Cerebellar Metabolic Connectivity during Treadmill Walking before and after Unilateral Dopamine Depletion in Rats
Abstract
1. Introduction
2. Results
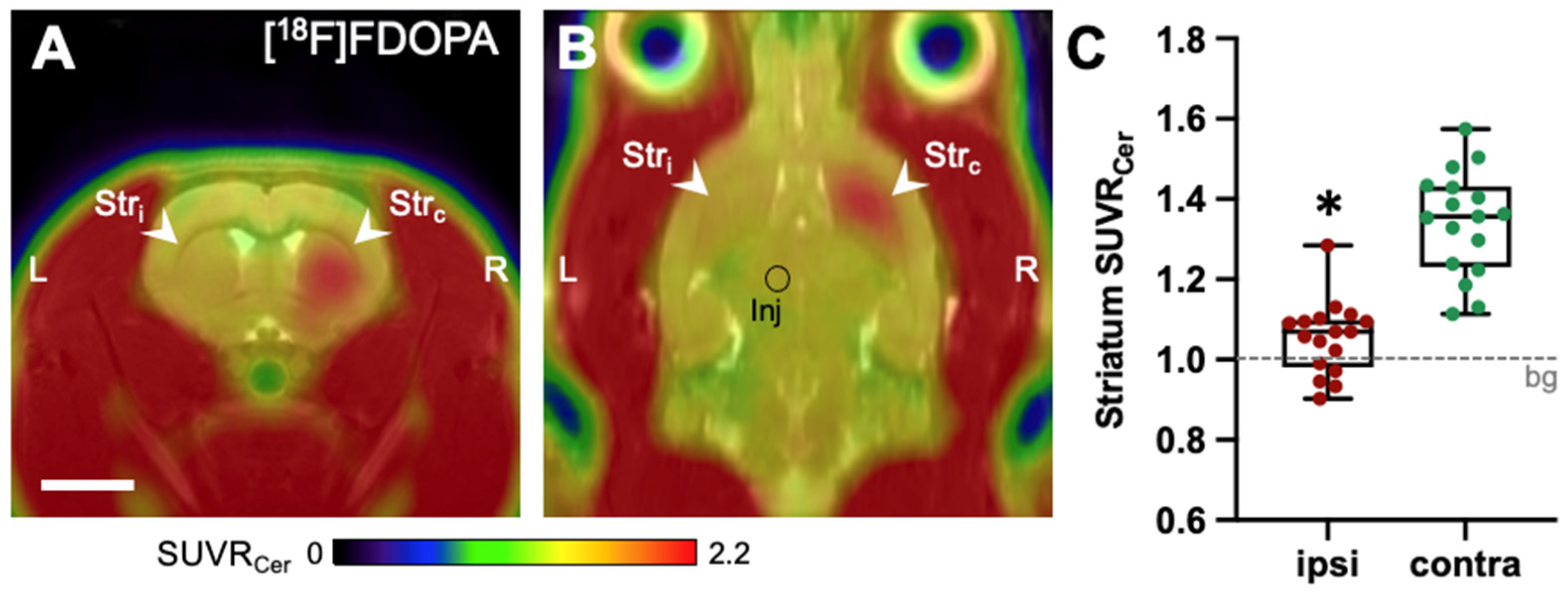
2.1. [18F]FDOPA-PET
2.2. [18F]FDG-PET
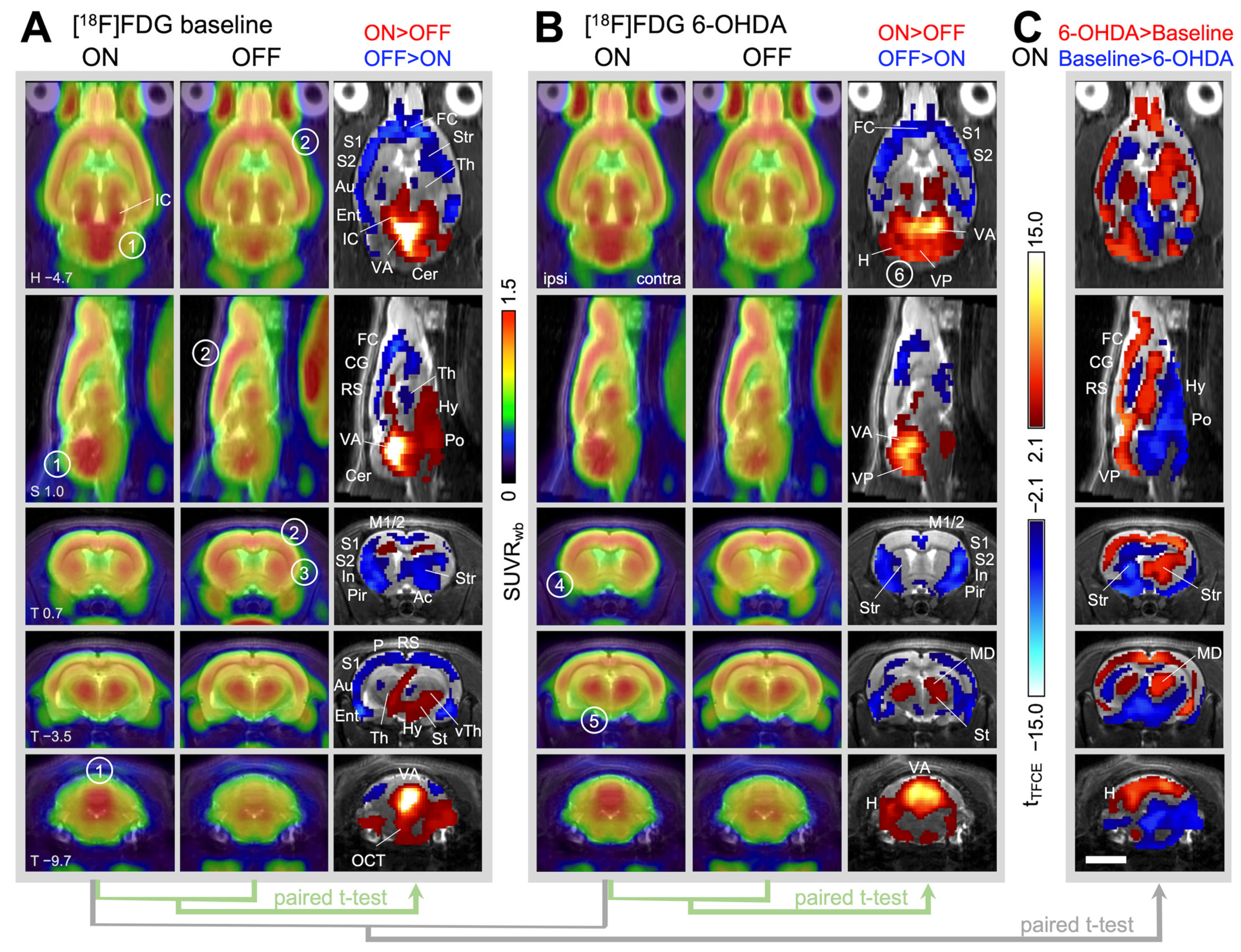
2.2.1. Brain Activity during Treadmill Walking in Healthy Animals (=Baseline)
2.2.2. Changes in Brain Activity during Treadmill Walking after Unilateral 6-OHDA Dopaminergic Lesion
2.2.3. Changes in Brain Activity during Rest after Unilateral 6-OHDA Dopaminergic Lesion
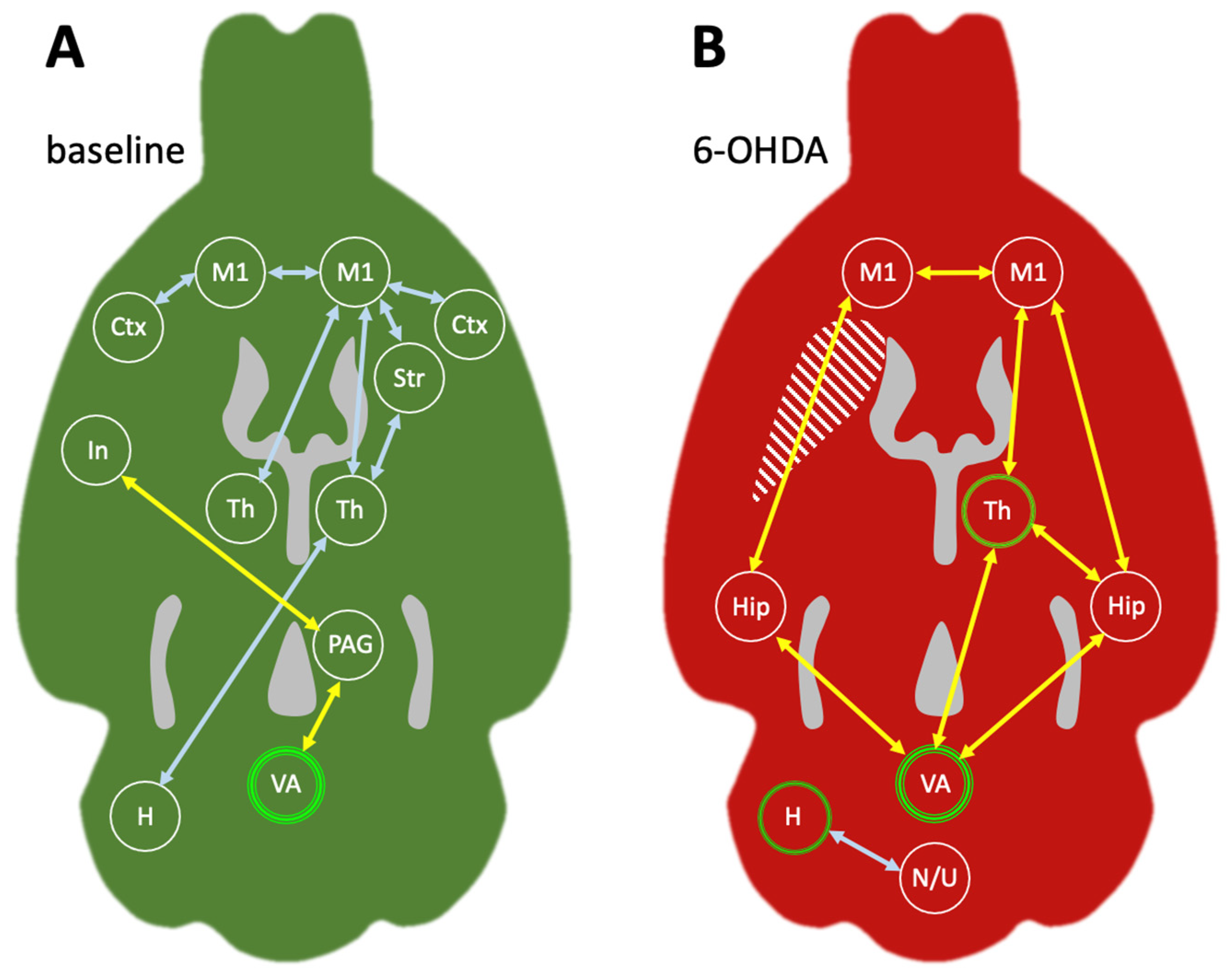
2.2.4. Metabolic Connectivity of the Cerebellum during Treadmill Walking
3. Discussion
3.1. Brain Activity during Treadmill Walking in Healthy Animals
3.2. Brain Activity during Treadmill Walking after Unilateral 6-OHDA Lesion (Chronic Phase)
3.3. Metabolic Connectivity of the Cerebellar Vermis during Treadmill Walking
3.4. Metabolic Connectivity of the Cerebellar Hemisphere during Treadmill Walking
3.5. Effects of Aging
4. Materials and Methods
4.1. Experimental Design
4.2. Animals
4.3. Baseline Treadmill Training
4.4. Surgery
4.5. Treadmill Training after 6-OHDA Lesion
4.6. PET Measurements
4.7. Image Reconstruction and Postprocessing
4.8. Image Statistics
4.9. Metabolic Connectivity Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hawkes, C.H. The prodromal phase of sporadic Parkinson’s disease: Does it exist and if so how long is it? Mov. Disord. 2008, 23, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Gregory, S.; Long, J.D.; Tabrizi, S.J.; Rees, G. Measuring compensation in neurodegeneration using MRI. Curr. Opin. Neurol. 2017, 30, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Sanjari Moghaddam, H.; Dolatshahi, M.; Mohebi, F.; Aarabi, M.H. Structural white matter alterations as compensatory mechanisms in Parkinson’s disease: A systematic review of diffusion tensor imaging studies. J. Neurosci. Res. 2020, 98, 1398–1416. [Google Scholar] [CrossRef] [PubMed]
- Thies, T.; Mucke, D.; Geerts, N.; Seger, A.; Fink, G.R.; Barbe, M.T.; Sommerauer, M. Compensatory articulatory mechanisms preserve intelligibility in prodromal Parkinson’s disease. Park. Relat. Disord. 2023, 112, 105487. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Trigo-Damas, I.; Dileone, M.; Del Rey, N.L.; Hernandez, L.F.; Obeso, J.A. Compensatory mechanisms in Parkinson’s disease: Circuits adaptations and role in disease modification. Exp. Neurol. 2017, 298, 148–161. [Google Scholar] [CrossRef] [PubMed]
- Tessitore, A.; Cirillo, M.; De Micco, R. Functional connectivity signatures of parkinson’s disease. J. Park. Dis. 2019, 9, 637–652. [Google Scholar] [CrossRef]
- Song, W.; Raza, H.K.; Lu, L.; Zhang, Z.; Zu, J.; Zhang, W.; Dong, L.; Xu, C.; Gong, X.; Lv, B.; et al. Functional MRI in Parkinson’s disease with freezing of gait: A systematic review of the literature. Neurol. Sci. 2021, 42, 1759–1771. [Google Scholar] [CrossRef]
- Wüllner, U.; Borghammer, P.; Choe, C.U.; Csoti, I.; Falkenburger, B.; Gasser, T.; Lingor, P.; Riederer, P. The heterogeneity of Parkinson’s disease. J. Neural Transm. 2023, 130, 827–838. [Google Scholar] [CrossRef]
- Shine, J.M.; Matar, E.; Bolitho, S.J.; Dilda, V.; Morris, T.R.; Naismith, S.L.; Moore, S.T.; Lewis, S.J. Modeling freezing of gait in Parkinson’s disease with a virtual reality paradigm. Gait Posture 2013, 38, 104–108. [Google Scholar] [CrossRef]
- Snijders, A.H.; Leunissen, I.; Bakker, M.; Overeem, S.; Helmich, R.C.; Bloem, B.R.; Toni, I. Gait-related cerebral alterations in patients with Parkinson’s disease with freezing of gait. Brain 2011, 134, 59–72. [Google Scholar] [CrossRef]
