The Impact of Thermal Treatment Intensity on Proteins, Fatty Acids, Macro/Micro-Nutrients, Flavor, and Heating Markers of Milk—A Comprehensive Review
Abstract
:1. Introduction
2. Impact of Thermal Treatment on Structure and Behavior of Main Milk Constituents
2.1. Whey Proteins
2.2. Casein
2.3. Milk Fat
3. Impact of Thermal Treatment on Flavor of Milk
4. Impact of Thermal Treatment on Macro/Micro-Nutrients in Milk
4.1. Minerals
4.2. Vitamins
| Vitamins | Heating Conditions (Whole Milk Unless Stated Otherwise) | Raw Milk | After Treatment | Ref. | |
|---|---|---|---|---|---|
| water-soluble vitamins | Vitamin B1 (thiamin) (μg/mL) | 77 °C/15 s | 0.45 ± 0.02 | No loss | [87] |
| 94 °C/420 s | 0.43 ± 0.03 | 6.34% loss | |||
| 129 °C/2 s, indirect | 0.44 ± 0.02 | No loss | |||
| 140 °C/4 s, direct | 0.46 ± 0.03 | No loss | |||
| 141 °C/4 s, indirect | 0.50 ± 0.03 | No loss | |||
| Vitamin B2 (riboflavin) | 77 °C/15 s | 1.42 ± 0.01 μg/mL | No loss | [87] | |
| 94 °C/420 s | 1.43 ± 0.01 μg/mL | 3.68% loss | |||
| 129 °C/2 s, indirect | 1.39 ± 0.01 μg/mL | 1.80% loss | |||
| 140 °C/4 s, direct | 1.42 ± 0.01 μg/mL | 1.41% loss | |||
| 141 °C/4 s, indirect | 1.35 ± 0.01 μg/mL | No loss | |||
| 75 °C/6 s | 0.17 ± 0.01 μmol/L | 5.9% loss | [98] | ||
| 85 °C/6 s | 0.17 ± 0.01 μmol/L | No loss | |||
| 92 °C/6 s | 0.17 ± 0.01 μmol/L | No loss | |||
| Vitamin B3 (niacin) | 62.5 °C/30 min | 218 μg/100 g | No loss | [97] | |
| 75 °C/15 min | 238 μg/100 g | No loss | |||
| 75 °C/6 s | 13.28 ± 0.44 μmol/L | No loss | [98] | ||
| 85 °C/6 s | 13.28 ± 0.44 μmol/L | No loss | |||
| 92 °C/6 s | 13.28 ± 0.44 μmol/L | No loss | |||
| Vitamin B5 (pantothenic acid) (μg/100 g) | 62.5 °C/30 min | 469 | No loss | [97] | |
| 75 °C/15 min | 610 | 4.8% loss | |||
| Vitamin B6 (pyridoxamine) (μmol/L) | 75 °C/6 s | 2.06 ± 0.60 | No loss | [98] | |
| 85 °C/6 s | 2.06 ± 0.60 | No loss | |||
| 92 °C/6 s | 2.06 ± 0.60 | No loss | |||
| Vitamin B7 (biotin) (μg//100 g) | 62.5 °C/30 min | 0.73 | No loss | [97] | |
| 75 °C/15 min | 0.95 | No loss | |||
| Vitamin B9 (folic acid) (μmol/L) | 75 °C/6 s | 0.75 ± 0.06 | No loss | [98] | |
| 85 °C/6 s | 0.75 ± 0.06 | No loss | |||
| 92 °C/6 s | 0.75 ± 0.06 | No loss | |||
| Vitamin B12 (cyanocobalamin) (ng/mL) | 77 °C/15 s | 3.60 ± 0.40 | No loss | [87] | |
| 94 °C/420 s | 3.30 ± 0.20 | 23.30% loss | |||
| 129 °C/2 s, indirect | 3.40 ± 0.60 | No loss | |||
| 140 °C/4 s, direct | 4.40 ± 0.30 | No loss | |||
| 141 °C/4 s, indirect | 3.30 ± 0.10 | No loss | |||
| 100 °C/1 h | 100 μM (standardized) | 10.2% loss | [100] | ||
| 100 °C/1 h (2% fat) | 100 μM (standardized) | 12.5% loss | |||
| 100 °C/1 h (fat-free) | 100 μM (standardized) | 14.4% loss | |||
| 76 °C/16 s | 0.31 μg 100 g−1 | No loss | [101] | ||
| 96 °C/5 min | 0.31 μg 100 g−1 | 3.2% loss | |||
| Vitamin C (ascorbic acid) (μg/mL) | 50 & 63 °C/10–60 min | 100 | ~15% loss at 10 min and up to 40% loss at 60 min | [99] | |
| 75 & 90 °C/0.25–10 min | 100 | ~12% loss at 0.25 min and up to ~35% loss at 10 min | |||
| fat-soluble vitamins | Vitamin A (retinol) (IU/L) | 63 °C/30 min | 325 ± 19.2 | 2% loss | [102] |
| 121 °C/15 min | 325 ± 19.2 | 36.6% loss | |||
| Vitamin D2 (ergocalciferol) (IU/L) | 63 °C/30 min | 594.28 ± 2.22 (fortified) | No loss | [103] | |
| 121 °C/15 min | 594.28 ± 2.22 (fortified) | No loss | |||
| Vitamin E (tocopherol) (μg/mL) | 77 °C/15 s | 0.97 ± 0.03 | No loss | [87] | |
| 94 °C/420 s | 0.96 ± 0.03 | No loss | |||
| 129 °C/2 s, indirect | 0.94 ± 0.04 | No loss | |||
| 140 °C/4 s, direct | 1.00 ± 0.06 | No loss | |||
| 141 °C/4 s, indirect | 0.98 ± 0.04 | No loss | |||
5. Influences of Heat Treatment on Heating Markers in Milk
5.1. Furosine and Lactulose
5.2. Alkaline Phosphatase (ALP)
5.3. Lactoperoxidase (LPO)
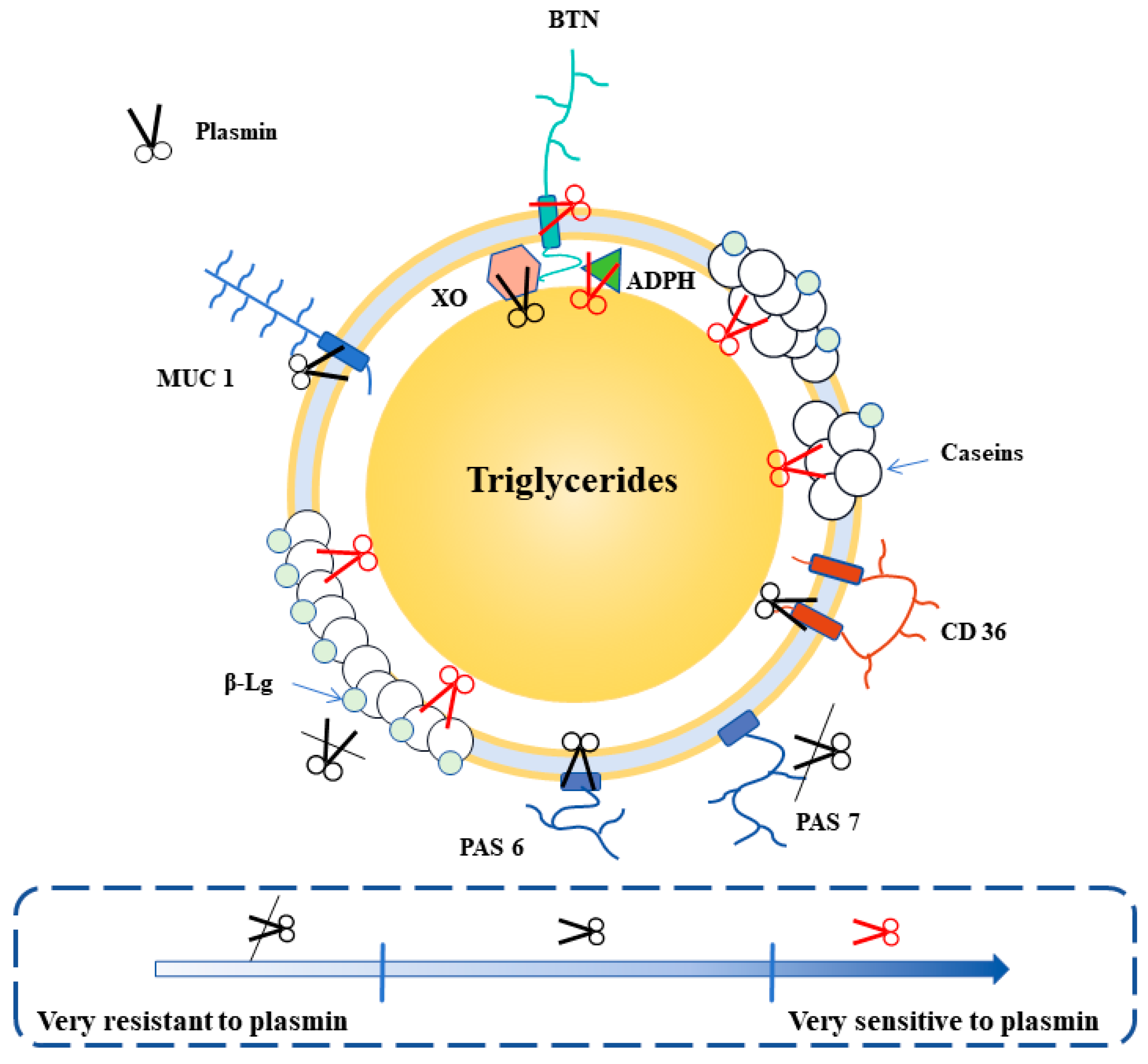
5.4. Plasmin
6. Impact of Heat Treatment on Other Properties of Milk
6.1. Age Gelation
6.2. Effect of Thermal Treatment on Unconventional Milk
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Syed, Q.A.; Hassan, A.; Sharif, S.; Ishaq, A.; Saeed, F.; Afzaal, M.; Hussain, M.; Anjum, F.M. Structural and functional properties of milk proteins as affected by heating, high pressure, Gamma and ultraviolet irradiation: A review. Int. J. Food Prop. 2021, 24, 871–884. [Google Scholar] [CrossRef]
- Tonolo, F.; Folda, A.; Cesaro, L.; Scalcon, V.; Marin, O.; Ferro, S.; Bindoli, A.; Rigobello, M.P. Milk-derived bioactive peptides exhibit antioxidant activity through the Keap1-Nrf2 signaling pathway. J. Funct. Foods 2020, 64, 103696–103703. [Google Scholar] [CrossRef]
- Chatterton, D.E.W.; Nguyen, D.N.; Bering, S.B.; Sangild, P.T. Anti-inflammatory mechanisms of bioactive milk proteins in the intestine of newborns. Int. J. Biochem. Cell Biol. 2013, 45, 1730–1747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mamet, T.; Guo, Y.; Li, C.; Yang, J. Yak milk promotes renal calcium reabsorption in mice with osteoporosis via the regulation of TRPV5. J. Dairy Sci. 2023, 106, 7396–7406. [Google Scholar] [CrossRef] [PubMed]
