Impact of Extrusion Parameters on the Formation of Nε-(Carboxymethyl)lysine, Nε-(Carboxyethyl)lysine and Acrylamide in Plant-Based Meat Analogues
Abstract
:1. Introduction
2. Results and Discussion
2.1. Moisture, Crude Protein and Amino Acids
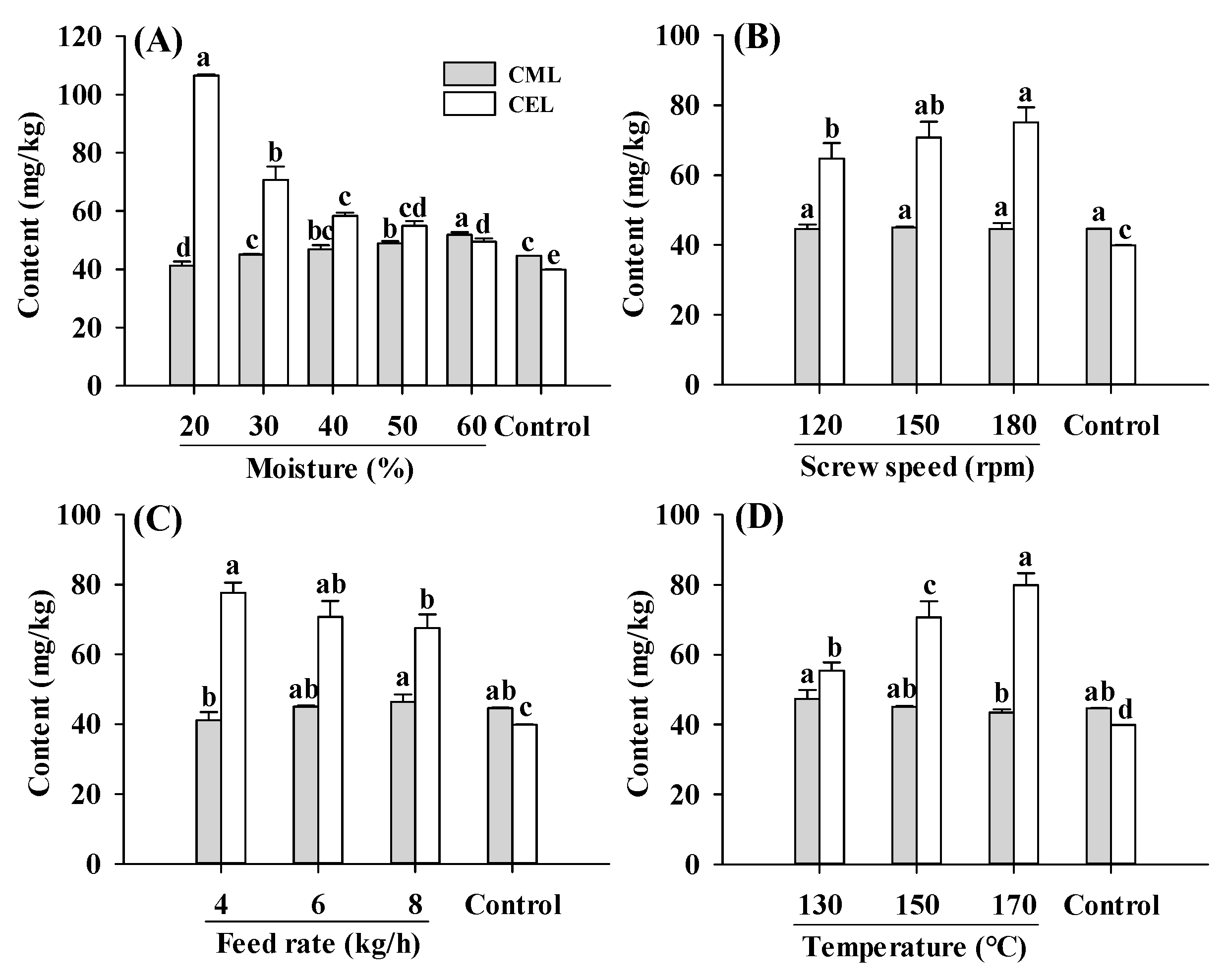
2.2. CML and CEL
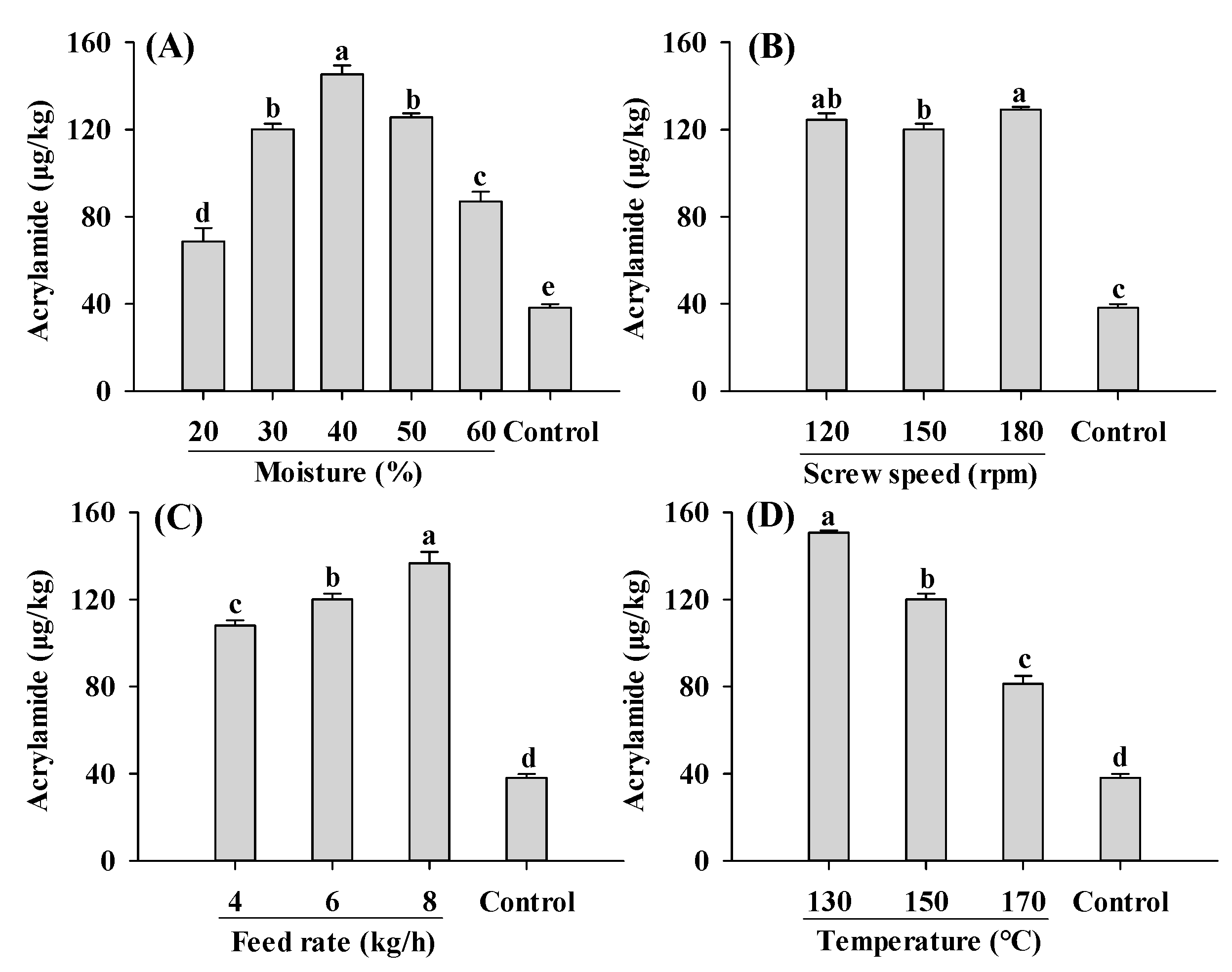
2.3. Acrylamide
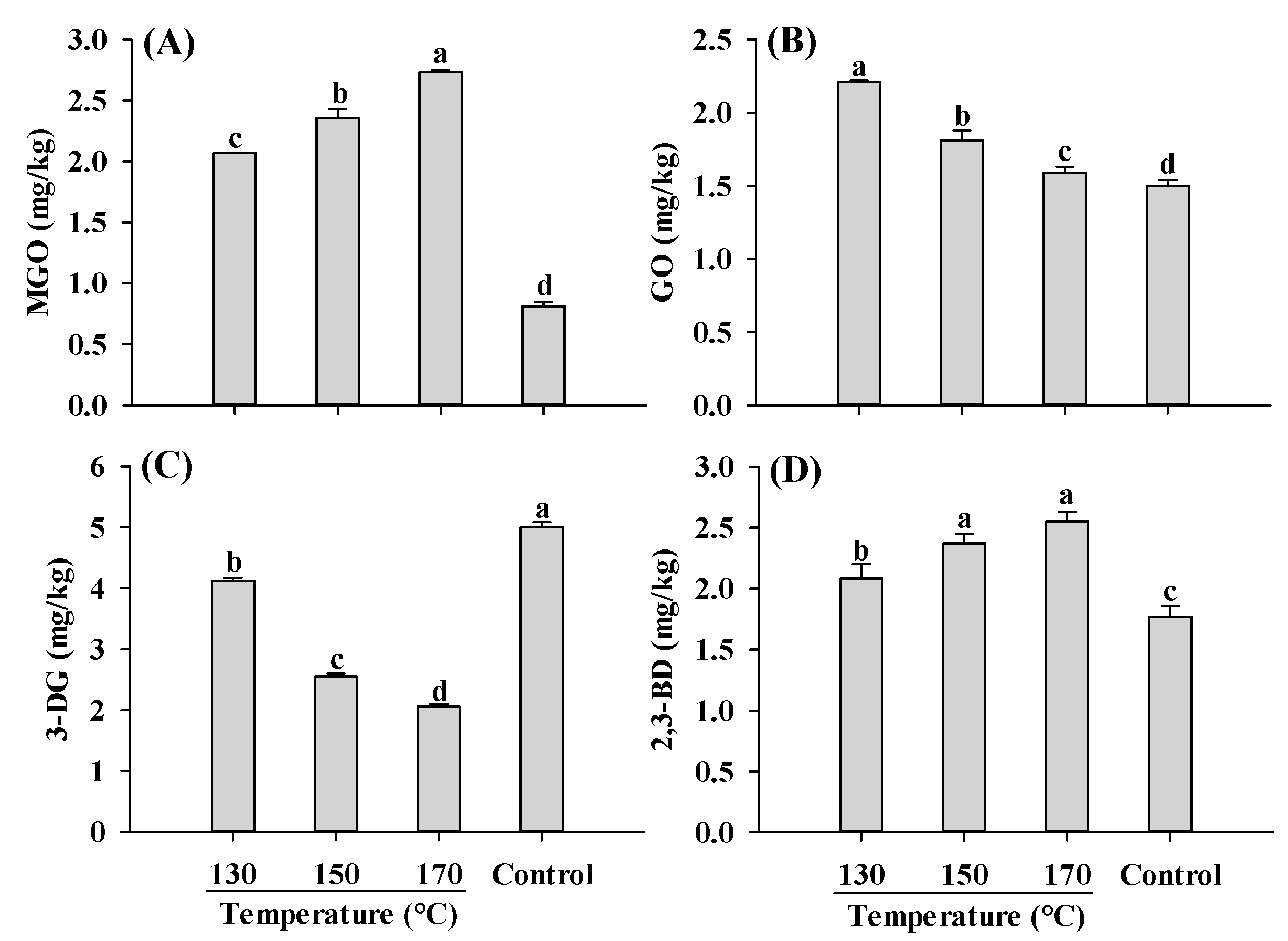
2.4. α-Dicarbonyl Compounds
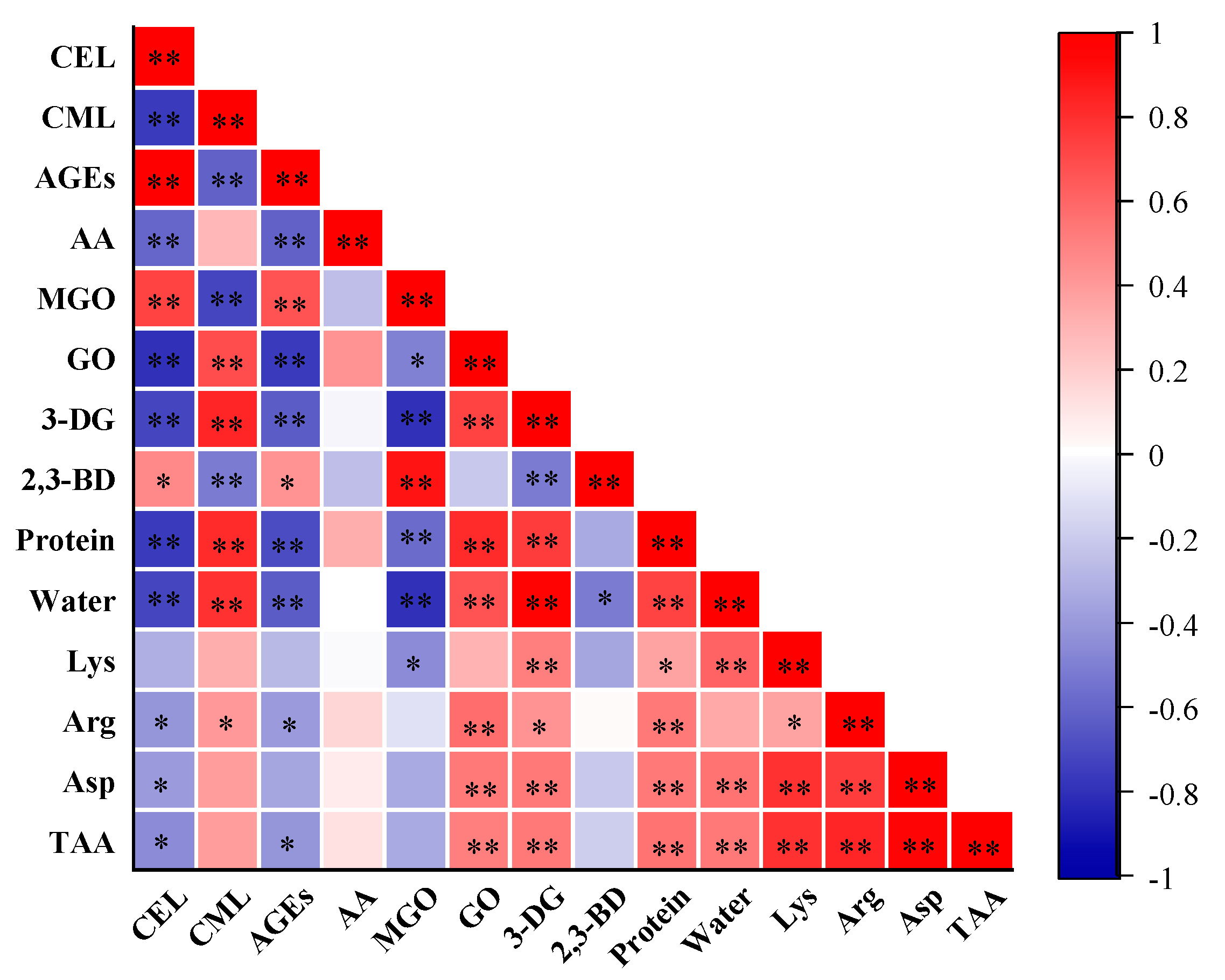
2.5. Correlation Analysis
3. Materials and Methods
3.1. Materials
3.2. Extrusion
3.3. Crude Protein, Moisture and Amino Acids Contents
3.4. CML and CEL
3.5. Acrylamide
3.6. α-Dicarbonyl Compounds
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, R.; Barden, A.; Mori, T.; Beilin, L. Advanced glycation end-products: A review. Diabetologia 2001, 44, 129–146. [Google Scholar] [CrossRef] [PubMed]
- Degen, J.; Hellwig, M.; Henle, T. 1,2-Dicarbonyl compounds in commonly consumed foods. J. Agric. Food Chem. 2012, 60, 7071–7079. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chai, M.; Zeng, M.; He, Z.; Chen, J. Effect of lipid oxidation on the formation of Nε-carboxymethyl-lysine and Nε-carboxyethyl-lysine in Chinese-style sausage during storage. Food Chem. 2018, 269, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Ueda, S.; Okuda, S. Food-derived advanced glycation end products (AGEs): A novel therapeutic target for various disorders. Curr. Pharm. Des. 2007, 13, 2832–2836. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H. Advanced glycation in health and disease: Role of the modern environment. Ann. N. Y. Acad. Sci. 2005, 1043, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Nie, C.; Liu, H.; Ma, Q.; Peng, B.; Zhang, M.; Chen, Z.; Li, J. Comparison of metabolic fate, target organs, and microbiota interactions of free and bound dietary advanced glycation end products. Crit. Rev. Food Sci. Nutr. 2023, 63, 3612–3633. [Google Scholar] [CrossRef] [PubMed]