- Fernandez-Del-Olmo, M.; Sanchez-Molina, J.A.; Novo-Ponte, S.; Fogelson, N. Directed connectivity in Parkinson’s disease patients during over-ground and treadmill walking. Exp. Gerontol. 2023, 178, 112220. [Google Scholar] [CrossRef]
- Metz, G.A.; Tse, A.; Ballermann, M.; Smith, L.K.; Fouad, K. The unilateral 6-OHDA rat model of Parkinson’s disease revisited: An electromyographic and behavioural analysis. Eur. J. Neurosci. 2005, 22, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Deumens, R.; Blokland, A.; Prickaerts, J. Modeling Parkinson’s disease in rats: An evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp. Neurol. 2002, 175, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Kordys, E.; Apetz, N.; Schneider, K.; Duncan, E.; Buschbell, B.; Rohleder, C.; Sue, M.; Drzezga, A.; Neumaier, B.; Timmermann, L.; et al. Motor impairment and compensation in a hemiparkinsonian rat model: Correlation between dopamine depletion severity, cerebral metabolism and gait patterns. EJNMMI Res. 2017, 7, 68. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.J.; Tan, E.K.; Chao, Y.X. Historical Perspective: Models of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 2464. [Google Scholar] [CrossRef] [PubMed]
- Dovonou, A.; Bolduc, C.; Soto Linan, V.; Gora, C.; Peralta Iii, M.R.; Levesque, M. Animal models of Parkinson’s disease: Bridging the gap between disease hallmarks and research questions. Transl. Neurodegener. 2023, 12, 36. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, B.M.; Fowler, J.S.; Gutterson, N.I.; MacGregor, R.R.; Wan, C.N.; Wolf, A.P. Metabolic trapping as a principle of oradiopharmaceutical design: Some factors resposible for the biodistribution of [18F] 2-deoxy-2-fluoro-D-glucose. J. Nucl. Med. 1978, 19, 1154–1161. [Google Scholar]
- Larson, S.M. Gallagher’s Principle of Metabolic Trapping. J. Nucl. Med. 2020, 61, 74S–76S. [Google Scholar] [CrossRef]
- Riedl, V.; Utz, L.; Castrillon, G.; Grimmer, T.; Rauschecker, J.P.; Ploner, M.; Friston, K.J.; Drzezga, A.; Sorg, C. Metabolic connectivity mapping reveals effective connectivity in the resting human brain. Proc. Natl. Acad. Sci. USA 2016, 113, 428–433. [Google Scholar] [CrossRef]
- Yakushev, I.; Drzezga, A.; Habeck, C. Metabolic connectivity: Methods and applications. Curr. Opin. Neurol. 2017, 30, 677–685. [Google Scholar] [CrossRef]
- Apetz, N.; Kordys, E.; Simon, M.; Mang, B.; Aswendt, M.; Wiedermann, D.; Neumaier, B.; Drzezga, A.; Timmermann, L.; Endepols, H. Effects of subthalamic deep brain stimulation on striatal metabolic connectivity in a rat hemiparkinsonian model. Dis. Model. Mech. 2019, 12, dmm039065. [Google Scholar] [CrossRef]
- Huo, B.B.; Zheng, M.X.; Hua, X.Y.; Shen, J.; Wu, J.J.; Xu, J.G. Metabolic brain network analysis with 18F-FDG PET in a rat model of neuropathic pain. Front. Neurol. 2021, 12, 566119. [Google Scholar] [CrossRef]
- Rohleder, C.; Wiedermann, D.; Neumaier, B.; Drzezga, A.; Timmermann, L.; Graf, R.; Leweke, F.M.; Endepols, H. The functional networks of prepulse inhibition: Neuronal connectivity analysis based on FDG-PET in awake and unrestrained rats. Front. Behav. Neurosci. 2016, 10, 148. [Google Scholar] [CrossRef]