- Coutinho, N.M.; Silveira, M.R.; Rocha, R.S.; Moraes, J.; Ferreira, M.V.S.; Pimentel, T.C.; Freitas, M.Q.; Silva, M.C.; Raices, R.S.L.; Ranadheera, C.S.; et al. Cold plasma processing of milk and dairy products. Trends Food Sci. Technol. 2018, 74, 56–68. [Google Scholar] [CrossRef]
- Munir, M.; Nadeem, M.; Qureshi, T.M.; Leong, T.S.H.; Gamlath, C.J.; Martin, G.J.O.; Ashokkumar, M. Effects of high pressure, microwave and ultrasound processing on proteins and enzyme activity in dairy systems—A review. Innov. Food Sci. Emerg. 2019, 57, 102192–102205. [Google Scholar] [CrossRef]
- Jo, Y.; Benoist, D.M.; Barbano, D.M.; Drake, M.A. Flavor and flavor chemistry differences among milks processed by high-temperature, short-time pasteurization or ultra-pasteurization. J. Dairy Sci. 2018, 101, 3812–3828. [Google Scholar] [CrossRef]
- Nikmaram, N.; Keener, K.M. The effects of cold plasma technology on physical, nutritional, and sensory properties of milk and milk products. LWT-Food Sci. Technol. 2022, 154, 112729–112736. [Google Scholar] [CrossRef]
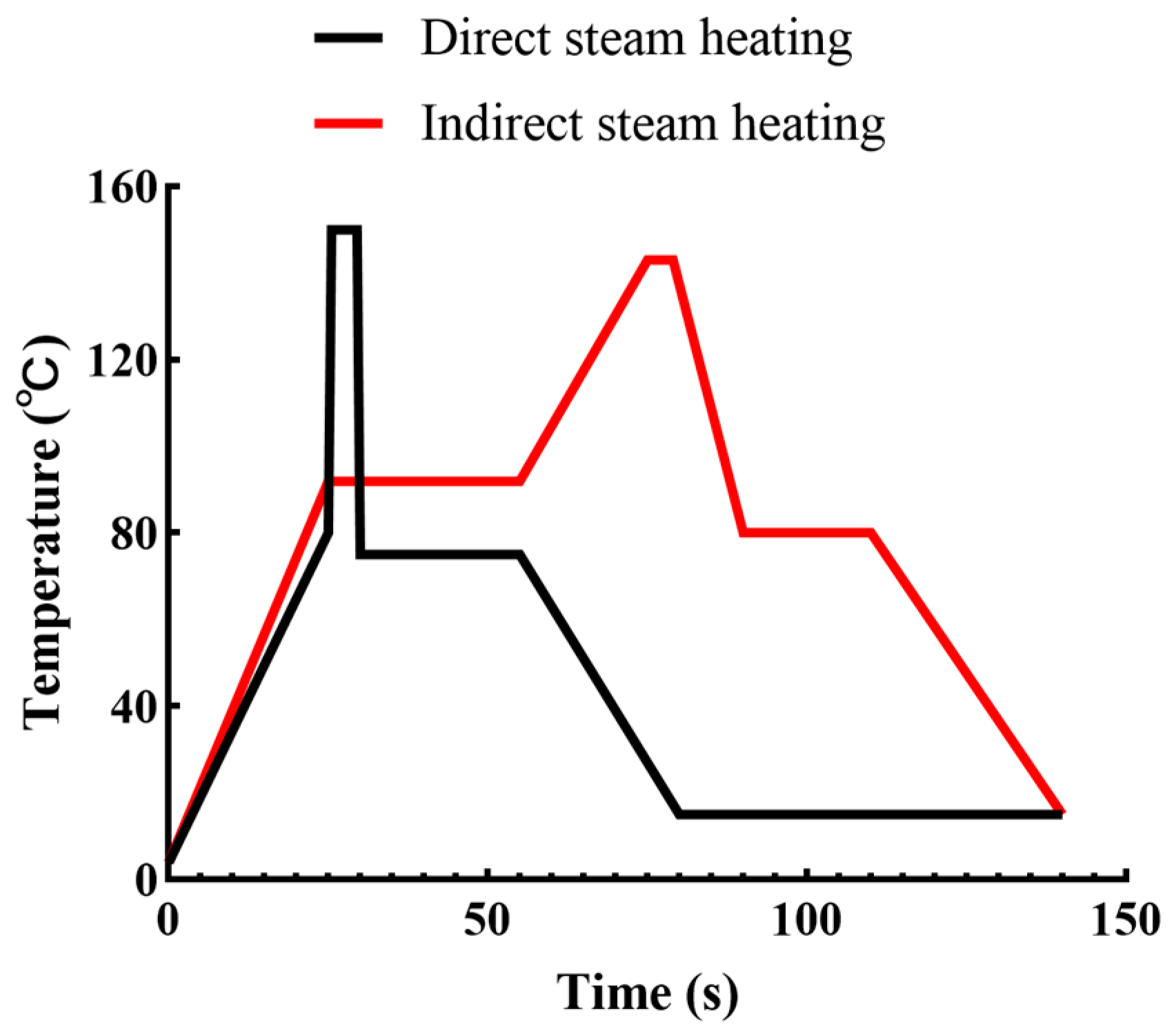
- Eisner, M.D. Direct and indirect heating of milk–A technological perspective beyond time–temperature profiles. Int. Dairy J. 2021, 122, 105145–105162. [Google Scholar] [CrossRef]
- Gao, T.; Sun, D.-W.; Tian, Y.; Zhu, Z. Gold–Silver Core-Shell Nanorods Based Time-Temperature Indicator for Quality Monitoring of Pasteurized Milk in the Cold Chain. J. Food Eng. 2021, 306, 110624. [Google Scholar] [CrossRef]
- Hanson, M.L.; Wendorff, W.L.; Houck, K.B. Effect of Heat Treatment of Milk on Activation of Bacillus Spores. J. Food Prot. 2005, 68, 1484–1486. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.M.; Dill, C.W. Direct-steam injection system for processing fluid milk products. J. Dairy Sci. 1962, 45, 937–940. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Z. A mini-review about direct steam heating and its application in dairy and plant protein processing. Food Chem. 2023, 408, 135233–135239. [Google Scholar] [CrossRef] [PubMed]
- Rauh, V. Impact of Plasmin Activity on the Shelf Life and Stability of UHT Milk. Ph.D. Thesis, Aarhus University, Aarhus, Denmark, 2014. [Google Scholar]
- Wang, Y.; Guo, M.; Wu, P.; Fan, K.; Zhang, W.; Chen, C.; Ren, F.; Wang, P.; Luo, J.; Yu, J.J.C.; et al. New insights into the destabilization of fat globules in ultra-instantaneous UHT milk induced by added plasmin: Molecular mechanisms and the effect of membrane structure on plasmin action. Colloids Surf. B Biointerfaces 2024, 240, 113987. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, C.M.; O’Mahony, J.A.; Kelly, A.L.; O’Callaghan, D.J.; Kilcawley, K.N.; McCarthy, N.A. The effect of direct and indirect heat treatment on the attributes of whey protein beverages. Int. Dairy J. 2018, 85, 144–152. [Google Scholar] [CrossRef]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant activity of proteins and peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ahlroos-Lehmus, A.; Pilvi, T.K.; Korpela, R.; Tossavainen, O.; Mervaala, E.M. Metabolic effects of a novel microfiltered native whey protein in diet-induced obese mice. J. Funct. Foods 2012, 4, 440–449. [Google Scholar] [CrossRef]
- Attaallah, W.; Yilmaz, A.M.; Erdogan, N.; Yalçin, A.S.; Aktan, A. Whey protein versus whey protein hydrolyzate for the protection of azoxymethane and dextran sodium sulfate induced colonic tumors in rats. Pathol. Oncol. Res. 2012, 18, 817–822. [Google Scholar] [CrossRef]
- Badr, G.; Ebaid, H.; Mohany, M.; Abuelsaad, A.S. Modulation of immune cell proliferation and chemotaxis towards CC chemokine ligand (CCL)-21 and CXC chemokine ligand (CXCL)-12 in undenatured whey protein-treated mice. J. Nutr. Biochem. 2012, 23, 1640–1646. [Google Scholar] [CrossRef]
- Arena, S.; Renzone, G.; D’Ambrosio, C.; Salzano, A.M.; Scaloni, A. Dairy products and the Maillard reaction: A promising future for extensive food characterization by integrated proteomics studies. Food Chem. 2017, 219, 477–489. [Google Scholar] [CrossRef]
- Wada, Y.; Lönnerdal, B. Effects of different industrial heating processes of milk on site-specific protein modifications and their relationship to in vitro and in vivo digestibility. J. Agric. Food Chem. 2014, 62, 4175–4185. [Google Scholar] [CrossRef] [PubMed]
- Krishna, T.C.; Najda, A.; Bains, A.; Tosif, M.M.; Paplinski, R.; Kaplan, M.; Chawla, P. Influence of ultra-heat treatment on properties of milk proteins. Polymers 2021, 13, 3164. [Google Scholar] [CrossRef] [PubMed]
- Pizzano, R.; Manzo, C.; Nicolai, M.A.; Addeo, F. Occurrence of major whey proteins in the pH 4.6 insoluble protein fraction from UHT-treated milk. J. Agric. Food Chem. 2012, 60, 8044–8050. [Google Scholar] [CrossRef] [PubMed]
- Datta, N.; Elliott, A.J.; Perkins, M.L.; Deeth, H.C. Ultra-high-temperature (UHT) treatment of milk: Comparison of direct and indirect modes of heating. Aust. J. Dairy Technol. 2002, 57, 211–227. [Google Scholar]
- Burton, H. Ultra-High-Temperature Processing of Milk and Milk Products; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Wang, X.J.; Zhao, Z.T. Structural and colloidal properties of whey protein aggregates produced by indirect tubular heating and direct steam injection. Food Struct.-Neth. 2023, 35, 100301. [Google Scholar] [CrossRef]
