Sex-Specific Differences in Kidney Function and Blood Pressure Regulation
Abstract
:1. Introduction
2. Sex and Hypertension
3. Kidney and Blood Pressure
4. Endothelins
5. Tubule Transporters
5.1. Epithelial Sodium Channel (ENaC)
5.2. Sodium–Hydrogen Exchanger 3 (NHE3)
5.3. Aquaporins
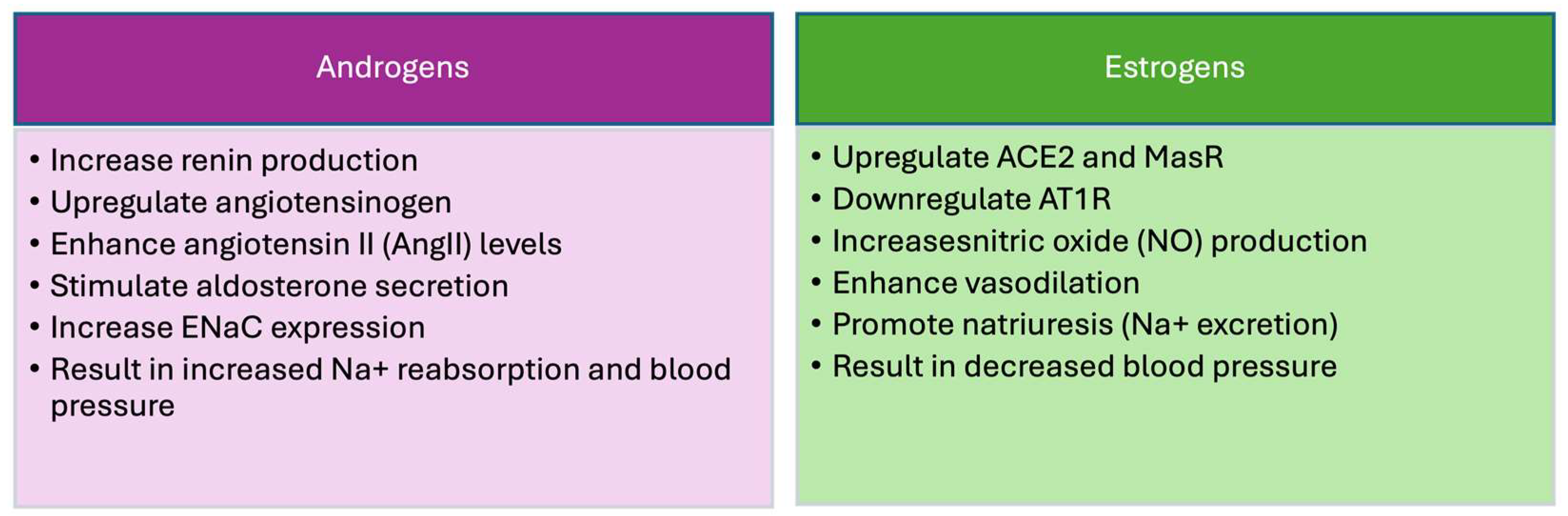
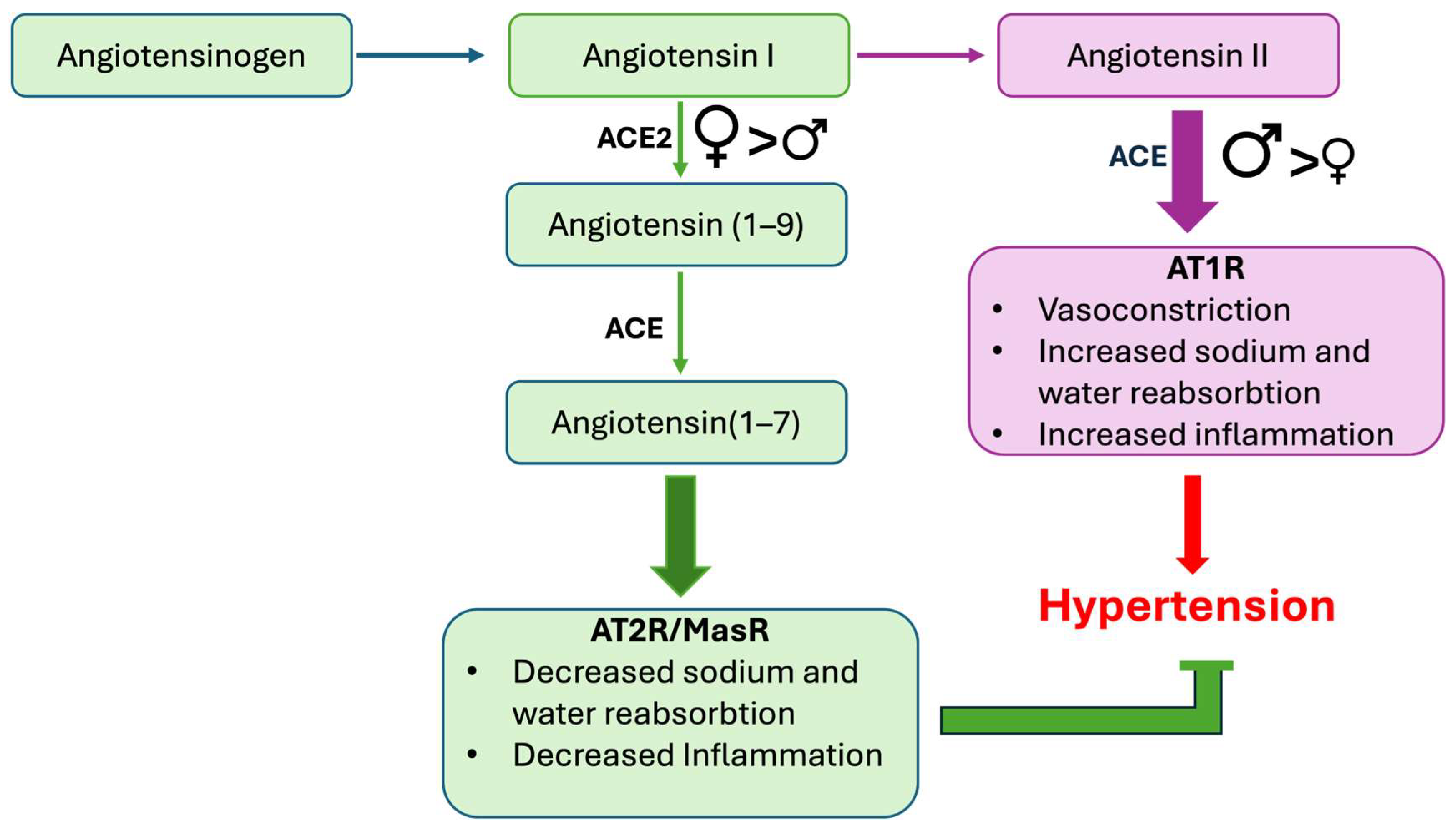
6. Renin–Angiotensin–Aldosterone System
7. Sex-Specific Genetic Susceptibilities in Hypertension: Insights from GWAS
8. Sex-Specific Nutraceutical Approaches
9. Conclusions
Funding
Conflicts of Interest
References
- Berg, U.B. Differences in decline in GFR with age between males and females. Reference data on clearances of inulin and PAH in potential kidney donors. Nephrol. Dial. Transpl. 2006, 21, 2577–2582. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, J.; Feldmer, M.; Ganten, U.; Stock, G.; Ganten, D. Sexual dimorphism of blood pressure: Possible role of the renin-angiotensin system. J. Steroid Biochem. Mol. Biol. 1991, 40, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Harshfield, G.A.; Alpert, B.S.; Pulliam, D.A.; Somes, G.W.; Wilson, D.K. Ambulatory blood pressure recordings in children and adolescents. Pediatrics 1994, 94, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Burt, V.L.; Whelton, P.; Roccella, E.J.; Brown, C.; Cutler, J.A.; Higgins, M.; Horan, M.J.; Labarthe, D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension 1995, 25, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Stamler, R.; Stamler, J.; Riedlinger, W.F.; Algera, G.; Roberts, R.H. Weight and blood pressure. Findings in hypertension screening of 1 million Americans. JAMA 1978, 240, 1607–1610. [Google Scholar] [CrossRef] [PubMed]
- Narkiewicz, K.; Phillips, B.G.; Kato, M.; Hering, D.; Bieniaszewski, L.; Somers, V.K. Gender-selective interaction between aging, blood pressure, and sympathetic nerve activity. Hypertension 2005, 45, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Balafa, O.; Fernandez-Fernandez, B.; Ortiz, A.; Dounousi, E.; Ekart, R.; Ferro, C.J.; Mark, P.B.; Valdivielso, J.M.; Del Vecchio, L.; Mallamaci, F. Sex disparities in mortality and cardiovascular outcomes in chronic kidney disease. Clin. Kidney J. 2024, 17, sfae044. [Google Scholar] [CrossRef]
- Chesnaye, N.C.; Carrero, J.J.; Hecking, M.; Jager, K.J. Differences in the epidemiology, management and outcomes of kidney disease in men and women. Nat. Rev. Nephrol. 2024, 20, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.B.; Pinkernell, B.H.; Jing, T.Y. The association of hypotestosteronemia with coronary artery disease in men. Arterioscler. Thromb. 1994, 14, 701–706. [Google Scholar] [CrossRef]
- Yanes, L.L.; Sartori-Valinotti, J.C.; Reckelhoff, J.F. Sex steroids and renal disease: Lessons from animal studies. Hypertension 2008, 51, 976–981. [Google Scholar] [CrossRef]
- Messerli, F.H.; Garavaglia, G.E.; Schmieder, R.E.; Sundgaard-Riise, K.; Nunez, B.D.; Amodeo, C. Disparate cardiovascular findings in men and women with essential hypertension. Ann. Intern. Med. 1987, 107, 158–161. [Google Scholar] [CrossRef] [PubMed]
- van Kesteren, P.J.M.; Kooistra, T.; Lansink, M.; van Kamp, G.J.; Asscheman, H.; Gooren, L.J.G.; Emeis, J.J.; Vischer, U.M.; Stehouwer, C.D.A. The effects of sex steroids on plasma levels of marker proteins of endothelial cell functioning. Thromb. Haemost. 1998, 79, 1029–1033. [Google Scholar] [CrossRef]
- Christakou, C.D.; Diamanti-Kandarakis, E. Role of androgen excess on metabolic aberrations and cardiovascular risk in women with polycystic ovary syndrome. Womens Health 2008, 4, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Srinivasan, S.R.; Li, S.; Boerwinkle, E.; Berenson, G.S. Gender-specific influence of NO synthase gene on blood pressure since childhood: The Bogalusa Heart Study. Hypertension 2004, 44, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.J.; Luke, R.G.; Dustan, H.P.; Kashgarian, M.; Whelchel, J.D.; Jones, P.; Diethelm, A.G. Remission of essential hypertension after renal transplantation. N. Engl. J. Med. 1983, 309, 1009–1015. [Google Scholar] [CrossRef] [PubMed]
- Zapater, P.; Novalbos, J.; Gallego-Sandín, S.; Hernández, F.T.; Abad-Santos, F. Gender differences in angiotensin-converting enzyme (ACE) activity and inhibition by enalaprilat in healthy volunteers. J. Cardiovasc. Pharmacol. 2004, 43, 737–744. [Google Scholar] [CrossRef]
- Ruggenenti, P.; Perna, A.; Zoccali, C.; Gherardi, G.; Benini, R.; Testa, A.; Remuzzi, G. Chronic proteinuric nephropathies. II. Outcomes and response to treatment in a prospective cohort of 352 patients: Differences between women and men in relation to the ACE gene polymorphism. Gruppo Italiano di Studi Epidemologici in Nefrologia (Gisen). J. Am. Soc. Nephrol. 2000, 11, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.B.; Jang, J.A. Genome-Wide Association Study of a Korean Population Identifies Genetic Susceptibility to Hypertension Based on Sex-Specific Differences. Genes 2021, 12, 1804. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Kim, J.; Kim, J. Gender Differences in the Association between Dietary Pattern and the Incidence of Hypertension in Middle-Aged and Older Adults. Nutrients 2018, 10, 252. [Google Scholar] [CrossRef]
- Kang, Y.; Kim, J. Association between fried food consumption and hypertension in Korean adults. Br. J. Nutr. 2016, 115, 87–94. [Google Scholar] [CrossRef]
- Reckelhoff, J.F.; Zhang, H.; Granger, J.P. Testosterone exacerbates hypertension and reduces pressure-natriuresis in male spontaneously hypertensive rats. Hypertension 1998, 31, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, J.F. Gender differences in the regulation of blood pressure. Hypertension 2001, 37, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Caplea, A.; Seachrist, D.; Dunphy, G.; Ely, D. Sodium-induced rise in blood pressure is suppressed by androgen receptor blockade. Am. J. Physiol. Heart Circ. Physiol. 2001, 280, H1793–H1801. [Google Scholar] [CrossRef] [PubMed]