- Verger, A.; Klesse, E.; Chawki, M.B.; Witjas, T.; Azulay, J.P.; Eusebio, A.; Guedj, E. Brain PET substrate of impulse control disorders in Parkinson’s disease: A metabolic connectivity study. Hum. Brain Mapp. 2018, 39, 3178–3186. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.T.; Lu, J.Y.; Sun, Y.M.; Li, L.; Yang, Y.J.; Zhao, J.; Ge, J.J.; Wu, P.; Jiang, J.H.; Wu, J.J.; et al. Dopaminergic dysfunction and glucose metabolism characteristics in parkin-induced early-onset Parkinson’s disease compared to genetically undetermined early-onset Parkinson’s disease. Phenomics 2023, 3, 22–33. [Google Scholar] [CrossRef]
- Zang, Z.; Song, T.; Li, J.; Nie, B.; Mei, S.; Zhang, C.; Wu, T.; Zhang, Y.; Lu, J. Simultaneous PET/fMRI revealed increased motor area input to subthalamic nucleus in Parkinson’s disease. Cereb. Cortex 2023, 33, 167–175. [Google Scholar] [CrossRef]
- Boccalini, C.; Carli, G.; Pilotto, A.; Padovani, A.; Perani, D. Gender-related vulnerability of dopaminergic neural networks in parkinson’s disease. Brain Connect. 2021, 11, 3–11. [Google Scholar] [CrossRef]
- Vo, A.; Schindlbeck, K.A.; Nguyen, N.; Rommal, A.; Spetsieris, P.G.; Tang, C.C.; Choi, Y.Y.; Niethammer, M.; Dhawan, V.; Eidelberg, D. Adaptive and pathological connectivity responses in Parkinson’s disease brain networks. Cereb. Cortex 2023, 33, 917–932. [Google Scholar] [CrossRef]
- Im, H.J.; Hahm, J.; Kang, H.; Choi, H.; Lee, H.; Hwang do, W.; Kim, E.E.; Chung, J.K.; Lee, D.S. Disrupted brain metabolic connectivity in a 6-OHDA-induced mouse model of Parkinson’s disease examined using persistent homology-based analysis. Sci. Rep. 2016, 6, 33875. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hallett, M. The cerebellum in Parkinson’s disease. Brain 2013, 136, 696–709. [Google Scholar] [CrossRef] [PubMed]
- Kyono, K.; Takashima, T.; Katayama, Y.; Kawasaki, T.; Zochi, R.; Gouda, M.; Kuwahara, Y.; Takahashi, K.; Wada, Y.; Onoe, H.; et al. Use of [18F]FDOPA-PET for in vivo evaluation of dopaminergic dysfunction in unilaterally 6-OHDA-lesioned rats. EJNMMI Res. 2011, 1, 25. [Google Scholar] [CrossRef]
- Morbelli, S.; Esposito, G.; Arbizu, J.; Barthel, H.; Boellaard, R.; Bohnen, N.I.; Brooks, D.J.; Darcourt, J.; Dickson, J.C.; Douglas, D.; et al. EANM practice guideline/SNMMI procedure standard for dopaminergic imaging in Parkinsonian syndromes 1.0. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1885–1912. [Google Scholar] [CrossRef]
- Raichle, M.E.; Mintun, M.A. Brain work and brain imaging. Annu. Rev. Neurosci. 2006, 29, 449–476. [Google Scholar] [CrossRef]
- Smith, S.M.; Nichols, T.E. Threshold-free cluster enhancement: Addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 2009, 44, 83–98. [Google Scholar] [CrossRef]
- Yang, J.; Sadler, T.R.; Givrad, T.K.; Maarek, J.M.; Holschneider, D.P. Changes in brain functional activation during resting and locomotor states after unilateral nigrostriatal damage in rats. Neuroimage 2007, 36, 755–773. [Google Scholar] [CrossRef][Green Version]
- Holschneider, D.P.; Maarek, J.M. Brain maps on the go: Functional imaging during motor challenge in animals. Methods 2008, 45, 255–261. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.; Myers, K.G.; Guo, Y.; Ocampo, M.A.; Pang, R.D.; Jakowec, M.W.; Holschneider, D.P. Functional reorganization of motor and limbic circuits after exercise training in a rat model of bilateral parkinsonism. PLoS ONE 2013, 8, e80058. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, Y.; Myers, K.G.; Heintz, R.; Holschneider, D.P. Recruitment of the prefrontal cortex and cerebellum in Parkinsonian rats following skilled aerobic exercise. Neurobiol. Dis. 2015, 77, 71–87. [Google Scholar] [CrossRef]
