Advances in Millimeter-Wave Treatment and Its Biological Effects Development
Abstract
:1. Introduction
2. Methodology
2.1. Literature Retrieval Strategy
2.2. Screening Criteria and Process
2.3. Quality Assessment and Data Extraction
3. Overview of MMWT and Its Biological Effects
4. Possibility of MMWT in Diseases
4.1. In Vivo and Vitro Studies of Cancer and Malignancy
4.2. In Vivo and Vitro Studies of Other Diseases
5. Study on the Mechanism of MMW Thermal Effect
| Cell/Tissue/Animal | Frequency | Power Density or SAR | Exposure Duration/Target Temperature | Research Group | Publication Year |
|---|---|---|---|---|---|
| Artificial lipid bilayer membrane | 54–74 GHz | 2000 W/kg | 5–25 min CW and Pulse modulation irradiation | Alekseev et al. [44] | 1995 |
| BP-4 pacemaker neuron of the pond snail Lymnaea stagnalis | 75 GHz | - | 12–22 min | Alekseev et al. [45] | 1997 |
| Human forearm and middle finger skin | 42.25 GHz | 55 and 208 mW/cm2 | up to 10, 20 min | Alekseev et al. [52] | 2005 |
| Lymnaea neurons | 60.22–62.22 GHz and 75 GHz | - | 5, 10, 20 min | Alekseev et al. [46] | 1999 |
| Sprague-Dawley rats | 35 GHz | 75 mW/cm2 | under a lethal temperature | Frei et al. [53] | 1995 |
| Healthy C57BL/6 mouse skin | 101 GHz PW | 0.5–1.5 kW | pulse duration 5–10 μs, 20–50 pulses | Furman et al. [61] | 2020 |
| Differentiating neuron-like cells | 60.4 GHz | 10 mW/cm2 | 24 h | Haas et al. [62] | 2016 |
| Neuron-like cells PC12 cells | 60.4 GHz | 10 mW/cm2 | 24 h | Haas et al. [63] | 2016 |
| NGF-treated PC12 cells | 60.4 GHz | 5 mW/cm2 | 24 h | Haas et al. [64] | 2017 |
| Primary culture of human keratinocytes | 60 GHz | 20 mW/cm2 | 3 h | Habauzit et al. [12] | 2014 |
| Rats skin | 35 GHz | 75 mW/cm2 | 0, 19, 38 min | Jauchem et al. [54] | 2016 |
| Dutch rabbit eyes | 60 GHz | 475 or 1898 mW/cm2 | 6, 30 min | Kojima et al. [56] | 2009 |
| Rabbit closed eyelid | 40, 60, 75, 90 and 162 GHz | ≈233 mW/cm2 | 6 min | Kojima et al. [58] | 2022 |
| Rabbit eyes | 40, 75 and 90 GHz | 10–600 mW/cm2 | 6 min | Kojima et al. [57] | 2019 |
| Skin cancer in DMBA-initiated SENCAR mice | 94 GHz | 1 or 333 mW/cm2 | 10 s/time, 2 times/week, 12 weeks | Mason et al. [65] | 2001 |
| Rats skin | 35 GHz | 75 mW/cm2 | 3–6 h or 24 | Millenbaugh et al. [48] | 2008 |
| Melanoma cells | 58.4 GHz CW or PW | 3.7 W | 49.2 °C | Orlacchio et al. [28] | 2019 |
| Keratinocyte and melanocyte cell lines | 60.4 GHz | 1–20 mW/cm2 | 20 min, 1 h, 6 h, 16 h, 24 h | Quément et al. [66] | 2014 |
| Leech interneuron | 60 GHz | around 100 mW | ≤40 °C | Romanenko et al. [67] | 2019 |
| Midbody ganglion nerve cells | 60 GHz | 1, 2, 4 mW/cm2 | 1 min | Romanenko et al. [47] | 2014 |
| Xenopus spinal cord neurons | 94 GHz | 31 mW/cm2 | 0.2–1 s | Samsonov et al. [68] | 2013 |
| Xenopus laevis oocytes | 60 GHz | 1–600 mW/cm2 | 1.66 min | Shapiro et al. [60] | 2013 |
| BHK-21/C13 cells | 41.8, 74.0 GHz | 320, 450 mW/cm2 | 42.0–44.5 °C | Stensaas et al. [43] | 1981 |
| Macrophage cells | 35 GHz | 75 mW/cm2 | 41–42 °C | Sypniewska et al. [23] | 2010 |
| B16F10 murine melanoma cells | 42.25 GHz | 0.74–1.48 mW/cm2 | 30 min | Szabo et al. [41] | 2004 |
| Human epidermal keratinocytes-HaCaT | 42.25 GHz (with high IPD), 61.2 GHz (with low IPD) | 29 mW/cm2 (low IPD), 1.67 W/cm2 (high IPD) | 30, 60 min | Szabo et al. [69] | 2001 |
| Neurons derived from mouse embryonic stem cells | 94 GHz | 18.6 kW/m2 | 60 min | Titushkin et al. [70] | 2009 |
| Human venous blood | 32.9–39.6 GHz | 10 mW/cm2 | 15 min | Vlasova et al. [71] | 2018 |
| Rats skin | 35 GHz | 0.5–7.5 W/cm2 | 0.5 min | Xie et al. [55] | 2011 |
6. Study of Mechanism of MMW Non-Thermal Effect
6.1. Cell Membrane Structure
6.2. Cell Morphology and Cell Cycle
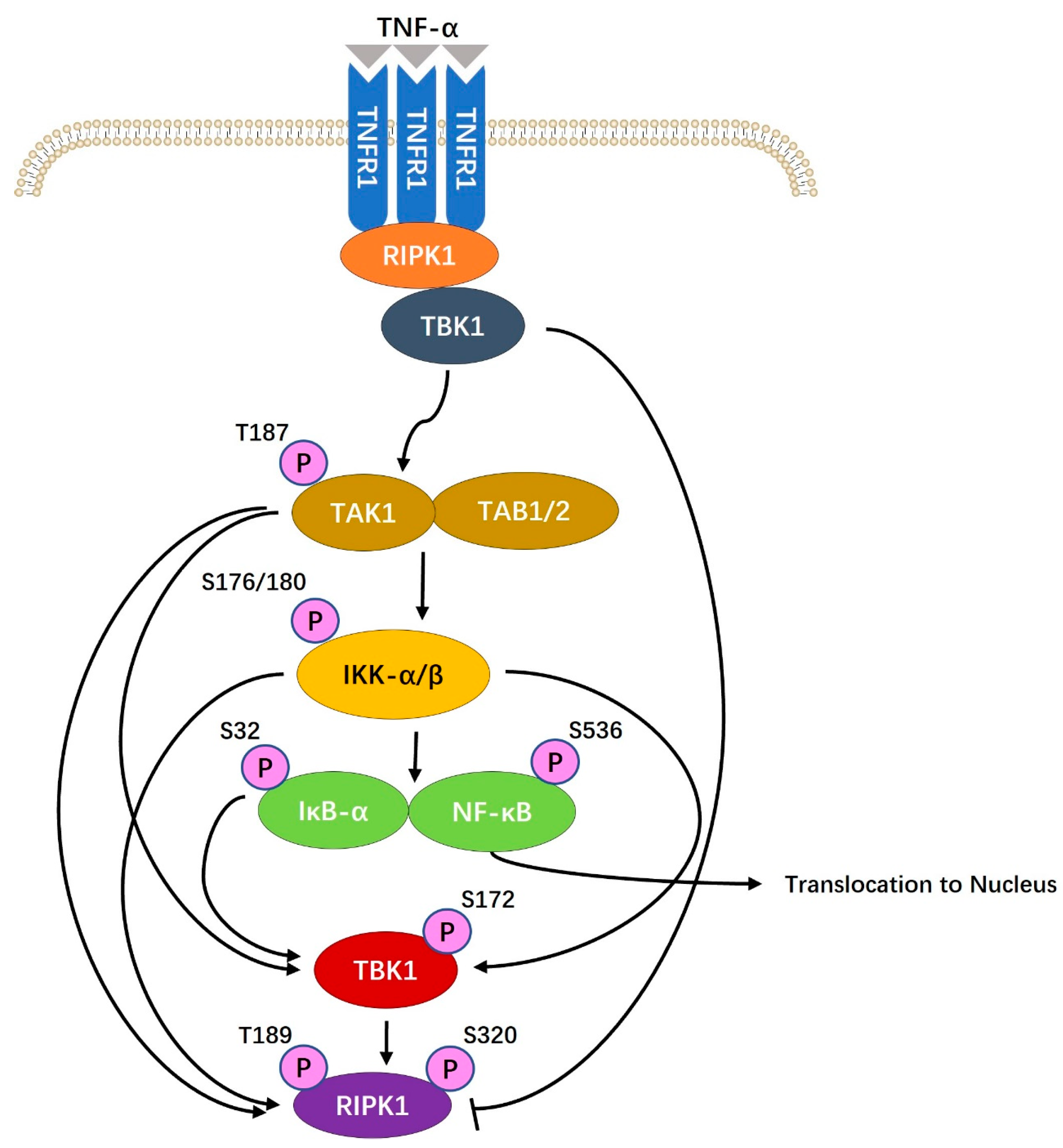
6.3. Cell Signal Pathway
7. Research on the Effect of MMW on the Nervous System and Immune System
7.1. Nervous System
7.2. Immune System
8. Research on MMW Biosafety
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Szabo, I.; Kappelmayer, J.; Alekseev, S.I.; Ziskin, M.C. Millimeter wave induced reversible externalization of phosphatidylserine molecules in cells exposed in vitro. Bioelectromagnetics 2006, 27, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Alekseev, S.; Ziskin, M. Influence of blood flow and millimeter wave exposure on skin temperature in different thermal models. Bioelectromagnetics 2009, 30, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Ziskin, M.C. Physiological mechanisms underlying millimeter wave therapy. In Bioelectromagnetics Current Concepts; Springer: Berlin/Heidelberg, Germany, 2006; pp. 241–251. [Google Scholar]
