schilirò, T.; Varese, G.C. Ecofriendly laccases treatment to challenge micropollutants issue in municipal wastewaters. Environ. Pollut. 2020, 257, 113579. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, A.; Erdogan Ozseker, E.; Akdogan, H.A. Degradation of dyes by laccase. Anal. Lett. 2016, 49, 790–798. [Google Scholar] [CrossRef]
- Preethi, P.S.; Vickram, S.; Das, R.; Hariharan, N.; Rameshpathy, M.; Subbaiya, R.; Karmegam, N.; Kim, W.; Govarthanan, M. Bioprospecting of novel peroxidase from Streptomyces coelicolor strain SPR7 for carcinogenic azo dyes decolorization. Chemosphere 2023, 310, 136836. [Google Scholar] [CrossRef] [PubMed]
- Caraene, I.D.; Gruchlik, Y.; Busetti, F.; Linge, K.L.; Joll, C.A. Degradation of selected pharmaceuticals detected in wastewater systems using an enzyme-mediator system and identification of resulting transformation products. Biocatal. Biotransform. 2023, 41, 133–144. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, B. Microbial enzymes for biodegradation and detoxification of pesticides. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2023; pp. 321–356. [Google Scholar]
- Saravanan, A.; Kumar, P.S.; Vo, D.-V.N.; Jeevanantham, S.; Karishma, S.; Yaashikaa, P. A review on catalytic-enzyme degradation of toxic environmental pollutants: Microbial enzymes. J. Hazard. Mater. 2021, 419, 126451. [Google Scholar] [CrossRef] [PubMed]
- Dave, S.; Das, J. Role of microbial enzymes for biodegradation and bioremediation of environmental pollutants: Challenges and future prospects. In Bioremediation for Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2021; pp. 325–346. [Google Scholar]
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef]
- Federsel, H.-J.; Moody, T.S.; Taylor, S.J. Recent trends in enzyme immobilization—Concepts for expanding the biocatalysis toolbox. Molecules 2021, 26, 2822. [Google Scholar] [CrossRef]
- Maghraby, Y.R.; El-Shabasy, R.M.; Ibrahim, A.H.; Azzazy, H.M.E.-S. Enzyme immobilization technologies and industrial applications. ACS Omega 2023, 8, 5184–5196. [Google Scholar] [CrossRef]
- Cavalcante, F.T.; Cavalcante, A.L.; de Sousa, I.G.; Neto, F.S.; dos Santos, J.C. Current status and future perspectives of supports and protocols for enzyme immobilization. Catalysts 2021, 11, 1222. [Google Scholar] [CrossRef]
- Ikeda, T.; Hata, Y.; Ninomiya, K.-i.; Ikura, Y.; Takeguchi, K.; Aoyagi, S.; Hirota, R.; Kuroda, A. Oriented immobilization of antibodies on a silicon wafer using Si-tagged protein A. Anal. Biochem. 2009, 385, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.A.; Son, R.G.; Park, K.S.; Pack, S.P. Oriented multivalent silaffin-affinity immobilization of recombinant lipase on diatom surface: Reliable loading and high performance of biocatalyst. Colloids Surf. B Biointerfaces 2022, 219, 112830. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.A.; Meligy, A.M.; Yeo, K.B.; Lee, C.-S.; Pack, S.P. Silaffin-3-derived pentalysine cluster as a new fusion tag for one-step immobilization and purification of recombinant Bacillus subtilis catalase on bare silica particles. Int. J. Biol. Macromol. 2020, 159, 1103–1112. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, S.; Poudel, D.K.; Marahatha, R.; Dawadi, S.; Khadayat, K.; Phuyal, S.; Shrestha, S.; Gaire, S.; Basnet, K.; Khadka, U. Microbial enzymes used in bioremediation. J. Chem. 2021, 2021, 8849512. [Google Scholar] [CrossRef]
- Varga, B.; Somogyi, V.; Meiczinger, M.; Kováts, N.; Domokos, E. Enzymatic treatment and subsequent toxicity of organic micropollutants using oxidoreductases—A review. J. Clean. Prod. 2019, 221, 306–322. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Świderska-Burek, U.; Polak, J.; Sulej, J.; Jarosz-Wilkołazka, A.; Paszczyński, A. Laccase properties, physiological functions, and evolution. Int. J. Mol. Sci. 2020, 21, 966. [Google Scholar] [CrossRef]
- Xu, P.F.; Du, H.; Peng, X.; Tang, Y.; Zhou, Y.Y.; Chen, X.Y.; Fei, J.; Meng, Y.; Yuan, L. Degradation of several polycyclic aromatic hydrocarbons by laccase in reverse micelle system. Sci. Total Environ. 2020, 708, 134970. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.; Chaturvedi, V.; Verma, P. Fungal laccase discovered but yet undiscovered. Bioresour. Bioprocess. 2018, 5, 4. [Google Scholar] [CrossRef]
- Gianfreda, L.; Xu, F.; Bollag, J.-M. Laccases: A useful group of oxidoreductive enzymes. Bioremediat. J. 1999, 3, 1–26. [Google Scholar] [CrossRef]
- Malhotra, M.; Suman, S.K. Laccase-mediated delignification and detoxification of lignocellulosic biomass: Removing obstacles in energy generation. Environ. Sci. Pollut. Res. 2021, 28, 58929–58944. [Google Scholar] [CrossRef]
- Gu, Y.; Yuan, L.; Jia, L.; Xue, P.; Yao, H. Recent developments of a co-immobilized laccase–mediator system: A review. RSC Adv. 2021, 11, 29498–29506. [Google Scholar] [CrossRef] [PubMed]
- Asadi, E.; Makhdoumi, A.; Asoodeh, A. Laccase mediator system obtained from a marine spore exhibits decolorization potential in harsh environmental conditions. Ecotoxicol. Environ. Saf. 2020, 191, 110184. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, N.; Solanki, V.S.; Gacem, A.; Hasan, M.A.; Pare, B.; Srivastava, A.; Singh, A.; Yadav, V.K.; Yadav, K.K.; Lee, C.; et al. Bacterial laccases as biocatalysts for the remediation of environmental toxic pollutants: A green and eco-friendly approach—A review. Water 2022, 14, 4068. [Google Scholar] [CrossRef]
- More, S.S.; Renuka, P.S.; Pruthvi, K.; Swetha, M.; Malini, S.; Veena, S.M. Isolation, purification, and characterization of fungal laccase from Pleurotus sp. Enzym. Res. 2011, 2011, 248735. [Google Scholar] [CrossRef] [PubMed]
- Moon-Jeong, H.; Hong-Gyu, S. Purification and characterization of laccase from the white rot fungus Trametes versicolor. J. Microbiol. 2005, 43, 555–560. [Google Scholar]
- Loi, M.; Glazunova, O.; Fedorova, T.; Logrieco, A.F.; Mulè, G. Fungal laccases: The forefront of enzymes for sustainability. J. Fungi 2021, 7, 1048. [Google Scholar] [CrossRef]
- Hachi, M.; Chergui, A.; Yeddou, A.R.; Selatnia, A.; Cabana, H. Removal of acetaminophen and carbamazepine in single and binary systems with immobilized laccase from Trametes hirsuta. Biocatal. Biotransform. 2017, 35, 51–62. [Google Scholar] [CrossRef]
- Guo, M.; Lu, F.P.; Liu, M.Y.; Li, T.P.; Pu, J.; Wang, N.; Liang, P.; Zhang, C.Y. Purification of recombinant laccase from Trametes versicolor in Pichia methanolica and its use for the decolorization of anthraquinone dye. Biotechnol. Lett. 2008, 30, 2091–2096. [Google Scholar] [CrossRef]
- Isanapong, J.; Suwannoi, K.; Lertlattanapong, S.; Panchal, S. Purification, characterization of laccase from Pleurotus ostreatus HK35, and optimization for congo red biodecolorization using Box-Behnken design. 3 Biotech 2024, 14, 73. [Google Scholar] [CrossRef]
- Liu, X.P.; Deng, W.; Yang, Y. Characterization of a novel laccase LAC-Yang1 from white-rot fungus Pleurotus ostreatus strain Yang1 with a strong ability to degrade and detoxify chlorophenols. Molecules 2021, 26, 473. [Google Scholar] [CrossRef] [PubMed]
- Givaudan, A.; Effosse, A.; Faure, D.; Potier, P.; Bouillant, M.-L.; Bally, R. Polyphenol oxidase in Azospirillum lipoferum isolated from rice rhizosphere: Evidence for laccase activity in non-motile strains of Azospirillum lipoferum. FEMS Microbiol. Lett. 1993, 108, 205–210. [Google Scholar] [CrossRef]
- Wang, H.; Huang, L.; Li, Y.; Ma, J.; Wang, S.; Zhang, Y.; Ge, X.; Wang, N.; Lu, F.; Liu, Y. Characterization and application of a novel laccase derived from Bacillus amyloliquefaciens. Int. J. Biol. Macromol. 2020, 150, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Machczynski, M.C.; Vijgenboom, E.; Samyn, B.; Canters, G.W. Characterization of SLAC: A small laccase from Streptomyces coelicolor with unprecedented activity. Protein Sci. 2004, 13, 2388–2397. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, L.; Guo, W.; Jia, L.; Fu, Y.; Gui, S.; Lu, F. Cloning, expression, and characterization of a thermostable and pH-stable laccase from Klebsiella pneumoniae and its application to dye decolorization. Process Biochem. 2017, 53, 125–134. [Google Scholar] [CrossRef]
