Effect of Synthetic Vitreous Fiber Exposure on TMEM16A Channels in a Xenopus laevis Oocyte Model
Abstract
1. Introduction
2. Results
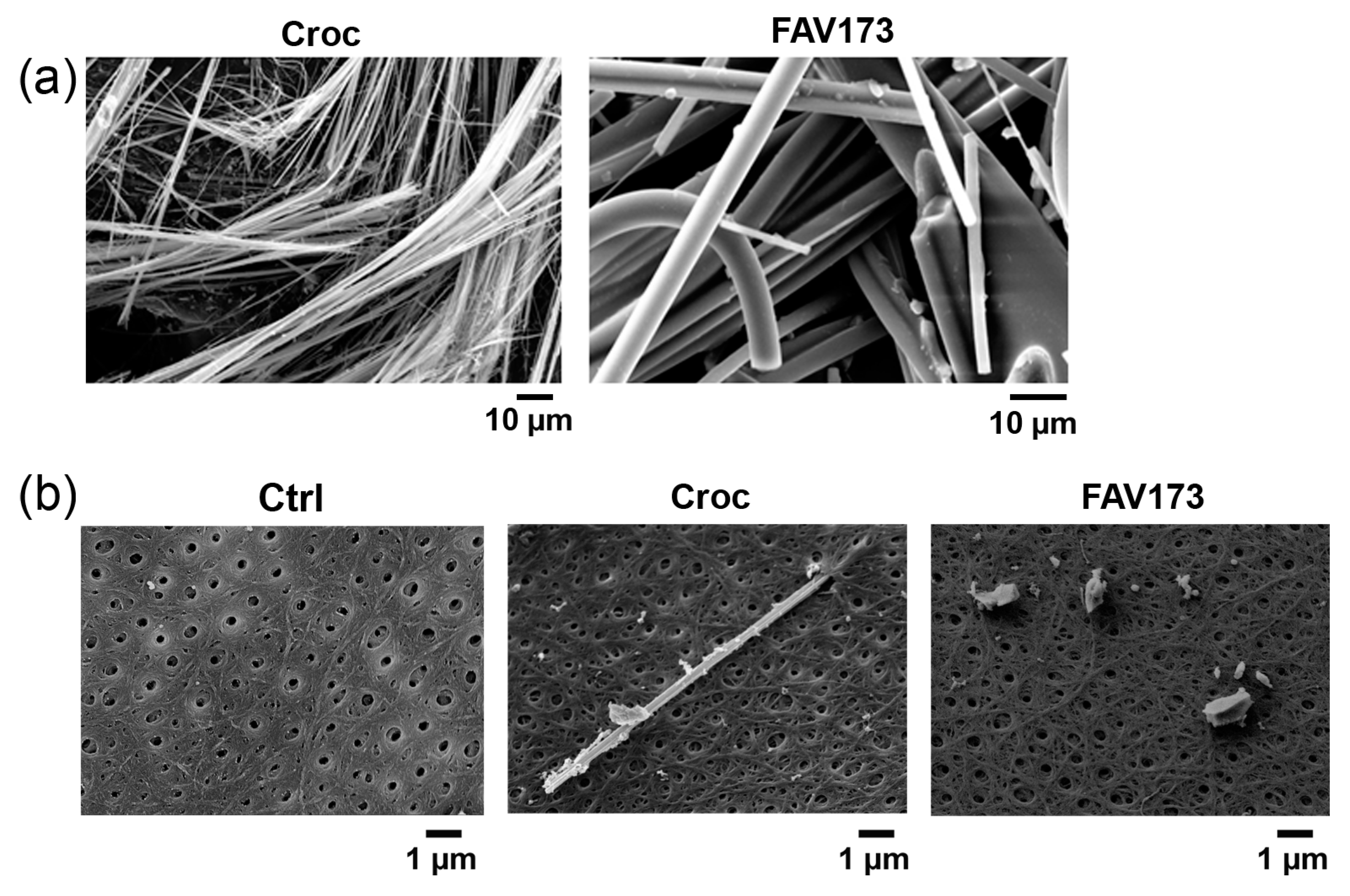
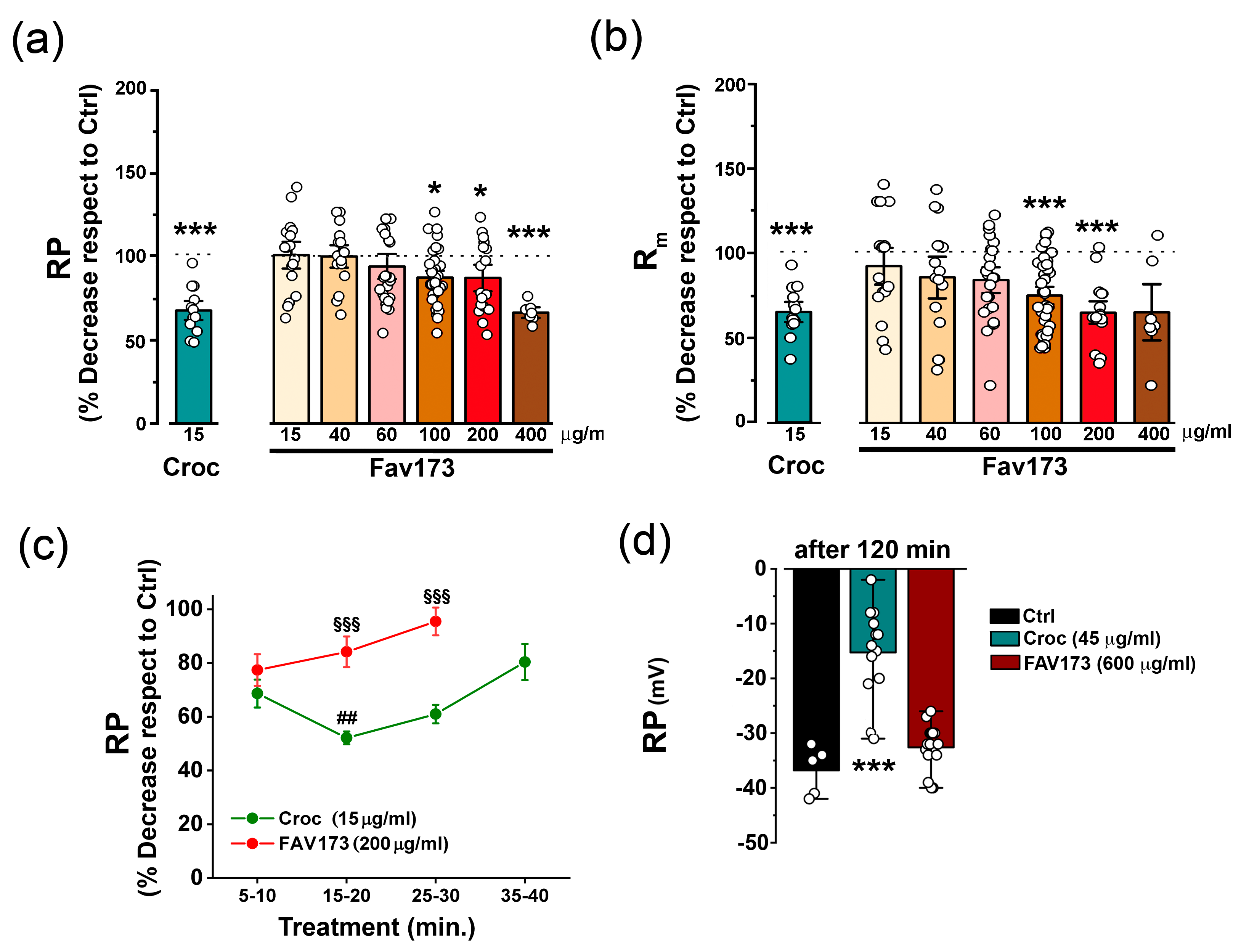