- Mi, L.; Liang, Q.; Zhang, W.B.; Zhen, S.B.; Song, X.M.; Wen, P.C.; Zhang, Y. Sterilization of yak milk by direct steam injection (DSI) and effects on milk quality. LWT-Food Sci. Technol. 2023, 189, 115450. [Google Scholar] [CrossRef]
- Paulsson, M.A.; Svensson, U.; Kishore, A.R.; Naidu, A.S. Thermal behavior of bovine lactoferrin in water and its relation to bacterial interaction and antibacterial activity. J. Dairy Sci. 1993, 76, 3711–3720. [Google Scholar] [CrossRef] [PubMed]
- Dannenberg, F.; Kessler, H.G. Reaction kinetics of the denaturation of whey proteins in milk. J. Food Sci. 1988, 53, 258–263. [Google Scholar] [CrossRef]
- Yang, Y.X.; Bu, D.P.; Zhao, X.W.; Sun, P.; Wang, J.Q.; Zhou, L.Y. Proteomic analysis of cow, yak, buffalo, goat and camel milk whey proteins: Quantitative differential expression patterns. J. Proteome Res. 2013, 12, 1660–1667. [Google Scholar] [CrossRef]
- Yang, M.; Cao, X.Y.; Wu, R.N.; Liu, B.A.; Ye, W.H.; Yue, X.Q.; Wu, J.R. Comparative proteomic exploration of whey proteins in human and bovine colostrum and mature milk using iTRAQ-coupled LC-MS/MS. Int. J. Food Sci. Nutr. 2017, 68, 671–681. [Google Scholar] [CrossRef]
- Li, W.X.; Li, M.H.; Cao, X.Y.; Han, H.J.; Kong, F.H.; Yue, X.Q. Comparative analysis of whey proteins in donkey colostrum and mature milk using quantitative proteomics. Food Res. Int. 2020, 127, 108741. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, M.X.; Qu, X.N.; Wang, S.C.; Ma, Z.N.; Zhang, R.M.; Li, H.J.; Liu, X.H.; Yu, J.H. Changes in the fat globule membrane protein components of pasteurized milk caused by different homogenization conditions determined using a label-free proteomic approach. LWT-Food Sci. Technol. 2019, 115, 108430. [Google Scholar] [CrossRef]
- Lucey, J.A.; Wilbanks, D.J.; Horne, D.S. Impact of heat treatment of milk on acid gelation. Int. Dairy J. 2022, 125, 105222. [Google Scholar] [CrossRef]
- Anema, S.G. On heating milk, the dissociation of κ-casein from the casein micelles can precede interactions with the denatured whey proteins. J. Dairy Res. 2008, 75, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Raikos, V. Effect of heat treatment on milk protein functionality at emulsion interfaces. A review. Food Hydrocolloids 2010, 24, 259–265. [Google Scholar] [CrossRef]
- Yun, G.U.; Gillies, G.; Ripberger, G.; Hashemizadeh, I.; Whitby, C.P.; Bronlund, J. Modelling the reaction kinetics of β-lactoglobulin and Κ-casein heat-induced interactions in skim milk. J. Food Eng. 2023, 344, 111391. [Google Scholar] [CrossRef]
- Anema, S.G.; Li, Y.M. Effect of pH on the association of denatured whey proteins with casein micelles in heated reconstituted skim milk. J. Agric. Food Chem. 2003, 51, 1640–1646. [Google Scholar] [CrossRef]
- Nicolai, T.; Chassenieux, C. Heat-induced gelation of casein micelles. Food Hydrocolloids 2021, 118, 106755. [Google Scholar] [CrossRef]
- Dalgleish, D.G. On the structural models of bovine casein micelles-review and possible improvements. Soft Matter 2011, 7, 2265–2272. [Google Scholar] [CrossRef]
- Dalgleish, D.G.; Corredig, M. The structure of the casein micelle of milk and its changes during processing. Annu. Rev. Food Sci. Technol. 2012, 3, 449–467. [Google Scholar] [CrossRef] [PubMed]
- Walstra, P. Casein sub-micelles: Do they exist? Int. Dairy J. 1999, 9, 189–192. [Google Scholar] [CrossRef]
- Home, D.S. Casein micelle structure: Models and muddles. Curr. Opin. Colloid. In. 2006, 11, 148–153. [Google Scholar] [CrossRef]
- Holt, C.; de Kruif, C.G.; Tuinier, R.; Timmins, P.A. Substructure of bovine casein micelles by small-angle X-ray and neutron scattering. Colloid Surf. A 2003, 213, 275–284. [Google Scholar] [CrossRef]
- Bhat, M.Y.; Dar, T.A.; Rajendrakumar Singh, L. Casein Proteins: Structural and Functional Aspects. In Milk Proteins—From Structure to Biological Properties and Health Aspects; IntechOpen: London, UK, 2016; ISBN 978-953-51-2537-2. [Google Scholar]
- Anema, S.G. Role of κ-casein in the association of denatured whey proteins with casein micelles in heated reconstituted skim milk. J. Agric. Food Chem. 2007, 55, 3635–3642. [Google Scholar] [CrossRef]
- Anema, S.G.; Li, Y.M. Association of denatured whey proteins with casein micelles in heated reconstituted skim milk and its effect on casein micelle size. J. Dairy Res. 2003, 70, 73–83. [Google Scholar] [CrossRef]
- Kelleher, C.M.; Tobin, J.T.; O’Mahony, J.A.; Kelly, A.L.; O’Callaghan, D.J.; McCarthy, N.A. A Comparison of Pilot-Scale Supersonic Direct Steam Injection to Conventional Steam Infusion and Tubular Heating Systems for the Heat Treatment of Protein-Enriched Skim Milk-Based Beverages. Innov. Food Sci. Emerg. Technol. 2019, 52, 282–290. [Google Scholar] [CrossRef]
- Oldfield, D.J.; Singh, H.; Taylor, M.W. Association of β-lactoglobulin and α-lactalbumin with the casein micelles in skim milk heated in an ultra-high temperature plant. Int. Dairy J. 1998, 8, 765–770. [Google Scholar] [CrossRef]