- Baltatu, O.; Cayla, C.A.A.; Iliescu, R.; Andreev, D.; Jordan, C.; Bader, M. Abolition of hypertension-induced end-organ damage by androgen receptor blockade in transgenic rats harboring the mouse ren-2 gene. J. Am. Soc. Nephrol. 2002, 13, 2681–2687. [Google Scholar] [CrossRef] [PubMed]
- Sherman, S.B.; Sarsour, N.; Salehi, M.; Schroering, A.; Mell, B.; Joe, B.; Hill, J.W. Prenatal androgen exposure causes hypertension and gut microbiota dysbiosis. Gut Microbes 2018, 9, 400–421. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Speed, J.S.; Hyndman, K.A.; O’Connor, P.M.; Pollock, D.M.; Douma, L.G.; Solocinski, K.; Holzworth, M.R.; Crislip, G.R.; Masten, S.H.; et al. Sex differences in ET-1 receptor expression and Ca2+ signaling in the IMCD. Am. J. Physiol. Renal. Physiol. 2013, 305, F1099-104. [Google Scholar] [CrossRef]
- Iams, S.G.; McMurthy, J.P.; Wexler, B.C. Aldosterone, deoxycorticosterone, corticosterone, and prolactin changes during the lifespan of chronically and spontaneously hypertensive rats. Endocrinology 1979, 104, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Kienitz, T.; Allolio, B.; Strasburger, C.; Quinkler, M. Sex-specific regulation of ENaC and androgen receptor in female rat kidney. Horm. Metab. Res. 2009, 41, 356–362. [Google Scholar] [CrossRef]
- Loh, S.Y.; Giribabu, N.; Salleh, N. Changes in plasma aldosterone and electrolytes levels, kidney epithelial sodium channel (ENaC) and blood pressure in normotensive WKY and hypertensive SHR rats following gonadectomy and chronic testosterone treatment. Steroids 2017, 128, 128–135. [Google Scholar] [CrossRef]
- Fortepiani, L.A.; Yanes, L.; Zhang, H.; Racusen, L.C.; Reckelhoff, J.F. Role of androgens in mediating renal injury in aging SHR. Hypertension 2003, 42, 952–955. [Google Scholar] [CrossRef]
- Müller, V.; Losonczy, G.; Heemann, U.; Vannay, Á.; Fekete, A.; Reusz, G.; Tulassay, T.; Szabó, A.J. Sexual dimorphism in renal ischemia-reperfusion injury in rats: Possible role of endothelin. Kidney Int. 2002, 62, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Vassileva, I.; Mountain, C.; Pollock, D.M. Functional role of ETB receptors in the renal medulla. Hypertension 2003, 41, 1359–1363. [Google Scholar] [CrossRef] [PubMed]
- Silva-Antonialli, M.M.; Tostes, R.C.; Fernandes, L.; Fior-Chadi, D.R.; Akamine, E.H.; Carvalho, M.H.C.; Fortes, Z.B.; Nigro, D. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc. Res. 2004, 62, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, P.J.; Michaelis, M.; Gotz, F.; Bartel, C.; Kienitz, T.; Quinkler, M. Flutamide increases aldosterone levels in gonadectomized male but not female Wistar rats. Am. J. Hypertens. 2012, 25, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Menini, S.; Mok, K.; Zheng, W.; Pesce, C.; Kim, J.; Mulroney, S.; Sandberg, K.; Zimmerman, M.A.; Hutson, D.D.; et al. Gonadal steroid regulation of renal injury in renal wrap hypertension. Am. J. Physiol. Ren. Physiol. 2005, 288, F513-20. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Ilhami, N.; Giudicelli, Y.; Dausse, J.-P. Testosterone dependence of salt-induced hypertension in Sabra rats and role of renal alpha(2)-adrenoceptor subtypes. J. Pharmacol. Exp. Ther. 2002, 300, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Masubuchi, Y.; Kumai, T.; Uematsu, A.; Komoriyama, K.; Hirai, M. Gonadectomy-induced reduction of blood pressure in adult spontaneously hypertensive rats. Acta Endocrinol. 1982, 101, 154–160. [Google Scholar] [CrossRef]
- Iams, S.G.; Wexler, B.C. Retardation in the development of spontaneous hypertension in SH rats by gonadectomy. J. Lab. Clin. Med. 1977, 90, 997–1003. [Google Scholar] [PubMed]
- Crofton, J.T.; Ota, M.; Share, L. Role of vasopressin, the renin-angiotensin system and sex in Dahl salt-sensitive hypertension. J. Hypertens 1993, 11, 1031–1038. [Google Scholar] [CrossRef]
- Chen, Y.F.; Meng, Q.C. Sexual dimorphism of blood pressure in spontaneously hypertensive rats is androgen dependent. Life Sci. 1991, 48, 85–96. [Google Scholar] [CrossRef]
- Hinojosa-Laborde, C.; Lange, D.L.; Haywood, J.R. Role of female sex hormones in the development and reversal of dahl hypertension. Hypertension 2000, 35, 484–489. [Google Scholar] [CrossRef]
- Vaněčková, I.; Husková, Z.; Vaňourková, Z.; Červenka, L. Castration has antihypertensive and organoprotective effects in male but not in female heterozygous Ren-2 rats. Kidney Blood Press Res. 2011, 34, 46–52. [Google Scholar] [CrossRef]
- Hu, J.; Tan, S.; Zhong, Y. Effects of testosterone on renal function in salt-loaded rats. Am. J. Med. Sci. 2011, 342, 38–43. [Google Scholar] [CrossRef]
- Dubey, R.K.; Jackson, E.K. Estrogen-induced cardiorenal protection: Potential cellular, biochemical, and molecular mechanisms. Am. J. Physiol. Renal. Physiol. 2001, 280, F365–F388. [Google Scholar] [CrossRef] [PubMed]
- Tostes, R.; Nigro, D.; Fortes, Z.; Carvalho, M. Effects of estrogen on the vascular system. Braz. J. Med. Biol. Res. 2003, 36, 1143–1158. [Google Scholar] [CrossRef] [PubMed]
- Khalil, R.A. Sex hormones as potential modulators of vascular function in hypertension. Hypertension 2005, 46, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Mankhey, R.W.; Bhatti, F.; Maric, C. 17beta-Estradiol replacement improves renal function and pathology associated with diabetic nephropathy. Am. J. Physiol. Renal. Physiol. 2005, 288, F399–F405. [Google Scholar] [CrossRef]
- Sakemi, T.; Ohtsuka, N.; Tomiyoshi, Y.; Morito, F. The ovaries attenuate the aggravating effect of testosterone on glomerular injury in Adriamycin-induced nephropathy of female rats. Kidney Blood Press Res. 1997, 20, 44–50. [Google Scholar] [CrossRef]