2.1. Effects of FAV173 Fiber Exposure on the Passive Membrane Properties of Xenopus Oocytes
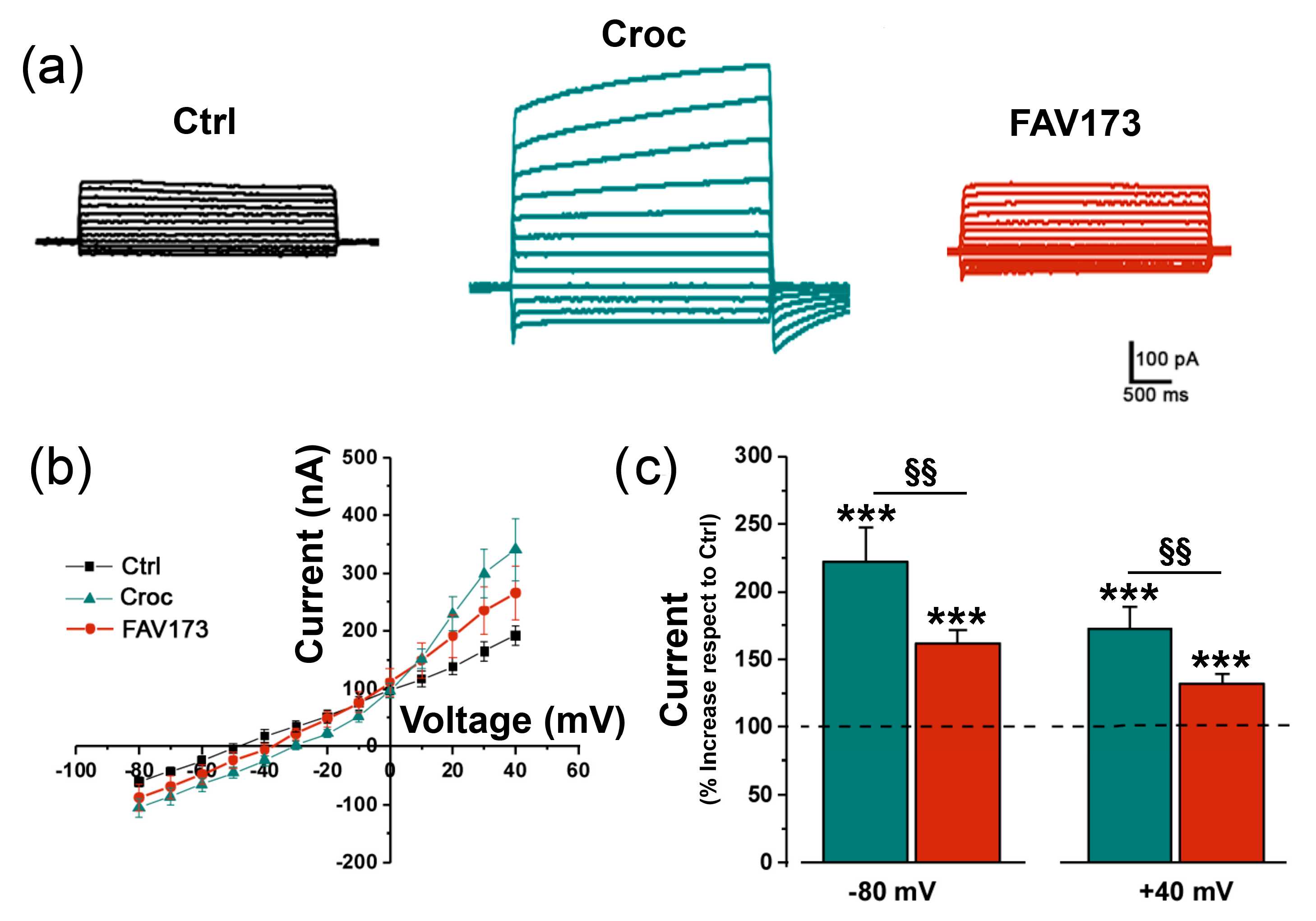
2.2. FAV173 Fiber Exposure Stimulates TMEM16A Channel Activity
