The Effect of Phototherapy on Systemic Inflammation Measured with Serum Vitamin D-Binding Protein and hsCRP in Patients with Inflammatory Skin Disease
Abstract
1. Introduction
2. Results
2.1. Demographics and Baseline Data
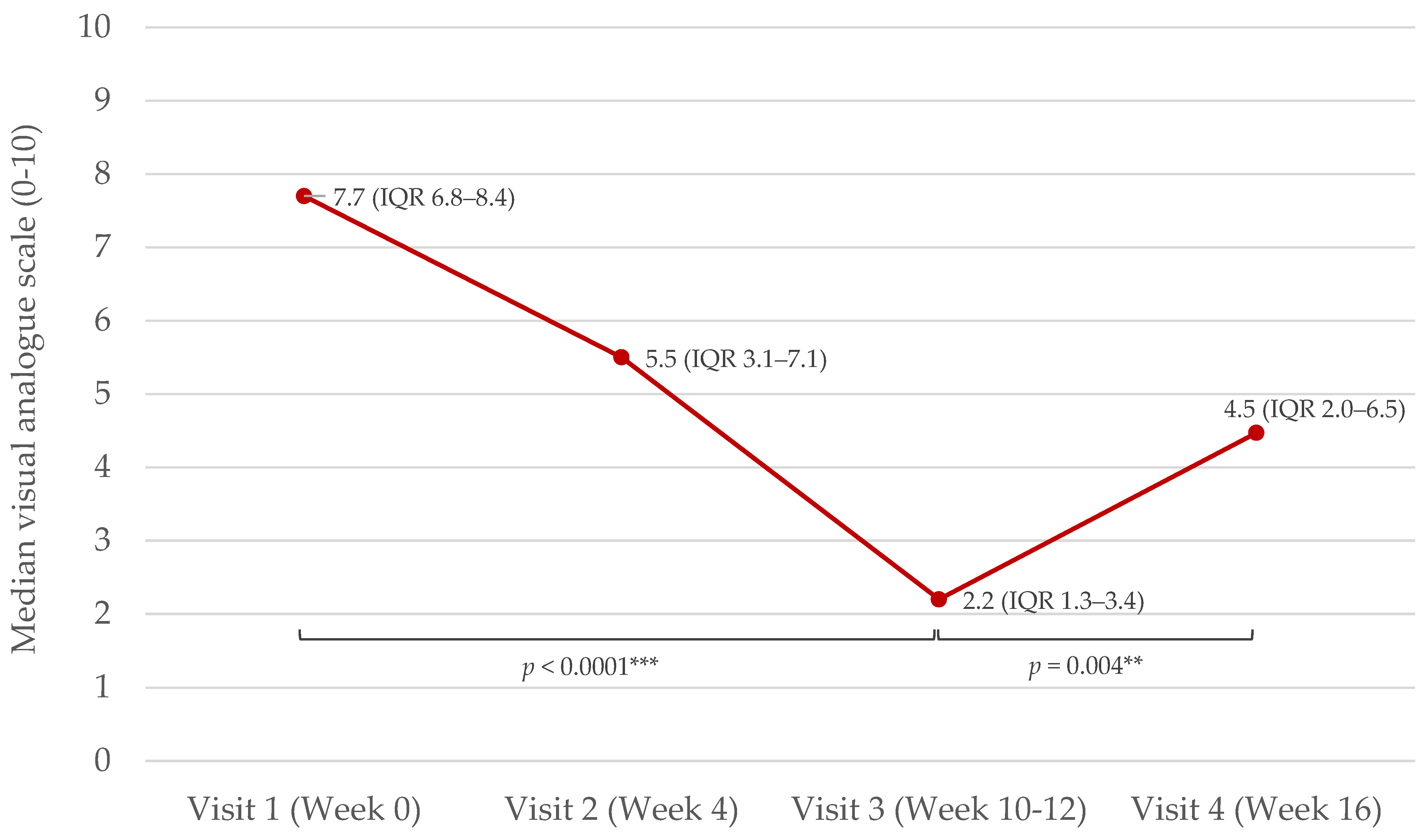
2.2. Effects of NB-UVB Phototherapy on Serum DBP, hsCRP, Vitamin D, and Disease Severity
2.2.1. Effect of NB-UVB Phototherapy on Disease Severity, DBP, hsCRP, and Vitamin D after Stratification by Baseline Vitamin D Levels
2.2.2. Effect of NB-UVB Phototherapy on Vitamin D, DBP, and hsCRP after Stratification by Baseline Body Mass Index (BMI)
2.2.3. Baseline DBP, hsCRP, and Disease Severity, and Effect of NB-UVB Phototherapy on DBP and hsCRP in Psoriasis Patients after Stratification by Self-Reported Arthropathy
2.3. Baseline Correlations
3. Discussion
4. Materials and Methods
4.1. Study Design, Setting, and Patients
4.2. Laboratory Analyses
4.3. Assessment of Disease Severity
4.4. Statistics
4.5. Ethical Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bikle, D.D. Vitamin D and the Skin: Physiology and Pathophysiology. Rev. Endocr. Metab. Disord. 2012, 13, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Brożyna, A.A.; Slominski, R.M.; Nedoszytko, B.; Zmijewski, M.A.; Slominski, A.T. Vitamin D Signaling in Psoriasis: Pathogenesis and Therapy. Int. J. Mol. Sci. 2022, 23, 8575. [Google Scholar] [CrossRef] [PubMed]
- Stanescu, A.M.A.; Simionescu, A.A.; Diaconu, C.C. Oral Vitamin D Therapy in Patients with Psoriasis. Nutrients 2021, 13, 163. [Google Scholar] [CrossRef]
- Jenssen, M.; Furberg, A.-S.; Jorde, R.; Wilsgaard, T.; Danielsen, K. Effect of Vitamin D Supplementation on Psoriasis Severity in Patients With Lower-Range Serum 25-Hydroxyvitamin D Levels: A Randomized Clinical Trial. JAMA Dermatol. 2023, 159, 518–525. [Google Scholar] [CrossRef]
- Bikle, D.; Christakos, S. New Aspects of Vitamin D Metabolism and Action—Addressing the Skin as Source and Target. Nat. Rev. Endocrinol. 2020, 16, 234–252. [Google Scholar] [CrossRef] [PubMed]
- Weiland, S.K.; Hüsing, A.; Strachan, D.P.; Rzehak, P.; Pearce, N.; ISAAC Phase One Study Group. Climate and the Prevalence of Symptoms of Asthma, Allergic Rhinitis, and Atopic Eczema in Children. Occup. Environ. Med. 2004, 61, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, S.-N.; Lee, Y.W.; Choe, Y.B.; Ahn, K.J. Vitamin D Status and Efficacy of Vitamin D Supplementation in Atopic Dermatitis: A Systematic Review and Meta-Analysis. Nutrients 2016, 8, E789. [Google Scholar] [CrossRef]