- Lebedeva, N. Neurophysiological mechanisms of biological effects of peripheral action of low-intensity nonionizing electromagnetic fields in humans. Millim. Waves Med. Biol. 1995, 1, 138–140. [Google Scholar]
- Pall, M.L. Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J. Cell. Mol. Med. 2013, 17, 958–965. [Google Scholar] [CrossRef]
- Bush, L.; Hill, D.; Riazi, A.; Stensaas, L.; Partlow, L.; Gandhi, O. Effects of millimeter-wave radiation on monolayer cell cultures. III. A search for frequency-specific athermal biological effects on protein synthesis. Bioelectromagnetics 1981, 2, 151–159. [Google Scholar] [CrossRef]
- Li, X.; Wu, G.; Wu, M.; Chen, W.; Liu, X. In vitro study of inhibitory millimeter wave treatment effects on the TNF-alpha-induced NF-kappaB signal transduction pathway. J. Mol. Med. 2011, 27, 71–78. [Google Scholar]
- Beneduci, A.; Chidichimo, G.; Tripepi, S.; Perrotta, E.; Cufone, F. Antiproliferative effect of millimeter radiation on human erythromyeloid leukemia cell line K562 in culture: Ultrastructural- and metabolic-induced changes. Bioelectrochemistry 2007, 70, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, H. The Biological Effects of Microwaves and Related Questions. Adv. Electron. Electron Phys. 1980, 53, 85–152. [Google Scholar]
- Pakhomov, A.G.; Akyel, Y.; Pakhomova, O.N.; Stuck, B.E.; Murphy, M.R. Current state and implications of research on biological effects of millimeter waves: A review of the literature. Bioelectromagnetics 1998, 19, 393–413. [Google Scholar] [CrossRef]
- Zhadobov, M.; Chahat, N.; Sauleau, R.; Le Quement, C.; Le Drean, Y. Millimeter-wave interactions with the human body: State of knowledge and recent advances. Int. J. Microw. Wirel. Technol. 2011, 3, 237–247. [Google Scholar] [CrossRef]
- Habauzit, D.; Le Quement, C.; Zhadobov, M.; Martin, C.; Aubry, M.; Sauleau, R.; Le Drean, Y. Transcriptome analysis reveals the contribution of thermal and the specific effects in cellular response to millimeter wave exposure. PLoS ONE 2014, 9, e109435. [Google Scholar] [CrossRef] [PubMed]
- Beneduci, A.; Chidichimo, G.; De Rose, R.; Filippelli, L.; Straface, S.V.; Venuta, S. Frequency and irradiation time-dependant antiproliferative effect of low-power millimeter waves on RPMI 7932 human melanoma cell line. Anticancer Res. 2005, 25, 1023–1028. [Google Scholar] [PubMed]
- Ni, J.-X.; Li, M.; Liang, L.-P.; Li, S.-L.; Yang, L.-Q.; Hu, J.-J.; Zhao, R.; Wang, X.-P.; Dou, Z.; Li, X.-H. Clinical effect of millimeter wave applied to acupoints on treatment of COVID-19. Chin. J. Nosocomial. 2020, 30, 2583–2587. [Google Scholar]
- Egot-Lemaire, S.J.P.; Ziskin, M.C. Dielectric properties of human skin at an acupuncture point in the 50–75 GHz frequency range: A pilot study. Bioelectromagnetics 2011, 32, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zeng, Q.L.; Lu, D.Q.; Chiang, H. Millimeter wave exposure reverses TPA suppression of gap junction intercellular communication in HaCaT human keratinocytes. Bioelectromagnetics 2004, 25, 1–4. [Google Scholar] [CrossRef]
- Radzievsky, A.A.; Gordiienko, O.V.; Szabo, I.; Alekseev, S.I.; Ziskin, M.C. Millimeter wave-induced suppression of B16 F10 melanoma growth in mice: Involvement of endogenous opioids. Bioelectromagnetics 2004, 25, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Beneduci, A. Evaluation of the potential in vitro antiproliferative effects of millimeter waves at some therapeutic frequencies on RPMI 7932 human skin malignant melanoma cells. Cell Biochem. Biophys. 2009, 55, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liu, Y.; Liu, S.; Luo, T.; Zhong, G.Y.; Liu, A.; Zeng, Q.; Xin, S.X. Apoptosis-Promoting Effects on A375 Human Melanoma Cells Induced by Exposure to 35.2-GHz Millimeter Wave. Technol. Cancer Res. Treat. 2020, 19, 1533033820934131. [Google Scholar] [CrossRef] [PubMed]
- Beneduci, A.; Chidichimo, G.; Tripepi, S.; Perrotta, E. Transmission electron microscopy study of the effects produced by wide-band low-power millimeter waves on MCF-7 human breast cancer cells in culture. Anticancer Res. 2005, 25, 1009–1013. [Google Scholar]
- Komoshvili, K.; Israel, K.; Levitan, J.; Yahalom, A.; Barbora, A.; Liberman-Aronov, S. W-Band Millimeter Waves Targeted Mortality of H1299 Human Lung Cancer Cells without Affecting Non-Tumorigenic MCF-10A Human Epithelial Cells In Vitro. Appl. Sci. 2020, 10, 4813. [Google Scholar] [CrossRef]
