Ss4368: Pathogen-Associated Molecular Pattern for Inducing Plant Cell Death and Resistance to Phytophthora capsici
Abstract
1. Introduction
2. Results
2.1. Conservation of Ss4368 Protein Sequence and Structure across Fungal Species
2.2. Ss4368 and Bc4368 Exhibit the Capacity to Induce Cell Death
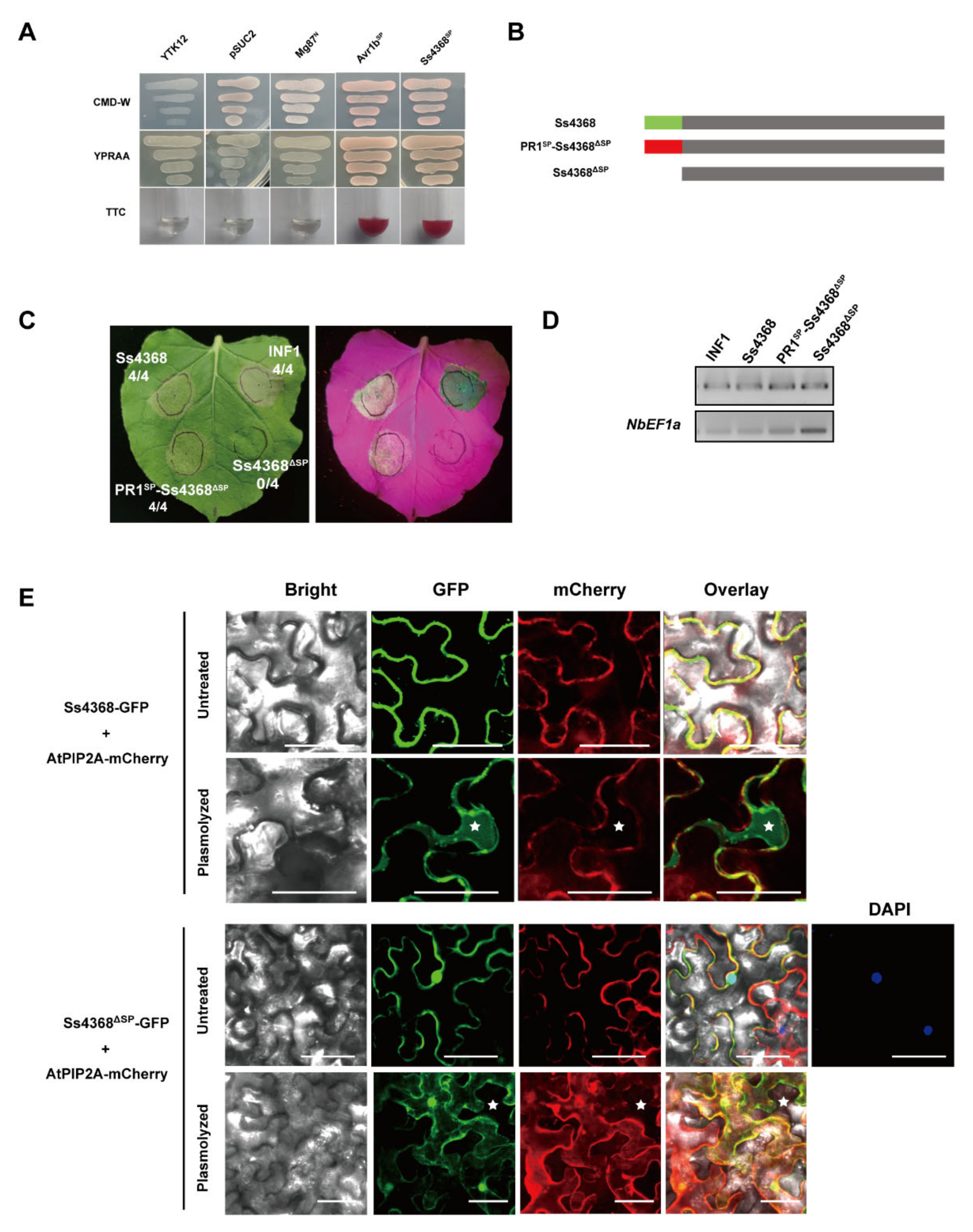
2.3. The Role of Ss4368 Signal Peptide in Protein Secretion, Localization, and Cell Death in N. benthamiana
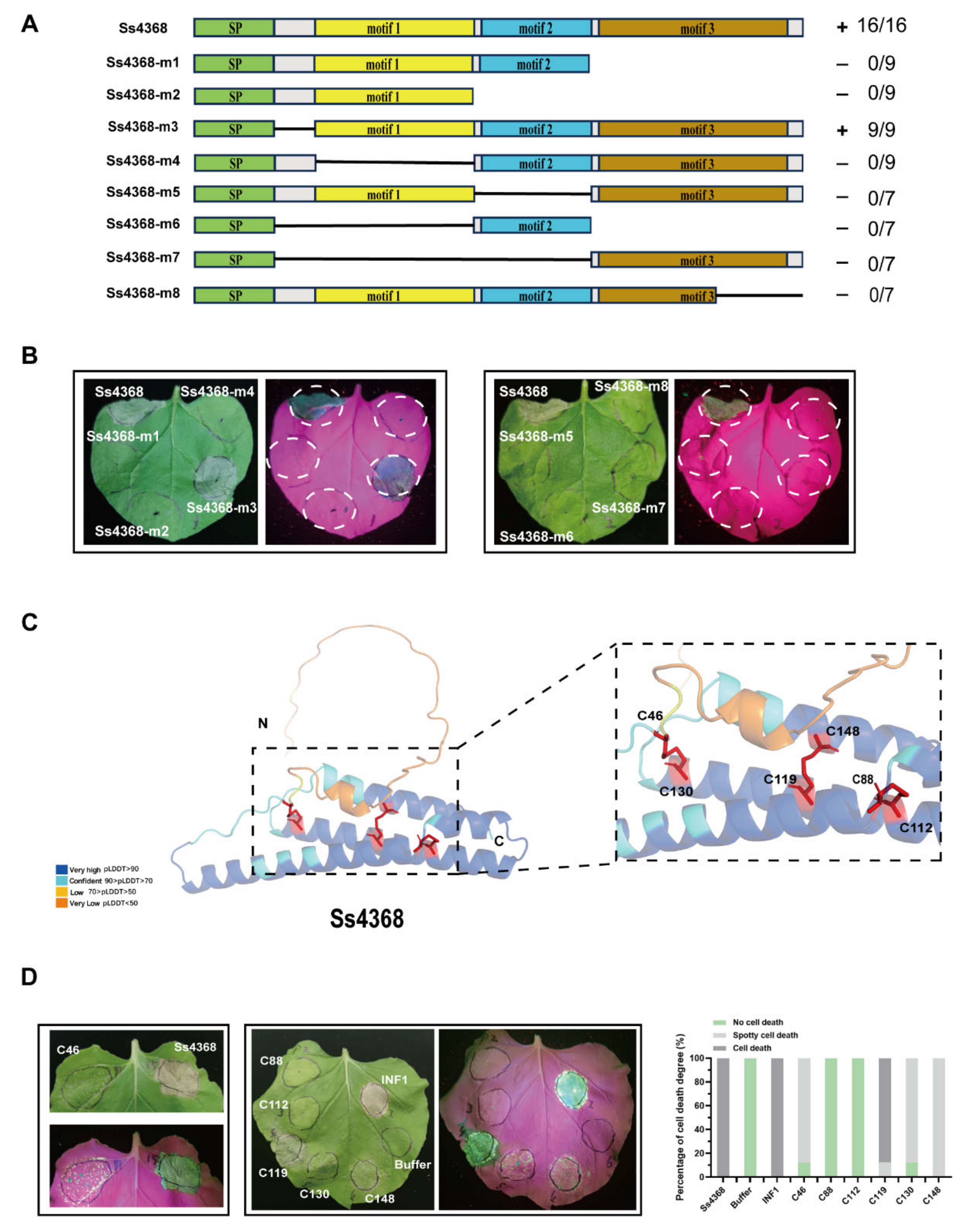
2.4. The Three Conserved Motifs of Ss4368 are Essential for Its Induction of Cell Death
2.5. Mutational Analysis of Cysteine Residues in Ss4368 Reveals Critical Disulfide Bonds for Cell Death Induction
2.6. Ss4368-Induce Immune Responses in N. benthamiana
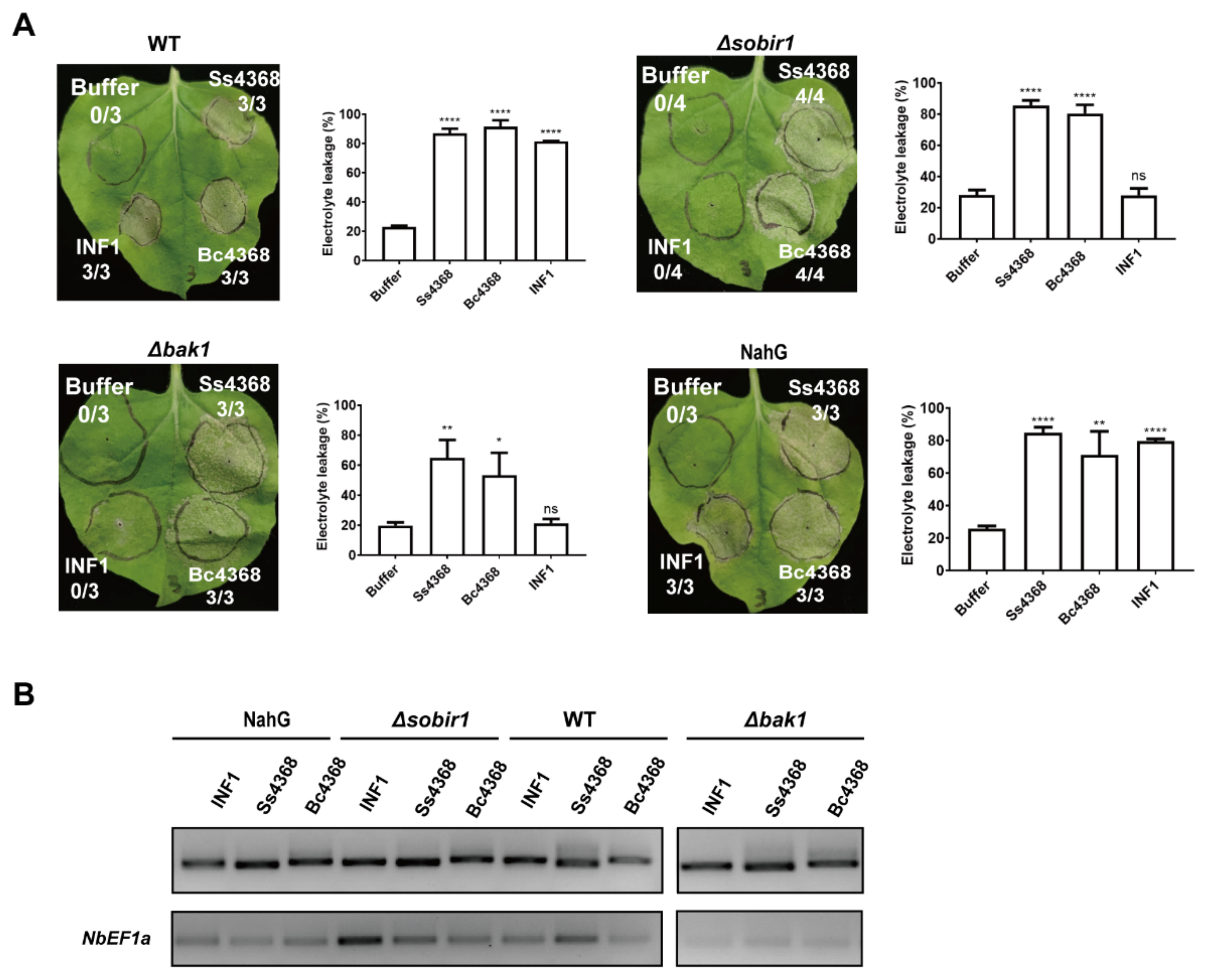
2.7. Ss4368/Bc4368-Mediated Cell Death: Independent from BAK1, SOBIR1, and Salicylic Acid Signaling Pathways
3. Discussion
3.1. Ss4368 Represents a Specific Elicitor Found in Fungi
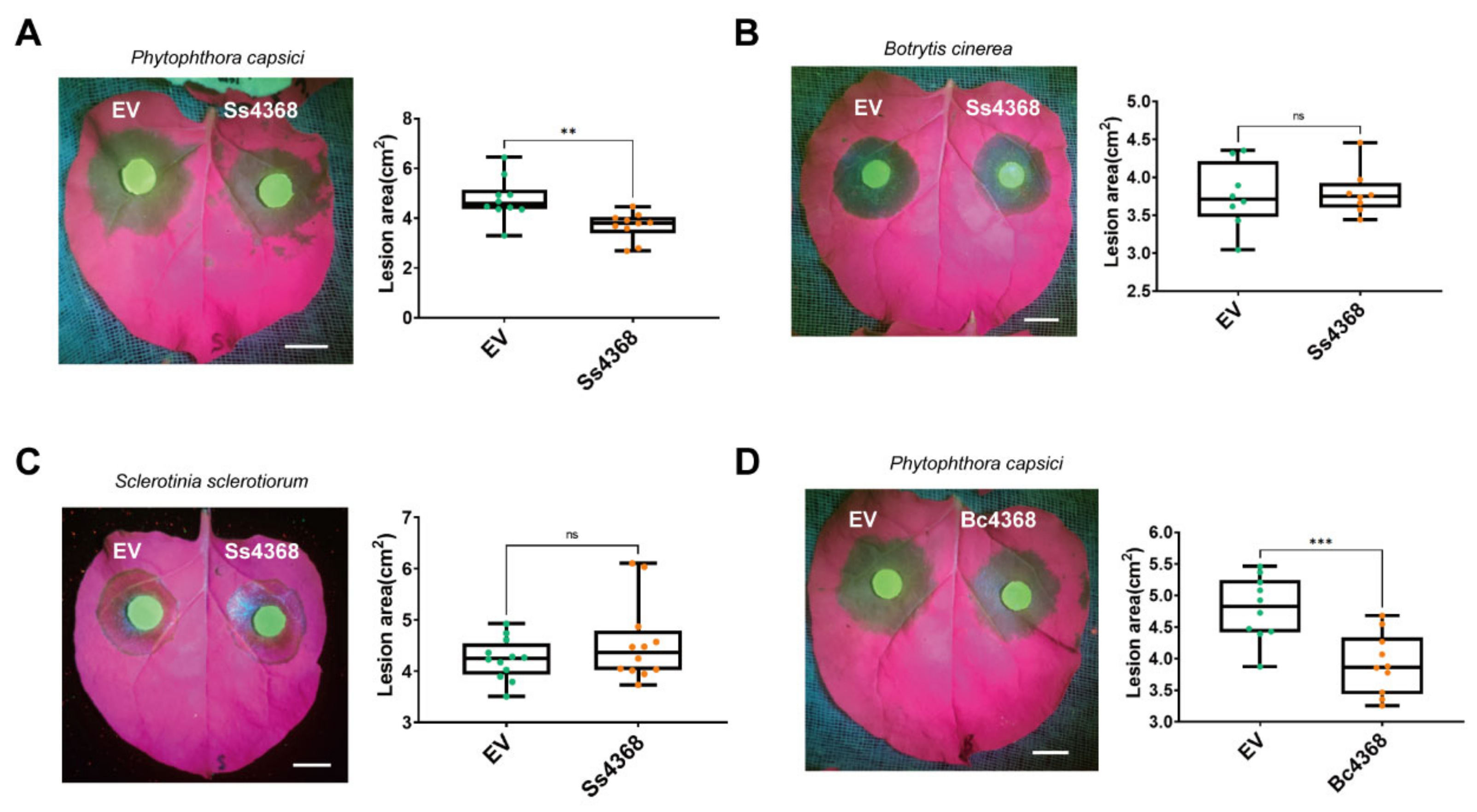
3.2. Ss4368 Is a Novel PAMP Elicitor in Inducing Resistance to Hemibiotrophic P. capsici
3.3. Cell Death Induced by Ss4368 Does Not Depend on Conventional Co-Receptors
4. Materials and Method
4.1. Plant and Strain Culture Conditions
4.2. Bioinformatic Analysis
4.3. Plasmid Construction
4.4. A. tumefaciens Agroinfiltration
4.5. Electrolyte Leakage Measurement
4.6. Confocal Microscopy
4.7. Analysis of Signal Peptide Function in Yeast
4.8. Inoculation Assay
4.9. RNA Isolation and Quantitative Reverse Transcription-PCR (qRT-PCR) Analysis
4.10. ROS and Callose Staining
4.11. Statistical Analysis
4.12. Accession Numbers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sánchez-Vallet, A.; Fouché, S.; Fudal, I.; Hartmann, F.E.; Soyer, J.L.; Tellier, A.; Croll, D. The Genome Biology of Effector Gene Evolution in Filamentous Plant Pathogens. Annu. Rev. Phytopathol. 