- Corona, G.; Giagulli, V.A.; Maseroli, E.; Vignozzi, L.; Aversa, A.; Zitzmann, M.; Saad, F.; Mannucci, E.; Maggi, M. Testosterone supplementation and body composition: Results from a meta-analysis of observational studies. J. Endocrinol. Invest. 2016, 39, 967–981. [Google Scholar] [CrossRef]
- Buvat, J.; Maggi, M.; Guay, A.; Torres, L.O. Testosterone deficiency in men: Systematic review and standard operating procedures for diagnosis and treatment. J. Sex. Med. 2013, 10, 245–284. [Google Scholar] [CrossRef]
- Liu, P.Y.; Death, A.K.; Handelsman, D.J. Androgens and cardiovascular disease. Endocr. Rev. 2003, 24, 313–340. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.; Hampton, D.; Turkes, A.; Newcombe, R.G.; Rees, D.A. Reduced total testosterone concentrations in young healthy South Asian men are partly explained by increased insulin resistance but not by altered adiposity. Clin. Endocrinol. 2010, 73, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, J.F. Androgens and Blood Pressure Control: Sex Differences and Mechanisms. Mayo Clin. Proc. 2019, 94, 536–543. [Google Scholar] [PubMed]
- Davis, D.D.; Lopez Ruiz, A.; Yanes, L.L.; Iliescu, R.; Yuan, K.; Moulana, M.; Racusen, L.C.; Reckelhoff, J.F. Testosterone supplementation in male obese Zucker rats reduces body weight and improves insulin sensitivity but increases blood pressure. Hypertension 2012, 59, 726–731. [Google Scholar] [CrossRef] [PubMed]
- Yanes, L.L.; Romero, D.G.; Moulana, M.; Lima, R.; Davis, D.D.; Zhang, H.; Lockhart, R.; Racusen, L.C.; Reckelhoff, J.F. Cardiovascular-renal and metabolic characterization of a rat model of polycystic ovary syndrome. Gend. Med. 2011, 8, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, J.F.; Granger, J.P. Role of androgens in mediating hypertension and renal injury. Clin. Exp. Pharmacol. Physiol. 1999, 26, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Guyton, A.C.; Coleman, T.G.; Cowley, A.W.; Scheel, K.W.; Manning, R.D.; Norman, R.A. Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am. J. Med. 1972, 52, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Wadei, H.M.; Textor, S.C. The role of the kidney in regulating arterial blood pressure. Nat. Rev. Nephrol. 2012, 8, 602–609. [Google Scholar] [CrossRef]
- Rettig, R.; Folberth, C.G.; Stauss, H.; Kopf, D.; Waldherr, R.; Baldauf, G.; Unger, T. Hypertension in rats induced by renal grafts from renovascular hypertensive donors. Hypertension 1990, 15, 429–435. [Google Scholar] [CrossRef]
- Hediger, M.A.; Romero, M.F.; Peng, J.B.; Rolfs, A.; Takanaga, H.; Bruford, E.A. The ABCs of solute carriers: Physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction. Pflugers Arch. 2004, 447, 465–468. [Google Scholar] [CrossRef]
- Morris, M.E.; Lee, H.J.; Predko, L.M. Gender differences in the membrane transport of endogenous and exogenous compounds. Pharmacol. Rev. 2003, 55, 229–240. [Google Scholar] [CrossRef]
- Rizwan, A.N.; Burckhardt, G. Organic anion transporters of the SLC22 family: Biopharmaceutical, physiological, and pathological roles. Pharm. Res. 2007, 24, 450–470. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.H. Role of organic cation transporters in the renal handling of therapeutic agents and xenobiotics. Toxicol. Appl. Pharmacol. 2005, 204, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Quinkler, M.; Bumke-Vogt, C.; Meyer, B.; Bähr, V.; Oelkers, W.; Diederich, S. The human kidney is a progesterone-metabolizing and androgen-producing organ. J. Clin. Endocrinol. Metab. 2003, 88, 2803–2809. [Google Scholar] [CrossRef] [PubMed]
- Grimont, A.; Bloch-Faure, M.; El Abida, B.; Crambert, G. Mapping of sex hormone receptors and their modulators along the nephron of male and female mice. FEBS Lett. 2009, 583, 1644–1648. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Mizuno, T.; Lasnitzki, I. Autoradiographic studies of androgen-binding sites in the rat urogenital sinus and postnatal prostate. J. Endocrinol. 1985, 104, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Boulkroun, S.; Le Moellic, C.; Blot-Chabaud, M.; Farman, N.; Courtois-Coutry, N. Expression of androgen receptor and androgen regulation of NDRG2 in the rat renal collecting duct. Pflugers Arch. 2005, 451, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Toot, J.; Jenkins, C.; Dunphy, G.; Boehme, S.; Hart, M.; Milsted, A.; Turner, M.; Ely, D. Testosterone influences renal electrolyte excretion in SHR/y and WKY males. BMC Physiol. 2008, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Khraibi, A.A.; Liang, M.; Berndt, T.J. Role of gender on renal interstitial hydrostatic pressure and sodium excretion in rats. Am. J. Hypertens. 2001, 14, 893–896. [Google Scholar] [CrossRef]
- Hilliard, L.M.; Nematbakhsh, M.; Kett, M.M.; Teichman, E.; Sampson, A.K.; Widdop, R.E.; Evans, R.G.; Denton, K.M. Gender differences in pressure-natriuresis and renal autoregulation: Role of the Angiotensin type 2 receptor. Hypertension 2011, 57, 275–282. [Google Scholar] [CrossRef]
- Hall, J.E.; Mizelle, H.L.; Hildebrandt, D.A.; Brands, M.W. Abnormal pressure natriuresis. A cause or a consequence of hypertension? Hypertension 1990, 15, 547–559. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, M.H.; Fineberg, N.S. Sodium and volume sensitivity of blood pressure. Age and pressure change over time. Hypertension 1991, 18, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Pechere-Bertschi, A.; Burnier, M. Gonadal steroids, salt-sensitivity and renal function. Curr. Opin. Nephrol. Hypertens. 2007, 16, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Maranon, R.; Lima, R.; Spradley, F.T.; Carmo, J.M.D.; Zhang, H.; Smith, A.D.; Bui, E.; Thomas, R.L.; Moulana, M.; Hall, J.E.; et al. Roles for the sympathetic nervous system, renal nerves, and CNS melanocortin-4 receptor in the elevated blood pressure in hyperandrogenemic female rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R708–R713. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, X.; Qu, H.Y.; Jiang, S.; Zhang, J.; Fu, L.; Buggs, J.; Pang, B.; Wei, J.; Liu, R. Role of Kidneys in Sex Differences in Angiotensin II-Induced Hypertension. Hypertension 2017, 70, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Inoue, A.; Yanagisawa, M.; Kimura, S.; Kasuya, Y.; Miyauchi, T.; Goto, K.; Masaki, T. The human endothelin family: Three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc. Natl. Acad. Sci. USA 1989, 86, 2863–2867. [Google Scholar] [CrossRef] [PubMed]