- Oldfield, D.J.; Singh, H.; Taylor, M.W.; Pearce, K.N. Kinetics of denaturation and aggregation of whey proteins in skim milk heated in an ultra-high temperature (UHT) pilot plant. Int. Dairy J. 1998, 8, 311–318. [Google Scholar] [CrossRef]
- Wu, P.P.; Guo, M.Y.; Wang, P.J.; Wang, Y.; Fan, K.; Zhou, H.; Qian, W.T.; Li, H.L.; Wang, M.H.; Wei, X.J.; et al. Age gelation in direct steam infusion ultra-high-temperature milk: Different heat treatments produce different gels. Foods 2024, 13, 1236. [Google Scholar] [CrossRef] [PubMed]
- Rauh, V.M.; Johansen, L.B.; Ipsen, R.; Paulsson, M.; Larsen, L.B.; Hammershoj, M. Plasmin activity in UHT milk: Relationship between proteolysis, age gelation, and bitterness. J. Agric. Food Chem. 2014, 62, 6852–6860. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Bijl, E.; Hettinga, K. Destabilization of UHT milk by protease AprX from Pseudomonas fluorescens and plasmin. Food Chem. 2018, 263, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Beliciu, C.M.; Sauer, A.; Moraru, C.I. The effect of commercial sterilization regimens on micellar casein concentrates. J. Dairy Sci. 2012, 95, 5510–5526. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.Y.; Wang, Y.; Wang, P.J.; Luo, J.; Qian, W.T.; Li, H.L.; Wang, M.H.; Yang, J.H.; Ren, F.Z. Multiscale structure analysis reveals changes in the structure of casein micelles treated with direct-steam-infusion UHT. Food Hydrocolloids 2024, 153, 110033. [Google Scholar] [CrossRef]
- Wang, M.Q.; Cao, C.J.; Wang, Y.; Li, H.B.; Li, H.J.; Yu, J.H. Comparison of bovine milk fat globule membrane protein retention by different ultrafiltration membranes using a label-free proteomic approach. LWT-Food Sci. Technol. 2021, 144, 111219. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, M.Y.; Ren, F.Z.; Wang, P.J.; Li, H.J.; Li, H.B.; Li, Y.X.; Luo, J.; Yu, J.H. A novel strategy to construct stable fat globules with all major milk fat globule membrane proteins to mimic breast milk fat emulsions at the protein level. Food Res. Int. 2023, 173, 113351. [Google Scholar] [CrossRef] [PubMed]
- Wiking, L.; Gregersen, S.B.; Hansen, S.F.; Hammershoj, M. Heat-induced changes in milk fat and milk fat globules and its derived effects on acid dairy gelation—A review. Int. Dairy J. 2022, 127, 105213. [Google Scholar] [CrossRef]
- Lee, S.J.E.; Sherbon, J.W. Chemical changes in bovine milk fat globule membrane caused by heat treatment and homogenization of whole milk. J. Dairy Res. 2002, 69, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.X.; Zheng, N.; Zhao, X.W.; Yang, J.H.; Zhang, Y.D.; Han, R.W.; Qi, Y.X.; Zhao, S.G.; Li, S.L.; Wen, F.; et al. Changes in bovine milk fat globule membrane proteins caused by heat procedures using a label-free proteomic approach. Food Res. Int. 2018, 113, 1–8. [Google Scholar] [CrossRef]
- Yan, D.; Zhang, L.; Zhu, Y.; Han, M.; Wang, Y.; Tang, J.; Zhou, P.J.F. Changes in caprine milk fat globule membrane proteins after heat treatment using a label-free proteomics technique. Foods 2022, 11, 2705. [Google Scholar] [CrossRef]
- Huang, Y.C.; Wei, T.; Chen, F.; Tan, C.L.; Gong, Z.Q.; Wang, F.X.; Deng, Z.Y.; Li, J. Effects of various thermal treatments on interfacial composition and physical properties of bovine milk fat globules. Food Res. Int. 2023, 167, 112580. [Google Scholar] [CrossRef]
- Kokkinidou, S.; Peterson, D.G. Control of Maillard-type off-flavor development in ultrahigh-temperature-processed bovine milk by phenolic chemistry. J. Agric. Food Chem. 2014, 62, 8023–8033. [Google Scholar] [CrossRef]
- Zhang, Y.; Yi, S.; Lu, J.; Pang, X.; Xu, X.; Lv, J.; Zhang, S. Effect of different heat treatments on the Maillard reaction products, volatile compounds and glycation level of milk. Int. Dairy J. 2021, 123, 105182. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Zhao, C.; Xue, Y.; Tan, D.; Wang, S.; Jia, M.; Wu, H.; Ma, A.; Chen, G. Milk lipids characterization in relation to different heat treatments using lipidomics. Food Res. Int. 2022, 157, 111345. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; He, J.; Zhao, Z.; Li, X.; Tang, J.; Liu, Q.; Wang, H. Effect of heat treatment on the physical stability, interfacial composition and protein-lipid co-oxidation of whey protein isolate-stabilised O/W emulsions. Food Res. Int. 2023, 172, 113126. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhou, B.; Wang, P.; Ren, F.; Mao, X. Physicochemical properties of fluid milk with different heat treatments and HS-GC-IMS identification of volatile organic compounds. Int. Dairy J. 2023, 142, 105654. [Google Scholar] [CrossRef]
- Cerny, C. The aroma side of the Maillard reaction. Ann. N. Y. Acad. Sci. 2008, 1126, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Wang, J.; Ji, X.; Min, W.; Yan, W. HS-GC-IMS detection of volatile organic compounds in yak milk powder processed by different drying methods. LWT-Food Sci. Technol. 2021, 141, 110855. [Google Scholar] [CrossRef]
- Jo, Y. Flavor and Flavor Chemistry of Fluid Milk. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2019. [Google Scholar]