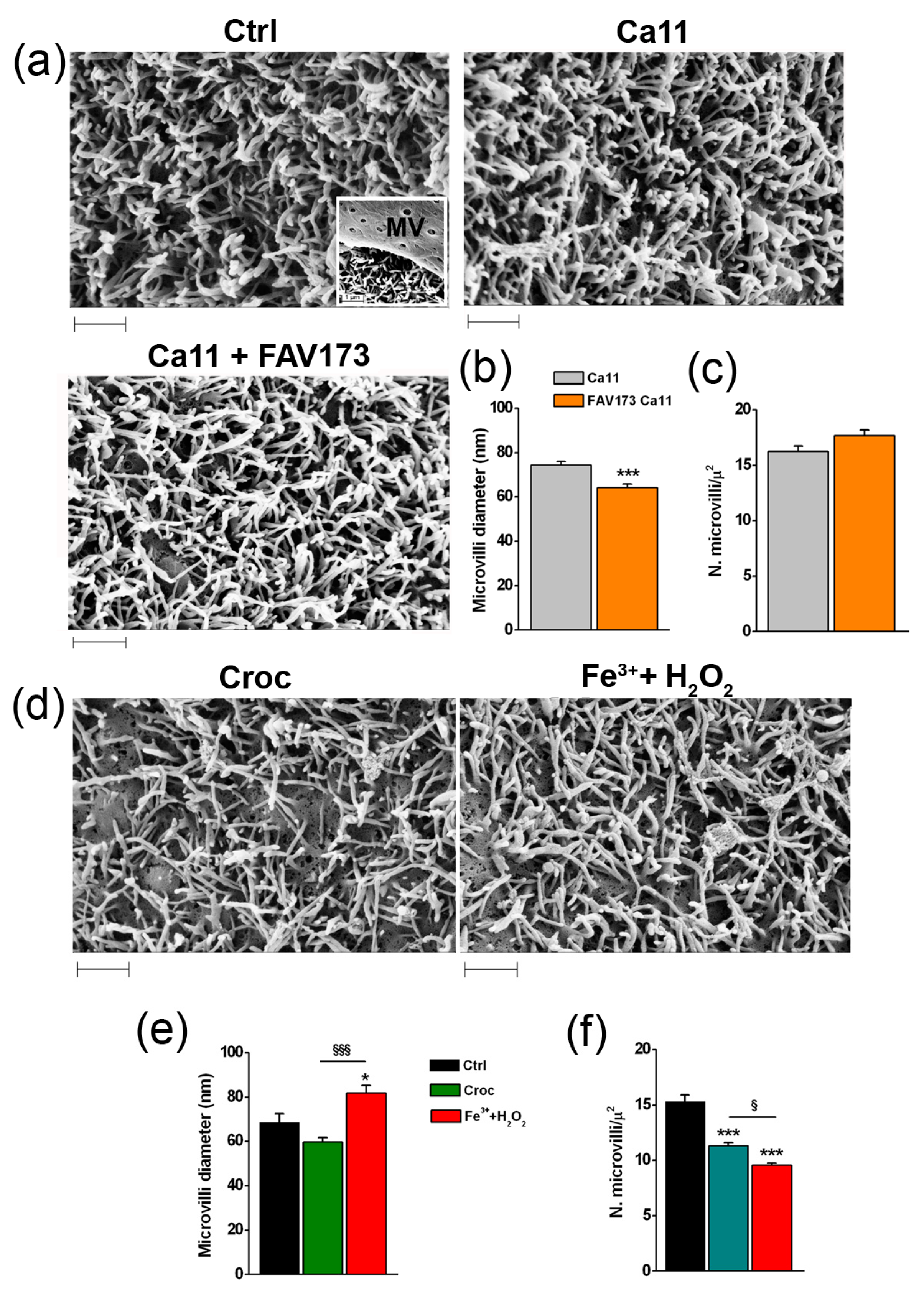
2.3. Effects of FAV173 Fiber Exposure on Cell Morphology
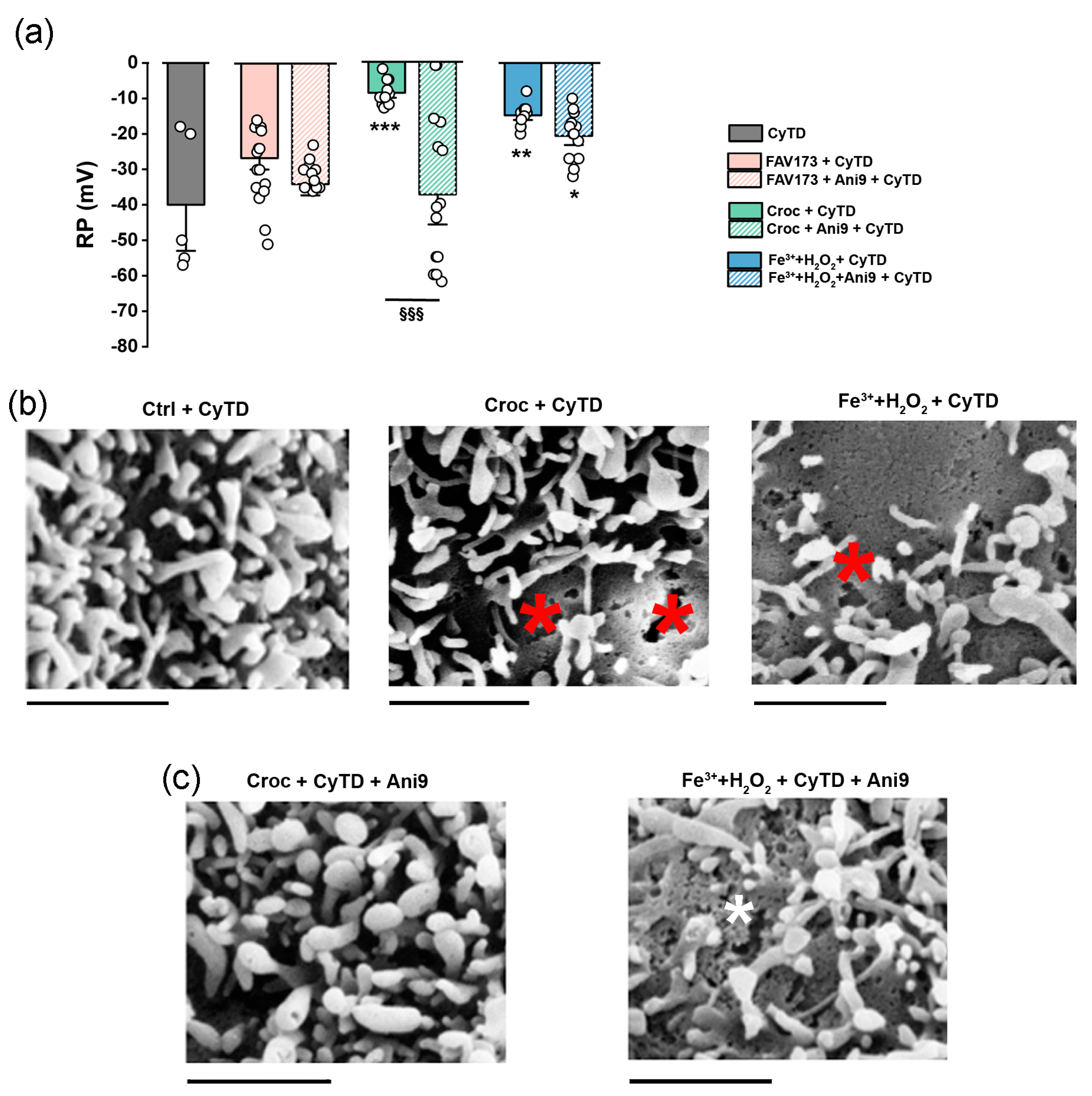
2.4. The Role of Actin in the TMEM16A-Mediated Effect Induced by FAV173 Fiber Exposure
3. Discussion
4. Materials and Methods
4.1. Isolation of Xenopus Oocytes
4.2. Electrophysiological Recordings
4.3. Asbestos and FAV173 Fibers
4.4. Scanning and Transmission Electron Microscopy
4.5. Chemicals
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IARC. Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1–42; International Agency for Research on Cancer: Lyon, France, 1987.