- Neifar, M.; Chouchane, H.; Mahjoubi, M.; Jaouani, A.; Cherif, A. Pseudomonas extremorientalis BU118: A new salt-tolerant laccase-secreting bacterium with biotechnological potential in textile azo dye decolourization. 3 Biotech 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Singh, D.; Rawat, S.; Waseem, M.; Gupta, S.; Lynn, A.; Nitin, M.; Ramchiary, N.; Sharma, K.K. Molecular modeling and simulation studies of recombinant laccase from Yersinia enterocolitica suggests significant role in the biotransformation of non-steroidal anti-inflammatory drugs. Biochem. Biophys. Res. Commun. 2016, 469, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, M.; Murugan, S.; Eva, A.; Devina, S.; Kalidass, S. Application of immobilized laccase from Bacillus subtilis MTCC 2414 on decolourization of synthetic dyes. Res. J. Microbiol. 2015, 10, 421–432. [Google Scholar]
- Chang, F.; Wu, L.; Xiong, Z.; Yang, Y.; Xia, X.; Wu, Q.; Ge, C.; Chen, H. Light-induced expression of a novel marine laccase in Escherichia coli from Marinomonas profundimaris and its application in synthetic dye decolorization. Protein Expr. Purif. 2022, 197, 106108. [Google Scholar] [CrossRef]
- Martins, L.O.; Durão, P.; Brissos, V.; Lindley, P.F. Laccases of prokaryotic origin: Enzymes at the interface of protein science and protein technology. Cell. Mol. Life Sci. 2015, 72, 911–922. [Google Scholar] [CrossRef]
- Akram, F.; Ashraf, S.; Haq, I.u.; Shah, F.I.; Aqeel, A. Eminent industrial and biotechnological applications of laccases from bacterial source: A current overview. Appl. Biochem. Biotechnol. 2022, 195, 2336–2356. [Google Scholar] [CrossRef] [PubMed]
- Hullo, M.-F.; Moszer, I.; Danchin, A.; Martin-Verstraete, I. CotA of Bacillus subtilis is a copper-dependent laccase. J. Bacteriol. 2001, 183, 5426–5430. [Google Scholar] [CrossRef] [PubMed]
- Passardi, F.; Zamocky, M.; Favet, J.; Jakopitsch, C.; Penel, C.; Obinger, C.; Dunand, C. Phylogenetic distribution of catalase-peroxidases: Are there patches of order in chaos? Gene 2007, 397, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Rasheed, T.; Iqbal, H.M.; Yan, Y. Peroxidases-assisted removal of environmentally-related hazardous pollutants with reference to the reaction mechanisms of industrial dyes. Sci. Total Environ. 2018, 644, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sellami, K.; Couvert, A.; Nasrallah, N.; Maachi, R.; Abouseoud, M.; Amrane, A. Peroxidase enzymes as green catalysts for bioremediation and biotechnological applications: A review. Sci. Total Environ. 2022, 806, 150500. [Google Scholar] [CrossRef] [PubMed]
- de Montellano, P.R.O. Catalytic mechanisms of heme peroxidases. In Biocatalysis Based on Heme Peroxidases: Peroxidases as Potential Industrial Biocatalysts; Springer: Berlin/Heidelberg, Germany, 2010; pp. 79–107. [Google Scholar]
- Banci, L. Structural properties of peroxidases. J. Biotechnol. 1997, 53, 253–263. [Google Scholar] [CrossRef]
- Tien, M.; Kirk, T.K. Lignin peroxidase of Phanerochaete chrysosporium. In Methods in Enzymology; Academic Press: Cambridge, MS, USA, 1988; Volume 161, pp. 238–249. [Google Scholar]
- Asgher, M.; Ramzan, M.; Bilal, M. Purification and characterization of manganese peroxidases from native and mutant Trametes versicolor IBL-04. Chin. J. Catal. 2016, 37, 561–570. [Google Scholar] [CrossRef]
- Fernández-Fueyo, E.; Ruiz-Dueñas, F.J.; Martínez, M.J.; Romero, A.; Hammel, K.E.; Medrano, F.J.; Martínez, A.T. Ligninolytic peroxidase genes in the oyster mushroom genome: Heterologous expression, molecular structure, catalytic and stability properties, and lignin-degrading ability. Biotechnol. Biofuels 2014, 7, 2. [Google Scholar] [CrossRef]
- Singh, R.; Wiseman, B.; Deemagarn, T.; Jha, V.; Switala, J.; Loewen, P.C. Comparative study of catalase-peroxidases (KatGs). Arch. Biochem. Biophys. 2008, 471, 207–214. [Google Scholar] [CrossRef]
- Lin, L.; Wang, X.; Cao, L.; Xu, M. Lignin catabolic pathways reveal unique characteristics of dye-decolorizing peroxidases in Pseudomonas putida. Environ. Microbiol. 2019, 21, 1847–1863. [Google Scholar] [CrossRef]
- Rekik, H.; Nadia, Z.J.; Bejar, W.; Kourdali, S.; Belhoul, M.; Hmidi, M.; Benkiar, A.; Badis, A.; Sallem, N.; Bejar, S.; et al. Characterization of a purified decolorizing detergent-stable peroxidase from Streptomyces griseosporeus SN9. Int. J. Biol. Macromol. 2015, 73, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Miramón, P.; Dunker, C.; Kasper, L.; Jacobsen, I.D.; Barz, D.; Kurzai, O.; Hube, B. A family of glutathione peroxidases contributes to oxidative stress resistance in Candida albicans. Med. Mycol. 2014, 52, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Park, S.G.; Cha, M.K.; Jeong, W.; Kim, I.H. Distinct physiological functions of thiol peroxidase isoenzymes in Saccharomyces cerevisiae. J. Biol. Chem. 2000, 275, 5723–5732. [Google Scholar] [CrossRef]
- Agarwal, P.; Singh, M.; Singh, J.; Singh, R. Microbial tyrosinases: A Novel enzyme, structural features, and applications. In Applied Microbiology and Bioengineering; Academic Press: Cambridge, MS, USA, 2019; pp. 3–19. [Google Scholar]
- Krishna, M.S.; Alfarhan, A.; Rajagopal, R.; Arya, P. 18 Microbial tyrosinases: Promising enzymes for pharmaceutical, food bioprocessing, and environmental industry. In Microbial Oxidative Enzymes: Biotechnological Applications; De Gruyter: Berlin, German, 2024; pp. 375–392. [Google Scholar]
- Rudrappa, M.; Santosh Kumar, M.; Basavarajappa, D.S.; Hiremath, H.; Hugar, A.; Almansour, A.I.; Kantli, G.B.; Nayaka, S. Bioproduction, purification, partial characterization and phenol removal efficacy of tyrosinase enzyme from Streptomyces sp. strain MR28. Environ. Res. 2024, 251, 118701. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, D.; Sharma, A.; Bansal, S. Thermo stable tyrosinase purified from Pleurotus djamor grown in biomimetic calcium carbonate: A biological strategy to industrial waste remediation. Environ. Technol. Innov. 2021, 21, 101294. [Google Scholar] [CrossRef]
- Silva, L.; Salgado, A.; Coelho, M. Agaricus bisporus as a source of tyrosinase for phenol detection for future biosensor development. Environ. Technol. 2010, 31, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, M.; Boll, S.; Kossuch, J.; Bielecki, J.; Uhl, S.; Kleiner, B.; Wichmann, R. Efficient immobilization of mushroom tyrosinase utilizing whole cells from Agaricus bisporus and its application for degradation of bisphenol A. Water Res. 2014, 57, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal structure of Agaricus bisporus mushroom tyrosinase: Identity of the tetramer subunits and interaction with tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef]
- Zhu, B.; Wei, N. Tyrosinase-functionalized polyhydroxyalkanoate bio-beads as a novel biocatalyst for degradation of bisphenol analogues. Environ. Int. 2022, 163, 107225. [Google Scholar] [CrossRef]
- van Beilen, J.B.; Duetz, W.A.; Schmid, A.; Witholt, B. Practical issues in the application of oxygenases. TRENDS Biotechnol. 2003, 21, 170–177. [Google Scholar] [CrossRef]
- Pazmiño, D.T.; Winkler, M.; Glieder, A.; Fraaije, M.W. Monooxygenases as biocatalysts: Classification, mechanistic aspects and biotechnological applications. J. Biotechnol. 2010, 146, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Chakraborty, J.; Dutta, T.K. Role of oxygenases in guiding diverse metabolic pathways in the bacterial degradation of low-molecular-weight polycyclic aromatic hydrocarbons: A review. Crit. Rev. Microbiol. 2011, 37, 64–90. [Google Scholar] [CrossRef]
- Takamura, N.; Yamazaki, A.; Sakuma, N.; Hirose, S.; Sakai, M.; Takani, Y.; Yamashita, S.; Oshima, M.; Kuroki, M.; Tozawa, Y. Catalytic promiscuity of rice 2-oxoglutarate/Fe (II)-dependent dioxygenases supports xenobiotic metabolism. Plant Physiol. 2021, 187, 816–828. [Google Scholar] [CrossRef]