- Liem, D.G.; Bolhuis, D.P.; Hu, X.; Keast, R.S.J. Short communication: Influence of labeling on Australian and Chinese consumers’ liking of milk with short (pasteurized) and long (UHT) shelf life. J. Dairy Sci. 2016, 99, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.Y.; Han, Z.S.; Zhang, Z.T.; Ding, H.; Lu, X.L.; Lu, C.; Ma, L.G.; Kang, Z.Y.; Wang, B.; Li, Y. Effect of steam infusion and steam injection ultra-high temperature treatment on active proteins and flavor compounds in milk. J. Food Sci. Technol. 2023, 41, 70–80. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Yuan, D.; Li, Y.; Zhang, L. Comparison of SDE and SPME for the analysis of volatile compounds in butters. Food Sci. Biotechnol. 2019, 29, 55–62. [Google Scholar] [CrossRef]
- Gaucheron, F. Milk salts | distribution and analysis. In Encyclopedia of Dairy Sciences, 2nd ed.; Fuquay, J.W., Ed.; Academic Press: San Diego, CA, USA, 2011; pp. 908–916. ISBN 978-0-12-374407-4. [Google Scholar]
- Kilic-Akyilmaz, M.; Ozer, B.; Bulat, T.; Topcu, A. Effect of heat treatment on micronutrients, fatty acids and some bioactive components of milk. Int. Dairy J. 2022, 126, 105231. [Google Scholar] [CrossRef]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Salts of milk. In Dairy Chemistry and Biochemistry; Fox, P.F., Uniacke-Lowe, T., McSweeney, P.L.H., O’Mahony, J.A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 241–270. ISBN 978-3-319-14892-2. [Google Scholar]
- Pouliot, Y.; Boulet, M.; Paquin, P. Observations on the heat-induced salt balance changes in milk I. Effect of heating time between 4 and 90 °C. J. Dairy Res. 1989, 56, 185–192. [Google Scholar] [CrossRef]
- Pouliot, Y.; Boulet, M.; Paquin, P. Experiments on the heat-induced salt balance changes in cow’s milk. J. Dairy Res. 1989, 56, 513–519. [Google Scholar] [CrossRef]
- Pouliot, Y.; Boulet, M.; Paquin, P. Observations on the heat-induced salt balance changes in milk II. Reversibility on cooling. J. Dairy Res. 1989, 56, 193–199. [Google Scholar] [CrossRef]
- Boiani, M.; Fenelon, M.; FitzGerald, R.J.; Kelly, P.M. Use of 31P NMR and FTIR to investigate key milk mineral equilibria and their interactions with micellar casein during heat treatment. Int. Dairy J. 2018, 81, 12–18. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, Y. Effect of temperature and pH on salts equilibria and calcium phosphate in bovine milk. Int. Dairy J. 2020, 110, 104713. [Google Scholar] [CrossRef]
- Britten, M.; Giroux, H.J. Rennet coagulation of heated milk: A review. Int. Dairy J. 2022, 124, 105179. [Google Scholar] [CrossRef]
- Jeurnink, T.; Walstra, P.; Kruif, D. Mechanisms of fouling in dairy processing. Neth. Milk Dairy J. 1996, 50, 407–426. [Google Scholar]
- Seiquer, I.; Delgado-Andrade, C.; Haro, A.; Navarro, M.P. Assessing the effects of severe heat treatment of milk on calcium bioavailability: In vitro and in vivo studies. J. Dairy Sci. 2010, 93, 5635–5643. [Google Scholar] [CrossRef]
- Hrubša, M.; Siatka, T.; Nejmanová, I.; Vopršalová, M.; Kujovská Krčmová, L.; Matoušová, K.; Javorská, L.; Macáková, K.; Mercolini, L.; Remião, F.; et al. Biological properties of vitamins of the B-complex, Part 1: Vitamins B1, B2, B3, and B5. Nutrients 2022, 14, 484. [Google Scholar] [CrossRef]
- Lalwani, S.; Lewerentz, F.; Håkansson, A.; Löfgren, R.; Eriksson, J.; Paulsson, M.; Glantz, M. Impact of Thermal Processing on Micronutrients and Physical Stability of Milk and Cream at Dairy Production Scale. Int. Dairy J. 2024, 153, 105901. [Google Scholar] [CrossRef]
- Lorenzen, P.C.; Clawin-Rädecker, I.; Einhoff, K.; Hammer, P.; Hartmann, R.; Hoffmann, W.; Martin, D.; Molkentin, J.; Walte, H.G.; Devrese, M. A survey of the quality of extended shelf life (ESL) milk in relation to HTST and UHT milk. Int. J. Dairy Technol. 2011, 64, 166–178. [Google Scholar] [CrossRef]
- Ryley, J.; Kajda, P. Vitamins in thermal processing. Food Chem. 1994, 49, 119–129. [Google Scholar] [CrossRef]
- Lalić, J.; Denić, M.; Sunarić, S.; Kocić, G.; Trutić, N.; Mitić, S.; Jovanović, T. Assessment of thiamine content in some dairy products and rice milk. CyTA-J. Food 2014, 12, 203–209. [Google Scholar] [CrossRef]
- Ottaway, P.B. 10—The stability of vitamins during food processing. In The Nutrition Handbook for Food Processors; Henry, C.J.K., Chapman, C., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2002; pp. 247–264. ISBN 978-1-85573-464-7. [Google Scholar]
- Guneser, O.; Karagul Yuceer, Y. Effect of ultraviolet light on water- and fat-soluble vitamins in cow and goat milk. J. Dairy Sci. 2012, 95, 6230–6241. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Kebede, B.; Chen, G.; McComb, K.; Frew, R. Effects of the vat pasteurization process and refrigerated storage on the bovine milk metabolome. J. Dairy Sci. 2020, 103, 2077–2088. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, S.; Parkin, K.L. (Eds.) Fennema’s Food Chemistry, 5th ed.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-315-37291-4. [Google Scholar]
- Saidi, B.; Warthesen, J.J. Effect of heat and homogenization on riboflavin photolysis in milk. Int. Dairy J. 1995, 5, 635–645. [Google Scholar] [CrossRef]