2018, 56, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Rocafort, M.; Fudal, I.; Mesarich, C.H. Apoplastic effector proteins of plant-associated fungi and oomycetes. Curr. Opin. Plant Biol. 2020, 56, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Stegmann, M.; Misas Villamil, J.C. The apoplast as battleground for plant-microbe interactions. New Phytol. 2016, 209, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, J.M. Plant immunity triggered by microbial molecular signatures. Mol. Plant 2010, 3, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Thomma, B.P.; Nürnberger, T.; Joosten, M.H. Of PAMPs and effectors: The blurred PTI-ETI dichotomy. Plant Cell 2011, 23, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Felix, G.; Duran, J.D.; Volko, S.; Boller, T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J. 1999, 18, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Kunze, G.; Zipfel, C.; Robatzek, S.; Niehaus, K.; Boller, T.; Felix, G. The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell 2004, 16, 3496–3507. [Google Scholar] [CrossRef] [PubMed]
- Felix, G.; Boller, T. Molecular sensing of bacteria in plants. The highly conserved RNA-binding motif RNP-1 of bacterial cold shock proteins is recognized as an elicitor signal in tobacco. J. Biol. Chem. 2003, 278, 6201–6208. [Google Scholar] [CrossRef]
- Gust, A.A.; Biswas, R.; Lenz, H.D.; Rauhut, T.; Ranf, S.; Kemmerling, B.; Götz, F.; Glawischnig, E.; Lee, J.; Felix, G.; et al. Bacteria-derived peptidoglycans constitute pathogen-associated molecular patterns triggering innate immunity in Arabidopsis. J. Biol. Chem. 2007, 282, 32338–32348. [Google Scholar] [CrossRef]
- Dow, M.; Newman, M.A.; von Roepenack, E. The Induction and Modulation of Plant Defense Responses by Bacterial Lipopolysaccharides. Annu. Rev. Phytopathol. 2000, 38, 241–261. [Google Scholar] [CrossRef] [PubMed]