- Arora, P.; Srivastava, A.; Singh, V. Application of monooxygenases in dehalogenation, desulphurization, denitrification and hydroxylation of aromatic compounds. J. Bioremed. Biodegrad. 2010, 1, 112. [Google Scholar] [CrossRef]
- van Berkel, W.J.H.; Kamerbeek, N.M.; Fraaije, M.W. Flavoprotein monooxygenases, a diverse class of oxidative biocatalysts. J. Biotechnol. 2006, 124, 670–689. [Google Scholar] [CrossRef]
- Emerson, J.P.; Kovaleva, E.G.; Farquhar, E.R.; Lipscomb, J.D.; Que, L., Jr. Swapping metals in Fe-and Mn-dependent dioxygenases: Evidence for oxygen activation without a change in metal redox state. Proc. Natl. Acad. Sci. USA 2008, 105, 7347–7352. [Google Scholar] [CrossRef] [PubMed]
- Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. Intradiol dioxygenases—The key enzymes in xenobiotics degradation. Biodegrad. Hazard. Spec. Prod. 2013, 7, 129–153. [Google Scholar]
- Eltoukhy, A.; Jia, Y.; Lamraoui, I.; Abo-Kadoum, M.; Atta, O.M.; Nahurira, R.; Wang, J.; Yan, Y. Transcriptome analysis and cytochrome P450 monooxygenase reveal the molecular mechanism of Bisphenol A degradation by Pseudomonas putida strain YC-AE1. BMC Microbiol. 2022, 22, 294. [Google Scholar] [CrossRef]
- Riebel, A.; de Gonzalo, G.; Fraaije, M.W. Expanding the biocatalytic toolbox of flavoprotein monooxygenases from Rhodococcus jostii RHA1. J. Mol. Catal. B Enzym. 2013, 88, 20–25. [Google Scholar] [CrossRef]
- Masuda, H.; McClay, K.; Steffan, R.; Zylstra, G. Characterization of three propane-inducible oxygenases in Mycobacterium sp. strain ENV421. Lett. Appl. Microbiol. 2012, 55, 175–181. [Google Scholar] [CrossRef]
- Gold, M.H.; Kuwahara, M.; Chiu, A.A.; Glenn, J.K. Purification and characterization of an extracellular H2O2-requiring diarylpropane oxygenase from the white rot basidiomycete, Phanerochaete chrysosporium. Arch. Biochem. Biophys. 1984, 234, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Ma, F.; Zhao, H.; Zhang, S.; Wang, L.; Zhang, X.; Yu, H. A lytic polysaccharide monooxygenase from a white-rot fungus drives the degradation of lignin by a versatile peroxidase. Appl. Environ. Microbiol. 2019, 85, e02803-18. [Google Scholar] [CrossRef] [PubMed]
- Eppink, M.; Boeren, S.A.; Vervoort, J.; Van Berkel, W. Purification and properties of 4-hydroxybenzoate 1-hydroxylase (decarboxylating), a novel flavin adenine dinucleotide-dependent monooxygenase from Candida parapsilosis CBS604. J. Bacteriol. 1997, 179, 6680–6687. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-C.; Li, Y.-K. Purification and characterization of a catechol 1, 2-dioxygenase from a phenol degrading Candida albicans TL3. Arch. Microbiol. 2007, 187, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Achermann, S.; Mansfeldt, C.B.; Müller, M.; Johnson, D.R.; Fenner, K. Relating metatranscriptomic profiles to the micropollutant biotransformation potential of complex microbial communities. Environ. Sci. Technol. 2019, 54, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Kennes-Veiga, D.M.; Gónzalez-Gil, L.; Carballa, M.; Lema, J.M. Enzymatic cometabolic biotransformation of organic micropollutants in wastewater treatment plants: A review. Bioresour. Technol. 2022, 344, 126291. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gudiukaite, R.; Gricajeva, A.; Sadauskas, M.; Malunavicius, V.; Kamyab, H.; Sharma, S.; Sharma, T.; Pant, D. Microbial lipolytic enzymes–promising energy-efficient biocatalysts in bioremediation. Energy 2020, 192, 116674. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, T.; Sharma, T.; Sharma, S.; Kanwar, S.S. Role of microbial hydrolases in bioremediation. In Microbes and Enzymes in Soil Health and Bioremediation; Springer: Singapore, 2019; pp. 149–164. [Google Scholar]
- Xu, Z. Research Progress on bacterial cutinases for plastic pollution. IOP Conf. Ser. Earth Environ. Sci. 2020, 450, 012077. [Google Scholar] [CrossRef]
- Akram, F.; Fatima, T.; Shabbir, I.; Haq, I.u.; Ibrar, R.; Mukhtar, H. Abridgement of microbial esterases and their eminent industrial endeavors. Mol. Biotechnol. 2024, 66, 1–17. [Google Scholar]
- Babic, M.; Jankovic, P.; Marchesan, S.; Mausa, G.; Kalafatovic, D. Esterase sequence composition patterns for the identification of catalytic triad microenvironment motifs. J. Chem. Inf. Model. 2022, 62, 6398–6410. [Google Scholar] [CrossRef]
- Rauwerdink, A.; Kazlauskas, R.J. How the same core catalytic machinery catalyzes 17 different reactions: The serine-histidine-aspartate catalytic triad of α/β-hydrolase fold enzymes. ACS Catal. 2015, 5, 6153–6176. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Du, H.; Zhou, M.-S.; Ji, Y.; Xie, Y.-Q.; Huang, H.-B.; Zhang, S.-H.; Li, F.; Xiang, L.; Cai, Q.-Y.; et al. Substrate-enzyme interactions and catalytic mechanism in a novel family VI esterase with dibutyl phthalate-hydrolyzing activity. Environ. Int. 2023, 178, 108054. [Google Scholar] [CrossRef]
- Sayali, K.; Sadichha, P.; Surekha, S. Microbial esterases: An overview. Int. J. Curr. Microbiol. Appl. Sci 2013, 2, 135–146. [Google Scholar]
- Bhatt, P.; Zhou, X.; Huang, Y.; Zhang, W.; Chen, S. Characterization of the role of esterases in the biodegradation of organophosphate, carbamate, and pyrethroid pesticides. J. Hazard. Mater. 2021, 411, 125026. [Google Scholar] [CrossRef] [PubMed]
- Godinho, L.F.; Reis, C.R.; Tepper, P.G.; Poelarends, G.J.; Quax, W.J. Discovery of an Escherichia coli esterase with high activity and enantioselectivity toward 1, 2-O-isopropylideneglycerol esters. Appl. Environ. Microbiol. 2011, 77, 6094–6099. [Google Scholar] [CrossRef]
- Noor, H.; Satti, S.M.; Din, S.u.; Farman, M.; Hasan, F.; Khan, S.; Badshah, M.; Shah, A.A. Insight on esterase from Pseudomonas aeruginosa strain S3 that depolymerize poly(lactic acid) (PLA) at ambient temperature. Polym. Degrad. Stab. 2020, 174, 109096. [Google Scholar] [CrossRef]
- Barbayianni, E.; Kokotos, C.G.; Bartsch, S.; Drakou, C.; Bornscheuer, U.T.; Kokotos, G. Bacillus subtilis esterase (BS2) and its double mutant have different selectivity in the removal of carboxyl protecting groups. Adv. Synth. Catal. 2009, 351, 2325–2332. [Google Scholar] [CrossRef]
- Dilokpimol, A.; Mäkelä, M.R.; Mansouri, S.; Belova, O.; Waterstraat, M.; Bunzel, M.; de Vries, R.P.; Hildén, K.S. Expanding the feruloyl esterase gene family of Aspergillus niger by characterization of a feruloyl esterase, FaeC. New Biotechnol. 2017, 37, 200–209. [Google Scholar] [CrossRef]
- García-Calvo, L.; Rodríguez-Castro, R.; Ullán, R.V.; Albillos, S.M.; Fernández-Aguado, M.; Vicente, C.M.; Degnes, K.F.; Sletta, H.; Barreiro, C. Penicillium chrysogenum as a fungal factory for feruloyl esterases. Appl. Microbiol. Biotechnol. 2023, 107, 691–717. [Google Scholar] [CrossRef]
- Hamid, A.; Hussain, Z.; Tayyab, M.; Zafar, A.; Nawaz, M.; Ali, S.; Rehman, A.; Aftab, M. Production and characterization of a thermostable extracellular esterase from Aspergillus niger. Rev. Mex. De Ing. Química 2021, 20, 839–852. [Google Scholar] [CrossRef]
- Barriuso, J.; Vaquero, M.E.; Prieto, A.; Martínez, M.J. Structural traits and catalytic versatility of the lipases from the Candida rugosa-like family: A review. Biotechnol. Adv. 2016, 34, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Mitsuhara, I.; Tabata, J.; Kugimiya, S.; Watanabe, T.; Suzuki, K.; Yoshida, S.; Kitamoto, H. Extracellular esterases of phylloplane yeast Pseudozyma antarctica induce defect on cuticle layer structure and water-holding ability of plant leaves. Appl. Microbiol. Biotechnol. 2015, 99, 6405–6415. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.S.; Jena, H.M. Biodegradation of diethyl phthalate from synthetic wastewater in a batch operated internal loop airlift bioreactor. Int. Biodeterior. Biodegrad. 2019, 143, 104728. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Factories 2020, 19, 169. [Google Scholar]