- Chiu, Y.E.; Havens, P.L.; Siegel, D.H.; Ali, O.; Wang, T.; Holland, K.E.; Galbraith, S.S.; Lyon, V.B.; Drolet, B.A. Serum 25-Hydroxyvitamin D Concentration Does Not Correlate with Atopic Dermatitis Severity. J. Am. Acad. Dermatol. 2013, 69, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Thuesen, B.H.; Heede, N.G.; Tang, L.; Skaaby, T.; Thyssen, J.P.; Friedrich, N.; Linneberg, A. No Association between Vitamin D and Atopy, Asthma, Lung Function or Atopic Dermatitis: A Prospective Study in Adults. Allergy 2015, 70, 1501–1504. [Google Scholar] [CrossRef]
- Sempos, C.T.; Heijboer, A.C.; Bikle, D.D.; Bollerslev, J.; Bouillon, R.; Brannon, P.M.; DeLuca, H.F.; Jones, G.; Munns, C.F.; Bilezikian, J.P.; et al. Vitamin D Assays and the Definition of Hypovitaminosis D: Results from the First International Conference on Controversies in Vitamin D. Br. J. Clin. Pharmacol. 2018, 84, 2194–2207. [Google Scholar] [CrossRef]
- Osmancevic, A.; Landin-Wilhelmsen, K.; Larkö, O.; Wennberg, A.-M.; Krogstad, A.L. Vitamin D Production in Psoriasis Patients Increases Less with Narrowband than with Broadband Ultraviolet B Phototherapy. Photodermatol. Photoimmunol. Photomed. 2009, 25, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Vähävihu, K.; Ala-Houhala, M.; Peric, M.; Karisola, P.; Kautiainen, H.; Hasan, T.; Snellman, E.; Alenius, H.; Schauber, J.; Reunala, T. Narrowband Ultraviolet B Treatment Improves Vitamin D Balance and Alters Antimicrobial Peptide Expression in Skin Lesions of Psoriasis and Atopic Dermatitis. Br. J. Dermatol. 2010, 163, 321–328. [Google Scholar] [CrossRef] [PubMed]
- El-Hamd, M.A.; El Saied, A.R.A.; Ahmed, S.H.; Ibrahim, H.M.; Hegazy, E.M. Effect of Narrow-Band Ultraviolet B Phototherapy, Methotrexate, and Combined Narrow-Band Ultraviolet B Phototherapy with Methotrexate on Serum Cathelicidin and Vitamin D in Patients with Psoriasis Vulgaris. J. Dermatol. Treat. 2022, 33, 408–414. [Google Scholar] [CrossRef]
- Kurz, B.; Berneburg, M.; Bäumler, W.; Karrer, S. Phototherapy: Theory and Practice. J. Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. JDDG 2023, 21, 882–897. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.M.; Ju, H.J.; Lee, R.W.; Oh, S.H.; Shin, J.H.; Kang, H.Y.; Park, J.H.; Kim, H.J.; Jeong, K.-H.; Lee, H.J.; et al. Evaluation for Skin Cancer and Precancer in Patients with Vitiligo Treated with Long-Term Narrowband UV-B Phototherapy. JAMA Dermatol. 2020, 156, 529–537. [Google Scholar] [CrossRef]
- Elmets, C.A.; Lim, H.W.; Stoff, B.; Connor, C.; Cordoro, K.M.; Lebwohl, M.; Armstrong, A.W.; Davis, D.M.R.; Elewski, B.E.; Gelfand, J.M.; et al. Joint American Academy of Dermatology-National Psoriasis Foundation Guidelines of Care for the Management and Treatment of Psoriasis with Phototherapy. J. Am. Acad. Dermatol. 2019, 81, 775–804. [Google Scholar] [CrossRef]