- Li, X.; Ye, H.; Cai, L.; Yu, F.; Chen, W.; Lin, R.; Zheng, C.; Xu, H.; Ye, J.; Wu, G.; et al. Millimeter wave radiation induces apoptosis via affecting the ratio of Bax/Bcl-2 in SW1353 human chondrosarcoma cells. Oncol. Rep. 2012, 27, 664–672. [Google Scholar] [CrossRef]
- Sypniewska, R.K.; Millenbaugh, N.J.; Kiel, J.L.; Blystone, R.V.; Ringham, H.N.; Mason, P.A.; Witzmann, F.A. Protein changes in macrophages induced by plasma from rats exposed to 35 GHz millimeter waves. Bioelectromagnetics 2010, 31, 656–663. [Google Scholar] [CrossRef]
- Makar, V.R.; Logani, M.K.; Bhanushali, A.; Alekseev, S.I.; Ziskin, M.C. Effect of cyclophosphamide and 61.22 GHz millimeter waves on T-cell, B-cell, and macrophage functions. Bioelectromagnetics 2006, 27, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Makar, V.; Logani, M.; Szabo, I.; Ziskin, M. Effect of millimeter waves on cyclophosphamide induced suppression of T cell functions. Bioelectromagnetics 2003, 24, 356–365. [Google Scholar] [CrossRef]
- Makar, V.R.; Logani, M.K.; Bhanushali, A.; Kataoka, M.; Ziskin, M.C. Effect of millimeter waves on natural killer cell activation. Bioelectromagnetics 2005, 26, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Komoshvili, K.; Becker, T.; Levitan, J.; Yahalom, A.; Barbora, A.; Liberman-Aronov, S. Morphological Changes in H1299 Human Lung Cancer Cells Following W-Band Millimeter-Wave Irradiation. Appl. Sci. 2020, 10, 3187. [Google Scholar] [CrossRef]
- Orlacchio, R.; Le Page, Y.; Le Drean, Y.; Le Guevel, R.; Sauleau, R.; Alekseev, S.; Zhadobov, M. Millimeter-wave pulsed heating in vitro: Cell mortality and heat shock response. Sci. Rep. 2019, 9, 15249. [Google Scholar] [CrossRef] [PubMed]
- Samoilov, V.O.; Shadrin, E.B.; Filippova, E.B.; Katsnelson, Y.; Backhoff, H.; Eventov, M. The effect of transcranial electromagnetic brain stimulation on the acquisition of the conditioned response in rats. Biophysics 2015, 60, 303–308. [Google Scholar] [CrossRef]
- Logani, M.K.; Anga, A.; Szabo, I.; Agelan, A.; Irizarry, A.R.; Ziskin, M.C. Effect of millimeter waves on cyclophosphamide induced suppression of the immune system. Bioelectromagnetics 2002, 23, 614–621. [Google Scholar] [CrossRef]
- Gapeyev, A.B.; Mikhailik, E.N.; Chemeris, N.K. Anti-inflammatory effects of low-intensity extremely high-frequency electromagnetic radiation: Frequency and power dependence. Bioelectromagnetics 2008, 29, 197–206. [Google Scholar] [CrossRef]
- Gapeyev, A.B.; Kulagina, T.P.; Aripovsky, A.V.; Chemeris, N.K. The role of fatty acids in anti-inflammatory effects of low-intensity extremely high-frequency electromagnetic radiation. Bioelectromagnetics 2011, 32, 388–395. [Google Scholar] [CrossRef]
- Rojavin, M.A.; Cowan, A.; Radzievsky, A.A.; Ziskin, M.C. Antipruritic effect of millimeter waves in mice: Evidence for opioid involvement. Bioelectromagnetics 1998, 63, PL251–PL257. [Google Scholar] [CrossRef]
- Rojavin, M.A.; Radzievsky, A.A.; Cowan, A.; Ziskin, M.C. Pain relief caused by millimeter waves in mice: Results of cold water tail flick tests. Int. J. Radiat. Biol. 2000, 76, 575–579. [Google Scholar] [PubMed]
- Radzievsky, A.A.; Gordiienko, O.V.; Alekseev, S.; Szabo, I.; Cowan, A.; Ziskin, M.C. Electromagnetic millimeter wave induced hypoalgesia: Frequency dependence and involvement of endogenous opioids. Bioelectromagnetics 2008, 29, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Luo, Q.-L.; Lin, H.-D.; Zhang, J.-L.; Guo, H.; He, C.-Q. The effect of different treatment time of millimeter wave on chondrocyte apoptosis, caspase-3, caspase-8, and MMP-13 expression in rabbit surgically induced model of knee osteoarthritis. Rheumatol. Int. 2012, 32, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Radzievsky, A.A.; Rojavin, M.A.; Cowan, A.; Alekseev, S.I.; Radzievsky, A.A., Jr.; Ziskin, M.C. Peripheral neural system involvement in hypoalgesic effect of electromagnetic millimeter waves. Life Sci. 2001, 68, 1143–1151. [Google Scholar] [CrossRef]
- Radzievsky, A.A.; Rojavin, M.A.; Cowan, A.; Ziskin, M.C. Suppression of pain sensation caused by millimeter waves: A double-blinded, cross-over, prospective human volunteer study. Anesth. Analg. 1999, 88, 836–840. [Google Scholar] [CrossRef] [PubMed]