- Fliegmann, J.; Mithofer, A.; Wanner, G.; Ebel, J. An ancient enzyme domain hidden in the putative beta-glucan elicitor receptor of soybean may play an active part in the perception of pathogen-associated molecular patterns during broad host resistance. J. Biol. Chem. 2004, 279, 1132–1140. [Google Scholar] [CrossRef] [PubMed]
- Brunner, F.; Rosahl, S.; Lee, J.; Rudd, J.J.; Geiler, C.; Kauppinen, S.; Rasmussen, G.; Scheel, D.; Nürnberger, T. Pep-13, a plant defense-inducing pathogen-associated pattern from Phytophthora transglutaminases. EMBO J. 2002, 21, 6681–6688. [Google Scholar] [CrossRef] [PubMed]
- Gaulin, E.; Dramé, N.; Lafitte, C.; Torto-Alalibo, T.; Martinez, Y.; Ameline-Torregrosa, C.; Khatib, M.; Mazarguil, H.; Villalba-Mateos, F.; Kamoun, S.; et al. Cellulose binding domains of a Phytophthora cell wall protein are novel pathogen-associated molecular patterns. Plant Cell 2006, 18, 1766–1777. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.M. Elicitins from Phytophthora and basic resistance in tobacco. Proc. Natl. Acad. Sci. USA 1995, 92, 4088–4094. [Google Scholar] [CrossRef] [PubMed]
- Shinya, T.; Nakagawa, T.; Kaku, H.; Shibuya, N. Chitin-mediated plant-fungal interactions: Catching, hiding and handshaking. Curr. Opin. Plant Biol. 2015, 26, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Rotblat, B.; Enshell-Seijffers, D.; Gershoni, J.M.; Schuster, S.; Avni, A. Identification of an essential component of the elicitation active site of the EIX protein elicitor. Plant J. 2002, 32, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Zhang, Y.; Zhang, Q.; Zhang, X.; Shen, D.; Yu, J.; Yu, M.; Pan, X.; Cao, H.; Yong, M.; et al. The N-terminus of an Ustilaginoidea virens Ser-Thr-rich glycosylphosphatidylinositol-anchored protein elicits plant immunity as a MAMP. Nat. Commun. 2021, 12, 2451. [Google Scholar] [CrossRef]
- Nie, J.; Yin, Z.; Li, Z.; Wu, Y.; Huang, L. A small cysteine-rich protein from two kingdoms of microbes is recognized as a novel pathogen-associated molecular pattern. New Phytol. 2019, 222, 995–1011. [Google Scholar]