- Slominski, R.M.; Chen, J.Y.; Raman, C.; Slominski, A.T. Photo-Neuro-Immuno-Endocrinology: How the Ultraviolet Radiation Regulates the Body, Brain, and Immune System. Proc. Natl. Acad. Sci. USA 2024, 121, e2308374121. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Schwartz, J. Vitamin D Binding Protein, Total and Free Vitamin D Levels in Different Physiological and Pathophysiological Conditions. Front. Endocrinol. 2019, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Henderson, C.M.; Fink, S.L.; Bassyouni, H.; Argiropoulos, B.; Brown, L.; Laha, T.J.; Jackson, K.J.; Lewkonia, R.; Ferreira, P.; Hoofnagle, A.N.; et al. Vitamin D-Binding Protein Deficiency and Homozygous Deletion of the GC Gene. N. Engl. J. Med. 2019, 380, 1150–1157. [Google Scholar] [CrossRef]
- Duchow, E.G.; Cooke, N.E.; Seeman, J.; Plum, L.A.; DeLuca, H.F. Vitamin D Binding Protein Is Required to Utilize Skin-Generated Vitamin D. Proc. Natl. Acad. Sci. USA 2019, 116, 24527–24532. [Google Scholar] [CrossRef]
- Vandikas, M.S.; Landin-Wilhelmsen, K.; Holmäng, A.; Gillstedt, M.; Osmancevic, A. High Levels of Serum Vitamin D-Binding Protein in Patients with Psoriasis: A Case-Control Study and Effects of Ultraviolet B Phototherapy. J. Steroid Biochem. Mol. Biol. 2021, 211, 105895. [Google Scholar] [CrossRef]
- Vandikas, M.S.; Landin-Wilhelmsen, K.; Gillstedt, M.; Osmancevic, A. Vitamin D-Binding Protein and the Free Hormone Hypothesis for Vitamin D in Bio-Naïve Patients with Psoriasis. Int. J. Mol. Sci. 2022, 23, 1302. [Google Scholar] [CrossRef]
- Robinson-Cohen, C.; Zelnick, L.R.; Hoofnagle, A.N.; Lutsey, P.L.; Burke, G.; Michos, E.D.; Shea, S.J.C.; Tracy, R.; Siscovick, D.S.; Psaty, B.; et al. Associations of Vitamin D-Binding Globulin and Bioavailable Vitamin D Concentrations with Coronary Heart Disease Events: The Multi-Ethnic Study of Atherosclerosis (MESA). J. Clin. Endocrinol. Metab. 2017, 102, 3075–3084. [Google Scholar] [CrossRef] [PubMed]
- Ghaly, S.; Murray, K.; Baird, A.; Martin, K.; Prosser, R.; Mill, J.; Simms, L.A.; Hart, P.H.; Radford-Smith, G.; Bampton, P.A.; et al. High Vitamin D-Binding Protein Concentration, Low Albumin, and Mode of Remission Predict Relapse in Crohn’s Disease. Inflamm. Bowel Dis. 2016, 22, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Walters, I.B.; Ozawa, M.; Cardinale, I.; Gilleaudeau, P.; Trepicchio, W.L.; Bliss, J.; Krueger, J.G. Narrowband (312-Nm) UV-B Suppresses Interferon Gamma and Interleukin (IL) 12 and Increases IL-4 Transcripts: Differential Regulation of Cytokines at the Single-Cell Level. Arch. Dermatol. 2003, 139, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Christen-Zäch, S.; Taieb, A.; Paul, C.; Thyssen, J.P.; de Bruin-Weller, M.; Vestergaard, C.; Seneschal, J.; Werfel, T.; Cork, M.J.; et al. ETFAD/EADV Eczema Task Force 2020 Position Paper on Diagnosis and Treatment of Atopic Dermatitis in Adults and Children. J. Eur. Acad. Dermatol. Venereol. JEADV 2020, 34, 2717–2744. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef]