- Sivachenko, I.B.; Medvedev, D.S.; Molodtsova, I.D.; Panteleev, S.S.; Sokolov, A.Y.; Lyubashina, O.A. Effects of Millimeter-Wave Electromagnetic Radiation on the Experimental Model of Migraine. Bull. Exp. Biol. Med. 2016, 160, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Partyla, T.; Hacker, H.; Edinger, H.; Leutzow, B.; Lange, J.; Usichenko, T. Remote effects of electromagnetic millimeter waves on experimentally induced cold pain: A double-blinded crossover investigation in healthy volunteers. Anesth. Analg. 2017, 124, 980–985. [Google Scholar] [CrossRef]
- Szabo, I.; Alekseev, S.I.; Acs, G.; Radzievsky, A.A.; Logani, M.K.; Makar, V.R.; Gordiienko, O.R.; Ziskin, M.C. Destruction of Cutaneous Melanoma With Millimeter Wave Hyperthermia in Mice. IEEE Trans. Plasma Sci. 2004, 32, 1653–1660. [Google Scholar] [CrossRef]
- Szabo, I.; Manning, M.R.; Radzievsky, A.A.; Wetzel, M.A.; Rogers, T.J.; Ziskin, M.C. Low power millimeter wave irradiation exerts no harmful effect on human keratinocytes in vitro. Bioelectromagnetics 2003, 24, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Stensaas, L.; Partlow, L.; Bush, L.; Iversen, P.; Hill, D.; Hagmann, M.; Gandhi, O. Effects of millimeter-wave radiation on monolayer cell cultures. II. Scanning and transmission electron microscopy. Bioelectromagnetics 1981, 2, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Alekseev, S.; Ziskin, M. Millimeter microwave effect on ion transport across lipid bilayer membranes. Bioelectromagnetics 1995, 16, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Alekseev, S.I.; Ziskin, M.C.; Kochetkova, N.V.; Bolshakov, M.A. Millimeter waves thermally alter the firing rate of the Lymnaea pacemaker neuron. Bioelectromagnetics 1997, 18, 89–98. [Google Scholar] [CrossRef]
- Alekseev, S.I.; Ziskin, M.C. Effects of millimeter waves on ionic currents of Lymnaea neurons. Bioelectromagnetics 1999, 20, 24–33. [Google Scholar] [CrossRef]
- Romanenko, S.; Siegel, P.H.; Wagenaar, D.A.; Pikov, V. Effects of millimeter wave irradiation and equivalent thermal heating on the activity of individual neurons in the leech ganglion. J. Neurophysiol. 2014, 112, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Millenbaugh, N.J.; Roth, C.; Sypniewska, R.; Chan, V.; Eggers, J.S.; Kiel, J.L.; Blystone, R.V.; Mason, P.A. Gene expression changes in the skin of rats induced by prolonged 35 GHz millimeter-wave exposure. J. Radiat. Res. 2008, 169, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Aronov, S.; Einat, M.; Furman, O.; Pilossof, M.; Komoshvili, K.; Ben-Moshe, R.; Yahalom, A.; Levitan, J. Millimeter-wave insertion loss of mice skin. J. Electromagn. Waves Appl. 2017, 32, 758–767. [Google Scholar] [CrossRef]
- Alekseev, S.; Radzievsky, A.; Logani, M.; Ziskin, M. Millimeter wave dosimetry of human skin. Bioelectromagnetics 2008, 29, 65–70. [Google Scholar] [CrossRef]
- Morelli, M.S.; Gallucci, S.; Siervo, B.; Hartwig, V. Numerical analysis of electromagnetic field exposure from 5G mobile communications at 28 GHZ in adults and children users for real-world exposure scenarios. Int. J. Environ. Res. Public Health 2021, 18, 1073. [Google Scholar] [CrossRef]
- Alekseev, S.I.; Radzievsky, A.A.; Szabo, I.; Ziskin, M.C. Local heating of human skin by millimeter waves: Effect of blood flow. Bioelectromagnetics 2005, 26, 489–501. [Google Scholar] [CrossRef] [PubMed]
- Frei, M.R.; Ryan, K.L.; Berger, R.E.; Jauchem, J.R. Sustained 35-GHz radiofrequency irradiation induces circulatory failure. Shock 1995, 4, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Jauchem, J.R.; Ryan, K.L.; Walters, T.J. Pathophysiological alterations induced by sustained 35-GHz radio-frequency energy heating. J. Basic Clin. Physiol. Pharmacol. 2016, 27, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Pei, J.; Cui, Y.; Zhang, J.; Qi, H.; Chen, S.; Qiao, D. EEG changes as heat stress reactions in rats irradiated by high intensity 35 GHz millimeter waves. Health Phys. 2011, 100, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Hanazawa, M.; Yamashiro, Y.; Sasaki, H.; Watanabe, S.; Taki, M.; Suzuki, Y.; Hirata, A.; Kamimura, Y.; Sasaki, K. Acute ocular injuries caused by 60-Ghz millimeter-wave exposure. Health Phys. 2009, 97, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Suzuki, Y.; Sasaki, K.; Taki, M.; Wake, K.; Watanabe, S.; Mizuno, M.; Tasaki, T.; Sasaki, H. Ocular effects of exposure to 40, 75, and 95 GHz millimeter waves. J. Infrared Millim. Terahertz Waves 2018, 39, 912–925. [Google Scholar] [CrossRef]