- Hamacher, D.; Herold, F.; Wiegel, P.; Hamacher, D.; Schega, L. Brain activity during walking: A systematic review. Neurosci. Biobehav. Rev. 2015, 57, 310–327. [Google Scholar] [CrossRef]
- Takakusaki, K. Functional neuroanatomy for posture and gait control. J. Mov. Disord. 2017, 10, 1–17. [Google Scholar] [CrossRef]
- Pernia-Andrade, A.J.; Wenger, N.; Esposito, M.S.; Tovote, P. Circuits for state-dependent modulation of locomotion. Front. Hum. Neurosci. 2021, 15, 745689. [Google Scholar] [CrossRef]
- Takakusaki, K.; Tomita, N.; Yano, M. Substrates for normal gait and pathophysiology of gait disturbances with respect to the basal ganglia dysfunction. J. Neurol. 2008, 255 (Suppl. S4), 19–29. [Google Scholar] [CrossRef]
- Pisotta, I.; Molinari, M. Cerebellar contribution to feedforward control of locomotion. Front. Hum. Neurosci. 2014, 8, 475. [Google Scholar] [CrossRef]
- D’Angelo, E. Physiology of the cerebellum. Handb. Clin. Neurol. 2018, 154, 85–108. [Google Scholar] [CrossRef]
- Ramnani, N. Automatic and controlled processing in the corticocerebellar system. Prog. Brain Res. 2014, 210, 255–285. [Google Scholar] [CrossRef]
- Schweighofer, N.; Lang, E.J.; Kawato, M. Role of the olivo-cerebellar complex in motor learning and control. Front. Neural Circuits 2013, 7, 94. [Google Scholar] [CrossRef]
- Whelan, P.J. Control of locomotion in the decerebrate cat. Prog. Neurobiol. 1996, 49, 481–515. [Google Scholar] [CrossRef]
- Kiehn, O.; Dougherty, K. Locomotion: Circuits and physiology. In Neuroscience in the 21st Century; Pfaff, D., Volkow, N., Eds.; Springer: New York, NY, USA, 2016; pp. 1337–1365. [Google Scholar]
- Scott, S.H. Inconvenient truths about neural processing in primary motor cortex. J. Physiol. 2008, 586, 1217–1224. [Google Scholar] [CrossRef]
- Suzuki, L.Y.; Meehan, S.K. Attention focus modulates afferent input to motor cortex during skilled action. Hum. Mov. Sci. 2020, 74, 102716. [Google Scholar] [CrossRef]
- Lanciego, J.L.; Luquin, N.; Obeso, J.A. Functional neuroanatomy of the basal ganglia. Cold Spring Harb. Perspect. Med. 2012, 2, a009621. [Google Scholar] [CrossRef]
- Yu, H.; Sternad, D.; Corcos, D.M.; Vaillancourt, D.E. Role of hyperactive cerebellum and motor cortex in Parkinson’s disease. Neuroimage 2007, 35, 222–233. [Google Scholar] [CrossRef]
- Wu, T.; Hallett, M. A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain 2005, 128, 2250–2259. [Google Scholar] [CrossRef]
- Tinaz, S.; Para, K.; Vives-Rodriguez, A.; Martinez-Kaigi, V.; Nalamada, K.; Sezgin, M.; Scheinost, D.; Hampson, M.; Louis, E.D.; Constable, R.T. Insula as the interface between body awareness and movement: A neurofeedback-guided kinesthetic motor imagery study in Parkinson’s disease. Front. Hum. Neurosci. 2018, 12, 496. [Google Scholar] [CrossRef]
- Jia, Z.; Yu, S.; Tang, W.; Zhao, D. Altered functional connectivity of the insula in a rat model of recurrent headache. Mol. Pain. 2020, 16, 1744806920922115. [Google Scholar] [CrossRef]
- Gogolla, N. The insular cortex. Curr. Biol. 2017, 27, R580–R586. [Google Scholar] [CrossRef] [PubMed]
- Dionisio, S.; Mayoglou, L.; Cho, S.M.; Prime, D.; Flanigan, P.M.; Lega, B.; Mosher, J.; Leahy, R.; Gonzalez-Martinez, J.; Nair, D. Connectivity of the human insula: A cortico-cortical evoked potential (CCEP) study. Cortex 2019, 120, 419–442. [Google Scholar] [CrossRef] [PubMed]
- Noga, B.R.; Whelan, P.J. The mesencephalic locomotor region: Beyond locomotor control. Front. Neural Circuits 2022, 16, 884785. [Google Scholar] [CrossRef]