- Nakano, D.; Pollock, D.M. Contribution of endothelin A receptors in endothelin 1-dependent natriuresis in female rats. Hypertension 2009, 53, 324–330. [Google Scholar] [CrossRef]
- Tostes, R.C.; Fortes, Z.B.; Callera, G.E.; Montezano, A.C.; Touyz, R.M.; Webb, R.C.; Carvalho, M.H.C. Endothelin, sex and hypertension. Clin. Sci. 2008, 114, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Kohan, D.E. The renal medullary endothelin system in control of sodium and water excretion and systemic blood pressure. Curr. Opin. Nephrol. Hypertens. 2006, 15, 34–40. [Google Scholar] [CrossRef]
- Granger, J.P.; Abram, S.; Stec, D.; Chandler, D.; Speed, J.; LaMarca, B. Endothelin, the kidney, and hypertension. Curr. Hypertens. Rep. 2006, 8, 298–303. [Google Scholar] [CrossRef]
- Ge, Y.; Bagnall, A.; Stricklett, P.K.; Webb, D.; Kotelevtsev, Y.; Kohan, D.E. Combined knockout of collecting duct endothelin A and B receptors causes hypertension and sodium retention. Am. J. Physiol. Renal. Physiol. 2008, 295, F1635–F1640. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Bagnall, A.; Stricklett, P.K.; Strait, K.; Webb, D.J.; Kotelevtsev, Y.; Kohan, D.E. Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am. J. Physiol. Renal. Physiol. 2006, 291, F1274–F1280. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.; Ge, Y.; Stricklett, P.K.; Gill, P.; Taylor, D.; Hughes, A.K.; Yanagisawa, M.; Miller, L.; Nelson, R.D.; Kohan, D.E. Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J. Clin. Invest. 2004, 114, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Pearson, L.J.; Yandle, T.G.; Nicholls, M.G.; Evans, J.J. Regulation of endothelin-1 release from human endothelial cells by sex steroids and angiotensin-II. Peptides 2008, 29, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; Dal Canton, A. Functional changes in the aging kidney. J. Nephrol. 2010, 23, S41–S45. [Google Scholar] [PubMed]
- Burrell, L.M.; Johnston, C.I. Angiotensin II receptor antagonists. Potential in elderly patients with cardiovascular disease. Drugs Aging 1997, 10, 421–434. [Google Scholar] [CrossRef] [PubMed]
- Benigni, A.; Corna, D.; Zoja, C.; Sonzogni, A.; Latini, R.; Salio, M.; Conti, S.; Rottoli, D.; Longaretti, L.; Cassis, P.; et al. Disruption of the Ang II type 1 receptor promotes longevity in mice. J. Clin. Invest. 2009, 119, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Basso, N.; Paglia, N.; Stella, I.; de Cavanagh, E.M.; Ferder, L.; Arnaiz, M.d.R.L.; Inserra, F. Protective effect of the inhibition of the renin-angiotensin system on aging. Regul. Pept. 2005, 128, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.S.; Black, M.J.; Widdop, R.E. Influence of Angiotensin II Subtype 2 Receptor (AT(2)R) Antagonist, PD123319, on Cardiovascular Remodelling of Aged Spontaneously Hypertensive Rats during Chronic Angiotensin II Subtype 1 Receptor (AT(1)R) Blockade. Int. J. Hypertens. 2012, 2012, 543062. [Google Scholar] [CrossRef]
- Polderman, K.H.; Stehouwer, C.D.A.; van Kamp, G.J.; Dekker, G.A.; Verheugt, F.W.A.; Gooren, L.J.G. Influence of sex hormones on plasma endothelin levels. Ann. Intern. Med. 1993, 118, 429–432. [Google Scholar] [CrossRef]
- Kumanov, P.; Tomova, A.; Kirilov, G. Testosterone replacement therapy in male hypogonadism is not associated with increase of endothelin-1 levels. Int. J. Androl. 2007, 30, 41–47. [Google Scholar] [CrossRef]
- Montezano, A.; Callera, G.; Mota, A.; Fortes, Z.; Nigro, D.; Carvalho, M.; Zorn, T.; Tostes, R. Endothelin-1 contributes to the sexual differences in renal damage in DOCA-salt rats. Peptides 2005, 26, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, D.L.; Liu, Y., Jr.; Pergola, P.E. Selected contribution: Gender differences in the endothelin-B receptor contribution to basal cutaneous vascular tone in humans. J. Appl. Physiol. 2001, 91, 2407–2411. [Google Scholar] [CrossRef]
- Kawanishi, H.; Hasegawa, Y.; Nakano, D.; Ohkita, M.; Takaoka, M.; Ohno, Y.; Matsumura, Y. Involvement of the endothelin ET(B) receptor in gender differences in deoxycorticosterone acetate-salt-induced hypertension. Clin. Exp. Pharmacol. Physiol. 2007, 34, 280–285. [Google Scholar] [CrossRef]
- Kittikulsuth, W.; Pollock, J.S.; Pollock, D.M. Sex differences in renal medullary endothelin receptor function in angiotensin II hypertensive rats. Hypertension 2011, 58, 212–218. [Google Scholar] [CrossRef]
- Rafiq, K.; Noma, T.; Fujisawa, Y.; Ishihara, Y.; Arai, Y.; Nabi, A.N.; Suzuki, F.; Nagai, Y.; Nakano, D.; Hitomi, H.; et al. Renal sympathetic denervation suppresses de novo podocyte injury and albuminuria in rats with aortic regurgitation. Circulation 2012, 125, 1402–1413. [Google Scholar] [CrossRef] [PubMed]
- Chun, T.-Y.; Bankir, L.; Eckert, G.J.; Bichet, D.G.; Saha, C.; Zaidi, S.-A.; Wagner, M.A.; Pratt, J.H. Ethnic differences in renal responses to furosemide. Hypertension 2008, 52, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Sarafidis, P.A.; Li, S.; Chen, S.-C.; Collins, A.J.; Brown, W.W.; Klag, M.J.; Bakris, G.L. Hypertension awareness, treatment, and control in chronic kidney disease. Am. J. Med. 2008, 121, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.G.; Speed, J.S.; Jin, C.; Pollock, D.M.; Ryan, M.J.; Sullivan, J.C.; Douma, L.G.; Solocinski, K.; Holzworth, M.R.; Crislip, G.R.; et al. Loss of endothelin B receptor function impairs sodium excretion in a time- and sex-dependent manner. Am. J. Physiol. Renal. Physiol. 2016, 311, F991–F998. [Google Scholar] [CrossRef]