- Koschinsky, T.; He, J.; Mitsuhashi, T.; Bucala, R.; Liu, C.; Buenting, C.; Heitmann, K.; Vlassara, H. Orally absorbed reactive glycation products (glycotoxins): An environmental risk factor in diabetic nephropathy. Proc. Natl. Acad. Sci. USA 1997, 94, 6474–6479. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Chen, X.; Li, L.; Li, B.; Yang, Z. The fate of dietary advanced glycation end products in the body: From oral intake to excretion. Crit. Rev. Food Sci. Nutr. 2020, 60, 3475–3491. [Google Scholar] [CrossRef] [PubMed]
- van Dongen, K.C.W.; Linkens, A.M.A.; Wetzels, S.M.W.; Wouters, K.; Vanmierlo, T.; van de Waarenburg, M.P.H.; Scheijen, J.L.J.M.; de Vos, W.M.; Belzer, C.; Schalkwijk, C.G. Dietary advanced glycation endproducts (AGEs) increase their concentration in plasma and tissues, result in inflammation and modulate gut microbial composition in mice; evidence for reversibility. Food Res. Int. 2021, 147, 110547. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Some Industrial Chemicals. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 1994; Volume 60, pp. 389–433. Available online: https://publications.iarc.fr/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Some-Industrial-Chemicals-1994 (accessed on 4 May 2024).
- Mottram, D.S.; Wedzicha, B.L.; Dodson, A.T. Acrylamide is formed in the Maillard reaction. Nature 2002, 419, 448–449. [Google Scholar] [CrossRef]
- Stadler, R.H.; Blank, I.; Varga, N.; Robert, F.; Hau, J.; Guy, P.A.; Robert, M.-C.; Riediker, S. Acrylamide from Maillard reaction products. Nature 2002, 419, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhao, G.; Chen, F.; Liu, J.; Wu, J.; Hu, X. Correlation of methylglyoxal with acrylamide formation in fructose/asparagine Maillard reaction model system. Food Chem. 2008, 108, 885–890. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration (FDA). Guidance for Industry: Acrylamide in Foods. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-acrylamide-foods (accessed on 10 May 2024).