- Zhao, J.; Ma, M.; Zeng, Z.; Wan, D.; Yan, X.; Xia, J.; Yu, P.; Gong, D. Production, purification, properties and current perspectives for modification and application of microbial lipases. Prep. Biochem. Biotechnol. 2024, 1–16. [Google Scholar] [CrossRef]
- Reyes-Reyes, A.L.; Valero Barranco, F.; Sandoval, G. Recent advances in lipases and their applications in the food and nutraceutical industry. Catalysts 2022, 12, 960. [Google Scholar] [CrossRef]
- Cavalcante, F.T.T.; Neto, F.S.; de Aguiar Falcão, I.R.; da Silva Souza, J.E.; de Moura Junior, L.S.; da Silva Sousa, P.; Rocha, T.G.; de Sousa, I.G.; de Lima Gomes, P.H.; de Souza, M.C.M. Opportunities for improving biodiesel production via lipase catalysis. Fuel 2021, 288, 119577. [Google Scholar] [CrossRef]
- Bracco, P.; van Midden, N.; Arango, E.; Torrelo, G.; Ferrario, V.; Gardossi, L.; Hanefeld, U. Bacillus subtilis lipase A—Lipase or Esterase? Catalysts 2020, 10, 308. [Google Scholar] [CrossRef]
- Griffin, J.M.; Atherton, J.H.; Page, M.I.; Powles, N.T. Lipase catalysed conversion of triglycerides to amides in liquid ammonia. J. Phys. Org. Chem. 2016, 29, 768–772. [Google Scholar] [CrossRef]
- Kapoor, M.; Gupta, M.N. Lipase promiscuity and its biochemical applications. Process Biochem. 2012, 47, 555–569. [Google Scholar] [CrossRef]
- Sarmah, N.; Revathi, D.; Sheelu, G.; Yamuna Rani, K.; Sridhar, S.; Mehtab, V.; Sumana, C. Recent advances on sources and industrial applications of lipases. Biotechnol. Prog. 2018, 34, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Ilesanmi, O.I.; Adekunle, A.E.; Omolaiye, J.A.; Olorode, E.M.; Ogunkanmi, A.L. Isolation, optimization and molecular characterization of lipase producing bacteria from contaminated soil. Sci. Afr. 2020, 8, e00279. [Google Scholar] [CrossRef]
- Bora, L.; Gohain, D.; Das, R. Recent advances in production and biotechnological applications of thermostable and alkaline bacterial lipases. J. Chem. Technol. Biotechnol. 2013, 88, 1959–1970. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, V.; Dubey, V.K.; Srivastava, A.; Garg, S.K.; Singh, V.P.; Arora, P.K. Industrial applications of fungal lipases: A review. Front. Microbiol. 2023, 14, 1142536. [Google Scholar] [CrossRef] [PubMed]
- da Silva, J.L.; Sales, M.B.; de Castro Bizerra, V.; Nobre, M.M.R.; de Sousa Braz, A.K.; da Silva Sousa, P.; Cavalcante, A.L.; Melo, R.L.; Gonçalves De Sousa Junior, P.; Neto, F.S. Lipase from Yarrowia lipolytica: Prospects as an industrial biocatalyst for biotechnological applications. Fermentation 2023, 9, 581. [Google Scholar] [CrossRef]
- Monteiro, R.R.; Virgen-Ortiz, J.J.; Berenguer-Murcia, A.; da Rocha, T.N.; dos Santos, J.C.; Alcantara, A.R.; Fernandez-Lafuente, R. Biotechnological relevance of the lipase A from Candida antarctica. Catal. Today 2021, 362, 141–154. [Google Scholar] [CrossRef]
- Dutta, K.; Sen, S.; Veeranki, V.D. Production, characterization and applications of microbial cutinases. Process Biochem. 2009, 44, 127–134. [Google Scholar] [CrossRef]
- Flores-Castañón, N.; Sarkar, S.; Banerjee, A. Structural, functional, and molecular docking analyses of microbial cutinase enzymes against polyurethane monomers. J. Hazard. Mater. Lett. 2022, 3, 100063. [Google Scholar] [CrossRef]
- Sahu, S.; Kaur, A.; Khatri, M.; Singh, G.; Arya, S.K. A review on cutinases enzyme in degradation of microplastics. J. Environ. Manag. 2023, 347, 119193. [Google Scholar] [CrossRef]
- Liang, X.; Zou, H. Biotechnological application of cutinase: A powerful tool in synthetic biology. SynBio 2022, 1, 54–64. [Google Scholar] [CrossRef]
- Chen, S.; Su, L.; Chen, J.; Wu, J. Cutinase: Characteristics, preparation, and application. Biotechnol. Adv. 2013, 31, 1754–1767. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Hiraga, K.; Takehana, T.; Taniguchi, I.; Yamaji, H.; Maeda, Y.; Toyohara, K.; Miyamoto, K.; Kimura, Y.; Oda, K. A bacterium that degrades and assimilates poly (ethylene terephthalate). Science 2016, 351, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Joo, S.; Cho, I.J.; Seo, H.; Son, H.F.; Sagong, H.-Y.; Shin, T.J.; Choi, S.Y.; Lee, S.Y.; Kim, K.-J. Structural insight into molecular mechanism of poly (ethylene terephthalate) degradation. Nat. Commun. 2018, 9, 382. [Google Scholar] [CrossRef] [PubMed]
- Austin, H.P.; Allen, M.D.; Donohoe, B.S.; Rorrer, N.A.; Kearns, F.L.; Silveira, R.L.; Pollard, B.C.; Dominick, G.; Duman, R.; El Omari, K. Characterization and engineering of a plastic-degrading aromatic polyesterase. Proc. Natl. Acad. Sci. USA 2018, 115, E4350–E4357. [Google Scholar] [CrossRef]
- Palm, G.J.; Reisky, L.; Böttcher, D.; Müller, H.; Michels, E.A.; Walczak, M.C.; Berndt, L.; Weiss, M.S.; Bornscheuer, U.T.; Weber, G. Structure of the plastic-degrading Ideonella sakaiensis MHETase bound to a substrate. Nat. Commun. 2019, 10, 1717. [Google Scholar] [CrossRef] [PubMed]
- Ang, T.-F.; Maiangwa, J.; Salleh, A.B.; Normi, Y.M.; Leow, T.C. Dehalogenases: From improved performance to potential microbial dehalogenation applications. Molecules 2018, 23, 1100. [Google Scholar] [CrossRef]
- Zakary, S.; Oyewusi, H.A.; Huyop, F. Dehalogenases for pollutant degradation: A mini review. J. Trop. Life Sci. 2021, 11, 17–24. [Google Scholar] [CrossRef]
- Oyewusi, H.A.; Wahab, R.A.; Huyop, F. Dehalogenase-producing halophiles and their potential role in bioremediation. Mar. Pollut. Bull. 2020, 160, 111603. [Google Scholar] [CrossRef] [PubMed]
- Adamu, A.; Wahab, R.A.; Aliyu, F.; Aminu, A.H.; Hamza, M.M.; Huyop, F. Haloacid dehalogenases of Rhizobium sp. and related enzymes: Catalytic properties and mechanistic analysis. Process Biochem. 2020, 92, 437–446. [Google Scholar] [CrossRef]
- Zinder, S.H. The genus dehalococcoides. In Organohalide-Respiring Bacteria; Springer: Berlin/Heidelberg, Germany, 2016; pp. 107–136. [Google Scholar]
- Mac Nelly, A.; Kai, M.; Svatoš, A.; Diekert, G.; Schubert, T. Functional heterologous production of reductive dehalogenases from Desulfitobacterium hafniense strains. Appl. Environ. Microbiol. 2014, 80, 4313–4322. [Google Scholar] [CrossRef]
- Huang, L.; Wang, W.; Zanaroli, G.; Xu, P.; Tang, H. Hexabromocyclododecanes are dehalogenated by CYP168A1 from Pseudomonas aeruginosa strain HS9. Appl. Environ. Microbiol. 2021, 87, e00826-21. [Google Scholar] [CrossRef]
- Invernizzi, G.; Casiraghi, L.; Grandori, R.; Lotti, M. Deactivation and unfolding are uncoupled in a bacterial lipase exposed to heat, low pH and organic solvents. J. Biotechnol. 2009, 141, 42–46. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Lu, Z.; Nian, B.; Yang, S.; Hu, Y. Enhanced stability and catalytic performance of laccase immobilized on magnetic graphene oxide modified with ionic liquids. J. Environ. Manag. 2023, 346, 118975. [Google Scholar] [CrossRef]
- Jesionowski, T.; Zdarta, J.; Krajewska, B. Enzyme immobilization by adsorption: A review. Adsorption 2014, 20, 801–821. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- Ashkan, Z.; Hemmati, R.; Homaei, A.; Dinari, A.; Jamlidoost, M.; Tashakor, A. Immobilization of enzymes on nanoinorganic support materials: An update. Int. J. Biol. Macromol. 2021, 168, 708–721. [Google Scholar] [CrossRef]
- Li, C.; Feng, X.; Sun, L.; Zhou, L.; Sun, J.; Wang, Z.; Liao, D.; Lan, P.; Lan, X. Non-covalent and covalent immobilization of papain onto Ti3C2 MXene nanosheets. Enzym. Microb. Technol. 2021, 148, 109817. [Google Scholar] [CrossRef]
- Bolibok, P.; Wiśniewski, M.; Roszek, K.; Terzyk, A.P. Controlling enzymatic activity by immobilization on graphene oxide. Sci. Nat. 2017, 104, 36. [Google Scholar] [CrossRef]
- Yandri, Y.; Ropingi, H.; Suhartati, T.; Irawan, B.; Hadi, S. Immobilization of α-amylase from Aspergillus fumigatus using adsorption method onto zeolite. Phys. Sci. Rev. 2024, 9, 909–920. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, W.; Cai, Y. Enzyme-enhanced adsorption of laccase immobilized graphene oxide for micro-pollutant removal. Sep. Purif. Technol. 2022, 294, 121178. [Google Scholar] [CrossRef]