- Thu Trang, V.; Kurogi, Y.; Katsuno, S.; Shimamura, T.; Ukeda, H. Protective effect of aminoreductone on photo-degradation of riboflavin. Int. Dairy J. 2008, 18, 344–348. [Google Scholar] [CrossRef]
- Friend, B.A.; Shahani, K.M.; Long, C.A.; Agel, E.N. Evaluation of freeze-drying, pasteurization, high-temperature heating and storage on selected enzymes, B-vitamins and lipids of mature human milk. J. Food Prot. 1983, 46, 330–334. [Google Scholar] [CrossRef]
- Matera, A.; Altieri, G.; Genovese, F.; Polidori, P.; Vincenzetti, S.; Perna, A.; Simonetti, A.; Rashvand Avei, M.; Calbi, A.; Di Renzo, G.C. Effect of Continuous Flow HTST Treatments on Donkey Milk Nutritional Quality. LWT-Food Sci. Technol. 2022, 153, 112444. [Google Scholar] [CrossRef]
- Bendicho, S.; Espachs, A.; Arántegui, J.; Martín, O. Effect of High Intensity Pulsed Electric Fields and Heat Treatments on Vitamins of Milk. J. Dairy Res. 2002, 69, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Johns, P.W.; Das, A.; Kuil, E.M.; Jacobs, W.A.; Schimpf, K.J.; Schmitz, D.J. Cocoa polyphenols accelerate vitamin B12 degradation in heated chocolate milk. Int. J. Food Sci. Technol. 2015, 50, 421–430. [Google Scholar] [CrossRef]
- Arkbåge, K.; Witthöft, C.; Fondén, R.; Jägerstad, M. Retention of vitamin B12 during manufacture of six fermented dairy products using a validated radio protein-binding assay. Int. Dairy J. 2003, 13, 101–109. [Google Scholar] [CrossRef]
- Sachdeva, B.; Kaushik, R.; Arora, S.; Khan, A. Effect of processing conditions on the stability of native vitamin A and fortified retinol acetate in milk. Int. J. Vitam. Nutr. Res. 2021, 91, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, R.; Sachdeva, B.; Arora, S. Vitamin D2 stability in milk during processing, packaging and storage. LWT—Food Sci. Technol. 2014, 56, 421–426. [Google Scholar] [CrossRef]
- Fox, P.F.; Uniacke-Lowe, T.; McSweeney, P.L.H.; O’Mahony, J.A. Vitamins in milk and dairy products. In Dairy Chemistry and Biochemistry; Fox, P.F., Uniacke-Lowe, T., McSweeney, P.L.H., O’Mahony, J.A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 271–297. ISBN 978-3-319-14892-2. [Google Scholar]
- Schmidt, A.; Mayer, H.K. Milk process authentication by vitamin B6 as a novel time temperature integrator. Food Control 2018, 91, 123–127. [Google Scholar] [CrossRef]
- Wigertz, K.; Hansen, I.; Høier-Madsen, M.; Holm, J.; Jägerstad, M. Effect of milk processing on the concentration of folate-binding protein (FBP), folate-binding capacity and retention of 5-methyltetrahydrofolate. Int. J. Food Sci. Nutr. 1996, 47, 315–322. [Google Scholar] [CrossRef] [PubMed]
- de Jong, R.J.; Verwei, M.; West, C.E.; van Vliet, T.; Siebelink, E.; van den Berg, H.; Castenmiller, J.J.M. Bioavailability of folic acid from fortified pasteurised and UHT-treated milk in humans. Eur. J. Clin. Nutr. 2005, 59, 906–913. [Google Scholar] [CrossRef]
- Riaz, M.N.; Asif, M.; Ali, R. Stability of vitamins during extrusion. Crit. Rev. Food Sci. Nutr. 2009, 49, 361–368. [Google Scholar] [CrossRef]
- Godoy, H.T.; Amaya-Farfan, J.; Rodriguez-Amaya, D.B. Chapter 8—Degradation of vitamins. In Chemical Changes During Processing and Storage of Foods; Rodriguez-Amaya, D.B., Amaya-Farfan, J., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 329–383. ISBN 978-0-12-817380-0. [Google Scholar]
- Khan, I.T.; Nadeem, M.; Imran, M.; Ayaz, M.; Ajmal, M.; Ellahi, M.Y.; Khalique, A. Antioxidant capacity and fatty acids characterization of heat treated cow and buffalo milk. Lipids Health Dis. 2017, 16, 163. [Google Scholar] [CrossRef]
- Andersson, I.; Öste, R. Loss of ascorbic acid, folacin and vitamin B12, and changes in oxygen content of UHT milk. I: Introduction and methods. Milchwiss.-Milk Sci. Int. 1992, 47, 223–224. [Google Scholar]
- Mehaia, M.A. Vitamin C and riboflavin content in camels milk: Effects of heat treatments. Food Chem. 1994, 50, 153–155. [Google Scholar] [CrossRef]
- Burch, R. 26—The stability and shelf life of vitamin-fortified foods. In Food and Beverage Stability and Shelf Life; Kilcast, D., Subramaniam, P., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2011; pp. 743–754. ISBN 978-1-84569-701-3. [Google Scholar]
- Berry Ottaway, P. 19—Stability of vitamins during food processing and storage. In Chemical Deterioration and Physical Instability of Food and Beverages; Skibsted, L.H., Risbo, J., Andersen, M.L., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2010; pp. 539–560. ISBN 978-1-84569-495-1. [Google Scholar]
- Lau, B.L.; Kakuda, Y.; Arnott, D.R. Effect of milk fat on the stability of vitamin A in ultra-high temperature milk. J. Dairy Sci. 1986, 69, 2052–2059. [Google Scholar] [CrossRef]
- Bezie, A. The effect of different heat treatment on the nutritional value of milk and milk products and shelf-life of milk products. A review. J. Dairy Vet. Sci. 2019, 11, 1–8. [Google Scholar] [CrossRef]
- Bonrath, W.; Gao, B.; Houston, P.; McClymont, T.; Müller, M.A.; Schäfer, C.; Schweiggert, C.; Schütz, J.; Medlock, J.A. 75 Years of vitamin A production: A historical and scientific overview of the development of new methodologies in chemistry, formulation, and biotechnology. Org. Process Res. Dev. 2023, 27, 1557–1584. [Google Scholar] [CrossRef]