- Elmelid, A.; Osmancevic, A.; Gillstedt, M.; Alsterholm, M. Effects of Phototherapy on Free Vitamin D Levels in Ten Patients with Atopic Dermatitis. Int. J. Transl. Med. 2022, 2, 586–596. [Google Scholar] [CrossRef]
- Elmelid, A.; Siekkeri Vandikas, M.; Gillstedt, M.; Osmancevic, A.; Alsterholm, M. The Effect of Narrow-Band Ultraviolet B Phototherapy on Free and Total Vitamin D Serum Levels in Mild to Severe Plaque Psoriasis. Biomolecules 2023, 13, 1018. [Google Scholar] [CrossRef]
- Ridker, P.M.; Morrow, D.A. C-Reactive Protein, Inflammation, and Coronary Risk. Cardiol. Clin. 2003, 21, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Mehta, N.N.; Shin, D.B.; Joshi, A.A.; Dey, A.K.; Armstrong, A.W.; Duffin, K.C.; Fuxench, Z.C.; Harrington, C.L.; Hubbard, R.A.; Kalb, R.E.; et al. Effect of 2 Psoriasis Treatments on Vascular Inflammation and Novel Inflammatory Cardiovascular Biomarkers: A Randomized Placebo-Controlled Trial. Circ. Cardiovasc. Imaging 2018, 11, e007394. [Google Scholar] [CrossRef]
- Sigurdardottir, G.; Ekman, A.-K.; Ståhle, M.; Bivik, C.; Enerbäck, C. Systemic Treatment and Narrowband Ultraviolet B Differentially Affect Cardiovascular Risk Markers in Psoriasis. J. Am. Acad. Dermatol. 2014, 70, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Le, P.; Tu, J.; Gebauer, K.; Brown, S. Serum 25-Hydroxyvitamin D Increases with NB-UVB and UVA/UVB Phototherapy in Patients with Psoriasis and Atopic Dermatitis in Western Australia. Australas. J. Dermatol. 2016, 57, 115–121. [Google Scholar] [CrossRef]
- Karampinis, E.; Goudouras, G.; Ntavari, N.; Bogdanos, D.P.; Roussaki-Schulze, A.-V.; Zafiriou, E. Serum Vitamin D Levels Can Be Predictive of Psoriasis Flares up after COVID-19 Vaccination: A Retrospective Case Control Study. Front. Med. 2023, 10, 1203426. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P. Guidelines for Optimizing Design and Analysis of Clinical Studies of Nutrient Effects. Nutr. Rev. 2014, 72, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Slominski, A.T.; Kim, T.-K.; Janjetovic, Z.; Slominski, R.M.; Li, W.; Jetten, A.M.; Indra, A.K.; Mason, R.S.; Tuckey, R.C. Biological Effects of CYP11A1-Derived Vitamin D and Lumisterol Metabolites in the Skin. J. Investig. Dermatol. 2024. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Hannen, R.F.; Michael, A.E.; Jaulim, A.; Bhogal, R.; Burrin, J.M.; Philpott, M.P. Steroid Synthesis by Primary Human Keratinocytes; Implications for Skin Disease. Biochem. Biophys. Res. Commun. 2011, 404, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Hannen, R.; Udeh-Momoh, C.; Upton, J.; Wright, M.; Michael, A.; Gulati, A.; Rajpopat, S.; Clayton, N.; Halsall, D.; Burrin, J.; et al. Dysfunctional Skin-Derived Glucocorticoid Synthesis Is a Pathogenic Mechanism of Psoriasis. J. Investig. Dermatol. 2017, 137, 1630–1637. [Google Scholar] [CrossRef]