- Vaaga, C.E.; Brown, S.T.; Raman, I.M. Cerebellar modulation of synaptic input to freezing-related neurons in the periaqueductal gray. eLife 2020, 9, e54302. [Google Scholar] [CrossRef] [PubMed]
- De Benedictis, A.; Rossi-Espagnet, M.C.; de Palma, L.; Carai, A.; Marras, C.E. Networking of the human cerebellum: From anatomo-functional development to neurosurgical implications. Front. Neurol. 2022, 13, 806298. [Google Scholar] [CrossRef]
- Manto, M.; Bower, J.M.; Conforto, A.B.; Delgado-Garcia, J.M.; da Guarda, S.N.; Gerwig, M.; Habas, C.; Hagura, N.; Ivry, R.B.; Marien, P.; et al. Consensus paper: Roles of the cerebellum in motor control--the diversity of ideas on cerebellar involvement in movement. Cerebellum 2012, 11, 457–487. [Google Scholar] [CrossRef]
- Pedersen, M.; Zalesky, A.; Omidvarnia, A.; Jackson, G.D. Multilayer network switching rate predicts brain performance. Proc. Natl. Acad. Sci. USA 2018, 115, 13376–13381. [Google Scholar] [CrossRef]
- Coffman, K.A.; Dum, R.P.; Strick, P.L. Cerebellar vermis is a target of projections from the motor areas in the cerebral cortex. Proc. Natl. Acad. Sci. USA 2011, 108, 16068–16073. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.M.; Dahmen, J.C.; Muir, D.R.; Imhof, F.; Martini, F.J.; Hofer, S.B. Thalamic nuclei convey diverse contextual information to layer 1 of visual cortex. Nat. Neurosci. 2016, 19, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Kraus, B.J.; Robinson, R.J., 2nd; White, J.A.; Eichenbaum, H.; Hasselmo, M.E. Hippocampal “time cells”: Time versus path integration. Neuron 2013, 78, 1090–1101. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Krook-Magnuson, E. Cognitive Collaborations: Bidirectional Functional Connectivity Between the Cerebellum and the Hippocampus. Front. Syst. Neurosci. 2015, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Voermans, N.C.; Petersson, K.M.; Daudey, L.; Weber, B.; Van Spaendonck, K.P.; Kremer, H.P.; Fernandez, G. Interaction between the human hippocampus and the caudate nucleus during route recognition. Neuron 2004, 43, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, M.; Yokofujita, J.; Murakami, K. An ultrastructural study of the neural circuit between the prefrontal cortex and the mediodorsal nucleus of the thalamus. Prog. Neurobiol. 1998, 54, 417–458. [Google Scholar] [CrossRef] [PubMed]
- Saalmann, Y.B. Intralaminar and medial thalamic influence on cortical synchrony, information transmission and cognition. Front. Syst. Neurosci. 2014, 8, 83. [Google Scholar] [CrossRef] [PubMed]
- Stokes, K.A.; Best, P.J. Mediodorsal thalamic lesions impair radial maze performance in the rat. Behav. Neurosci. 1988, 102, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, F.; Marchand, A.R.; Courtand, G.; Coutureau, E.; Wolff, M. Parallel inputs from the mediodorsal thalamus to the prefrontal cortex in the rat. Eur. J. Neurosci. 2016, 44, 1972–1986. [Google Scholar] [CrossRef]
- Georgescu, I.A.; Popa, D.; Zagrean, L. The anatomical and functional heterogeneity of the mediodorsal thalamus. Brain Sci. 2020, 10, 624. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.; Spruston, N.; Bloss, E.B. Hippocampal and thalamic afferents form distinct synaptic microcircuits in the mouse infralimbic frontal cortex. Cell Rep. 2021, 37, 109837. [Google Scholar] [CrossRef] [PubMed]