- Nakano, D.; Pollock, J.S.; Pollock, D.M. Renal medullary ETB receptors produce diuresis and natriuresis via NOS1. Am. J. Physiol. Renal. Physiol. 2008, 294, F1205-11. [Google Scholar] [CrossRef]
- Gohar, E.Y.; Speed, J.S.; Kasztan, M.; Jin, C.; Pollock, D.M.; Becker, B.K. Activation of purinergic receptors (P2) in the renal medulla promotes endothelin-dependent natriuresis in male rats. Am. J. Physiol. Renal. Physiol. 2016, 311, F260–F267. [Google Scholar] [CrossRef] [PubMed]
- Gohar, E.Y.; Kasztan, M.; Becker, B.K.; Speed, J.S.; Pollock, D.M. Ovariectomy uncovers purinergic receptor activation of endothelin-dependent natriuresis. Am. J. Physiol. Renal. Physiol. 2017, 313, F361–F369. [Google Scholar] [CrossRef] [PubMed]
- Oloyo, A.K.; Sofola, O.A.; Yakubu, M.A. Orchidectomy attenuates high-salt diet-induced increases in blood pressure, renovascular resistance, and hind limb vascular dysfunction: Role of testosterone. Clin. Exp. Pharmacol. Physiol. 2016, 43, 825–833. [Google Scholar] [CrossRef]
- Taylor, T.A.; Gariepy, C.E.; Pollock, D.M.; Pollock, J.S. Gender differences in ET and NOS systems in ETB receptor-deficient rats: Effect of a high salt diet. Hypertension 2003, 41, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Brinson, K.N.; Rafikova, O.; Sullivan, J.C. Female sex hormones protect against salt-sensitive hypertension but not essential hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R149–R157. [Google Scholar] [CrossRef] [PubMed]
- Gohar, E.Y.; Cook, A.K.; Pollock, D.M.; Inscho, E.W. Afferent arteriole responsiveness to endothelin receptor activation: Does sex matter? Biol. Sex. Differ. 2019, 10, 1. [Google Scholar] [CrossRef]
- Saleh, M.A.; Sandoval, R.M.; Rhodes, G.J.; Campos-Bilderback, S.B.; Molitoris, B.A.; Pollock, D.M. Chronic endothelin-1 infusion elevates glomerular sieving coefficient and proximal tubular albumin reuptake in the rat. Life Sci. 2012, 91, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.A.; Boesen, E.I.; Pollock, J.S.; Savin, V.J.; Pollock, D.M. Endothelin-1 increases glomerular permeability and inflammation independent of blood pressure in the rat. Hypertension 2010, 56, 942–949. [Google Scholar] [CrossRef]
- Heimlich, J.B.; Speed, J.S.; Bloom, C.J.; O’Connor, P.M.; Pollock, J.S.; Pollock, D.M. ET-1 increases reactive oxygen species following hypoxia and high-salt diet in the mouse glomerulus. Acta Physiol. 2015, 213, 722–730. [Google Scholar] [CrossRef]
- Schneider, M.P.; Ge, Y.; Pollock, D.M.; Pollock, J.S.; Kohan, D.E. Collecting duct-derived endothelin regulates arterial pressure and Na excretion via nitric oxide. Hypertension 2008, 51, 1605–1610. [Google Scholar] [CrossRef]
- Kalk, P.; Thöne-Reineke, C.; Schwarz, A.; Godes, M.; Bauer, C.; Pfab, T.; Hocher, B. Renal phenotype of ET-1 transgenic mice is modulated by androgens. Eur. J. Med. Res. 2009, 14, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Stringer, K.D.; Komers, R.; Osman, S.A.; Oyama, T.T.; Lindsley, J.N.; Anderson, S. Gender hormones and the progression of experimental polycystic kidney disease. Kidney Int. 2005, 68, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, M.; Yuba, M.; Fujii, T.; Ohkita, M.; Matsumura, Y. Oestrogen protects against ischaemic acute renal failure in rats by suppressing renal endothelin-1 overproduction. Clin. Sci. 2002, 103, 434S–437S. [Google Scholar] [CrossRef]
- De Miguel, C.; Speed, J.S.; Kasztan, M.; Gohar, E.Y.; Pollock, D.M. Endothelin-1 and the kidney: New perspectives and recent findings. Curr. Opin. Nephrol. Hypertens. 2016, 25, 35–41. [Google Scholar] [CrossRef]
- Yanes, L.L.; Romero, D.G.; Cucchiarelli, V.E.; Fortepiani, L.A.; Gomez-Sanchez, C.E.; Santacruz, F.; Reckelhoff, J.F. Role of endothelin in mediating postmenopausal hypertension in a rat model. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R229–R233. [Google Scholar] [CrossRef]
- Kontula, K.K.; Seppanen, P.J.; VAN Duyne, P.; Bardin, C.W.; Janne, O.A. Effect of a nonsteroidal antiandrogen, flutamide, on androgen receptor dynamics and ornithine decarboxylase gene expression in mouse kidney. Endocrinology 1985, 116, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Riazi, S.; Madala-Halagappa, V.K.; Hu, X.; Ecelbarger, C.A. Sex and body-type interactions in the regulation of renal sodium transporter levels, urinary excretion, and activity in lean and obese Zucker rats. Gend. Med. 2006, 3, 309–327. [Google Scholar] [CrossRef]
- Veiras, L.C.; Girardi, A.C.; Curry, J.; Pei, L.; Ralph, D.L.; Tran, A.; Castelo-Branco, R.C.; Pastor-Soler, N.; Arranz, C.T.; Yu, A.S.; et al. Sexual Dimorphic Pattern of Renal Transporters and Electrolyte Homeostasis. J. Am. Soc. Nephrol. 2017, 28, 3504–3517. [Google Scholar] [CrossRef]
- Wilson, D.K.; Bayer, L.; Sica, D.A. Variability in salt sensitivity classifications in black male versus female adolescents. Hypertension 1996, 28, 250–255. [Google Scholar] [CrossRef]
- Crofton, J.T.; Share, L. Gonadal hormones modulate deoxycorticosterone-salt hypertension in male and female rats. Hypertension 1997, 29, 494–499. [Google Scholar] [CrossRef]
- Quan, A.; Chakravarty, S.; Chen, J.-C.; Loleh, S.; Saini, N.; Harris, R.C.; Capdevila, J.; Quigley, R. Androgens augment proximal tubule transport. Am. J. Physiol. Renal. Physiol. 2004, 287, F452–F459. [Google Scholar] [CrossRef] [PubMed]
- Quinkler, M.; Bujalska, I.J.; Kaur, K.; Onyimba, C.U.; Buhner, S.; Allolio, B.; Hughes, S.V.; Hewison, M.; Stewart, P.M. Androgen receptor-mediated regulation of the alpha-subunit of the epithelial sodium channel in human kidney. Hypertension 2005, 46, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Loh, S.Y.; Giribabu, N.; Salleh, N. Sub-chronic testosterone treatment increases the levels of epithelial sodium channel (ENaC)-alpha, beta and gamma in the kidney of orchidectomized adult male Sprague-Dawley rats. PeerJ 2016, 4, e2145. [Google Scholar] [CrossRef] [PubMed]