- Qutob, D.; Kemmerling, B.; Brunner, F.; Küfner, I.; Engelhardt, S.; Gust, A.A.; Luberacki, B.; Seitz, H.U.; Stahl, D.; Rauhut, T.; et al. Phytotoxicity and innate immune responses induced by Nep1-like proteins. Plant Cell 2006, 18, 3721–3744. [Google Scholar] [CrossRef]
- Oome, S.; Raaymakers, T.M.; Cabral, A.; Samwel, S.; Böhm, H.; Albert, I.; Nürnberger, T.; Van den Ackerveken, G. Nep1-like proteins from three kingdoms of life act as a microbe-associated molecular pattern in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 16955–16960. [Google Scholar] [CrossRef]
- Böhm, H.; Albert, I.; Oome, S.; Raaymakers, T.M.; Van den Ackerveken, G.; Nürnberger, T. A conserved peptide pattern from a widespread microbial virulence factor triggers pattern-induced immunity in Arabidopsis. PLoS Pathog. 2014, 10, e1004491. [Google Scholar] [CrossRef]
- Ma, Z.; Song, T.; Zhu, L.; Ye, W.; Wang, Y.; Shao, Y.; Dong, S.; Zhang, Z.; Dou, D.; Zheng, X.; et al. A Phytophthora sojae Glycoside Hydrolase 12 Protein Is a Major Virulence Factor during Soybean Infection and Is Recognized as a PAMP. Plant Cell 2015, 27, 2057–2072. [Google Scholar] [CrossRef]
- Yang, B.; Yang, S.; Zheng, W.; Wang, Y. Plant immunity inducers: From discovery to agricultural application. Stress Biol. 2022, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Dewen, Q.; Yijie, D.; Yi, Z.; Shupeng, L.; Fachao, S. Plant Immunity Inducer Development and Application. Mol. Plant-Microbe Interact. MPMI 2017, 30, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Reglinski, T.; Havis, N.; Rees, H.J.; de Jong, H. The Practical Role of Induced Resistance for Crop Protection. Phytopathology 2023, 113, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.M.; Laby, R.J.; Zumoff, C.H.; Bauer, D.W.; He, S.Y.; Collmer, A.; Beer, S.V. Harpin, elicitor of the hypersensitive response produced by the plant pathogen Erwinia amylovora. Science 1992, 257, 85–88. [Google Scholar] [CrossRef]