- Aumann, T.D.; Rawson, J.A.; Finkelstein, D.I.; Horne, M.K. Projections from the lateral and interposed cerebellar nuclei to the thalamus of the rat: A light and electron microscopic study using single and double anterograde labelling. J. Comp. Neurol. 1994, 349, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, N.; Mori, F.; Shoumura, K. A di-synaptic projection from the lateral cerebellar nucleus to the laterodorsal part of the striatum via the central lateral nucleus of the thalamus in the rat. Brain Res. 2000, 880, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Novello, M.; Bosman, L.W.J.; De Zeeuw, C.I. A systematic review of direct outputs from the cerebellum to the brainstem and diencephalon in mammals. Cerebellum 2022, 23, 210–239. [Google Scholar] [CrossRef] [PubMed]
- McAfee, S.S.; Liu, Y.; Sillitoe, R.V.; Heck, D.H. Cerebellar coordination of neuronal communication in cerebral cortex. Front. Syst. Neurosci. 2021, 15, 781527. [Google Scholar] [CrossRef] [PubMed]
- Sharp, F.R.; Gonzalez, M.F. Multiple vibrissae sensory regions in rat cerebellum: A (14C) 2-deoxyglucose study. J. Comp. Neurol. 1985, 234, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Odeh, F.; Ackerley, R.; Bjaalie, J.G.; Apps, R. Pontine maps linking somatosensory and cerebellar cortices are in register with climbing fiber somatotopy. J. Neurosci. 2005, 25, 5680–5690. [Google Scholar] [CrossRef] [PubMed]
- Bubic, A.; von Cramon, D.Y.; Jacobsen, T.; Schroger, E.; Schubotz, R.I. Violation of expectation: Neural correlates reflect bases of prediction. J. Cogn. Neurosci. 2009, 21, 155–168. [Google Scholar] [CrossRef]
- Voogd, J.; Gerrits, N.M.; Ruigrok, T.J. Organization of the vestibulocerebellum. Ann. N. Y. Acad. Sci. 1996, 781, 553–579. [Google Scholar] [CrossRef]
- Kheradmand, A.; Zee, D.S. Cerebellum and ocular motor control. Front. Neurol. 2011, 2, 53. [Google Scholar] [CrossRef]
- Ioffe, M.E. Cerebellar control of posture. In Handbook of the Cerebellum and Cerebellar Disorders; Manto, M., Schmahmann, J.D., Rossi, F., Gruol, D.L., Koibuchi, N., Eds.; Springer: Dordrecht, The Netherlands, 2013; pp. 1221–1240. [Google Scholar]
- Laurens, J. The otolith vermis: A systems neuroscience theory of the Nodulus and Uvula. Front. Syst. Neurosci. 2022, 16, 886284. [Google Scholar] [CrossRef]
- Arata, A.; Ito, M. Purkinje cell functions in the in vitro cerebellum isolated from neonatal rats in a block with the pons and medulla. Neurosci. Res. 2004, 50, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Kebschull, J.M.; Richman, E.B.; Ringach, N.; Friedmann, D.; Albarran, E.; Kolluru, S.S.; Jones, R.C.; Allen, W.E.; Wang, Y.; Cho, S.W.; et al. Cerebellar nuclei evolved by repeatedly duplicating a conserved cell-type set. Science 2020, 370, eabd5059. [Google Scholar] [CrossRef] [PubMed]
- Musienko, P.E.; Deliagina, T.G.; Gerasimenko, Y.P.; Orlovsky, G.N.; Zelenin, P.V. Limb and trunk mechanisms for balance control during locomotion in quadrupeds. J. Neurosci. 2014, 34, 5704–5716. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, R. Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol. Aging 2002, 17, 85–100. [Google Scholar] [CrossRef]
- Przybyla, A.; Haaland, K.Y.; Bagesteiro, L.B.; Sainburg, R.L. Motor asymmetry reduction in older adults. Neurosci. Lett. 2011, 489, 99–104. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, X.; Li, Q.; Wang, Z.; Gu, X.; Zang, J.; Ge, R.; Liu, H.; Fan, Z.; Bu, L. The effects of age on brain cortical activation and functional connectivity during video game-based finger-to-thumb opposition movement: A functional near-infrared spectroscopy study. Neurosci. Lett. 2021, 746, 135668. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, T.S.; King, B.R.; Zivari Adab, H.; Mantini, D.; Swinnen, S.P. Age-related differences in network flexibility and segregation at rest and during motor performance. Neuroimage 2019, 194, 93–104. [Google Scholar] [CrossRef]