- Bernstein, D.M. Synthetic Vitreous Fibers: A Review Toxicology, Epidemiology and Regulations. Crit. Rev. Toxicol. 2007, 37, 839–886. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.P.; Harrison, P.T.C. Crystalline Silica in Heated Man-Made Vitreous Fibres: A Review. Regul. Pharmacol. 2014, 68, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, M. Man-Made Mineral Fibers (MMMF): Human Exposures and Health Risk Assessment. Toxicol. Ind. Health 1990, 6, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Maxim, L.D.; Hadley, J.G.; Potter, R.M.; Niebo, R. The Role of Fiber Durability/Biopersistence of Silica-Based Synthetic Vitreous Fibers and Their Influence on Toxicology. Regul. Toxicol. Pharmacol. 2006, 46, 42–62. [Google Scholar] [CrossRef] [PubMed]
- Guldberg, M.; Jensen, S.L.; Knudsen, T.; Steenberg, T.; Kamstrup, O. High-Alumina Low-Silica HT Stone Wool Fibers: A Chemical Compositional Range with High Biosolubility. Regul. Toxicol. Pharmacol. 2002, 35, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Suder Egnot, N.; Allen, H.; Hazan, R.; Vater, M.F.; Denic-Roberts, H.; LeClaire, R.; Marsh, G.M. Systematic Review of Epidemiological Studies Evaluating the Association between Exposure to Man-Made Vitreous Fibers and Non Malignant Respiratory Diseases. Regul. Toxicol. Pharmacol. 2023, 139, 105361. [Google Scholar] [CrossRef]
- Mattson, S.M. Glass Fiber Dissolution in Simulated Lung Fluid and Measures Needed to Improve Consistency and Correspondence to In Vivo Dissolution. Environ. Health Perspect. 1994, 102, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Baccarelli, A.; Tretiakova, M.; Gorbanev, S.; Lomtev, A.; Klimkina, I.; Tchibissov, V.; Averkina, O.; Rice, C.; Dosemeci, M. Risk of Lung Cancer from Exposure to Dusts and Fibers in Leningrad Province, Russia. Ann. Epidemiol. 2004, 14, 593. [Google Scholar] [CrossRef]
- Gaze, R. The Physical and Molecular Structure of Asbestos. Ann. N. Y. Acad. Sci. 1965, 132, 23–30. [Google Scholar] [CrossRef]
- Aust, A.E.; Cook, P.M.; Dodson, R.F. Morphological and Chemical Mechanisms of Elongated Mineral Particle Toxicities. J. Toxicol. Environ. Health B Crit. Rev. 2011, 14, 40–75. [Google Scholar] [CrossRef]
- Ceppi, M.; Smolkova, B.; Staruchova, M.; Kazimirova, A.; Barancokova, M.; Volkovova, K.; Collins, A.; Kocan, A.; Dzupinkova, Z.; Horska, A.; et al. Genotoxic Effects of Occupational Exposure to Glass Fibres—A Human Biomonitoring Study. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2023, 885, 503572. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Huang, L.; Huo, T.; Dong, F.; Wang, G.; Zhang, Q. Man-Made Mineral Fiber Effects on the Expression of Anti-Oncogenes P53 and P16 and Oncogenes C-JUN and C-FOS in the Lung Tissue of Wistar Rats. Toxicol. Ind. Health 2019, 35, 431–444. [Google Scholar] [CrossRef]
- Gilmour, P.S.; Beswick, P.H.; Brown, D.M.; Donaldson, K. Detection of Surface Free Radical Activity of Respirable Industrial Fibres Using Supercoiled Phi X174 RF1 Plasmid DNA. Carcinogenesis 1995, 16, 2973–2979. [Google Scholar] [CrossRef] [PubMed]
- Enterline, P.E. Carcinogenic Effects of Man-Made Vitreous Fibers. Annu. Rev. Public. Health 1991, 12, 459–480. [Google Scholar] [CrossRef]
- Oberdörster, G. Determinants of the Pathogenicity of Man-Made Vitreous Fibers (MMVF). Int. Arch. Occup. Environ. Health 2000, 73, S60–S68. [Google Scholar] [CrossRef] [PubMed]