- Dong, H.; Delaney, T.P.; Bauer, D.W.; Beer, S.V. Harpin induces disease resistance in Arabidopsis through the systemic acquired resistance pathway mediated by salicylic acid and the NIM1 gene. Plant J. 1999, 20, 207–215. [Google Scholar] [CrossRef]
- Fontanilla, M.; Montes, M.; De Prado, R. Effects of the foliar-applied protein “Harpin(Ea)” (messenger) on tomatoes infected with Phytophthora infestans. Commun. Agric. Appl. Biol. Sci. 2005, 70, 41–45. [Google Scholar]
- Sands, L.B.; Cheek, T.; Reynolds, J.; Ma, Y.; Berkowitz, G.A. Effects of Harpin and Flg22 on Growth Enhancement and Pathogen Defense in Cannabis sativa Seedlings. Plants 2022, 11, 1178. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, X.; Qiu, D.; Guo, L.; Zeng, H.; Mao, J.; Gao, Q. PeaT1-induced systemic acquired resistance in tobacco follows salicylic acid-dependent pathway. Mol. Biol. Rep. 2011, 38, 2549–2556. [Google Scholar] [CrossRef] [PubMed]
- Macho, A.P.; Zipfel, C. Plant PRRs and the activation of innate immune signaling. Mol. Cell 2014, 54, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Couto, D.; Zipfel, C. Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 2016, 16, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Y.; Zhang, X.; Chen, Z.; Xia, Y.; Wang, L.; Sun, Y.; Zhang, M.; Xiao, Y.; Han, Z.; et al. Plant receptor-like protein activation by a microbial glycoside hydrolase. Nature 2022, 610, 335–342. [Google Scholar] [PubMed]
- Wang, Y.; Xu, Y.; Sun, Y.; Wang, H.; Qi, J.; Wan, B.; Ye, W.; Lin, Y.; Shao, Y.; Dong, S.; et al. Leucine-rich repeat receptor-like gene screen reveals that Nicotiana RXEG1 regulates glycoside hydrolase 12 MAMP detection. Nat. Commun. 2018, 9, 594. [Google Scholar] [PubMed]
- Nie, J.; Zhou, W.; Liu, J.; Tan, N.; Zhou, J.M.; Huang, L. A receptor-like protein from Nicotiana benthamiana mediates VmE02 PAMP-triggered immunity. New Phytol. 2021, 229, 2260–2272. [Google Scholar] [PubMed]