- Quinn, S.; Harvey, B.J.; Thomas, W. Rapid aldosterone actions on epithelial sodium channel trafficking and cell proliferation. Steroids 2014, 81, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, J.F.; Zhang, H.; Srivastava, K.; Granger, J.P. Gender differences in hypertension in spontaneously hypertensive rats: Role of androgens and androgen receptor. Hypertension 1999, 34, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Masilamani, S.; Kim, G.H.; Mitchell, C.; Wade, J.B.; Knepper, M.A. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J. Clin. Invest. 1999, 104, R19–R23. [Google Scholar] [CrossRef]
- Riazi, S.; Maric, C.; Ecelbarger, C.A. 17-beta Estradiol attenuates streptozotocin-induced diabetes and regulates the expression of renal sodium transporters. Kidney Int. 2006, 69, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Gambling, L.; Dunford, S.; Wilson, C.A.; McArdle, H.J.; Baines, D.L. Estrogen and progesterone regulate alpha, beta, and gammaENaC subunit mRNA levels in female rat kidney. Kidney Int. 2004, 65, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-T.; Sun, C.-Y.; Pong, C.-Y.; Chen, Y.-C.; Lin, G.-P.; Chang, T.-C.; Wu, M.-S. Interaction of estrogen and progesterone in the regulation of sodium channels in collecting tubular cells. Chang Gung Med. J. 2007, 30, 305–312. [Google Scholar] [PubMed]
- Arias-Loza, P.A.; Muehlfelder, M.; Elmore, S.A.; Maronpot, R.; Hu, K.; Blode, H.; Hegele-Hartung, C.; Fritzemeier, K.H.; Ertl, G.; Pelzer, T. Differential effects of 17beta-estradiol and of synthetic progestins on aldosterone-salt-induced kidney disease. Toxicol. Pathol. 2009, 37, 969–982. [Google Scholar] [CrossRef]
- Pearce, D.; Soundararajan, R.; Trimpert, C.; Kashlan, O.B.; Deen, P.M.; Kohan, D.E. Collecting duct principal cell transport processes and their regulation. Clin. J. Am. Soc. Nephrol. 2015, 10, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Loh, S.Y.; Giribabu, N.; Salleh, N. Effects of gonadectomy and testosterone treatment on aquaporin expression in the kidney of normotensive and hypertensive rats. Exp. Biol. Med. 2017, 242, 1376–1386. [Google Scholar] [CrossRef] [PubMed]
- Loh, S.Y.; Giribabu, N.; Gholami, K.; Salleh, N. Effects of testosterone on mean arterial pressure and aquaporin (AQP)-1, 2, 3, 4, 6 and 7 expressions in the kidney of orchidectomized, adult male Sprague-Dawley rats. Arch. Biochem. Biophys. 2017, 614, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Ellis, B.; Li, X.C.; Miguel-Qin, E.; Gu, V.; Zhuo, J.L. Evidence for a functional intracellular angiotensin system in the proximal tubule of the kidney. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, R494–R509. [Google Scholar] [CrossRef] [PubMed]
- Mačković, M.; Zimolo, Z.; Burckhardt, G.; Sabolić, I. Isolation of renal brush-border membrane vesicles by a low-speed centrifugation; effect of sex hormones on Na+-H+ exchange in rat and mouse kidney. Biochim. Biophys. Acta 1986, 862, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Carrisoza-Gaytan, R.; Ray, E.C.; Flores, D.; Marciszyn, A.L.; Wu, P.; Liu, L.; Subramanya, A.R.; Wang, W.; Sheng, S.; Nkashama, L.J.; et al. Intercalated cell BKalpha subunit is required for flow-induced K+ secretion. JCI Insight 2020, 5, e130553. [Google Scholar] [CrossRef] [PubMed]
- Ong, A.C.; Fine, L.G. Loss of glomerular function and tubulointerstitial fibrosis: Cause or effect? Kidney Int. 1994, 45, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.; Neilson, E.G. Molecular mechanisms of tubulointerstitial hypertrophy and hyperplasia. Kidney Int. 1991, 39, 401–420. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.C.; Chan, L.; Schrier, R.W. Remnant kidney hypermetabolism and progression of chronic renal failure. Am. J. Physiol. 1988, 254, F267-76. [Google Scholar] [CrossRef]
- Ellison, K.E.; Ingelfinger, J.R.; Pivor, M.; Dzau, V.J. Androgen regulation of rat renal angiotensinogen messenger RNA expression. J. Clin. Invest. 1989, 83, 1941–1945. [Google Scholar] [CrossRef]
- Givens, J.R.; Andersen, R.N.; Ragland, J.B.; Wiser, W.L.; Umstot, E.S. Adrenal function in hirsutism I. Diurnal change and response of plasma androstenedione, testosterone, 17-hydroxyprogesterone, cortisol, LH and FSH to dexamethasone and 1/2 unit of ACTH. J. Clin. Endocrinol. Metab. 1975, 40, 988–1000. [Google Scholar] [CrossRef]
- Chen, Y.F.; Naftilan, A.J.; Oparil, S. Androgen-dependent angiotensinogen and renin messenger RNA expression in hypertensive rats. Hypertension 1992, 19, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Yanes, L.L.; Sartori-Valinotti, J.C.; Iliescu, R.; Romero, D.G.; Racusen, L.C.; Zhang, H.; Reckelhoff, J.F. Testosterone-dependent hypertension and upregulation of intrarenal angiotensinogen in Dahl salt-sensitive rats. Am. J. Physiol. Renal. Physiol. 2009, 296, F771–F779. [Google Scholar] [CrossRef]
- James, G.D.; Sealey, J.E.; Müller, F.; Alderman, M.; Madhavan, S.; Laragh, J.H. Renin relationship to sex, race and age in a normotensive population. J. Hypertens. Suppl. 1986, 4, S387–S389. [Google Scholar]
- Kienitz, T.; Quinkler, M. Testosterone and blood pressure regulation. Kidney Blood Press Res. 2008, 31, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Kau, M.-M.; Lo, M.-J.; Wang, S.-W.; Tsai, S.-C.; Chen, J.-J.; Chiao, Y.-C.; Yeh, J.-Y.; Lin, H.; Shum, A.Y.-C.; Fang, V.S.; et al. Inhibition of aldosterone production by testosterone in male rats. Metabolism 1999, 48, 1108–1114. [Google Scholar] [CrossRef]