- Hoffstaedter, F.; Grefkes, C.; Roski, C.; Caspers, S.; Zilles, K.; Eickhoff, S.B. Age-related decrease of functional connectivity additional to gray matter atrophy in a network for movement initiation. Brain Struct. Funct. 2015, 220, 999–1012. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, Y.S.; Lee, J.J.; Song, H.J.; Yoo, D.S.; Lee, H.J.; Kim, H.J.; Chang, Y. Functional magnetic resonance imaging reveals age-related alterations to motor networks in weighted elbow flexion-extension movement. Neurol. Res. 2010, 32, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Straathof, M.; Sinke, M.R.T.; van der Toorn, A.; Weerheim, P.L.; Otte, W.M.; Dijkhuizen, R.M. Differences in structural and functional networks between young adult and aged rat brains before and after stroke lesion simulations. Neurobiol. Dis. 2019, 126, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Wu, J.J.; Huo, B.B.; Xing, X.X.; Ma, J.; Li, Y.L.; Zheng, M.X.; Hua, X.Y.; Xu, J.G. Age-related alterations of brain metabolic network based on [18F]FDG-PET of rats. Aging 2022, 14, 923–942. [Google Scholar] [CrossRef] [PubMed]
- Colon-Perez, L.M.; Turner, S.M.; Lubke, K.N.; Pompilus, M.; Febo, M.; Burke, S.N. Multiscale Imaging Reveals Aberrant Functional Connectome Organization and Elevated Dorsal Striatal Arc Expression in Advanced Age. eNeuro 2019, 6, ENEURO.0047-19.2019. [Google Scholar] [CrossRef] [PubMed]
- Borzykh, A.A.; Gaynullina, D.K.; Shvetsova, A.A.; Kiryukhina, O.O.; Kuzmin, I.V.; Selivanova, E.K.; Nesterenko, A.M.; Vinogradova, O.L.; Tarasova, O.S. Voluntary wheel exercise training affects locomotor muscle, but not the diaphragm in the rat. Front. Physiol. 2022, 13, 1003073. [Google Scholar] [CrossRef] [PubMed]
- Fadaei Chafy, M.R.; Bagherpour Tabalvandani, M.M.; Elmieh, A.; Arabzadeh, E. Determining the range of aerobic exercise on a treadmill for male Wistar rats at different ages: A pilot study. J. Exerc. Organ Cross Talk 2022, 2, 96–100. [Google Scholar] [CrossRef]
- Real, C.C.; Doorduin, J.; Kopschina Feltes, P.; Vallez Garcia, D.; de Paula Faria, D.; Britto, L.R.; de Vries, E.F. Evaluation of exercise-induced modulation of glial activation and dopaminergic damage in a rat model of Parkinson’s disease using [(11)C]PBR28 and [(18)F]FDOPA PET. J. Cereb. Blood Flow Metab. 2019, 39, 989–1004. [Google Scholar] [CrossRef] [PubMed]
- Snow, B.J.; Tooyama, I.; McGeer, E.G.; Yamada, T.; Calne, D.B.; Takahashi, H.; Kimura, H. Human positron emission tomographic [18F]fluorodopa studies correlate with dopamine cell counts and levels. Ann. Neurol. 1993, 34, 324–330. [Google Scholar] [CrossRef]
- Pate, B.D.; Kawamata, T.; Yamada, T.; McGeer, E.G.; Hewitt, K.A.; Snow, B.J.; Ruth, T.J.; Calne, D.B. Correlation of striatal fluorodopa uptake in the MPTP monkey with dopaminergic indices. Ann. Neurol. 1993, 34, 331–338. [Google Scholar] [CrossRef]
- Panagopoulos, N.T.; Papadopoulos, G.C.; Matsokis, N.A. Dopaminergic innervation and binding in the rat cerebellum. Neurosci. Lett. 1991, 130, 208–212. [Google Scholar] [CrossRef]
- Raichle, M.E. Neuroscience. The brain’s dark energy. Science 2006, 314, 1249–1250. [Google Scholar] [CrossRef]
- Friston, K.J. Functional and effective connectivity: A review. Brain Connect. 2011, 1, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, K.; Krishnamurthy, V.; Cabanban, R.; Crosson, B.A. Hubs of anticorrelation in high-resolution resting-state functional connectivity network architecture. Brain Connect. 2015, 5, 267–275. [Google Scholar] [CrossRef] [PubMed]