- Yang, B.; Wang, Y.; Tian, M.; Dai, K.; Zheng, W.; Liu, Z.; Yang, S.; Liu, X.; Shi, D.; Zhang, H.; et al. Fg12 ribonuclease secretion contributes to Fusarium graminearum virulence and induces plant cell death. J. Integr. Plant Biol. 2021, 63, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Hu, S.; Jin, M.; Xu, Y.; Jiang, Q.; Ma, J.; Zhang, Y.; Qi, P.; Chen, G.; Jiang, Y.; et al. The N-terminus of a Fusarium graminearum-secreted protein enhances broad-spectrum disease resistance in plants. Mol. Plant Pathol. 2022, 23, 1751–1764. [Google Scholar] [CrossRef]
- Li, H.; Yang, Z.; Zeng, Q.; Wang, S.; Luo, Y.; Huang, Y.; Xin, Y.; He, N. Abnormal expression of bHLH3 disrupts a flavonoid homeostasis network, causing differences in pigment composition among mulberry fruits. Hortic. Res. 2020, 7, 83. [Google Scholar] [CrossRef]
- Jan, B.; Parveen, R.; Zahiruddin, S.; Khan, M.U.; Mohapatra, S.; Ahmad, S. Nutritional constituents of mulberry and their potential applications in food and pharmaceuticals: A review. Saudi J. Biol. Sci. 2021, 28, 3909–3921. [Google Scholar] [CrossRef]
- Lv, Z.; He, Z.; Hao, L.; Kang, X.; Ma, B.; Li, H.; Luo, Y.; Yuan, J.; He, N. Genome Sequencing Analysis of Scleromitrula shiraiana, a Causal Agent of Mulberry Sclerotial Disease With Narrow Host Range. Front. Microbiol. 2021, 11, 603927. [Google Scholar] [CrossRef]
- Hong, S.K.; Kim, W.G.; Sung, G.B.; Nam, S.H. Identification and distribution of two fungal species causing sclerotial disease on mulberry fruits in Korea. Mycobiology 2007, 35, 87–90. [Google Scholar] [CrossRef][Green Version]
- Luderer, R.; De Kock, M.J.; Dees, R.H.; De Wit, P.J.; Joosten, M.H. Functional analysis of cysteine residues of ECP elicitor proteins of the fungal tomato pathogen Cladosporium fulvum. Mol. Plant Pathol. 2002, 3, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tian, L.; Zhang, D.D.; Song, J.; Song, S.S.; Yin, C.M.; Zhou, L.; Liu, Y.; Wang, B.L.; Kong, Z.Q.; et al. Functional analyses of small secreted cysteine-rich proteins identified candidate effectors in Verticillium dahliae. Mol. Plant Pathol. 