- Kojima, M.; Tasaki, T.; Suzuki, Y.; Kamijo, T.; Hada, A.; Kik, A.; Ikehata, M.; Sasaki, H. Threshold for Millimeter-Wave (60 GHz)-Induced Ocular Injury. J. Infrared Millim. Terahertz Waves 2022, 43, 260–271. [Google Scholar] [CrossRef]
- Papaioannou, A.; Samaras, T. Numerical model of heat transfer in the rabbit eye exposed to 60-GHz millimeter wave radiation. IEEE. Trans. Biomed. Eng. 2011, 58, 2582–2588. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, M.G.; Priest, M.F.; Siegel, P.H.; Bezanilla, F. Thermal mechanisms of millimeter wave stimulation of excitable cells. Biophysics 2013, 104, 2622–2628. [Google Scholar] [CrossRef]
- Furman, O.; Komoshvili, K.; Levitan, J.; Yahalom, A.; Marks, H.; Borodin, D.; Liberman-Aronov, S. The Lack of Toxic Effect of High-Power Short-Pulse 101 GHz Millimeter Waves on Healthy Mice. Bioelectromagnetics 2020, 41, 188–199. [Google Scholar] [CrossRef]
- Haas, A.J.; Le Page, Y.; Zhadobov, M.; Boriskin, A.; Sauleau, R.; Le Drean, Y. Impact of 60-GHz millimeter waves on stress and pain-related protein expression in differentiating neuron-like cells. Bioelectromagnetics 2016, 37, 444–454. [Google Scholar] [CrossRef]
- Haas, A.J.; Le Page, Y.; Zhadobov, M.; Sauleau, R.; Le Dréan, Y. Effects of 60-GHz millimeter waves on neurite outgrowth in PC12 cells using high-content screening. Neurosci. Lett. 2016, 618, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Haas, A.J.; Le Page, Y.; Zhadobov, M.; Sauleau, R.; Drean, Y.L.; Saligaut, C. Effect of acute millimeter wave exposure on dopamine metabolism of NGF-treated PC12 cells. J. Radiat. Res. 2017, 58, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Mason, P.A.; Walters, T.J.; DiGiovanni, J.; Beason, C.W.; Jauchem, J.R.; Dick, E.J., Jr.; Mahajan, K.; Dusch, S.J.; Shields, B.A.; Merritt, J.H.; et al. Lack of effect of 94 GHz radio frequency radiation exposure in an animal model of skin carcinogenesis. J. Carcinog. 2001, 22, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Le Quément, C.; Nicolaz, C.N.; Habauzit, D.; Zhadobov, M.; Sauleau, R.; Le Dréan, Y. Impact of 60-GHz millimeter waves and corresponding heat effect on endoplasmic reticulum stress sensor gene expression. Bioelectromagnetics 2014, 35, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Romanenko, S.; Harvey, A.R.; Hool, L.; Fan, S.; Wallace, V.P. Millimeter Wave Radiation Activates Leech Nociceptors via TRPV1-Like Receptor Sensitization. Biophys. J. 2019, 116, 2331–2345. [Google Scholar] [CrossRef] [PubMed]
- Samsonov, A.; Popov, S.V. The effect of a 94 GHz electromagnetic field on neuronal microtubules. Bioelectromagnetics 2013, 34, 133–144. [Google Scholar] [CrossRef]
- Szabo, I.; Rojavin, M.A.; Rogers, T.J.; Ziskin, M.C. Reactions of keratinocytes to in vitro millimeter wave exposure. Bioelectromagnetics 2001, 22, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Titushkin, I.A.; Rao, V.S.; Pickard, W.F.; Moros, E.G.; Shafirstein, G.; Cho, M.R. Altered calcium dynamics mediates P19-derived neuron-like cell responses to millimeter-wave radiation. Radiat. Res. 2009, 172, 725–736. [Google Scholar] [CrossRef]
- Vlasova, I.I.; Mikhalchik, E.V.; Gusev, A.A.; Balabushevich, N.G.; Gusev, S.A.; Kazarinov, K.D. Extremely high-frequency electromagnetic radiation enhances neutrophil response to particulate agonists. Bioelectromagnetics 2018, 39, 144–155. [Google Scholar] [CrossRef]
- Kremer, F.; Koschnitzke, C.; Santo, L.; Quick, P.; Poglitsch, A. The Non-Thermal Effect on Millimeter Wave Radiation on the Puffing of Giant Chromosomes. In Coherent Excitations in Biological Systems; Springer: Berlin/Heidelberg, Germany, 1983; pp. 10–20. [Google Scholar]
- Le Pogam, P.; Le Page, Y.; Habauzit, D.; Doué, M.; Zhadobov, M.; Sauleau, R.; Le Dréan, Y.; Rondeau, D. Untargeted metabolomics unveil alterations of biomembranes permeability in human HaCaT keratinocytes upon 60 GHz millimeter-wave exposure. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Binhi, V.J.B. Primary physical mechanism of the biological effects of weak magnetic fields. Biophys. J. 2016, 61, 170–176. [Google Scholar] [CrossRef]
- Binhi, V.N.; Prato, F.S. A physical mechanism of magnetoreception: Extension and analysis. Bioelectromagnetics 2017, 38, 41–52. [Google Scholar] [CrossRef]