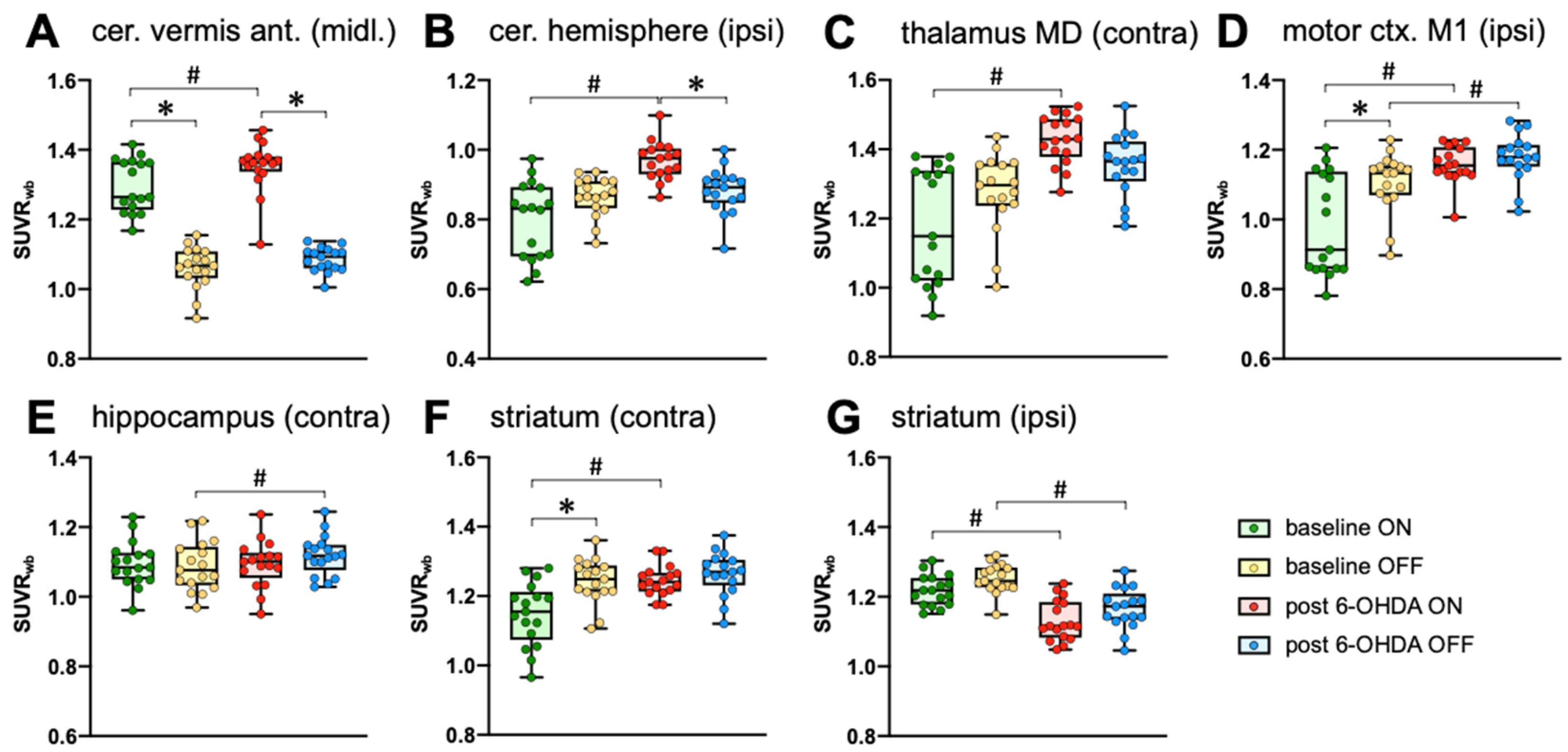

| [18F]FDG SUVRwb | Cerebellum VA Midline | Cerebellum Hem. Ipsi | Motor Cortex M1 Ipsi | Hippocampus Contra | Striatum Ipsi | Striatum Contra | Thalamus MD Contra |
|---|---|---|---|---|---|---|---|
| Baseline ON | 1.30 ± 0.08 | 0.80 ± 0.11 | 0.98 ± 0.14 | 1.09 ± 0.07 | 1.22 ± 0.04 | 1.15 ± 0.09 | 1.18 ± 0.17 |
| Baseline OFF | 1.06 ± 0.06 | 0.87 ± 0.06 | 1.11 ± 0.09 | 1.09 ± 0.07 | 1.25 ± 0.04 | 1.24 ± 0.06 | 1.27 ± 0.11 |
| post 6-OHDA ON | 1.35 ± 0.07 | 0.97 ± 0.06 | 1.16 ± 0.05 | 1.09 ± 0.07 | 1.13 ± 0.06 | 1.24 ± 0.04 | 1.43 ± 0.07 |
| post 6-OHDA OFF | 1.08 ± 0.03 | 0.88 ± 0.06 | 1.18 ± 0.07 | 1.12 ± 0.06 | 1.17 ± 0.06 | 1.26 ± 0.06 | 1.35 ± 0.09 |
| main effect treadmill | F(1,16) = 268.2 p < 0.0001 | F(1,16) = 0.25 p = 0.6262 | F(1,16) = 17.9 p = 0.0006 | F(1,16) = 1.1 p = 0.3017 | F(1,16) = 17.4 p = 0.0007 | F(1,16) = 23.0 p = 0.0002 | F(1,16) = 0.4 p = 0.5226 |
| main effect lesion | F(1,16) = 6.0 p = 0.0257 | F(1,16) = 26.7 p < 0.0001 | F(1,16) = 31.1 p < 0.0001 | F(1,16) = 2.3 p = 0.1478 | F(1,16) = 35.9 p < 0.0001 | F(1,16) = 22.3 p = 0.0002 | F(1,16) = 30.6 p < 0.0001 |
| factor interaction | F(1,16) = 1.5 p = 0.2367 | F(1,16) = 15.4 p = 0.0012 | F(1,16) = 10.0 p = 0.0061 | F(1,16) = 5.2 p = 0.0373 | F(1,16) = 0.005 p = 0.9464 | F(1,16) = 5.5 p = 0.0327 | F(1,16) = 9.8 p = 0.0065 |
| Baseline ON vs. Baseline OFF | d = 3.41 p < 0.0001 | d = 0.78 p = 0.1071 | d = 1.04 p = 0.0004 | d = 0.11 p = 0.8944 | d = 0.81 p = 0.0511 | d = 1.21 p < 0.0001 | d = 0.67 p = 0.0956 |
| 6-OHDA ON vs. 6-OHDA OFF | d = 4.66 p < 0.0001 | d = 0.49 p = 0.0320 | d = 0.33 p = 0.8171 | d = 0.41 p = 0.0964 | d = 0.61 p = 0.0425 | d = 0.37 p = 0.7284 | d = 0.90 p = 0.2625 |
| Baseline ON vs. 6-OHDA ON | d = 0.74 p = 0.0482 | d = 0.92 p < 0.0001 | d = 1.60 p < 0.0001 | d = 0.02 p = 0.9994 | d = 1.69 p < 0.0001 | d = 1.34 p < 0.0001 | d = 1.92 p < 0.0001 |
| Baseline OFF vs. 6-OHDA OFF | d = 0.47 p = 0.7744 | d = 0.28 p = 0.9245 | d = 0.92 p = 0.0346 | d = 0.49 p = 0.0319 | d = 1.72 p < 0.0001 | d = 0.32 p = 0.7106 | d = 0.76 p = 0.2097 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endepols, H.; Apetz, N.; Vieth, L.; Lesser, C.; Schulte-Holtey, L.; Neumaier, B.; Drzezga, A. Cerebellar Metabolic Connectivity during Treadmill Walking before and after Unilateral Dopamine Depletion in Rats. Int. J. Mol. Sci. 2024, 25, 8617. https://doi.org/10.3390/ijms25168617
Endepols H, Apetz N, Vieth L, Lesser C, Schulte-Holtey L, Neumaier B, Drzezga A. Cerebellar Metabolic Connectivity during Treadmill Walking before and after Unilateral Dopamine Depletion in Rats. International Journal of Molecular Sciences. 2024; 25(16):8617. https://doi.org/10.3390/ijms25168617
Chicago/Turabian StyleEndepols, Heike, Nadine Apetz, Lukas Vieth, Christoph Lesser, Léon Schulte-Holtey, Bernd Neumaier, and Alexander Drzezga. 2024. "Cerebellar Metabolic Connectivity during Treadmill Walking before and after Unilateral Dopamine Depletion in Rats" International Journal of Molecular Sciences 25, no. 16: 8617. https://doi.org/10.3390/ijms25168617
APA StyleEndepols, H., Apetz, N., Vieth, L., Lesser, C., Schulte-Holtey, L., Neumaier, B., & Drzezga, A. (2024). Cerebellar Metabolic Connectivity during Treadmill Walking before and after Unilateral Dopamine Depletion in Rats. International Journal of Molecular Sciences, 25(16), 8617. https://doi.org/10.3390/ijms25168617