- Balci, E.; Rosales, E.; Pazos, M.; Sofuoglu, A.; Sanromán, M.A. Immobilization of esterase from Bacillus subtilis on Halloysite nanotubes and applications on dibutyl phthalate degradation. Environ. Technol. Innov. 2023, 30, 103113. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.; Yeo, K.B.; Ki, M.-R.; Pack, S.P. Self-encapsulation and controlled release of recombinant proteins using novel silica-forming peptides as fusion linkers. Int. J. Biol. Macromol. 2019, 125, 1175–1183. [Google Scholar] [CrossRef]
- Rehman, H.U.; Nawaz, M.A.; Pervez, S.; Jamal, M.; Attaullah, M.; Aman, A.; Qader, S.A.U. Encapsulation of pectinase within polyacrylamide gel: Characterization of its catalytic properties for continuous industrial uses. Heliyon 2020, 6, e04578. [Google Scholar] [CrossRef]
- Traffano-Schiffo, M.V.; Aguirre Calvo, T.R.; Castro-Giraldez, M.; Fito, P.J.; Santagapita, P.R. Alginate beads containing lactase: Stability and microstructure. Biomacromolecules 2017, 18, 1785–1792. [Google Scholar] [CrossRef]
- Bilal, M.; Iqbal, H.M.; Hu, H.; Wang, W.; Zhang, X. Enhanced bio-catalytic performance and dye degradation potential of chitosan-encapsulated horseradish peroxidase in a packed bed reactor system. Sci. Total Environ. 2017, 575, 1352–1360. [Google Scholar] [CrossRef]
- El-Komy, H.M.; Al-Dosary, S.K.; El-Naghy, M.A.; Abdelhamid, M.A.; Immam, M.M. Optimization of azo-keratin hydrolysis by alginate-immobilized Keratinase produced from Bacillus licheniforms. J. Adv. Biomed. Pharm. Sci. 2019, 2, 41–46. [Google Scholar]
- Klein, W.P.; Thomsen, R.P.; Turner, K.B.; Walper, S.A.; Vranish, J.; Kjems, J.; Ancona, M.G.; Medintz, I.L. Enhanced catalysis from multienzyme cascades assembled on a DNA origami triangle. ACS Nano 2019, 13, 13677–13689. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, K.-R.; Ahn, D.-R.; Lee, J.E.; Yang, E.G.; Kim, S.Y. Reversible regulation of enzyme activity by pH-responsive encapsulation in DNA nanocages. ACS Nano 2017, 11, 9352–9359. [Google Scholar] [CrossRef]
- Caparco, A.A.; Dautel, D.R.; Champion, J.A. Protein Mediated Enzyme Immobilization. Small 2022, 18, 2106425. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.; Ki, M.-R.; El-Hafeez, A.A.A.; Son, R.G.; Pack, S.P. Tailored functionalized protein nanocarriers for cancer therapy: Recent developments and prospects. Pharmaceutics 2023, 15, 168. [Google Scholar] [CrossRef]
- Luckarift, H.R.; Spain, J.C.; Naik, R.R.; Stone, M.O. Enzyme immobilization in a biomimetic silica support. Nat. Biotechnol. 2004, 22, 211–213. [Google Scholar] [CrossRef]
- Lee, C.H.; Jin, E.S.; Lee, J.H.; Hwang, E.T. Immobilization and stabilization of enzyme in biomineralized calcium carbonate microspheres. Front. Bioeng. Biotechnol. 2020, 8, 553591. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.; Pack, S.P. Biomimetic and bioinspired silicifications: Recent advances for biomaterial design and applications. Acta Biomater. 2021, 120, 38–56. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.A.; Son, R.G.; Ki, M.-R.; Pack, S.P. Biosilica-coated carbonic anhydrase displayed on Escherichia coli: A novel design approach for efficient and stable biocatalyst for CO2 sequestration. Int. J. Biol. Macromol. 2024, 277, 134058. [Google Scholar] [CrossRef]
- Min, K.-H.; Son, R.G.; Ki, M.-R.; Choi, Y.S.; Pack, S.P. High expression and biosilica encapsulation of alkaline-active carbonic anhydrase for CO2 sequestration system development. Chemosphere 2016, 143, 128–134. [Google Scholar] [CrossRef]
- Ryu, Y.H.; Yeo, K.B.; Ki, M.-R.; Kim, Y.J.; Pack, S.P. Improved stability and reusability of endoglucanase from Clostridium thermocellum by a biosilica-based auto-encapsulation method. Biochem. Eng. J. 2016, 105, 144–149. [Google Scholar] [CrossRef]
- Vetrano, A.; Gabriele, F.; Germani, R.; Spreti, N. Characterization of lipase from Candida rugosa entrapped in alginate beads to enhance its thermal stability and recyclability. New J. Chem. 2022, 46, 10037–10047. [Google Scholar] [CrossRef]
- Schoonen, L.; Nolte, R.J.; van Hest, J.C. Highly efficient enzyme encapsulation in a protein nanocage: Towards enzyme catalysis in a cellular nanocompartment mimic. Nanoscale 2016, 8, 14467–14472. [Google Scholar] [CrossRef]
- Jiang, S.; Ren, D.; Wang, Z.; Zhang, S.; Zhang, X.; Chen, W. Improved stability and promoted activity of laccase by One-Pot encapsulation with Cu (PABA) nanoarchitectonics and its application for removal of Azo dyes. Ecotoxicol. Environ. Saf. 2022, 234, 113366. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M.; Cheng, H.R.; Yan, Y.J.; Iqbal, H.M.N. Multi-point enzyme immobilization, surface chemistry, and novel platforms: A paradigm shift in biocatalyst design. Crit. Rev. Biotechnol. 2019, 39, 202–219. [Google Scholar] [CrossRef] [PubMed]
- Zucca, P.; Sanjust, E. Inorganic Materials as Supports for Covalent Enzyme Immobilization: Methods and Mechanisms. Molecules 2014, 19, 14139–14194. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, Q.; Wang, J.; Liang, Q.; Quan, J. Oriented Immobilization of G Protein-Coupled Receptors. In G Protein-Coupled Receptors: Immobilization and Applications in Drug Discovery; Zhao, X., Li, Q., Wang, J., Liang, Q., Quan, J., Eds.; Springer Nature: Singapore, 2023; pp. 41–63. [Google Scholar]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Carballares, D.; Morellon-Sterling, R.; Fernandez-Lafuente, R. Stabilization of enzymes via immobilization: Multipoint covalent attachment and other stabilization strategies. Biotechnol. Adv. 2021, 52, 107821. [Google Scholar] [CrossRef]
- Lou, X.; Zhi, F.; Sun, X.; Wang, F.; Hou, X.; Lv, C.; Hu, Q. Construction of co-immobilized laccase and mediator based on MOFs membrane for enhancing organic pollutants removal. Chem. Eng. J. 2023, 451, 138080. [Google Scholar] [CrossRef]
- Othman, A.M.; Sanromán, Á.; Moldes, D. Laccase multi-point covalent immobilization: Characterization, kinetics, and its hydrophobicity applications. Appl. Microbiol. Biotechnol. 2023, 107, 719–733. [Google Scholar] [CrossRef]
- Nagatani, A.; Mokhtar, A.; Okobira, T. A highly efficient laccase-immobilized bioreactor prepared by radiation induced graft polymerization for removal of persistent pollutants. Bioresour. Technol. Rep. 2023, 22, 101444. [Google Scholar] [CrossRef]
- Gao, Y.; Truong, Y.B.; Cacioli, P.; Butler, P.; Kyratzis, I.L. Bioremediation of pesticide contaminated water using an organophosphate degrading enzyme immobilized on nonwoven polyester textiles. Enzym. Microb. Technol. 2014, 54, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Chang, B.; Shi, N.; Yan, W.; Lu, F.; Liu, F. Cross-linked enzyme aggregates immobilization: Preparation, characterization, and applications. Crit. Rev. Biotechnol. 2023, 43, 369–383. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.D.; Jia, S.R. Optimization protocols and improved strategies of cross-linked enzyme aggregates technology: Current development and future challenges. Crit. Rev. Biotechnol. 2015, 35, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhang, H.; Fu, X.; Li, X.; Guo, Q.; Yang, T.; Li, X. Immobilization of esterase SulE in cross-linked gelatin/chitosan and its application in remediating soils polluted with tribenuron-methyl and metsulfuron-methyl. Process Biochem. 2020, 98, 217–223. [Google Scholar] [CrossRef]
- George, J.; Rajendran, D.S.; Senthil Kumar, P.; Sonai Anand, S.; Vinoth Kumar, V.; Rangasamy, G. Efficient decolorization and detoxification of triarylmethane and azo dyes by porous-cross-linked enzyme aggregates of Pleurotus ostreatus laccase. Chemosphere 2023, 313, 137612. [Google Scholar] [CrossRef]
- Sanchez, J.M.; Lopez-Laguna, H.; Serna, N.; Unzueta, U.; Clop, P.D.; Villaverde, A.; Vazquez, E. Engineering the performance of artificial inclusion bodies built of catalytic β-galactosidase. ACS Sustain. Chem. Eng. 2021, 9, 2552–2558. [Google Scholar] [CrossRef]