- Slominski, R.M.; Raman, C.; Elmets, C.; Jetten, A.M.; Slominski, A.T.; Tuckey, R.C. The Significance of CYP11A1 Expression in Skin Physiology and Pathology. Mol. Cell. Endocrinol. 2021, 530, 111238. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, S.; Slominski, R.M.; Raman, C.; Slominski, A.T. Novel CYP11A1-Derived Vitamin D and Lumisterol Biometabolites for the Management of COVID-19. Nutrients 2022, 14, 4779. [Google Scholar] [CrossRef]
- Oleröd, G.; Hultén, L.M.; Hammarsten, O.; Klingberg, E. The Variation in Free 25-Hydroxy Vitamin D and Vitamin D-Binding Protein with Season and Vitamin D Status. Endocr. Connect. 2017, 6, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Vandikas, M.S.; Landin-Wilhelmsen, K.; Polesie, S.; Gillstedt, M.; Osmancevic, A. Impact of Etanercept on Vitamin D Status and Vitamin D-Binding Protein in Bio-Naïve Patients with Psoriasis. Acta Derm. Venereol. 2021, 101, 359. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.H.; Van Alstyne, E.L.; Galbraith, R.M. Evidence of a Novel Association of Unsaturated Fatty Acids with Gc (Vitamin D-Binding Protein). Biochem. Biophys. Res. Commun. 1988, 153, 1019–1024. [Google Scholar] [CrossRef]
- Bouillon, R.; Xiang, D.Z.; Convents, R.; Van Baelen, H. Polyunsaturated Fatty Acids Decrease the Apparent Affinity of Vitamin D Metabolites for Human Vitamin D-Binding Protein. J. Steroid Biochem. Mol. Biol. 1992, 42, 855–861. [Google Scholar] [CrossRef] [PubMed]
- Ghazizadeh, H.; Mansoori, A.; Sahranavard, T.; Nasrabadi, M.; Hadiloo, K.; Andalibi, N.S.; Azmon, M.; Tavallaei, S.; Timar, A.; Ferns, G.A.; et al. The Associations of Oxidative Stress and Inflammatory Markers with Obesity in Iranian Population: MASHAD Cohort Study. BMC Endocr. Disord. 2024, 24, 56. [Google Scholar] [CrossRef] [PubMed]
- Golzarand, M.; Hollis, B.W.; Mirmiran, P.; Wagner, C.L.; Shab-Bidar, S. Vitamin D Supplementation and Body Fat Mass: A Systematic Review and Meta-Analysis. Eur. J. Clin. Nutr. 2018, 72, 1345–1357. [Google Scholar] [CrossRef] [PubMed]
- Haghighat, N.; Sohrabi, Z.; Bagheri, R.; Akbarzadeh, M.; Esmaeilnezhad, Z.; Ashtary-Larky, D.; Barati-Boldaji, R.; Zare, M.; Amini, M.; Hosseini, S.V.; et al. A Systematic Review and Meta-Analysis of Vitamin D Status of Patients with Severe Obesity in Various Regions Worldwide. Obes. Facts 2023, 16, 519–539. [Google Scholar] [CrossRef]