- Gordon, M.H. Fat-soluble vitamin assays in food analysis. Food Chem. 1990, 36, 87. [Google Scholar] [CrossRef]
- Fennema, O. Chemical changes in food during processing–An overview. In Chemical Changes in Food during Processing; Richardson, T., Finley, J.W., Eds.; Springer: Boston, MA, USA, 1985; pp. 1–16. ISBN 978-1-4613-2265-8. [Google Scholar]
- Pandya, J.K.; DeBonee, M.; Corradini, M.G.; Camire, M.E.; McClements, D.J.; Kinchla, A.J. Development of vitamin E-enriched functional foods: Stability of tocotrienols in food systems. Int. J. Food Sci. Technol. 2019, 54, 3196–3204. [Google Scholar] [CrossRef]
- García-Martínez, C.; Holgado, F.; Velasco, J.; Márquez-Ruiz, G. Effect of classic sterilization on lipid oxidation in model liquid milk-based infant and follow-on formulas. Eur. J. Lipid Sci. Technol. 2012, 114, 1373–1380. [Google Scholar] [CrossRef]
- Li, H.; Yang, H.; Li, P.; Li, M.; Yao, Q.; Min, L.; Zhang, Y.; Wang, J.; Zheng, N. Maillard reaction products with furan ring, like furosine, cause kidney injury through triggering ferroptosis pathway. Food Chem. 2020, 319, 126368. [Google Scholar] [CrossRef]
- Katsuno, S.; Shimamura, T.; Kashiwagi, T.; Izawa, N.; Ukeda, H. Effects of dissolved oxygen on the Maillard reaction during heat treatment of milk. Int. Dairy J. 2013, 33, 34–37. [Google Scholar] [CrossRef]
- Elliott, A.J.; Datta, N.; Amenu, B.; Deeth, H.C. Heat-induced and other chemical changes in commercial UHT milks. J. Dairy Res. 2005, 72, 442–446. [Google Scholar] [CrossRef]
- Corzo, N.; Delgado, T.; Troyano, E.; Olano, A. Ratio of lactulose to furosine as indicator of quality of commercial milks. J. Food Prot. 1994, 57, 737–739. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Fandiño, R.; Corzo, N.; Villamiel, M.; Delgado, T.; Olano, A.; Ramos, M. Assessment of quality of commercial UHT milks by chromatographic and electrophoretic methods. J. Food Prot. 1993, 56, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Van Renterghem, R.; De Block, J. Furosine in consumption milk and milk powders. Int. Dairy J. 1996, 6, 371–382. [Google Scholar] [CrossRef]
- Lee, A.P.; Barbano, D.M.; Drake, M.A. The influence of ultra-pasteurization by indirect heating versus direct steam injection on skim and 2% fat milks. J. Dairy Sci. 2017, 100, 1688–1701. [Google Scholar] [CrossRef] [PubMed]
- Rauh, V.M.; Johansen, L.B.; Bakman, M.; Ipsen, R.; Paulsson, M.; Larsen, L.B.; Hammershøj, M. Protein lactosylation in UHT milk during storage measured by liquid chromatography–mass spectrometry and quantification of furosine. Int. J. Dairy Technol. 2015, 68, 486–494. [Google Scholar] [CrossRef]
- Ding, H.; Han, Z.; Wang, B.; Wang, Y.; Ran, Y.; Zhang, L.; Li, Y.; Lu, C.; Lu, X.; Ma, L. Effect of direct steam injection and instantaneous ultra-high-temperature (DSI-IUHT) sterilization on the physicochemical quality and volatile flavor components of milk. Molecules 2023, 28, 3543. [Google Scholar] [CrossRef]
- Marconi, E.; Messia, M.C.; Amine, A.; Moscone, D.; Vernazza, F.; Stocchi, F.; Palleschi, G. Heat-Treated Milk Differentiation by a Sensitive Lactulose Assay. Food Chem. 2004, 84, 447–450. [Google Scholar] [CrossRef]
- Fox, P.F.; Kelly, A.L. Indigenous enzymes in milk: Overview and historical aspects—Part 2. Int. Dairy J. 2006, 16, 517–532. [Google Scholar] [CrossRef]
- Obaidi, A.; Ahmad, A.H. Role of airway lactoperoxidase in scavenging of hydrogen peroxide damage in asthma. Ann. Thorac. Med. 2007, 2, 107. [Google Scholar] [CrossRef]
- Dickow, J.A.; Nielsen, M.T.; Hammershøj, M. Effect of lenient steam injection (LSI) heat treatment of bovine milk on the activities of some enzymes, the milk fat globule and pH. Int. J. Dairy Technol. 2012, 65, 191–200. [Google Scholar] [CrossRef]
- Griffiths, M.W. Use of milk enzymes as indices of heat treatment. J. Food Prot. 1986, 49, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Villamiel, M.; López-Fandiño, R.; Corzo, N.; Olano, A. Denaturation of β-Lactoglobulin and native enzymes in the plate exchanger and holding tube section during continuous flow pasteurization of milk. Food Chem. 1997, 58, 49–52. [Google Scholar] [CrossRef]
- Lan, X.Y.; Wang, J.Q.; Bu, D.P.; Shen, J.S.; Zheng, N.; Sun, P. Effects of heating temperatures and addition of reconstituted milk on the heat indicators in milk. J. Food Sci. 2010, 75, C653–C658. [Google Scholar] [CrossRef] [PubMed]
- van Asselt, A.J.; Sweere, A.P.J.; Rollema, H.S.; de Jong, P. Extreme high-temperature treatment of milk with respect to plasmin inactivation. Int. Dairy J. 2008, 18, 531–538. [Google Scholar] [CrossRef]
- Leite, J.A.S.; Montoya, C.A.; Loveday, S.M.; Maes, E.; Mullaney, J.A.; McNabb, W.C.; Roy, N.C. Heat-treatments affect protease activities and peptide profiles of ruminants’ milk. Front. Nutr. 2021, 8, 626475. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, R.; Li, Q.; Ma, Y. Influence of storage time on protein composition and simulated digestion of UHT milk and centrifugation presterilized UHT milk in vitro. J. Dairy Sci. 2023, 106, 3109–3122. [Google Scholar] [CrossRef] [PubMed]