2020, 21, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Ngou, B.P.M.; Jones, J.D.G.; Ding, P. Plant immune networks. Trends Plant Sci. 2022, 27, 255–273. [Google Scholar] [CrossRef]
- Wang, N.; Yin, Z.; Zhao, Y.; Wang, J.; Pei, Y.; Ji, P.; Daly, P.; Li, Z.; Dou, D.; Wei, L. An F-box protein attenuates fungal xylanase-triggered immunity by destabilizing LRR-RLP NbEIX2 in a SOBIR1-dependent manner. New Phytol. 2022, 236, 2202–2215. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Shen, C.; Fu, Y.; Xie, J.; Jiang, D.; Li, G.; Cheng, J. A Small Secreted Virulence-Related Protein Is Essential for the Necrotrophic Interactions of Sclerotinia sclerotiorum with Its Host Plants. PLoS Pathog. 2016, 12, e1005435. [Google Scholar] [CrossRef]
- Doehlemann, G.; van der Linde, K.; Assmann, D.; Schwammbach, D.; Hof, A.; Mohanty, A.; Jackson, D.; Kahmann, R. Pep1, a secreted effector protein of Ustilago maydis, is required for successful invasion of plant cells. PLoS Pathog. 2009, 5, e1000290. [Google Scholar] [CrossRef]
- Yang, G.; Tang, L.; Gong, Y.; Xie, J.; Fu, Y.; Jiang, D.; Li, G.; Collinge, D.B.; Chen, W.; Cheng, J. A cerato-platanin protein SsCP1 targets plant PR1 and contributes to virulence of Sclerotinia sclerotiorum. New Phytol. 2018, 217, 739–755. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, X.; Chen, X.; Xia, Y.; Liu, J.; Zhou, G. Plant immune receptors interact with hemibiotrophic pathogens to activate plant immunity. Front. Microbiol. 2023, 14, 1252039. [Google Scholar] [CrossRef]