- Herak-Kramberger, C.M.; Breljak, D.; Ljubojević, M.; Matokanović, M.; Lovrić, M.; Rogić, D.; Brzica, H.; Vrhovac, I.; Karaica, D.; Micek, V.; et al. Sex-dependent expression of water channel AQP1 along the rat nephron. Am. J. Physiol. Renal. Physiol. 2015, 308, F809–F821. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimian, T.; He, Y.; Schiffrin, E.L.; Touyz, R.M. Differential regulation of thioredoxin and NAD(P)H oxidase by angiotensin II in male and female mice. J. Hypertens. 2007, 25, 1263–1271. [Google Scholar] [CrossRef]
- Tatchum-Talom, R.; Eyster, K.M.; Martin, D.S. Sexual dimorphism in angiotensin II-induced hypertension and vascular alterations. Can. J. Physiol. Pharmacol. 2005, 83, 413–422. [Google Scholar] [CrossRef]
- Xue, B.; Pamidimukkala, J.; Hay, M. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am. J. Physiol. Heart. Circ. Physiol. 2005, 288, H2177–H2184. [Google Scholar] [CrossRef]
- Sartori-Valinotti, J.C.; Iliescu, R.; Yanes, L.L.; Dorsett-Martin, W.; Reckelhoff, J.F. Sex differences in the pressor response to angiotensin II when the endogenous renin-angiotensin system is blocked. Hypertension 2008, 51, 1170–1176. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Anacta, L.A.; Cattran, D.C. Impact of gender on the renal response to angiotensin II. Kidney Int. 1999, 55, 278–285. [Google Scholar] [CrossRef]
- Howard, C.G.; Mitchell, K.D. Renal functional responses to selective intrarenal renin inhibition in Cyp1a1-Ren2 transgenic rats with ANG II-dependent malignant hypertension. Am. J. Physiol. Renal. Physiol. 2012, 302, F52–F59. [Google Scholar] [CrossRef]
- Machnik, A.; Neuhofer, W.; Jantsch, J.; Dahlmann, A.; Tammela, T.; Machura, K.; Park, J.-K.; Beck, F.-X.; Müller, D.N.; Derer, W.; et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat. Med. 2009, 15, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Rands, V.F.; Seth, D.M.; Kobori, H.; Prieto, M.C. Sexual dimorphism in urinary angiotensinogen excretion during chronic angiotensin II-salt hypertension. Gend. Med. 2012, 9, 207–218. [Google Scholar] [CrossRef]
- Ji, H.; Menini, S.; Zheng, W.; Pesce, C.; Wu, X.; Sandberg, K. Role of angiotensin-converting enzyme 2 and angiotensin(1-7) in 17beta-oestradiol regulation of renal pathology in renal wrap hypertension in rats. Exp. Physiol. 2008, 93, 648–657. [Google Scholar] [CrossRef]
- Brosnihan, K.B.; Li, P.; Ganten, D.; Ferrario, C.M. Estrogen protects transgenic hypertensive rats by shifting the vasoconstrictor-vasodilator balance of RAS. Am. J. Physiol. 1997, 273, R1908–R1915. [Google Scholar] [CrossRef]
- Sampson, A.K.; Moritz, K.M.; Denton, K.M. Postnatal ontogeny of angiotensin receptors and ACE2 in male and female rats. Gend. Med. 2012, 9, 21–32. [Google Scholar] [CrossRef]
- Sampson, A.K.; Moritz, K.M.; Jones, E.S.; Flower, R.L.; Widdop, R.E.; Denton, K.M. Enhanced angiotensin II type 2 receptor mechanisms mediate decreases in arterial pressure attributable to chronic low-dose angiotensin II in female rats. Hypertension 2008, 52, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Baiardi, G.; Macova, M.; Armando, I.; Ando, H.; Tyurmin, D.; Saavedra, J.M. Estrogen upregulates renal angiotensin II AT1 and AT2 receptors in the rat. Regul. Pept. 2005, 124, 7–17. [Google Scholar] [CrossRef]
- Dean, S.A.; Tan, J.; O’Brien, E.R.; Leenen, F.H. 17beta-estradiol downregulates tissue angiotensin-converting enzyme and ANG II type 1 receptor in female rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R759–R766. [Google Scholar] [CrossRef] [PubMed]
- Hilliard, L.M.; Sampson, A.K.; Brown, R.D.; Denton, K.M. The “his and hers” of the renin-angiotensin system. Curr. Hypertens. Rep. 2013, 15, 71–79. [Google Scholar] [CrossRef]
- Abadir, P.M.; Carey, R.M.; Siragy, H.M. Angiotensin AT2 receptors directly stimulate renal nitric oxide in bradykinin B2-receptor-null mice. Hypertension 2003, 42, 600–604. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.D.; Hilliard, L.M.; Head, G.A.; Jones, E.S.; Widdop, R.E.; Denton, K.M. Sex differences in the pressor and tubuloglomerular feedback response to angiotensin II. Hypertension 2012, 59, 129–135. [Google Scholar] [CrossRef]
- Os, I.; Franco, V.; Kjeldsen, S.E.; Manhem, K.; Devereux, R.B.; Gerdts, E.; Hille, D.A.; Lyle, P.A.; Okin, P.M.; Dahlof, B.; et al. Effects of losartan in women with hypertension and left ventricular hypertrophy: Results from the Losartan Intervention for Endpoint Reduction in Hypertension Study. Hypertension 2008, 51, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Hudson, M.; Rahme, E.; Behlouli, H.; Sheppard, R.; Pilote, L. Sex differences in the effectiveness of angiotensin receptor blockers and angiotensin converting enzyme inhibitors in patients with congestive heart failure--a population study. Eur. J. Heart Fail. 2007, 9, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Reckelhoff, J.F.; Zhang, H.; Srivastava, K. Gender differences in development of hypertension in spontaneously hypertensive rats: Role of the renin-angiotensin system. Hypertension 2000, 35, 480–483. [Google Scholar] [CrossRef]
- Nakagawa, K.; Marji, J.S.; Schwartzman, M.L.; Waterman, M.R.; Capdevila, J.H. Androgen-mediated induction of the kidney arachidonate hydroxylases is associated with the development of hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1055–R1062. [Google Scholar] [CrossRef] [PubMed]
- Maier, K.G.; Roman, R.J. Cytochrome P450 metabolites of arachidonic acid in the control of renal function. Curr. Opin. Nephrol. Hypertens. 2001, 10, 81–87. [Google Scholar] [CrossRef]
- Wu, C.C.; Schwartzman, M.L. The role of 20-HETE in androgen-mediated hypertension. Prostaglandins Other Lipid Mediat. 2011, 96, 45–53. [Google Scholar] [CrossRef]
- Cheema, M.U.; Irsik, D.L.; Wang, Y.; Miller-Little, W.; Hyndman, K.A.; Marks, E.S.; Frøkiær, J.; Boesen, E.I.; Norregaard, R. Estradiol regulates AQP2 expression in the collecting duct: A novel inhibitory role for estrogen receptor alpha. Am. J. Physiol. Renal. Physiol. 2015, 309, F305–F317. [Google Scholar] [CrossRef] [PubMed]