- Fu, S.; Ma, Y.; Wang, Y.; Sun, C.; Chen, F.; Cheng, K.-W.; Liu, B. Contents and correlations of Nε-(carboxymethyl)lysine, Nε-(carboxyethyl)lysine, acrylamide and nutrients in plant-based meat analogs. Foods 2023, 12, 1967. [Google Scholar] [CrossRef] [PubMed]
- Hull, G.L.J.; Woodside, J.V.; Ames, J.M.; Cuskelly, G.J. Nε-(carboxymethyl)lysine content of foods commonly consumed in a Western style diet. Food Chem. 2012, 131, 170–174. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain. Scientific opinion on acrylamide in food. EFSA J. 2015, 13, 4104. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, Q.; Kaplan, D.L.; Wang, Q. High-moisture extruded protein fiber formation toward plant-based meat sub-sttutes applications: Science, technology, and prospect. Trends Food Sci. Technol. 2022, 128, 202–216. [Google Scholar] [CrossRef]
- Deng, P.; Chen, Y.; Xie, S.; Xue, C.; He, Z.; Chen, Q.; Wang, Z.; Qin, F.; Chen, J.; Zeng, M. Accumulation of heterocyclic amines and advanced glycation end products in various processing stages of plant-based burgers by UHPLC-MS/MS. J. Agric. Food Chem. 2022, 70, 14771–14783. [Google Scholar] [CrossRef] [PubMed]
- Palermo, M.; Fiore, A.; Fogliano, V. Okara promoted acrylamide and carboxymethyl-lysine formation in bakery products. J. Agric. Food Chem. 2012, 60, 10141–10146. [Google Scholar] [CrossRef] [PubMed]
- van Rooijen, C.; Bosch, G.; van der Poel, A.F.B.; Wierenga, P.A.; Alexander, L.; Hendriks, W.H. Quantitation of Maillard reaction products in commercially available pet Foods. J. Agric. Food Chem. 2014, 62, 8883–8891. [Google Scholar] [CrossRef]