- D’Agostino, S.; Della Monica, C.; Palizzi, E.; Di Pietrantonio, F.; Benetti, M.; Cannatà, D.; Cavagnaro, M.; Sardari, D.; Stano, P.; Ramundo-Orlando, A. Extremely high frequency electromagnetic fields facilitate electrical signal propagation by increasing transmembrane potassium efflux in an artificial axon model. Sci. Rep. 2018, 8, 9299. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, C.; Liang, W.; Ye, H.; Chen, W.; Lin, R.; Li, Z.; Liu, X.; Wu, M. Millimeter wave promotes the synthesis of extracellular matrix and the proliferation of chondrocyte by regulating the voltage-gated K+ channel. J. Bone Miner. Metab. 2014, 32, 367–377. [Google Scholar] [CrossRef]
- Sun, S.; Titushkin, I.; Varner, J.; Cho, M. Millimeter wave-induced modulation of calcium dynamics in an engineered skin co-culture model: Role of secreted ATP on calcium spiking. J. Radiat. Res. 2012, 53, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wu, G.; Li, X.; Li, Z.; Zheng, C.; Liu, X.; Ye, H. Millimeter Wave Treatment Inhibits Apoptosis of Chondrocytes via Regulation Dynamic Equilibrium of Intracellular Free Ca2+. Evid. Based Complement. Altern. Med. 2015, 2015, 464161. [Google Scholar] [CrossRef]
- Simkó, M.; Mattsson, M.-O. Activation of the intracellular temperature and ROS sensor membrane protein STIM1 as a mechanism underpinning biological effects of low-level low frequency magnetic fields. Med. Hypotheses 2019, 122, 68–72. [Google Scholar] [CrossRef]
- Geletyuk, V.I.; Kazachenko, V.N.; Chemeris, N.K.; Fesenko, E.E. Dual effects of microwaves on single Ca2+-activated K+ channels in cultured kidney cells Vero. FEBS Lett. 1995, 359, 85–88. [Google Scholar] [CrossRef]
- Albini, M.; Dinarelli, S.; Pennella, F.; Romeo, S.; Zampetti, E.; Girasole, M.; Morbiducci, U.; Massa, R.; Ramundo-Orlando, A. Induced movements of giant vesicles by millimeter wave radiation. Biochim. Biophys. Acta 2014, 1838, 1710–1718. [Google Scholar] [CrossRef]
- Shckorbatov, Y.G.; Grigoryeva, N.; Shakhbazov, V.; Grabina, V.; Bogoslavsky, A. Microwave irradiation influences on the state of human cell nuclei. Bioelectromagnetics 1998, 19, 414–419. [Google Scholar] [CrossRef]
- De Amicis, A.; Sanctis, S.D.; Cristofaro, S.D.; Franchini, V.; Lista, F.; Regalbuto, E.; Giovenale, E.; Gallerano, G.P.; Nenzi, P.; Bei, R.; et al. Biological effects of in vitro THz radiation exposure in human foetal fibroblasts. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2015, 793, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Du, M.; Liu, X.; Wu, M.; Ye, H.; Lin, J.; Chen, W.; Wu, G. Millimeter wave treatment inhibits NO-induced apoptosis of chondrocytes through the p38MAPK pathway. J. Mol. Med. 2010, 25, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ye, H.; Yu, F.; Cai, L.; Li, H.; Chen, J.; Wu, M.; Chen, W.; Lin, R.; Li, Z.; et al. Millimeter wave treatment promotes chondrocyte proliferation via G1/S cell cycle transition. J. Mol. Med. 2012, 29, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Sferra, T.; Chen, X.; Chen, Y.; Wu, M.; Xu, H.; Peng, J.; Liu, X. Millimeter wave treatment inhibits the mitochondrion-dependent apoptosis pathway in chondrocytes. Mol. Med. Rep. 2011, 4, 1001–1006. [Google Scholar] [PubMed]
- Burlaka, A.; Selyuk, M.; Gafurov, M.; Lukin, S.; Potaskalova, V.; Sidorik, E. Changes in mitochondrial functioning with electromagnetic radiation of ultra high frequency as revealed by electron paramagnetic resonance methods. Int. J. Radiat. Biol. 2014, 90, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Soubere Mahamoud, Y.; Aite, M.; Martin, C.; Zhadobov, M.; Sauleau, R.; Le Dréan, Y.; Habauzit, D. Additive effects of millimeter waves and 2-deoxyglucose co-exposure on the human keratinocyte transcriptome. PLoS ONE 2016, 11, e0160810. [Google Scholar] [CrossRef] [PubMed]
- Narinyan, L.; Ayrapetyan, S. Cyclic AMP-dependent signaling system is a primary metabolic target for non-thermal effect of microwaves on heart muscle hydration. Electromagn. Biol. Med. 2017, 36, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Alekseev, S.I.; Gordiienko, O.V.; Radzievsky, A.A.; Ziskin, M.C. Millimeter wave effects on electrical responses of the sural nerve in vivo. Bioelectromagnetics 2010, 31, 180–190. [Google Scholar] [CrossRef]
- Pakhomov, A.G.; Prol, H.K.; Mathur, S.P.; Akyel, Y.; Campbell, C.B.G. Frequency-Specific Effects of Millimeter-Wavelength Electromagnetic Radiation in Isolated Nerve. Electron Magnetobiol. 2009, 16, 43–57. [Google Scholar] [CrossRef]
- Pikov, V.; Arakaki, X.; Harrington, M.; Fraser, S.E.; Siegel, P.H. Modulation of neuronal activity and plasma membrane properties with low-power millimeter waves in organotypic cortical slices. J. Neural Eng. 2010, 7, 045003. [Google Scholar] [CrossRef]