- Luoto, K.; Holopainen, M.; Sarataho, M.; Savolainen, K. Comparison of Cytotoxicity of Man-Made Vitreous Fibres. Ann. Occup. Hyg. 1997, 41, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Bernareggi, A.; Conte, G.; Constanti, A.; Borelli, V.; Vita, F.; Zabucchi, G. On the Mechanism of the Electrophysiological Changes and Membrane Lesions Induced by Asbestos Fiber Exposure in Xenopus Laevis Oocytes. Sci. Rep. 2019, 9, 2014. [Google Scholar] [CrossRef]
- Bernareggi, A.; Zangari, M.; Constanti, A.; Zacchi, P.; Borelli, V.; Mangogna, A.; Lorenzon, P.; Zabucchi, G. Asbestos Fibers Enhance the TMEM16A Channel Activity in Xenopus Oocytes. Membranes 2023, 13, 180. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, B.C.; Cheng, T.; Jan, Y.N.; Jan, L.Y. Expression Cloning of TMEM16A as a Calcium-Activated Chloride Channel Subunit. Cell 2008, 134, 1019–1029. [Google Scholar] [CrossRef]
- Wozniak, K.L.; Phelps, W.A.; Tembo, M.; Lee, M.T.; Carlson, A.E. The TMEM16A Channel Mediates the Fast Polyspermy Block in Xenopus Laevis. J. Gen. Physiol. 2018, 150, 1249–1259. [Google Scholar] [CrossRef]
- Courjaret, R.; Hodeify, R.; Hubrack, S.; Ibrahim, A.; Dib, M.; Daas, S.; Machaca, K. The Ca2+-Activated Cl− Channel Ano1 Controls Microvilli Length and Membrane Surface Area in the Oocyte. J. Cell Sci. 2016, 129, 2548–2558. [Google Scholar] [CrossRef]
- Hu, C.; Zhang, R.; Jiang, D. TMEM16A as a Potential Biomarker in the Diagnosis and Prognosis of Lung Cancer. Arch. Iran. Med. 2019, 22, 32–38. [Google Scholar] [PubMed]
- Newman, H.A.; Saat, Y.A.; Hart, R.W. Putative Inhibitory Effects of Chrysotile, Crocidolite, and Amosite Mineral Fibers on the More Complex Surface Membrane Glycolipids and Glycoproteins. Environ. Health Perspect. 1980, 34, 103–111. [Google Scholar] [CrossRef]
- Murthy, S.; Larson-Casey, J.L.; Ryan, A.J.; He, C.; Kobzik, L.; Carter, A.B. Alternative Activation of Macrophages and Pulmonary Fibrosis Are Modulated by Scavenger Receptor, Macrophage Receptor with Collagenous Structure. FASEB J. 2015, 29, 3527–3536. [Google Scholar] [CrossRef]
- Zanella, C.L.; Timblin, C.R.; Cummins, A.; Jung, M.; Goldberg, J.; Raabe, R.; Tritton, T.R.; Mossman, B.T. Asbestos-Induced Phosphorylation of Epidermal Growth Factor Receptor Is Linked to c-Fos and Apoptosis. Am. J. Physiol.-Lung Cell. Mol. Physiol. 1999, 277, L684–L693. [Google Scholar] [CrossRef]
- Bernareggi, A.; Ren, E.; Borelli, V.; Vita, F.; Constanti, A.; Zabucchi, G. Xenopus Laevis Oocytes as a Model System or Studying the Interaction Between Asbestos Fibres and Cell Membranes. Toxicol. Sci. 2015, 145, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Caputo, A.; Caci, E.; Ferrera, L.; Pedemonte, N.; Barsanti, C.; Sondo, E.; Pfeffer, U.; Ravazzolo, R.; Zegarra-Moran, O.; Galietta, L.J.V. TMEM16A, A Membrane Protein Associated with Calcium-Dependent Chloride Channel Activity. Science 2008, 322, 590–594. [Google Scholar] [CrossRef]
- Seo, Y.; Lee, H.K.; Park, J.; Jeon, D.; Jo, S.; Jo, M.; Namkung, W. Ani9, A Novel Potent Small-Molecule ANO1 Inhibitor with Negligible Effect on ANO2. PLoS ONE 2016, 11, e0155771. [Google Scholar] [CrossRef] [PubMed]
- Perez-Cornejo, P.; Gokhale, A.; Duran, C.; Cui, Y.; Xiao, Q.; Hartzell, H.C.; Faundez, V. Anoctamin 1 (Tmem16A) Ca2+-Activated Chloride Channel Stoichiometrically Interacts with an Ezrin–Radixin–Moesin Network. Proc. Natl. Acad. Sci. USA 2012, 109, 10376–10381. [Google Scholar] [CrossRef]