- Snekvik, I.; Smith, C.H.; Nilsen, T.I.L.; Langan, S.M.; Modalsli, E.H.; Romundstad, P.R.; Saunes, M. Obesity, Waist Circumference, Weight Change, and Risk of Incident Psoriasis: Prospective Data from the HUNT Study. J. Investig. Dermatol. 2017, 137, 2484–2490. [Google Scholar] [CrossRef] [PubMed]
- Paroutoglou, K.; Papadavid, E.; Christodoulatos, G.S.; Dalamaga, M. Deciphering the Association Between Psoriasis and Obesity: Current Evidence and Treatment Considerations. Curr. Obes. Rep. 2020, 9, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jing, D.; Zhou, G.; Xiao, Y.; Shen, M.; Chen, X.; Liu, H. Evidence of a Causal Relationship between Vitamin D Status and Risk of Psoriasis from the UK Biobank Study. Front. Nutr. 2022, 9, 807344. [Google Scholar] [CrossRef] [PubMed]
- Jenssen, M.; Furberg, A.-S.; Jorde, R.; Wilsgaard, T.; Danielsen, K. The Association between Serum 25-Hydroxyvitamin D Levels and Psoriasis in a Large Population-Based Cohort: A Cross-Sectional Analysis of The Tromsø Study 2015-16. Br. J. Dermatol. 2024, 190, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Ekwaru, J.P.; Zwicker, J.D.; Holick, M.F.; Giovannucci, E.; Veugelers, P.J. The Importance of Body Weight for the Dose Response Relationship of Oral Vitamin D Supplementation and Serum 25-Hydroxyvitamin D in Healthy Volunteers. PLoS ONE 2014, 9, e111265. [Google Scholar] [CrossRef]
- Batista, M.C.; Menegat, F.D.; Ferreira, C.E.S.; Faulhaber, A.C.L.; Campos, D.A.L.S.; Mangueira, C.L.P. Analytical and Clinical Validation of the New Roche Elecsys Vitamin D Total II Assay. Clin. Chem. Lab. Med. 2018, 56, e298–e301. [Google Scholar] [CrossRef]
- Flytström, I.; Stenberg, B.; Svensson, Å.; Bergbrant, I.-M. Patients’ Visual Analogue Scale: A Useful Method for Assessing Psoriasis Severity. Acta Derm. Venereol. 2012, 92, 347–348. [Google Scholar] [CrossRef] [PubMed]

| Mean | Min–Max | n | |
|---|---|---|---|
| Age (years) | 39 | 20–70 | 30 |
| Age women (years) | 34 | 20–65 | 14 |
| Age men (years) | 43 | 21–70 | 16 |
| Weight (kg) | 74 | 49–111 | 30 |
| Height (cm) | 172 | 154–189 | 30 |
| BMI (kg/m2) | 25.1 | 19.3–33.5 | 30 |
| Systolic blood pressure (mmHg) | 122 | 90–165 | 30 |
| Diastolic blood pressure (mmHg) | 77 | 60–95 | 30 |
| Disease duration of psoriasis or atopic dermatitis (years) | 21 | 1–46 | 30 |
| n (%) | |||
| Current smokers | 10 (33%) | ||
| Omega 3 use | 2 (7%) | ||
| Obesity (BMI > 30 kg/m2) | 5 (17%) | ||
| Skin type | |||
| II | 11 (37%) | ||
| III | 18 (60%) | ||
| IV | 1 (3%) | ||
| Concomitant medication | |||
| Antilipidemic use | 1 (3%) | ||
| Antihypertensive use | 3 (10%) | ||
| Antidiabetic use | 1 (3%) | ||
| Antidepressant use | 2 (7%) | ||
| Painkiller use | 2 (7%) | ||
| Thyroid hormone use | 2 (7%) | ||
| Hormonal contraception | 1 (3%) | ||