- Taniguchi, K.; Nomura, K.; Hata, Y.; Nishimura, T.; Asami, Y.; Kuroda, A. The Si-tag for immobilizing proteins on a silica surface. Biotechnol. Bioeng. 2007, 96, 1023–1029. [Google Scholar] [CrossRef]
- Freitas, A.I.; Domingues, L.; Aguiar, T.Q. Tag-mediated single-step purification and immobilization of recombinant proteins toward protein-engineered advanced materials. J. Adv. Res. 2022, 36, 249–264. [Google Scholar] [CrossRef]
- Ikeda, T.; Ninomiya, K.-i.; Hirota, R.; Kuroda, A. Single-step affinity purification of recombinant proteins using the silica-binding Si-tag as a fusion partner. Protein Expr. Purif. 2010, 71, 91–95. [Google Scholar] [CrossRef]
- Abdelhamid, M.A.; Motomura, K.; Ikeda, T.; Ishida, T.; Hirota, R.; Kuroda, A. Affinity purification of recombinant proteins using a novel silica-binding peptide as a fusion tag. Appl. Microbiol. Biotechnol. 2014, 98, 5677–5684. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.A.; Ikeda, T.; Motomura, K.; Tanaka, T.; Ishida, T.; Hirota, R.; Kuroda, A. Application of volcanic ash particles for protein affinity purification with a minimized silica-binding tag. J. Biosci. Bioeng. 2016, 122, 633–638. [Google Scholar] [CrossRef]
- Ikeda, T.; Motomura, K.; Agou, Y.; Ishida, T.; Hirota, R.; Kuroda, A. The silica-binding Si-tag functions as an affinity tag even under denaturing conditions. Protein Expr. Purif. 2011, 77, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Kimple, M.E.; Brill, A.L.; Pasker, R.L. Overview of affinity tags for protein purification. Curr. Protoc. Protein Sci. 2013, 73, 9. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Abdelhamid, M.A.; Pack, S.P. Direct immobilization and recovery of recombinant proteins from cell lysates by using EctP1-peptide as a short fusion tag for silica and titania supports. Int. J. Biol. Macromol. 2019, 135, 969–977. [Google Scholar] [CrossRef]
- Soto-Rodríguez, J.; Coyle, B.L.; Samuelson, A.; Aravagiri, K.; Baneyx, F. Affinity purification of Car9-tagged proteins on silica matrices: Optimization of a rapid and inexpensive protein purification technology. Protein Expr. Purif. 2017, 135, 70–77. [Google Scholar] [CrossRef]
- Wang, J.; Yu, S.; Feng, F.; Lu, L. Simultaneous purification and immobilization of laccase on magnetic zeolitic imidazolate frameworks: Recyclable biocatalysts with enhanced stability for dye decolorization. Biochem. Eng. J. 2019, 150, 107285. [Google Scholar] [CrossRef]
- Girelli, A.M.; Astolfi, M.L.; Scuto, F.R. Agro-industrial wastes as potential carriers for enzyme immobilization: A review. Chemosphere 2020, 244, 125368. [Google Scholar] [CrossRef] [PubMed]
- Bijoy, G.; Rajeev, R.; Benny, L.; Jose, S.; Varghese, A. Enzyme immobilization on biomass-derived carbon materials as a sustainable approach towards environmental applications. Chemosphere 2022, 307, 135759. [Google Scholar] [CrossRef] [PubMed]
- de Cazes, M.; Belleville, M.P.; Petit, E.; Salomo, M.; Bayer, S.; Czaja, R.; De Gunzburg, J.; Sanchez-Marcano, J. Erythromycin degradation by esterase (EreB) in enzymatic membrane reactors. Biochem. Eng. J. 2016, 114, 70–78. [Google Scholar] [CrossRef]
- Ni, S.; Li, C.; Yu, Y.; Niu, D.; Zhu, J.; Yin, D.; Wang, C.; Zhang, W.; Jiang, X.; Ren, J. Immobilization of EreB on acid-modified palygorskite for highly efficient degradation of erythromycin. Int. J. Environ. Res. Public Health 2022, 19, 11064. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Li, C.; Zhang, W.; Niu, D.; Zhi, J.; Wang, C.; Jiang, X.; Ren, J. Immobilization of purified enzyme EreB in metalorganic framework (MOF) mesopores for erythromycin degradation. Environ. Res. 2023, 237, 117023. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Liu, Z.; Zeng, G.; Liu, Y.; Yang, X.; Zhou, C.; Chen, M.; Liu, Y.; Jiang, Y.; Yan, M. Immobilization of laccase on hollow mesoporous carbon nanospheres: Noteworthy immobilization, excellent stability and efficacious for antibiotic contaminants removal. J. Hazard. Mater. 2019, 362, 318–326. [Google Scholar] [CrossRef] [PubMed]
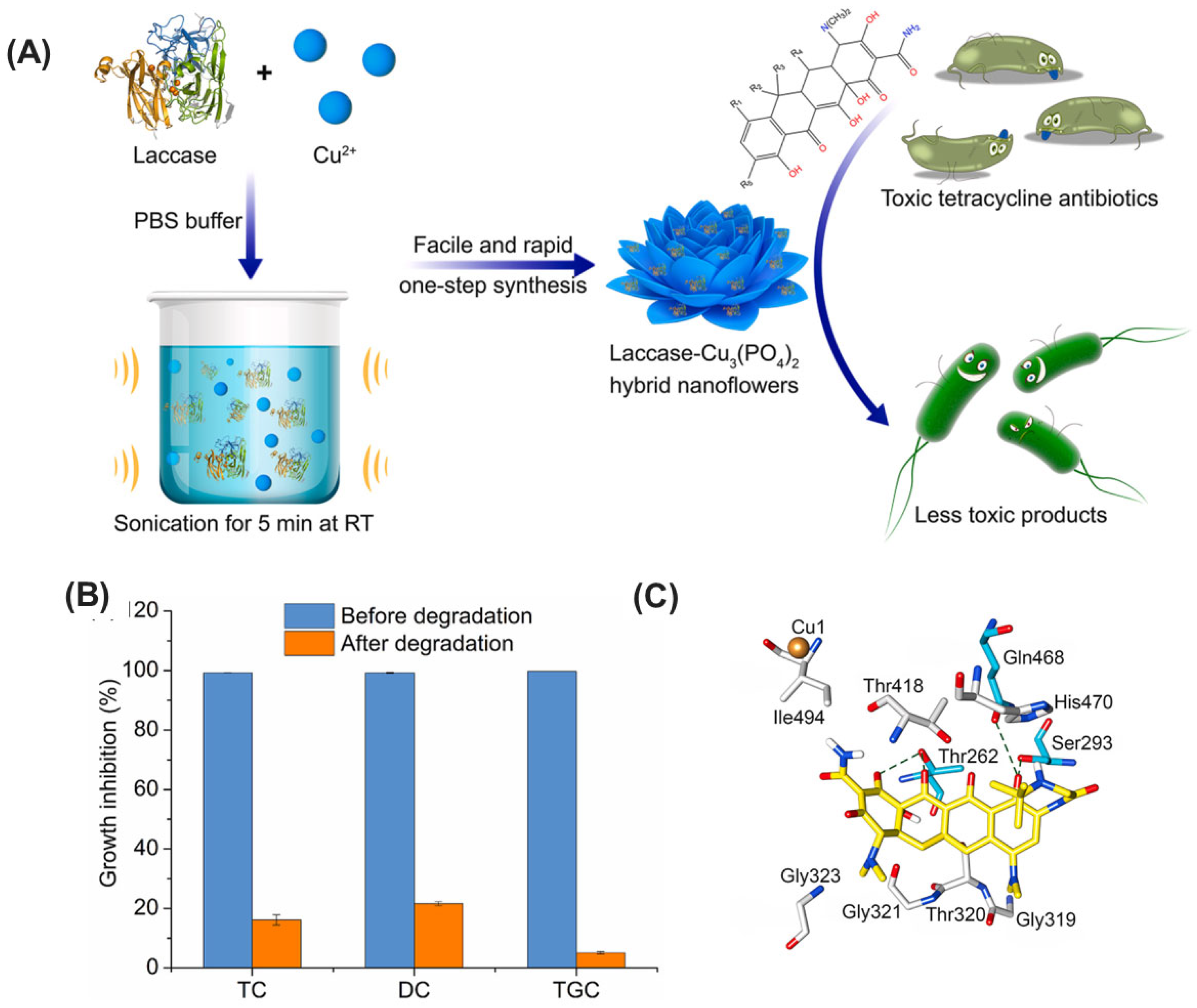
- Han, Z.; Wang, H.; Zheng, J.; Wang, S.; Yu, S.; Lu, L. Ultrafast synthesis of laccase-copper phosphate hybrid nanoflowers for efficient degradation of tetracycline antibiotics. Environ. Res. 2023, 216, 114690. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lin, Y.; Yang, X.; Ng, T.B.; Ye, X.; Lin, J. Degradation of tetracycline by immobilized laccase and the proposed transformation pathway. J. Hazard. Mater. 2017, 322, 525–531. [Google Scholar] [CrossRef]
- Zou, M.; Tian, W.; Chu, M.; Lu, Z.; Liu, B.; Xu, D. Magnetically separable laccase-biochar composite enable highly efficient adsorption-degradation of quinolone antibiotics: Immobilization, removal performance and mechanisms. Sci. Total Environ. 2023, 879, 163057. [Google Scholar] [CrossRef]
- Rybarczyk, A.; Smułek, W.; Grzywaczyk, A.; Kaczorek, E.; Jesionowski, T.; Nghiem, L.D.; Zdarta, J. 3D printed polylactide scaffolding for laccase immobilization to improve enzyme stability and estrogen removal from wastewater. Bioresour. Technol. 2023, 381, 129144. [Google Scholar] [CrossRef] [PubMed]
- Touahar, I.E.; Haroune, L.; Ba, S.; Bellenger, J.-P.; Cabana, H. Characterization of combined cross-linked enzyme aggregates from laccase, versatile peroxidase and glucose oxidase, and their utilization for the elimination of pharmaceuticals. Sci. Total Environ. 2014, 481, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Kalsoom, U.; Ahsan, Z.; Bhatti, H.N.; Amin, F.; Nadeem, R.; Aftab, K.; Bilal, M. Iron oxide nanoparticles immobilized Aspergillus flavus manganese peroxidase with improved biocatalytic, kinetic, thermodynamic, and dye degradation potentialities. Process Biochem. 2022, 117, 117–133. [Google Scholar] [CrossRef]
- Jiménez Vizcarra, M.J.; Mahendra, S.; Wang, M. A Co-immobilized enzyme-mediator system for facilitating manganese peroxidase catalysis in solution free of divalent manganese ions. Bioresour. Technol. 2023, 390, 129897. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, Z.; Sun, Y. Facile co-immobilization of laccase and mediator by polyethyleneimine-induced biomimetic mineralization for dye removal. Biochem. Eng. J. 2023, 199, 109067. [Google Scholar] [CrossRef]
- Yavaşer, R.; Aktaş Uygun, D.; Karagözler, A.A. Removal of selected azo dyes and phenolic compounds via tyrosinase immobilized magnetic iron oxide silver nanoparticles. Catal. Lett. 2023, 153, 1265–1277. [Google Scholar] [CrossRef]
- Yang, X.; Tang, X.; Dong, F.; Lin, L.; Wei, W.; Wei, D. Facile one-pot immobilization of a novel thermostable carboxylesterase from Geobacillus uzenensis for continuous pesticide degradation in a packed-bed column reactor. Catalysts 2020, 10, 518. [Google Scholar] [CrossRef]
- Vitola, G.; Mazzei, R.; Giorno, L. Biohybrid membranes for organophosphate pesticides degradation: Hyperactivation of immobilized phosphotriesterase by surfactants. Environ. Technol. Innov. 2023, 30, 103053. [Google Scholar] [CrossRef]
- Vidal-Limon, A.; García Suárez, P.C.; Arellano-García, E.; Contreras, O.E.; Aguila, S.A. Enhanced degradation of pesticide dichlorophen by laccase immobilized on nanoporous materials: A cytotoxic and molecular simulation investigation. Bioconjug. Chem. 2018, 29, 1073–1080. [Google Scholar] [CrossRef]
- Salem, T.; Fetyan, N.A.; Malhat, F.; Abdelhafez, A.A.; Ramadan, A.I. A novel multi-enzyme immobilized biocatalyst for Biodegradation of p, p′-DDT. Egypt. J. Chem. 2024. [Google Scholar] [CrossRef]