- Safronova, V.G.; Gabdoulkhakova, A.G.; Santalov, B.F. Immunomodulating action of low intensity millimeter waves on primed neutrophils. Bioelectromagnetics 2002, 23, 599–606. [Google Scholar] [CrossRef]
- Radzievsky, A.A.; Rojavin, M.A.; Cowan, A.; Alekseev, S.I.; Ziskin, M.C. Hypoalgesic effect of millimeter waves in mice: Dependence on the site of exposure. Life Sci. 2000, 66, 2101–2111. [Google Scholar] [CrossRef] [PubMed]
- Radzievsky, A.A.; Cowan, A.; Byrd, C.; Radzievsky, A.A., Jr.; Ziskin, M.C. Single millimeter wave treatment does not impair gastrointestinal transit in mice. Life Sci. 2002, 71, 1763–1770. [Google Scholar] [CrossRef]
- Radzievsky, A.; Gordiienko, O.; Cowan, A.; Alekseev, S.I.; Ziskin, M.C. Millimeter-Wave-Induced Hypoalgesia in Mice: Dependence on Type of Experimental Pain. IEEE Trans. Plasma Sci. 2004, 32, 1634–1643. [Google Scholar] [CrossRef]
- Gapeyev, A.; Safronova, V.; Chemeris, N.; Fesenko, E.E. Inhibition of the production of reactive oxygen species in mouse peritoneal neutrophils by millimeter wave radiation in the near and far field zones of the radiator. Bioelectrochem. Bioenerg. 1997, 43, 217–220. [Google Scholar] [CrossRef]
- Logani, M.K.; Agelan, A.; Ziskin, M.C. Effect of millimeter wave radiation on catalase activity. Electromagn. Biol. Med. 2002, 21, 303–308. [Google Scholar] [CrossRef]
- Logani, M.K.; Alekseev, S.; Bhopale, M.K.; Slovinsky, W.S.; Ziskin, M.C. Effect of millimeter waves and cyclophosphamide on cytokine regulation. Immunopharmacol. Immunotoxicol. 2012, 34, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Korenstein-Ilan, A.; Barbul, A.; Hasin, P.; Eliran, A.; Gover, A.; Korenstein, R. Terahertz radiation increases genomic instability in human lymphocytes. Radiat. Res. 2008, 170, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Logani, M.; Yi, L.; Ziskin, M.C. Millimeter waves enhance delayed-type hypersensitivity in mouse skin. Electro Magnetobiol. 1999, 18, 165–176. [Google Scholar] [CrossRef]
- Novoselova, E.; Ogaĭ, V.; Sinotova, O.; Glushkova, O.; Sorokina, O.; Fesenko, E.J.B. Effect oh millimeter waves on the immune system in mice with experimental tumors. Biofizika 2002, 47, 933–942. [Google Scholar] [PubMed]
- Chatterjee, I.; Yoon, J.; Wiese, R.; Luongo, S.; Mastin, P.; Sadovnik, L.; Craviso, G.L. Millimeter wave bioeffects at 94 GHz on skeletal muscle contraction. In Proceedings of the 2013 IEEE Topical Conference on Biomedical Wireless Technologies, Networks, and Sensing Systems, Austin, TX, USA, 20–23 January 2013; pp. 67–69. [Google Scholar]
- Subbotina, T.; Tereshkina, O.; Khadartsev, A.; Yashin, A. Effect of low-intensity extremely high frequency radiation on reproductive function in Wistar rats. Bull. Exp. Biol. Med. 2006, 142, 189–190. [Google Scholar] [CrossRef] [PubMed]
- Deghoyan, A.; Heqimyan, A.; Nikoghosyan, A.; Dadasyan, E.; Ayrapetyan, S. Cell bathing medium as a target for non thermal effect of millimeter waves. Electromagn. Biol. Med. 2012, 31, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Nicolaz, C.N.; Zhadobov, M.; Desmots, F.; Ansart, A.; Sauleau, R.; Thouroude, D.; Michel, D.; Le Drean, Y. Study of narrow band millimeter-wave potential interactions with endoplasmic reticulum stress sensor genes. Bioelectromagnetics 2009, 30, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Partlow, L.; Bush, L.; Stensaas, L.; Hill, D.; Riazi, A.; Gandhi, O. Effects of millimeter-wave radiation on monolayer cell cultures. I. Design and validation of a novel exposure system. Bioelectromagnetics 1981, 2, 123–140. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Narita, E.; Shimizu, Y.; Suzuki, Y.; Shiina, T.; Taki, M.; Shinohara, N.; Miyakoshi, J. Effects of Long-Term Exposure to 60 GHz Millimeter-Wavelength Radiation on the Genotoxicity and Heat Shock Protein (Hsp) Expression of Cells Derived from Human Eye. Int. J. Environ. Res. Public Health 2016, 13, 802. [Google Scholar] [CrossRef] [PubMed]
- Zhadobov, M.; Sauleau, R.; Le Coq, L.; Debure, L.; Thouroude, D.; Michel, D.; Le Drean, Y. Low-power millimeter wave radiations do not alter stress-sensitive gene expression of chaperone proteins. Bioelectromagnetics 2007, 28, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Nicolas Nicolaz, C.; Zhadobov, M.; Desmots, F.; Sauleau, R.; Thouroude, D.; Michel, D.; Le Drean, Y. Absence of direct effect of low-power millimeter-wave radiation at 60.4 GHz on endoplasmic reticulum stress. Cell Biol. Toxicol. 2009, 25, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Zhadobov, M.; Nicolaz, C.N.; Sauleau, R.; Desmots, F.; Thouroude, D.; Michel, D.; Le Dréan, Y. Evaluation of the potential biological effects of the 60-GHz millimeter waves upon human cells. Antennas Propag. 2009, 57, 2949–2956. [Google Scholar] [CrossRef]