- Emin, M.A.; Wittek, P.; Schwegler, Y. Numerical analysis of thermal and mechanical stress profile during the extrusion processing of plasticized starch by non-isothermal flow simulation. J. Food Eng. 2021, 294, 110407. [Google Scholar] [CrossRef]
- Palanisamy, M.; Franke, K.; Berger, R.G.; Heinz, V.; Toepfl, S. High moisture extrusion of lupin protein: Influence of extrusion parameters on extruder responses and product properties. J. Sci. Food Agric. 2019, 99, 2175–2185. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Ren, K.; Cao, X.; Peng, X.; Zheng, L.; Dai, S.; Tong, X.; Zeng, Q.; Qiu, S.; Wang, H.; et al. High moisture extrusion of soybean-wheat co-precipitation protein: Mechanism of fibrosis based on different extrusion energy regulation. Food Hydrocoll. 2023, 144, 108950. [Google Scholar] [CrossRef]
- Singkhornart, S.; Gu, B.-J.; Ryu, G.H. Physicochemical properties of extruded germinated wheat and barley as modified by CO2 injection and difference extrusion conditions. Int. J. Food Sci. Technol. 2013, 48, 290–299. [Google Scholar] [CrossRef]
- Cho, H.; Park, M.K.; Kim, Y.-S. Study on volatile compounds formed from the thermal interaction of hydrolyzed vegetable proteins with reducing sugars. Food Sci. Biotechnol. 2023, 32, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Camire, M.E.; Camire, A.; Krumhar, K. Chemical and nutritional changes in foods during extrusion. Crit. Rev. Food Sci. Nutr. 1990, 29, 35–57. [Google Scholar] [CrossRef] [PubMed]
- Srey, C.; Hull, G.L.J.; Connolly, L.; Elliott, C.T.; del Castillo, M.D.; Ames, J.M. Effect of inhibitor compounds on Nε-(carboxymethyl)lysine (CML) and Nε-(carboxyethyl)lysine (CEL) formation in model foods. J. Agric. Food Chem. 2010, 58, 12036–12041. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Gao, C.; Zeng, M.; He, Z.; Wang, L.; Zhang, S.; Chen, J. Effects of raw meat and process procedure on Nε-carboxymethyllysine and Nε-carboxyethyl-lysine formation in meat products. Food Sci. Biotechnol. 2016, 25, 1163–1168. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.L.; Wei, Y.M.; Zhang, B.; Ojokoh, A.O. System parameters and product properties response of soybean protein extruded at wide moisture range. J. Food Eng. 2010, 96, 208–213. [Google Scholar] [CrossRef]
- Zhu, Z.; Cheng, Y.; Huang, S.; Yao, M.; Lei, Y.; Khan, I.A.; Huang, M.; Zhou, X. Formation of Nε-carboxymethyllysine and Nε-carboxyethyllysine in prepared chicken breast by pan frying. J. Food Prot. 2019, 82, 2154–2160. [Google Scholar] [CrossRef]
- Singh, S.; Gamlath, S.; Wakeling, L. Nutritional aspects of food extrusion: A review. Int. J. Food Sci. Technol. 2007, 42, 916–929. [Google Scholar] [CrossRef]
- Zhao, D.; Li, L.; Le, T.T.; Larsen, L.B.; Su, G.; Liang, Y.; Li, B. Digestibility of glyoxal-glycated β-casein and β-lactoglobulin and distribution of peptide-bound advanced glycation end products in gastrointestinal digests. J. Agric. Food Chem. 2017, 65, 5778–5788. [Google Scholar] [CrossRef] [PubMed]
- Žilić, S.; Mogol, B.A.; Akıllıoğlu, G.; Serpen, A.; Delić, N.; Gökmen, V. Effects of extrusion, infrared and microwave processing on Maillard reaction products and phenolic compounds in soybean. J. Sci. Food Agric. 2014, 94, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Mulla, M.Z.; Bharadwaj, V.R.; Annapure, U.S.; Singhal, R.S. Effect of formulation and processing parameters on acrylamide formation: A case study on extrusion of blends of potato flour and semolina. LWT-Food Sci. Technol. 2011, 44, 1643–1648. [Google Scholar] [CrossRef]