- Limon, A.; Mattei, C. The Xenopus Oocyte: A Tool for Membrane Biology. Membranes 2023, 13, 831. [Google Scholar] [CrossRef]
- Zhang, A.; Yan, X.; Li, H.; Gu, Z.; Zhang, P.; Zhang, H.; Li, Y.; Yu, H. TMEM16A Protein Attenuates Lipopolysaccharide-Mediated Inflammatory Response of Human Lung Epithelial Cell Line A549. Exp. Lung Res. 2014, 40, 237–250. [Google Scholar] [CrossRef]
- Guo, S.; Bai, X.; Shi, S.; Deng, Y.; Kang, X.; An, H. TMEM16A, a Homoharringtonine Receptor, as a Potential Endogenic Target for Lung Cancer Treatment. Int. J. Mol. Sci. 2021, 22, 10930. [Google Scholar] [CrossRef]
- Jiang, L.; Nagai, H.; Ohara, H.; Hara, S.; Tachibana, M.; Hirano, S.; Shinohara, Y.; Kohyama, N.; Akatsuka, S.; Toyokuni, S. Characteristics and Modifying Factors of Asbestos-Induced Oxidative DNA Damage. Cancer Sci. 2008, 99, 2142–2151. [Google Scholar] [CrossRef]
- Jung, H.-S.; Park, E.-K.; Cha, J.-S.; Lee, J.-W.; Lee, J.-C.; Jang, J.; Kim, S.; Oak, C.; Yates, D.H.; Kim, H. Characteristics of Asbestos Fibers in Lung Tissue from Occupational and Environmental Asbestos Exposure of Lung Cancer Patients in Busan, Korea. Sci. Rep. 2020, 10, 20359. [Google Scholar] [CrossRef]
- Oberdörster, G. Toxicokinetics and Effects of Fibrous and Nonfibrous Particles. Inhal. Toxicol. 2002, 14, 29–56. [Google Scholar] [CrossRef] [PubMed]
- Cardinali, G.; Kovacs, D.; Maresca, V.; Flori, E.; Dell’Anna, M.L.; Campopiano, A.; Casciardi, S.; Spagnoli, G.; Torrisi, M.R.; Picardo, M. Differential in Vitro Cellular Response Induced by Exposure to Synthetic Vitreous Fibers (SVFs) and Asbestos Crocidolite Fibers. Exp. Mol. Pathol. 2006, 81, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, A.K.; Varshney, A.; Mathew, M.K. Resting Membrane Potential as a Marker of Apoptosis: Studies onenopus Oocytes Microinjected with Cytochrome c. Cell Death Differ. 2001, 8, 63–69. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zangari, M.; Zabucchi, G.; Conti, M.; Lorenzon, P.; Borelli, V.; Constanti, A.; Dellisanti, F.; Leone, S.; Vaccari, L.; Bernareggi, A. Effect of Synthetic Vitreous Fiber Exposure on TMEM16A Channels in a Xenopus laevis Oocyte Model. Int. J. Mol. Sci. 2024, 25, 8661. https://doi.org/10.3390/ijms25168661
Zangari M, Zabucchi G, Conti M, Lorenzon P, Borelli V, Constanti A, Dellisanti F, Leone S, Vaccari L, Bernareggi A. Effect of Synthetic Vitreous Fiber Exposure on TMEM16A Channels in a Xenopus laevis Oocyte Model. International Journal of Molecular Sciences. 2024; 25(16):8661. https://doi.org/10.3390/ijms25168661
Chicago/Turabian StyleZangari, Martina, Giuliano Zabucchi, Martina Conti, Paola Lorenzon, Violetta Borelli, Andrew Constanti, Francesco Dellisanti, Sara Leone, Lisa Vaccari, and Annalisa Bernareggi. 2024. "Effect of Synthetic Vitreous Fiber Exposure on TMEM16A Channels in a Xenopus laevis Oocyte Model" International Journal of Molecular Sciences 25, no. 16: 8661. https://doi.org/10.3390/ijms25168661
APA StyleZangari, M., Zabucchi, G., Conti, M., Lorenzon, P., Borelli, V., Constanti, A., Dellisanti, F., Leone, S., Vaccari, L., & Bernareggi, A. (2024). Effect of Synthetic Vitreous Fiber Exposure on TMEM16A Channels in a Xenopus laevis Oocyte Model. International Journal of Molecular Sciences, 25(16), 8661. https://doi.org/10.3390/ijms25168661