- Jia, Y.; Samak, N.A.; Hao, X.; Chen, Z.; Yang, G.; Zhao, X.; Mu, T.; Yang, M.; Xing, J. Nano-immobilization of PETase enzyme for enhanced polyethylene terephthalate biodegradation. Biochem. Eng. J. 2021, 176, 108205. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, G.; Chen, Y.; Yi, Z.; Jin, W.; Zhang, G. A versatile tag for simple preparation of cutinase towards enhanced biodegradation of polyethylene terephthalate. Int. J. Biol. Macromol. 2023, 225, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Han, H.; Song, P.; Jiang, Y.; Fan, J.; Khan, A.; Liu, P.; Mašek, O.; Li, X. Biochar immobilized hydrolase degrades PET microplastics and alleviates the disturbance of soil microbial function via modulating nitrogen and phosphorus cycles. J. Hazard. Mater. 2024, 474, 134838. [Google Scholar] [CrossRef] [PubMed]
- Rincon, I.; Hidalgo, T.; Armani, G.; Rojas, S.; Horcajada, P. Enzyme_Metal-Organic Framework Composites as Novel Approach for Microplastic Degradation. ChemSusChem 2024, n/a, e202301350. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Yan, J.; Yang, Y.; Wu, Q.; Hu, X. Immobilization of laccase on magnetic mesoporous silica as a recoverable biocatalyst for the efficient degradation of benzo[a]pyrene. Chemosphere 2024, 346, 140642. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Guo, M.; Hu, S.; Fang, X.; Jin, Z.; Wu, R. Nanosurface-immobilized lipase and its degradation of phthalate wastewater. Mol. Catal. 2022, 529, 112522. [Google Scholar] [CrossRef]
- Awad, G.; Mohamed, E.F. Immobilization of P450 BM3 monooxygenase on hollow nanosphere composite: Application for degradation of organic gases pollutants under solar radiation lamp. Appl. Catal. B Environ. 2019, 253, 88–95. [Google Scholar] [CrossRef]
- Miri, S.; Perez, J.A.E.; Brar, S.K.; Rouissi, T.; Martel, R. Sustainable production and co-immobilization of cold-active enzymes from Pseudomonas sp. for BTEX biodegradation. Environ. Pollut. 2021, 285, 117678. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Fang, R.; Gul, I.; Aer, L.; Zhao, Y.; Guo, J.; Tang, L. Halohydrin dehalogenase immobilization in magnetic biochar for sustainable halocarbon biodegradation and biotransformation. Environ. Technol. Innov. 2022, 27, 102759. [Google Scholar] [CrossRef]
- Lacerda, M.F.A.R.; Lopes, F.M.; Sartoratto, A.; Ponezi, A.N.; Thomaz, D.V.; Schimidt, F.; Santiago, M.F. Stability of immobilized laccase on Luffa cylindrica fibers and assessment of synthetic hormone degradation. Prep. Biochem. Biotechnol. 2019, 49, 58–63. [Google Scholar] [CrossRef]
- JO, U.; Moropeng, R.C.; Momba, M. Novel bio-catalytic degradation of endocrine disrupting compounds in wastewater. Front. Bioeng. Biotechnol. 2022, 10, 996566. [Google Scholar]
- Bhardwaj, P.; Sharma, S.; Khatri, M.; Singh, G.; Arya, S.K. Eradication of ibuprofen and diclofenac via in situ synthesized and immobilized bacterial laccase to Cu-based metal organic framework. J. Water Process Eng. 2023, 54, 104023. [Google Scholar] [CrossRef]
- Rafaqat, S.; Perveen, B.; Raqba; Imran, W.; Hussain, A.; Ali, N. Development of manganese peroxidase based voltammetric biosensor for detection of textile Azo dyes RR 195 & RB 221. Mater. Chem. Phys. 2024, 312, 128647. [Google Scholar] [CrossRef]
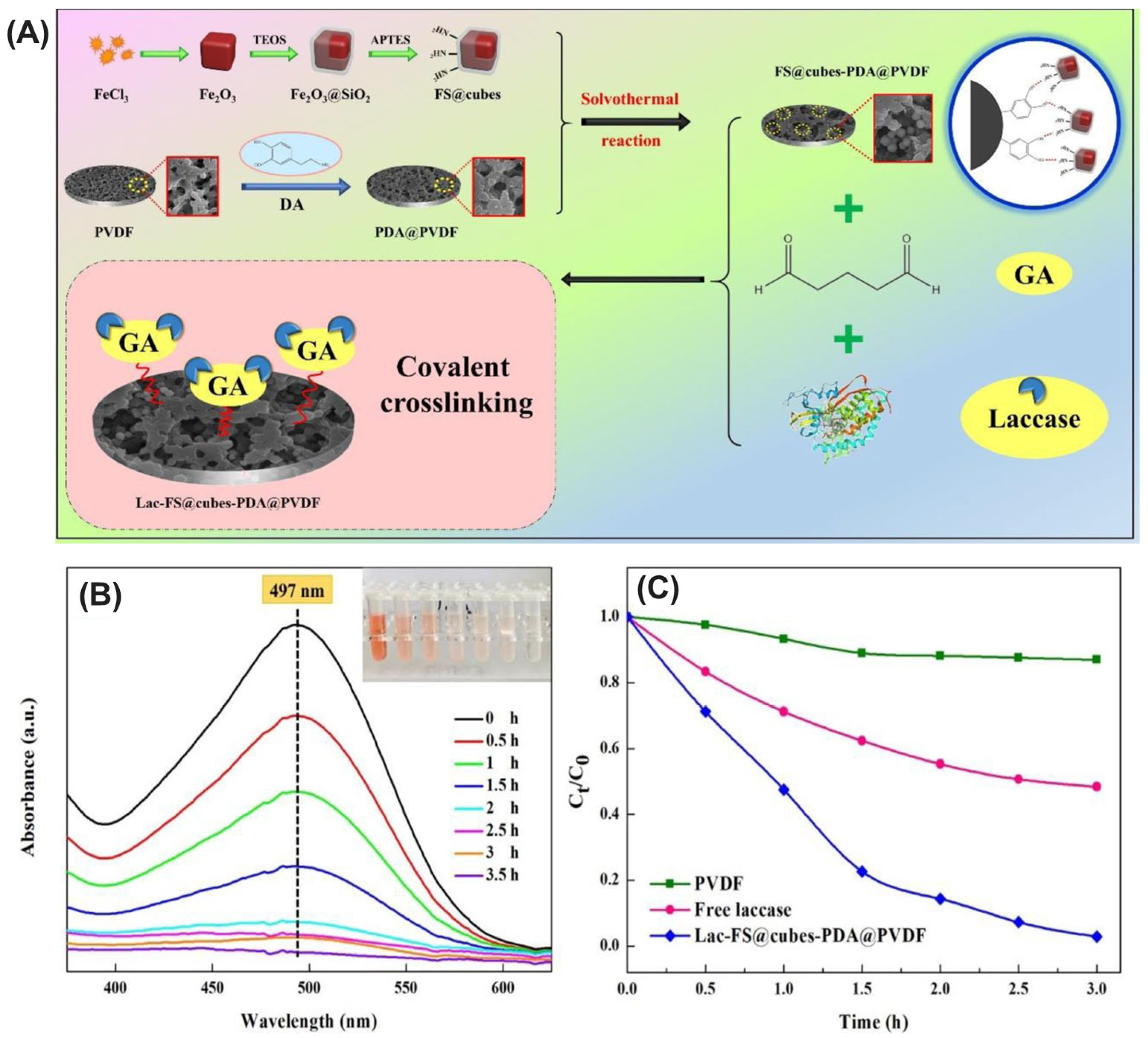
- Zhu, Y.; Qiu, F.; Rong, J.; Zhang, T.; Mao, K.; Yang, D. Covalent laccase immobilization on the surface of poly(vinylidene fluoride) polymer membrane for enhanced biocatalytic removal of dyes pollutants from aqueous environment. Colloids Surf. B Biointerfaces 2020, 191, 111025. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, X.; Wang, H.; Cavaco-Paulo, A.; Su, J. Co-immobilizing laccase-mediator system by in-situ synthesis of MOF in PVA hydrogels for enhanced laccase stability and dye decolorization efficiency. J. Environ. Manag. 2024, 353, 120114. [Google Scholar] [CrossRef]
- Srinivasan, P.; Selvankumar, T.; Paray, B.A.; Rehman, M.U.; Kamala-Kannan, S.; Govarthanan, M.; Kim, W.; Selvam, K. Chlorpyrifos degradation efficiency of Bacillus sp. laccase immobilized on iron magnetic nanoparticles. 3 Biotech 2020, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.; Su, W.; Xie, Q.; Gu, Q.; Deng, X.; Ren, Y.; Li, H. Expression, characterization, and immobilization of a novel SGNH esterase Est882 and its potential for pyrethroid degradation. Front. Microbiol. 2022, 13, 1069754. [Google Scholar] [CrossRef]
- Hernández, A.F.; Parrón, T.; Tsatsakis, A.M.; Requena, M.; Alarcón, R.; López-Guarnido, O. Toxic effects of pesticide mixtures at a molecular level: Their relevance to human health. Toxicology 2013, 307, 136–145. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Arica, M.Y. Biodegradation of methylene blue and carbaryl by Trametes versicolor laccase preparations in the presence of a mediator compound. J. Macromol. Sci. Part A 2019, 56, 277–285. [Google Scholar] [CrossRef]
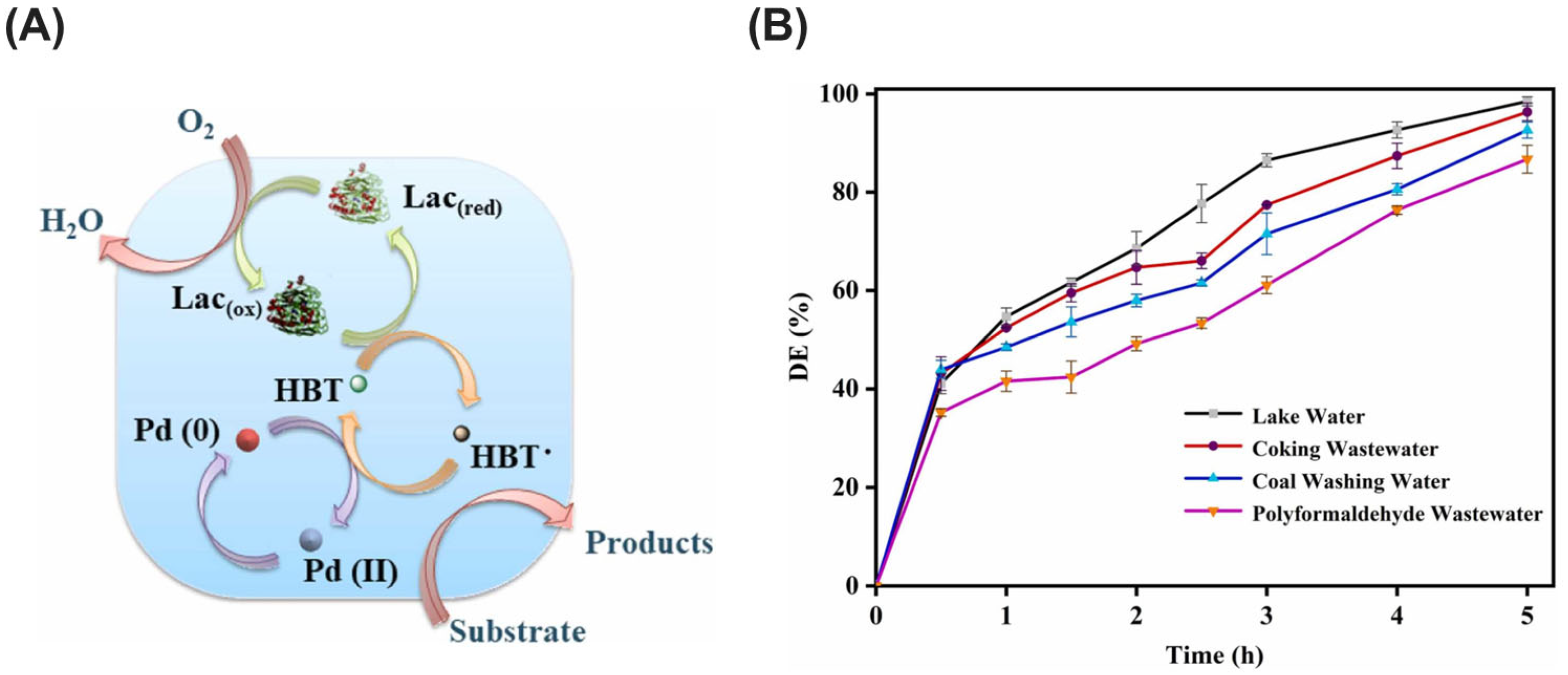
- Jia, J.; Xue, P.; Ma, L.; Li, P.; Xu, C. Deep degradation of atrazine in water using co-immobilized laccase-1-hydroxybenzotriazole-Pd as composite biocatalyst. J. Hazard. Mater. 2024, 468, 133779. [Google Scholar] [CrossRef]
- Yue, W.; Wang, X.; Zhang, J.; Bao, J.; Yao, M. Construction of immobilized laccase system based on ZnO and degradation of mesotrione. Toxics 2024, 12, 434. [Google Scholar] [CrossRef]
- Lee, Y.L.; Jaafar, N.R.; Ling, J.G.; Huyop, F.; Abu Bakar, F.D.; Rahman, R.A.; Illias, R.M. Cross-linked enzyme aggregates of polyethylene terephthalate hydrolyse (PETase) from Ideonella sakaiensis for the improvement of plastic degradation. Int. J. Biol. Macromol. 2024, 263, 130284. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, J.; Wang, X.; Liu, D.; Tian, Q.; Shen, H.; Zhang, J.; Song, D.; Dong, W.; Yu, Z. Microreactor-enhanced enzymatic breakdown: Janus microspheres with cutinase for nanoplastic removal during water treatment. Chem. Eng. Sci. 2024, 298, 120398. [Google Scholar] [CrossRef]
- Zayed, M.E.M.; Obaid, A.Y.; Almulaiky, Y.Q.; El-Shishtawy, R.M. Enhancing the sustainable immobilization of laccase by amino-functionalized PMMA-reinforced graphene nanomaterial. J. Environ. Manag. 2024, 351, 119503. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, M.; Song, J.; Zhang, Y.; Nian, B.; Hu, Y. Spacer arm of ionic liquids facilitated laccase immobilization on magnetic graphene enhancing its stability and catalytic performance. Chemosphere 2024, 362, 142735. [Google Scholar] [CrossRef]
- Balci, E.; Rosales, E.; Pazos, M.; Sofuoglu, A.; Sanroman, M.A. Continuous treatment of diethyl hexyl and dibutyl phthalates by fixed-bed reactor: Comparison of two esterase bionanocomposites. Bioresour. Technol. 2022, 363, 127990. [Google Scholar] [CrossRef]
- Dvorak, P.; Bidmanova, S.; Damborsky, J.; Prokop, Z. Immobilized synthetic pathway for biodegradation of toxic recalcitrant pollutant 1,2,3-trichloropropane. Environ. Sci. Technol. 2014, 48, 6859–6866. [Google Scholar] [CrossRef]
- Shahar, H.; Tan, L.L.; Ta, G.C.; Heng, L.Y. Detection of halogenated hydrocarbon pollutants using enzymatic reflectance biosensor. Sens. Actuators B Chem. 2019, 281, 80–89. [Google Scholar] [CrossRef]
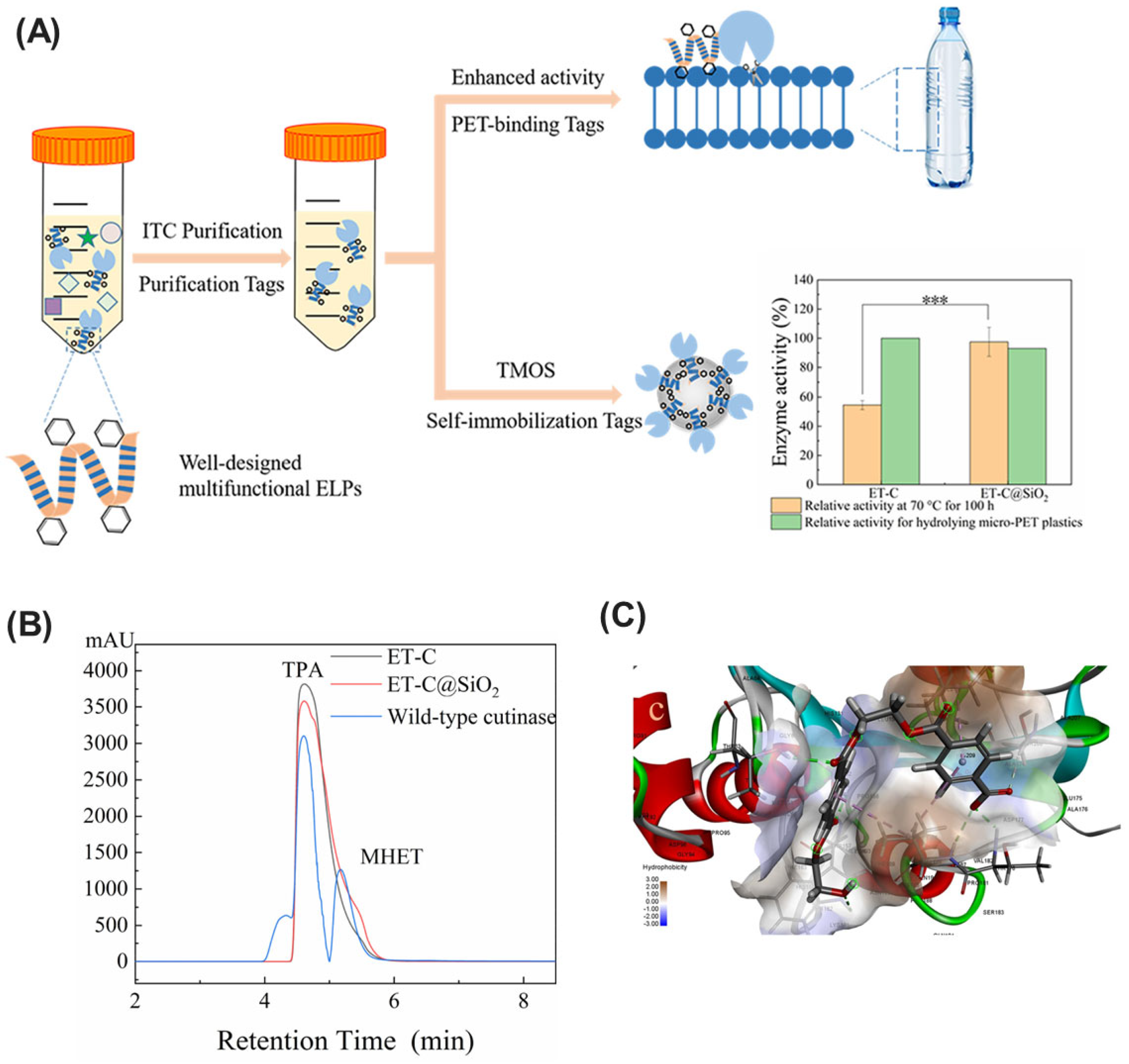

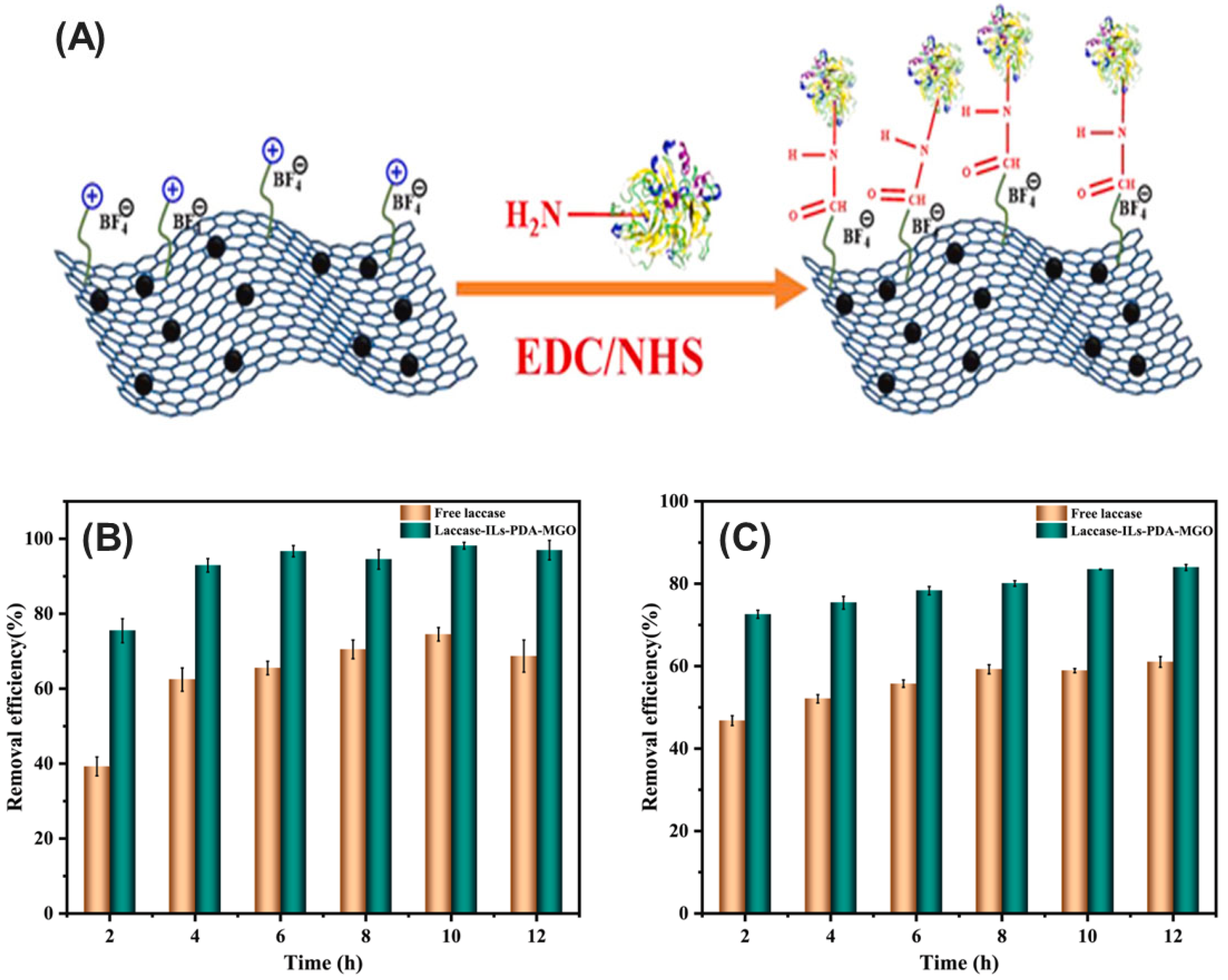


| Enzyme | Microbial Source | Support Matrix | Immobilization Method | Advantages of Immobilization | Mitigation Application | References |
|---|---|---|---|---|---|---|
| EreB esterase | E. coli, recombinantly produced | Enzymatic membrane reactor (EMR) | Adsorption | Long-term stability, continuous degradation | Continuous erythromycin degradation at a rate of 15.8 mg/h for 100 h | [195] |
| EreB esterase | E. coli, recombinantly produced | Palygorskite (acid-modified) | Cross-linking | Enhanced stability (temperature and pH), increased activity, reusability (45% after 10 cycles) | Erythromycin degradation in polluted water (20 mg/L)—achieved degradation within 300 min | [196] |
| EreB esterase | E. coli, recombinantly produced | Cu-BTC MOF | Adsorption | Improved heat tolerance (25–45 °C), improved pH tolerance (6.5–10), increased substrate affinity, reusability (57% activity after 10 cycles) | Complete inactivation of erythromycin in wastewater | [197] |