- Makowska, A.; Cais-Sokolinska, D.; Waskiewicz, A.; Tokarczyk, G.; Paschke, H. Quality and nutritional properties of corn snacks enriched with nanofiltered whey powder. Czech J. Food Sci. 2016, 34, 154–159. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, J.; Zhang, G.; Huang, C.; Ou, S. The formation of acrylamide from and its reduction by 3-aminopropanamide occur simultaneously during thermal treatment. J. Food Sci. 2018, 83, 2662–2668. [Google Scholar] [CrossRef] [PubMed]
- Granvogl, M.; Schieberle, P. Thermally generated 3-aminopropionamide as a transient intermediate in the formation of acrylamide. J. Agric. Food Chem. 2006, 54, 5933–5938. [Google Scholar] [CrossRef] [PubMed]
- de Vleeschouwer, K.; van der Plancken, I.; van Loey, A.; Hendrickx, M.E. Investigation of the influence of different moisture levels on acrylamide formation/elimination reactions using multiresponse analysis. J. Agric. Food Chem. 2008, 56, 6460–6470. [Google Scholar] [CrossRef] [PubMed]
- Koutsidis, G.; Simons, S.P.J.; Thong, Y.H.; Haldoupis, Y.; Mojica-Lazaro, J.; Wedzicha, B.L.; Mottram, D.S. Investigations on the effect of amino acids on acrylamide, pyrazines, and michael addition products in model systems. J. Agric. Food Chem. 2009, 57, 9011–9015. [Google Scholar] [CrossRef] [PubMed]
- Quan, W.; Li, Y.; Jiao, Y.; Xue, C.; Liu, G.; Wang, Z.; He, Z.; Qin, F.; Zeng, M.; Chen, J. Simultaneous generation of acrylamide, beta-carboline heterocyclic amines and advanced glycation ends products in an aqueous Maillard reaction model system. Food Chem. 2020, 332, 127387. [Google Scholar] [CrossRef]
- Solina, M.; Johnson, R.L.; Whitfield, F.B. Effects of glucose and acid-hydrolysed vegetable protein on the volatile components of extruded wheat starch. Food Chem. 2007, 100, 678–692. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Xu, D.; Hu, H.; Huang, Y.; Liu, Y.; Nie, S.; Li, C.; Xie, M. Investigation on the contents of heat-induced hazards in commercial nuts. Food Res. Int. 2023, 163, 112041. [Google Scholar] [CrossRef] [PubMed]