- Koyama, S.; Narita, E.; Suzuki, Y.; Shiina, T.; Taki, M.; Shinohara, N.; Miyakoshi, J. Long-term exposure to a 40-GHz electromagnetic field does not affect genotoxicity or heat shock protein expression in HCE-T or SRA01/04 cells. J. Radiat. Res. 2019, 60, 417–423. [Google Scholar] [CrossRef]
- Vijayalaxmi; Logani, M.K.; Bhanushali, A.; Ziskin, M.C.; Prihoda, T.J. Micronuclei in peripheral blood and bone marrow cells of mice exposed to 42 GHz electromagnetic millimeter waves. Radiat. Res. 2004, 161, 341–345. [Google Scholar] [PubMed]
- Koyama, S.; Narita, E.; Shimizu, Y.; Shiina, T.; Taki, M.; Shinohara, N.; Miyakoshi, J. Twenty Four-Hour Exposure to a 0.12 THz Electromagnetic Field Does Not Affect the Genotoxicity, Morphological Changes, or Expression of Heat Shock Protein in HCE-T Cells. Int. J. Environ. Res. Public Health 2016, 13, 793. [Google Scholar] [CrossRef] [PubMed]
- Yaekashiwa, N.; Otsuki, S.; Hayashi, S.I.; Kawase, K. Investigation of the non-thermal effects of exposing cells to 70–300 GHz irradiation using a widely tunable source. J. Radiat. Res. 2018, 59, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Volkova, N.A.; Pavlovich, E.V.; Gapon, A.A.; Nikolov, O.T. Effects of millimeter-wave electromagnetic exposure on the morphology and function of human cryopreserved spermatozoa. Bull. Exp. Biol. Med. 2014, 157, 574–576. [Google Scholar] [CrossRef]
- Kues, H.A.; D’Anna, S.A.; Osiander, R.; Green, W.R.; Monahan, J.C. Absence of ocular effects after either single or repeated exposure to 10 mW/cm2 from a 60 GHz CW source. Bioelectromagnetics 1999, 20, 463–473. [Google Scholar] [CrossRef]

| Tumor Cell or Tissue | Frequency | Power Density | Exposure Duration | Research Result | Research Group | Publication Year |
|---|---|---|---|---|---|---|
| RPMI 7932 human melanoma cells | 52–78 GHz | - | 1 h/s day, 3 h/day, up to 7 days | Growth inhibition | Beneduci et al. [13] | 2005 |
| MCF-7 human breast cancer cell | 52–78 GHz | 0.07 μW/cm2 | - | Growth inhibition | Beneduci et al. [20] | 2005 |
| RPMI 7932 human melanoma cells | 42.20, 53.57 GHz | 0.3 mW/cm2 | 1 h/day, 4 days | No influence | Beneduci et al. [18] | 2009 |
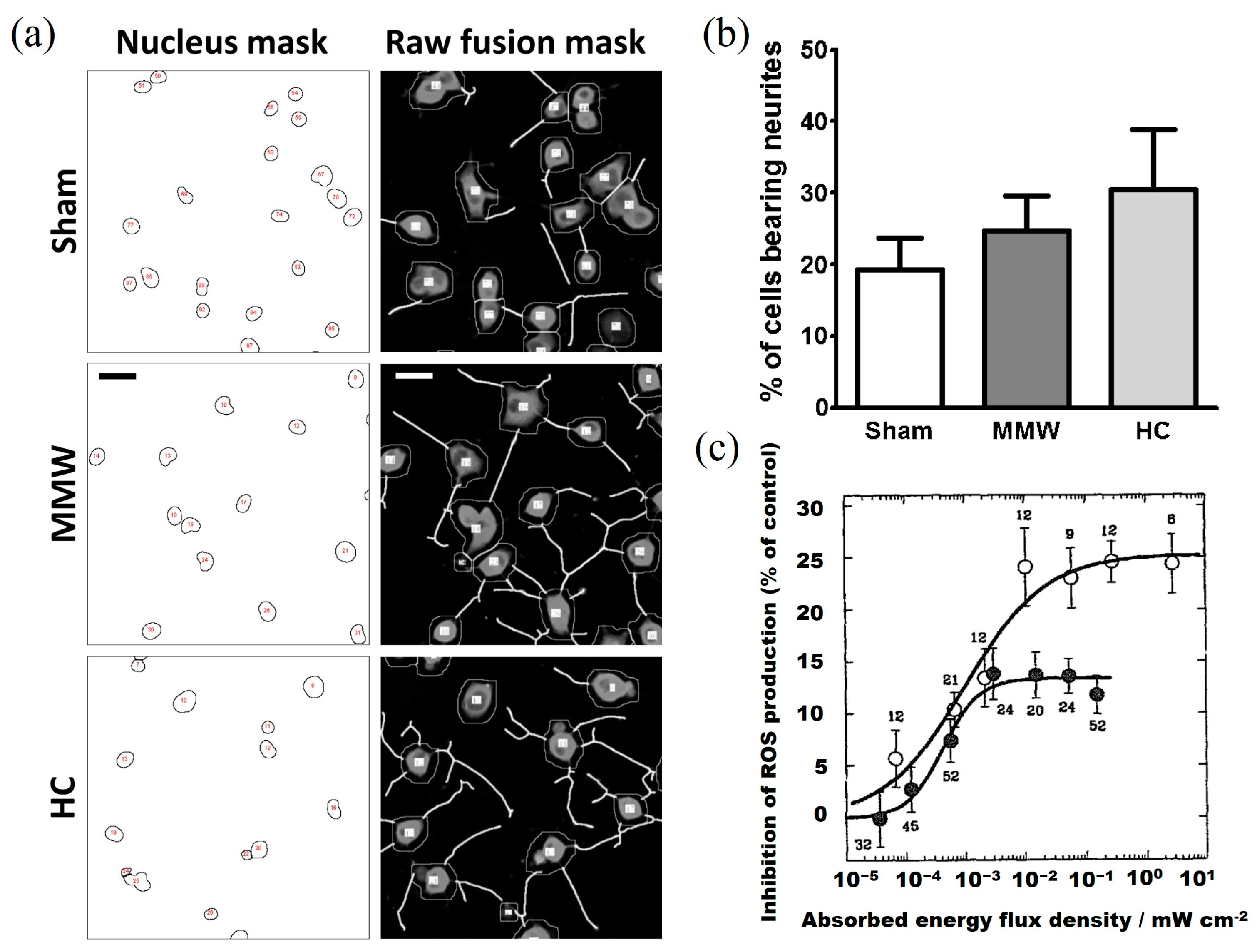
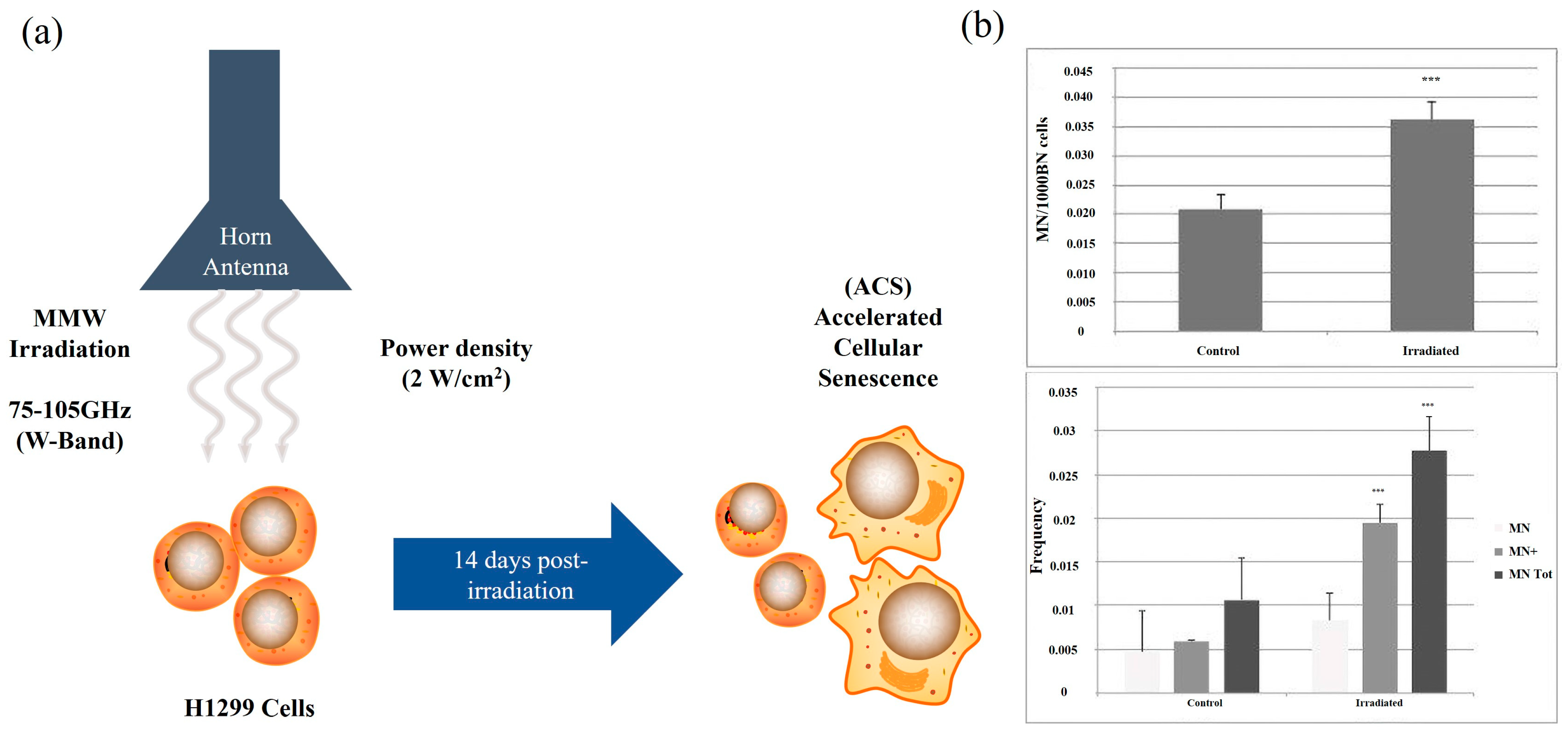
| H1299 human lung cancer cells | 75–105 GHz | 0.2 mW/cm2 | 2, 4, or 10 min | Increase cell mortality and aging | Komoshvili et al. [27] | 2020 |
| H1299 human lung cancer cells and Non-tumorigenic MCF-10A epithelial cells | 75–105 GHz | 0.2 mW/cm2 | 2, 4, or 10 min | H1299 cells died, but MCF-10A cells had no effect | Komoshvili et al. [21] | 2020 |
| SW1353 human chondrosarcoma cells | 33.2 ± 3 mm–45.6 ± 4 mm | 4 mW/cm2 | 15, 30, 60, 90, and 120 min | Inhibit activity | Li et al. [22] | 2012 |
| Melanoma cells | 58.4 GHz CW or PW | 3.7 W | 90 min pulse sequence (pulse duration 1.5 s and period 20 s) | Inducing cell damage | Orlacchio et al. [28] | 2019 |
| B16 F10 mouse melanoma cells | 61.22 GHz | 13.3 mW/cm2 | 15 min, 15 min/5 days | Growth inhibition | Radzievsky et al. [17] | 2004 |
| MCF-7 human breast cancer cell | 50–75 GHz | Low-power | - | Growth inhibition | Samoilov et al. [29] | 2015 |
| A375 human melanoma cells | 35.2 GHz | 0.16 mW/cm2 | 90 min | Inhibit vitality and induce apoptosis | Zhao et al. [19] | 2020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jing, R.; Jiang, Z.; Tang, X. Advances in Millimeter-Wave Treatment and Its Biological Effects Development. Int. J. Mol. Sci. 2024, 25, 8638. https://doi.org/10.3390/ijms25168638
Jing R, Jiang Z, Tang X. Advances in Millimeter-Wave Treatment and Its Biological Effects Development. International Journal of Molecular Sciences. 2024; 25(16):8638. https://doi.org/10.3390/ijms25168638
Chicago/Turabian StyleJing, Rui, Zhenqi Jiang, and Xiaoying Tang. 2024. "Advances in Millimeter-Wave Treatment and Its Biological Effects Development" International Journal of Molecular Sciences 25, no. 16: 8638. https://doi.org/10.3390/ijms25168638
APA StyleJing, R., Jiang, Z., & Tang, X. (2024). Advances in Millimeter-Wave Treatment and Its Biological Effects Development. International Journal of Molecular Sciences, 25(16), 8638. https://doi.org/10.3390/ijms25168638