| Total 25(OH)D < 75 nmol/L (deficiency/insufficiency) Total 25(OH)D ≥ 75 nmol/L (sufficiency) | 19 (63%) 11 (37%) |
| Before NB-UVB (Visit 1 = Week 0) | After NB-UVB (Visit 3 = Week 10–12) | p-Value | |
|---|---|---|---|
| Psoriasis patients | n = 20 | n = 15 | |
| DBP (μg/mL) | 218.0 ± 38.9 | 201.2 ± 16.7 | 0.031 * |
| hsCRP (mg/L) | 2.6 ± 2.7 | 2.0 ± 1.9 | 0.31 |
| AD patients | n = 10 | n = 8 | |
| DBP (μg/mL) | 247.6 ± 39.8 | 248.6 ± 47.0 | 0.58 |
| hsCRP (mg/L) | 3.3 ± 3.4 | 2.5 ± 1.9 | 0.68 |
| All patients | n = 30 | n = 23 | |
| DBP (μg/mL) | 227.8 ± 41.0 | 201.2 ± 16.7 | 0.20 |
| hsCRP (mg/L) | 2.9 ± 2.9 | 2.2 ± 1.9 | 0.29 |
| Variable | DBP | ||
|---|---|---|---|
| All Patients (n = 30) | Psoriasis Patients (n = 20) | AD Patients (n = 10) | |
| Age | 0.62 (−0.09) | 0.20 (0.30) | 0.71 (−0.14) |
| BMI | 0.62 (−0.09) | 0.85 (−0.05) | 0.95 (0.03) |
| hsCRP | 0.25 (0.22) | 0.96 (−0.01) | 0.054 (0.64) |
| Total 25(OH)D | 0.031 (0.39) * | 0.21 (0.29) | 0.080 (0.59) |
| Free 25(OH)D | 0.045 (0.37) * | 0.23 (0.28) | 0.53 (0.23) |
| Percentage of free 25(OH)D | 0.19 (−0.25) | 0.34 (−0.23) | 0.28 (−0.38) |
| 1,25(OH)2D | 0.0001 (0.65) *** | 0.006 (0.59) ** | 0.30 (0.36) |
| iPTH | 0.073 (−0.33) | 0.63 (0.11) | 0.028 (−0.71) * |
| VAS | 0.11 (0.29) | 0.11 (0.36) | 0.58 (0.20) |
| PASI | N/A | 0.92 (−0.02) | N/A |
| Objective SCORAD | N/A | N/A | 0.84 (−0.07) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmelid, A.; Vandikas, M.S.; Gillstedt, M.; Alsterholm, M.; Osmancevic, A. The Effect of Phototherapy on Systemic Inflammation Measured with Serum Vitamin D-Binding Protein and hsCRP in Patients with Inflammatory Skin Disease. Int. J. Mol. Sci. 2024, 25, 8632. https://doi.org/10.3390/ijms25168632
Elmelid A, Vandikas MS, Gillstedt M, Alsterholm M, Osmancevic A. The Effect of Phototherapy on Systemic Inflammation Measured with Serum Vitamin D-Binding Protein and hsCRP in Patients with Inflammatory Skin Disease. International Journal of Molecular Sciences. 2024; 25(16):8632. https://doi.org/10.3390/ijms25168632
Chicago/Turabian StyleElmelid, Andrea, Maria Siekkeri Vandikas, Martin Gillstedt, Mikael Alsterholm, and Amra Osmancevic. 2024. "The Effect of Phototherapy on Systemic Inflammation Measured with Serum Vitamin D-Binding Protein and hsCRP in Patients with Inflammatory Skin Disease" International Journal of Molecular Sciences 25, no. 16: 8632. https://doi.org/10.3390/ijms25168632
APA StyleElmelid, A., Vandikas, M. S., Gillstedt, M., Alsterholm, M., & Osmancevic, A. (2024). The Effect of Phototherapy on Systemic Inflammation Measured with Serum Vitamin D-Binding Protein and hsCRP in Patients with Inflammatory Skin Disease. International Journal of Molecular Sciences, 25(16), 8632. https://doi.org/10.3390/ijms25168632