| Laccase | Trametes versicolor | HMCs | Covalent interaction and physical adsorption | Improved stability (temperature, pH, storage), reusability, high removal efficiency for antibiotics (TCH and CPH) | Bioremediation of antibiotic pollutants in environmental application | [198] |
| Laccase | Bacillus amyloliquefaciens, recombinantly produced in E. coli | Inorganic hNFs | Sonication-mediated self-encapsulation | Enhanced thermal and storage stability, superior degradation performance for various TCs (including tigecycline), reusability, reduced TC toxicity | Removal of tetracycline antibiotics (TCs) from the environment | [199] |
| Laccase | Cerrena unicolor | Magnetic CLEAs | Cross-linking | Potentially improved stability and reusability | Complete degradation of tetracycline (TC) and oxytetracycline (OTC) (100 μg/mL) within 48 h (pH 6, 25 °C), reduced antimicrobial activity of TC and OTC | [200] |
| Laccase | Trametes versicolor | Magnetic biochar | Adsorption and cross-linking method | Improved pH, thermal, storage, and operational stability | Synergistic effect of adsorption by MBC and degradation by laccase for higher removal of quinolone antibiotics from wastewater | [201] |
| Laccase | Trametes versicolor | 3D printed PLA scaffolds | Physical adsorption | Improved chemical and thermal stability, reusability | Removal of estrogens (estradiol and ethinylestradiol) from real wastewater | [202] |
| Laccase, versatile peroxidase, and glucose oxidase | Trametes versicolor (for laccase); Bjerkandera adusta (for peroxidase); Aspergillus niger (for glucose oxidase) | - | Cross-linking (Combi-CLEAs) | Retained enzyme activity (30–40% each), broader degradation range | Treatment of municipal wastewater effluents | [203] |
| Manganese peroxidase | Aspergillus flavus | Iron oxide nanoparticles | Physical adsorption | Enhanced thermal stability, wider pH and temperature range, improved catalytic activity, magnetic separation and reusability | Textile wastewater treatment, Direct Red 31 (complete decolorization), Acid Black 234 (92% decolorization) | [204] |
| Manganese peroxidase | Phanerochaete chrysosporium | Silica gels | Encapsulation through the sol-gel method | 4-fold increase in enzymatic activity via co-immobilization with Mn3+ | Dye decolorization (the co-immobilized system degraded 81.1% of RB19 and 32.7% of AO7) | [205] |
| Laccase | Bacillus subtilis | Calcium phosphate | Co-encapsulation of laccase and ABTS via biomineralization | High activity recovery, enhanced pH tolerance, improved storage stability | MG dye degradation in wastewater | [206] |
| Tyrosinase | Agaricus bisporus | 3-mercaptopropionic acid modified silver-coated Fe3O4 nanoparticles | EDC/NHS chemistry (covalent) | Increased substrate affinity (1.4x), improved storage stability (68.3% after 84 days), reusability (48.9% after 6 cycles) | Azo dyes (Reactive Green 19, Congo Red), phenolic compounds (phenol, bisphenol F, bisphenol A, p-cresol, phenyl acetate, chlorophenols) | [207] |
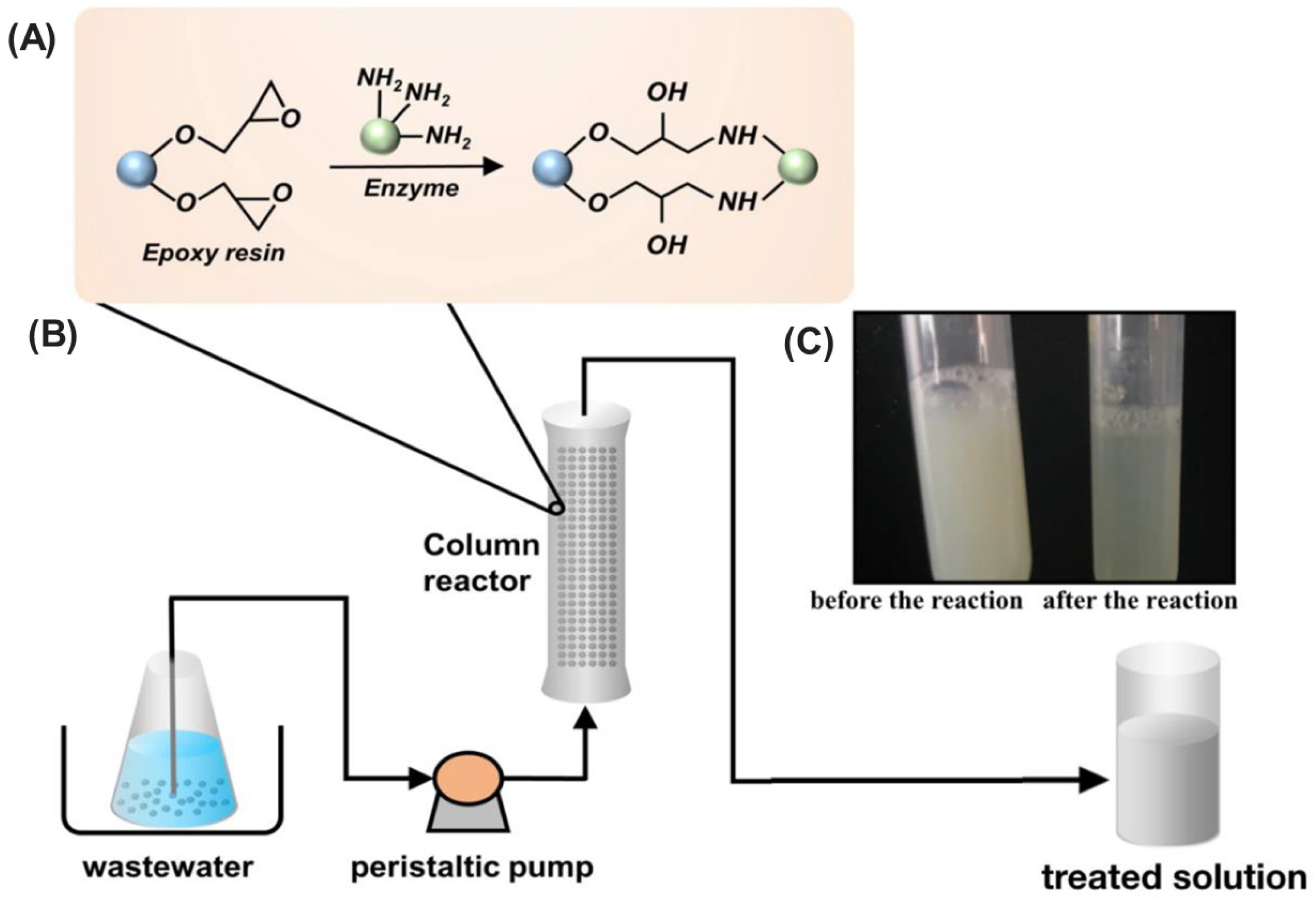
| EstM160K (engineered esterase) | Geobacillus uzenensis | Epoxy resin lx-105s | Covalent bonding | Enhanced thermostability (T½ 36.8 h at 70 °C), reusability | Malathion pesticide: 95.8% at 20 mg/L, Bifenthrin pesticide: 90.4% at 500 mg/L (packed-bed column reactor) | [208] |
| PTE | Sulfolobus solfataricus | Specialized biocatalytic membrane | Covalent immobilization | Maintain activity through cycles with surfactant reloading, 96% paraoxon conversion rate in biocatalytic reactor | Degradation of paraoxon (organophosphate pesticide) | [209] |
| Laccase | Coriollopsis gallica | MSU-F | Physical adsorption | Potentially reduces genotoxicity and apoptotic effects, reduces binding to hormone receptors | Degradation of dichlorophen pesticide | [210] |
| Laccase, Aryl alcohol oxidase, Lignin peroxidase, Manganese peroxidase | Pleurotus ostreatus | Nano-silica particles | Covalent immobilization | Enhanced stability, reusability, wide pH and temperature range (4–9, 20–55 °C) | Complete elimination of p,p′-DDT within 12 h (pH 5, 30 °C) | [211] |
| PETase | Ideonella sakaiensis | Co3(PO4)2 nanostructures | Encapsulation through biomimetic mineralization | Increased enzyme loading, improved stability (broader temperature and pH tolerance), reusability | Bioremediation of PET plastic pollution (by degrading to terephthalic acid) | [212] |
| ELP-tagged cutinase | Synthetic construct, recombinantly produced in E. coli | Biomimetic silica | Self-immobilization via ELPs | Superior loading capacity, activity, and thermal stability | Bioremediation of PET plastic pollution | [213] |
| PET hydrolase | Recombinant E. coli expressed LCC-FDS | Magnetic biochar | Covalent immobilization | Enhanced relative activity, improved reusability | Bioremediation of PET microplastics in soil | [214] |
| Lipase | Candida rugosa | MOFs | Physical adsorption | Enhanced BHET removal efficiency, reusability | Bioremediation of BHET from microplastic pollution in wastewater treatment | [215] |
| Tyrosinase | Bacillus megaterium | Self-assembled biopolymer beads | Genetic immobilization | Degrades various BPA analogues, reduces estrogenic activity, exceptional reusability and stability | Sustainable water treatment for BPA and similar contaminants | [77] |
| Laccase | Trametes versicolor | Multilayer core–shell magnetic mesoporous silica | Physical adsorption | High loading capacity (567 mg/g), improved pH, thermal, and storage stability, easy magnetic recovery and good reusability (58.2% activity after 10 cycles) | Bioremediation of BaP-contaminated sites (high BaP removal efficiency (99.0% within 1 h)) | [216] |
| Lipase | Candida lipolytica | Molecularly imprinted halloysite nanotubes (MIP-HNTs) | Cross-linking | Improved recognition of PAEs, high lipase loading efficiency (76%), good stability and reusability | Degradation of PAE pollutants (specifically DEHP) | [217] |
| Cytochrome P450 BM3 monooxygenase | Bacillus megaterium, recombinantly produced in E. coli | Hollow TiO2-Cu nanospheres (<50 nm) | Physical adsorption | Increased enzyme activity (doubled compared to free enzyme), enhanced stability, high degradation efficiency (95% of isopropanol) | Air pollution remediation (mitigation of isopropanol pollutants) | [218] |
| ToMO and C1,2D enzymes | Pseudomonas S2TR-14 | Micro/nano biochar-chitosan | Physical adsorption and covalent bonding | Enhanced storage stability (>50% activity after 30 days), effective degradation of BTEX in groundwater and soil (over 80% removal at 10 °C) | Biodegradation of BTEX pollutants in cold environments | [219] |
| Halohydrin Dehalogenase | Agrobacterium radiobacter AD1, recombinantly produced in E. coli | Functionalized magnetic biochar | Covalent immobilization | Exceptional storage stability (50% activity after 70 days at 4 °C), organic solvent tolerance, excellent reusability (over 70% activity after 30 cycles), easy separation (magnetic) | Biodegradation of halogenated pollutants | [220] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdelhamid, M.A.A.; Khalifa, H.O.; Yoon, H.J.; Ki, M.-R.; Pack, S.P. Microbial Immobilized Enzyme Biocatalysts for Multipollutant Mitigation: Harnessing Nature’s Toolkit for Environmental Sustainability. Int. J. Mol. Sci. 2024, 25, 8616. https://doi.org/10.3390/ijms25168616
Abdelhamid MAA, Khalifa HO, Yoon HJ, Ki M-R, Pack SP. Microbial Immobilized Enzyme Biocatalysts for Multipollutant Mitigation: Harnessing Nature’s Toolkit for Environmental Sustainability. International Journal of Molecular Sciences. 2024; 25(16):8616. https://doi.org/10.3390/ijms25168616
Chicago/Turabian StyleAbdelhamid, Mohamed A. A., Hazim O. Khalifa, Hyo Jik Yoon, Mi-Ran Ki, and Seung Pil Pack. 2024. "Microbial Immobilized Enzyme Biocatalysts for Multipollutant Mitigation: Harnessing Nature’s Toolkit for Environmental Sustainability" International Journal of Molecular Sciences 25, no. 16: 8616. https://doi.org/10.3390/ijms25168616