- Kocadağlı, T.; Gökmen, V. Effect of sodium chloride on α-dicarbonyl compound and 5-hydroxymethyl-2-furfural formations from glucose under caramelization conditions: A multiresponse kinetic modeling approach. J. Agric. Food Chem. 2016, 64, 6333–6342. [Google Scholar] [CrossRef] [PubMed]
- Berk, E.; Aktag, I.G.; Gokmen, V. Formation of alpha-dicarbonyl compounds and glycation products in sesame (Sesamum indicum L.) seeds during roasting: A multiresponse kinetic modelling approach. Eur. Food Res. Technol. 2021, 247, 2285–2298. [Google Scholar] [CrossRef]
- Kou, Y.; Song, Z.; Jing, Y.; Li, H.; Wei, X.; Xie, J.; Shen, M. Key Maillard intermediates—α-dicarbonyl compounds in foods: Occurrence, analysis, toxicity, and mitigation strategies. Food Control 2024, 166, 110652. [Google Scholar] [CrossRef]
- Wang, Y.; Lyu, B.; Fu, H.L.; Li, J.X.; Ji, L.; Gong, H.; Zhang, R.N.; Liu, J.S.; Yu, H.S. The development process of plant-based meat alternatives: Raw material formulations and processing strategies. Food Res. Int. 2023, 167, 112689. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Tang, J.; Wang, J.; Rasco, B.A.; Lai, K.; Huang, Y. Formation of free and protein-bound carboxymethyllysine and carboxyethyllysine in meats during commercial sterilization. Meat Sci. 2016, 116, 1–7. [Google Scholar] [CrossRef]
- Deng, S.; Cui, H.; Hayat, K.; Hussain, S.; Tahir, M.U.; Zhai, Y.; Zhang, Q.; Zhang, X.; Ho, C.-T. Effect of methionine on the thermal degradation of N-(1-deoxy-d-fructos-1-yl)-methionine affecting browning formation. J. Agric. Food Chem. 2021, 69, 5167–5177. [Google Scholar] [CrossRef]



| Parameter | Content | |||
|---|---|---|---|---|
| Water (%) | Protein (%) | TAA (mg/g) | ||
| Moisture (%) | 20 | 15.5 ± 0.4 e | 79.2 ± 0.5 c | 678 ± 3 b |