- Lamour, K.H.; Stam, R.; Jupe, J.; Huitema, E. The oomycete broad-host-range pathogen Phytophthora capsici. Mol. Plant Pathol. 2012, 13, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yan, J.; Fu, Z.; Shi, W.; Ninkuu, V.; Li, G.; Yang, X.; Zeng, H. FoEG1, a secreted glycoside hydrolase family 12 protein from Fusarium oxysporum, triggers cell death and modulates plant immunity. Mol. Plant Pathol. 2021, 22, 522–538. [Google Scholar] [CrossRef] [PubMed]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef] [PubMed]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, P.; Deng, Y.; Zhang, Z.; Situ, J.; Huang, J.; Li, M.; Xi, P.; Jiang, Z.; Kong, G. Litchi aspartic protease LcAP1 enhances plant resistance via suppressing cell death triggered by the pectate lyase PlPeL8 from Peronophythora litchii. New Phytol. 2024, 242, 2682–2701. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Chen, T.; Lin, Y.; Luo, C. Functional Evaluation of the Signal Peptides of Secreted Proteins. Bio Protoc. 2018, 8, e2839. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Z.; Peng, S.; Yin, Q.; Huang, Y.; Deng, T.; Luo, Y.; He, N. Ss4368: Pathogen-Associated Molecular Pattern for Inducing Plant Cell Death and Resistance to Phytophthora capsici. Int. J. Mol. Sci. 2024, 25, 8674. https://doi.org/10.3390/ijms25168674
He Z, Peng S, Yin Q, Huang Y, Deng T, Luo Y, He N. Ss4368: Pathogen-Associated Molecular Pattern for Inducing Plant Cell Death and Resistance to Phytophthora capsici. International Journal of Molecular Sciences. 2024; 25(16):8674. https://doi.org/10.3390/ijms25168674
Chicago/Turabian StyleHe, Ziwen, Shufang Peng, Qingqing Yin, Yuanyuan Huang, Ting Deng, Yiwei Luo, and Ningjia He. 2024. "Ss4368: Pathogen-Associated Molecular Pattern for Inducing Plant Cell Death and Resistance to Phytophthora capsici" International Journal of Molecular Sciences 25, no. 16: 8674. https://doi.org/10.3390/ijms25168674
APA StyleHe, Z., Peng, S., Yin, Q., Huang, Y., Deng, T., Luo, Y., & He, N. (2024). Ss4368: Pathogen-Associated Molecular Pattern for Inducing Plant Cell Death and Resistance to Phytophthora capsici. International Journal of Molecular Sciences, 25(16), 8674. https://doi.org/10.3390/ijms25168674