- Leite, J.A.S.; Montoya, C.A.; Loveday, S.M.; Mullaney, J.A.; Loo, T.S.; McNabb, W.C.; Roy, N.C. The impact of heating and drying on protease activities of ruminant milk before and after in vitro infant digestion. Food Chem. 2023, 429, 136979. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Wu, P.; Guo, M.; Wang, Y.; Cao, Y.; Wang, P.; Renb, F.; Luo, J. Destabilization of ultra-instantaneous UHT sterilization milk stored at different temperatures. J. Dairy Sci. 2024, 107, 5460–5472. [Google Scholar] [CrossRef]
- Kiełczewska, K.; Jankowska, A.; Dąbrowska, A.; Wachowska, M.; Ziajka, J. The effect of high pressure treatment on the dispersion of fat globules and the fatty acid profile of caprine milk. Int. Dairy J. 2020, 102, 104607. [Google Scholar] [CrossRef]
- Gao, Y.; Ma, Y.; Pan, L.; Li, W.; Peng, X.; Zhang, M.; Dong, L.; Wang, J.; Gu, R. Comparative analysis of whey proteins in yak milk from different breeds in China using a data-independent acquisition proteomics method. J. Dairy Sci. 2023, 106, 3791–3806. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, F.; Wang, P.; Liang, Q.; Peng, Y.; Song, L.; Wen, P. The influence of yak casein micelle size on rennet-induced coagulation properties. J. Sci. Food Agric. 2021, 101, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Gammoh, S.; Alu’datt, M.H.; Tranchant, C.C.; Al-U’datt, D.G.; Alhamad, M.N.; Rababah, T.; Kubow, S.; Haddadin, M.S.Y.; Ammari, Z.; Maghaydah, S.; et al. Modification of the functional and bioactive properties of camel milk casein and whey proteins by ultrasonication and fermentation with Lactobacillus Delbrueckii Subsp. Lactis. LWT-Food Sci. Technol. 2020, 129, 109501. [Google Scholar] [CrossRef]
- Barłowska, J.; Szwajkowska, M.; Litwińczuk, Z.; Król, J. Nutritional value and technological suitability of milk from various animal species used for dairy production. Compr. Rev. Food Sci. Food Saf. 2011, 10, 291–302. [Google Scholar] [CrossRef]
- Heilig, A.; Çelik, A.; Hinrichs, J. Suitability of dahlem cashmere goat milk towards pasteurisation, ultrapasteurisation and UHT-heating with regard to sensory properties and storage stability. Small Rumin. Res. 2008, 78, 152–161. [Google Scholar] [CrossRef]
- Liu, Z.; Suolang, Q.; Wang, J.; Li, L.; Luo, Z.; Shang, P.; Chen, X.D.; Wu, P. Formation of structured clots, gastric emptying and hydrolysis kinetics of yak milk during in vitro dynamic gastrointestinal digestion: Impact of different heat treatments. Food Res. Int. 2022, 162, 111958. [Google Scholar] [CrossRef] [PubMed]
- Farah, Z.; Atkins, D. Heat coagulation of camel milk. J. Dairy Res. 1992, 59, 229–231. [Google Scholar] [CrossRef]
- Farah, Z. Composition and characteristics of camel milk. J. Dairy Res. 1993, 60, 603–626. [Google Scholar] [CrossRef] [PubMed]
- Sagar, S.P.; Mehta, B.M.; Wadhwani, K.N.; Darji, V.B.; Aparnathi, K.D. Evaluation of camel milk for selected processing related parameters and comparisons with cow and buffalo milk. Int. J. Health Anim. Sci. Food Saf. 2016, 3, 27–37. [Google Scholar] [CrossRef]
- Genene, A.; Hansen, E.B.; Eshetu, M.; Hailu, Y.; Ipsen, R. Effect of heat treatment on denaturation of whey protein and resultant rennetability of camel milk. LWT-Food Sci. Technol. 2019, 101, 404–409. [Google Scholar] [CrossRef]
- Zhao, X.; Guo, Y.; Zhang, Y.; Pang, X.; Wang, Y.; Lv, J.; Zhang, S. Effects of different heat treatments on maillard reaction products and volatile substances of camel milk. Front. Nutr. 2023, 10, 1072261. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, G.; Faye, B.; Serikbayeva, A.; Montet, D. Enzymes ability to serve as markers of pasteurized camel milk. In Proceedings of the Conference on New Horizons in Biotechnology, Trivandrum, India, 18–21 April 2001. [Google Scholar]
- Tayefi-Nasrabadi, H.; Hoseinpour-fayzi, M.A.; Mohasseli, M. Effect of heat treatment on lactoperoxidase activity in camel milk: A comparison with bovine lactoperoxidase. Small Rumin. Res. 2011, 99, 187–190. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Xiao, R.; Liu, S.; Wang, P.; Zhu, Y.; Niu, T.; Chen, H. The Impact of Thermal Treatment Intensity on Proteins, Fatty Acids, Macro/Micro-Nutrients, Flavor, and Heating Markers of Milk—A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 8670. https://doi.org/10.3390/ijms25168670
Wang Y, Xiao R, Liu S, Wang P, Zhu Y, Niu T, Chen H. The Impact of Thermal Treatment Intensity on Proteins, Fatty Acids, Macro/Micro-Nutrients, Flavor, and Heating Markers of Milk—A Comprehensive Review. International Journal of Molecular Sciences. 2024; 25(16):8670. https://doi.org/10.3390/ijms25168670
Chicago/Turabian StyleWang, Yi, Ran Xiao, Shiqi Liu, Pengjie Wang, Yinhua Zhu, Tianjiao Niu, and Han Chen. 2024. "The Impact of Thermal Treatment Intensity on Proteins, Fatty Acids, Macro/Micro-Nutrients, Flavor, and Heating Markers of Milk—A Comprehensive Review" International Journal of Molecular Sciences 25, no. 16: 8670. https://doi.org/10.3390/ijms25168670