| 30 | 21.5 ± 0.5 d | 80.3 ± 0.3 c | 679 ± 7 b | |
| 40 | 35.1 ± 0.5 c | 81.8 ± 1.1 b | 701 ± 12 a | |
| 50 | 44.3 ± 0.8 b | 82.8 ± 0.4 ab | 704 ± 6 a | |
| 60 | 56.3 ± 0.1 a | 83.5 ± 0.8 ab | 712 ± 3 a | |
| Raw material | 6.8 ± 0.1 f | 84.1 ± 0.4 a | 702 ± 7 a | |
| Screw speed (rpm) | 120 | 21.1 ± 0.1 a | 80.4 ± 1 b | 686 ± 10 a |
| 150 | 21.5 ± 0.5 a | 80.3 ± 0.3 b | 679 ± 7 a | |
| 180 | 20.4 ± 0.1 b | 80.4 ± 0.8 b | 687 ± 13 a | |
| Raw material | 6.8 ± 0.1 c | 84.1 ± 0.4 a | 701 ± 7 a | |
| Feed rate (kg/h) | 4 | 21.3 ± 0.2 ab | 79.7 ± 0.9 b | 646 ± 37 b |
| 6 | 21.5 ± 0.5 a | 80.3 ± 0.3 b | 679 ± 7 ab | |
| 8 | 20.8 ± 0.3 b | 80.8 ± 0.2 b | 684 ± 6 ab | |
| Raw material | 6.8 ± 0.1 c | 84.1 ± 0.4 a | 701 ± 7 a | |
| Barrel | 130 | 25.0 ± 0.4 a | 83.7 ± 0.3 a | 715 ± 6 a |
| temperature (°C) | 150 | 21.5 ± 0.5 b | 80.3 ± 0.3 b | 679 ± 7 b |
| 170 | 19.8 ± 0.5 c | 80.7 ± 0.4 b | 687 ± 13 b | |
| Raw material | 6.8 ± 0.1 d | 84.1 ± 0.4 a | 701 ± 7 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Fu, S.; Cheng, K.-W.; Liu, B. Impact of Extrusion Parameters on the Formation of Nε-(Carboxymethyl)lysine, Nε-(Carboxyethyl)lysine and Acrylamide in Plant-Based Meat Analogues. Int. J. Mol. Sci. 2024, 25, 8668. https://doi.org/10.3390/ijms25168668
Ma Y, Fu S, Cheng K-W, Liu B. Impact of Extrusion Parameters on the Formation of Nε-(Carboxymethyl)lysine, Nε-(Carboxyethyl)lysine and Acrylamide in Plant-Based Meat Analogues. International Journal of Molecular Sciences. 2024; 25(16):8668. https://doi.org/10.3390/ijms25168668
Chicago/Turabian StyleMa, Yurong, Shuang Fu, Ka-Wing Cheng, and Bin Liu. 2024. "Impact of Extrusion Parameters on the Formation of Nε-(Carboxymethyl)lysine, Nε-(Carboxyethyl)lysine and Acrylamide in Plant-Based Meat Analogues" International Journal of Molecular Sciences 25, no. 16: 8668. https://doi.org/10.3390/ijms25168668