- O’Donald, P. “Haldane’s dilemma” and the rate of natural selection. Nature 1969, 221, 815–817. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.T.; Aslibekyan, S.; Tiwari, H.K.; Zhi, D.; Sung, Y.J.; Hunt, S.C.; Rao, D.C.; Broeckel, U.; Judd, S.E.; Muntner, P.; et al. PCSK9 variation and association with blood pressure in African Americans: Preliminary findings from the HyperGEN and REGARDS studies. Front. Genet. 2015, 6, 136. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, T.; Lindgren, A.; Engström, G.; Jern, C.; Melander, O. A stop-codon of the phosphodiesterase 11A gene is associated with elevated blood pressure and measures of obesity. J. Hypertens. 2016, 34, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Han, B.G.; KoGES Group. Cohort Profile: The Korean Genome and Epidemiology Study (KoGES) Consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef] [PubMed]
- Yasukochi, Y.; Sakuma, J.; Takeuchi, I.; Kato, K.; Oguri, M.; Fujimaki, T.; Horibe, H.; Yamada, Y. Longitudinal exome-wide association study to identify genetic susceptibility loci for hypertension in a Japanese population. Exp. Mol. Med. 2017, 49, e409. [Google Scholar] [CrossRef] [PubMed]
- Knopp, R.H.; Paramsothy, P.; Retzlaff, B.M.; Fish, B.; Walden, C.; Dowdy, A.; Tsunehara, C.; Aikawa, K.; Cheung, M.C. Gender differences in lipoprotein metabolism and dietary response: Basis in hormonal differences and implications for cardiovascular disease. Curr. Atheroscler. Rep. 2005, 7, 472–479. [Google Scholar] [CrossRef]
- Lapointe, A.; Balk, E.M.; Lichtenstein, A.H. Gender differences in plasma lipid response to dietary fat. Nutr. Rev. 2006, 64, 234–249. [Google Scholar] [CrossRef] [PubMed]
- Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Kokkinos, P.; Marinakis, N.; Stefanadis, C.; Toutouzas, P.K. Gender differences on the risk evaluation of acute coronary syndromes: The CARDIO2000 study. Prev. Cardiol. 2003, 6, 71–77. [Google Scholar] [CrossRef]
- Abramson, B.L.; Melvin, R.G. Cardiovascular risk in women: Focus on hypertension. Can. J. Cardiol. 2014, 30, 553–559. [Google Scholar] [CrossRef]
| Study | Population | Key Findings |
|---|---|---|
| Messerli, F.H. et al. [11] | Male and female patients with hypertension | Found that men have higher blood pressure compared to women starting at puberty, with this trend reversing after menopause. |
| van Kesteren, P.J.M [12] | M—>F and F→M transexuals | Male-to-female transsexuals exhibit lower ET levels after estrogen supplementation, whereas testosterone treatment in female-to-male transsexuals leads to an increase in ET levels. |
| Phillips, G.B. et al. [9] | Men with coronary artery disease | Found an association of hypotestosteronemia with coronary artery disease in men. |
| Christakou, C.D. et al. [13] | Women with polycystic ovary syndrome | Discussed the role of androgen excess on metabolic aberrations and cardiovascular risk in women with PCOS. |
| Chen, W. et al. [14] | Men and women without hypertension at baseline | Suggested that the endothelial NO synthase gene may contribute to the predisposition of females for hypertension |
| Curtis, J.J. et al. [15] | Hypertensive patients undergoing renal transplantation | Found remission of essential hypertension after renal transplantation. |
| Zapater P. et al. [16] | Men and women with hypertension | Found that women had lower blood pressure and angiotensin converting enzyme (ACE) activities than men |
| Ruggenenti P. et al. [17] | Men and women with hypertension | Found that men exhibit a lower response to ACE inhibitor treatment |
| Cho, S.B. et al. [18] | GWAS | Indicated a sex-specific genetic susceptibility to hypertension |
| Song, S. et al. [19] | Men and women without hypertension at baseline | Found that a diet rich in grains and legumes is inversely associated with the risk of hypertension in Korean women |
| Kang, Y. et al. [20] | Men and women without hypertension at baseline | Found that frequent fried food consumption is associated with hypertension in Korean women |
| Animal Model | Sex-Specific Findings | References |
|---|---|---|
| Spontaneously Hypertensive Rats (SHRs) | Testosterone mediates hypertension via AR; flutamide decreases blood pressure | [21,22,23,24,25] |
| Deoxycorticosterone Acetate (DOCA)-Salt Rats | Higher blood pressure elevation in males; Endothelin B (ETB) receptor knockout abolishes blood pressure differences | [26] |
| Ren2 Gene-Expressing Rats | Flutamide lowers blood pressure and reduces organ damage | [27] |
| Orchidectomized Sprague Dawley Rats | Testosterone increases ENaC expression; castration decreases blood pressure | [28,29] |
| Ovariectomized Rats | Salt-independent hypertension in DS rats post-ovariectomy | [30,31] |
| Angiotensin II (Ang II)-Infused Mice | Blood pressure increase in females with male kidney; Angiotensing 1 receptor (AT1R) and endothelin 1 (ET-1) mRNA differences | [32] |
| High-Fat Diet (HFD)-Induced Hypertension | HFD induces hypertension in males; losartan prevents this; and females resistant due to Ang (1–7) | [33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stamellou, E.; Sterzer, V.; Alam, J.; Roumeliotis, S.; Liakopoulos, V.; Dounousi, E. Sex-Specific Differences in Kidney Function and Blood Pressure Regulation. Int. J. Mol. Sci. 2024, 25, 8637. https://doi.org/10.3390/ijms25168637
Stamellou E, Sterzer V, Alam J, Roumeliotis S, Liakopoulos V, Dounousi E. Sex-Specific Differences in Kidney Function and Blood Pressure Regulation. International Journal of Molecular Sciences. 2024; 25(16):8637. https://doi.org/10.3390/ijms25168637
Chicago/Turabian StyleStamellou, Eleni, Viktor Sterzer, Jessica Alam, Stefanos Roumeliotis, Vassilios Liakopoulos, and Evangelia Dounousi. 2024. "Sex-Specific Differences in Kidney Function and Blood Pressure Regulation" International Journal of Molecular Sciences 25, no. 16: 8637. https://doi.org/10.3390/ijms25168637






