Granzyme B Expression in Conjunctiva of Patients with Pterygium
Abstract
:1. Introduction
2. Results
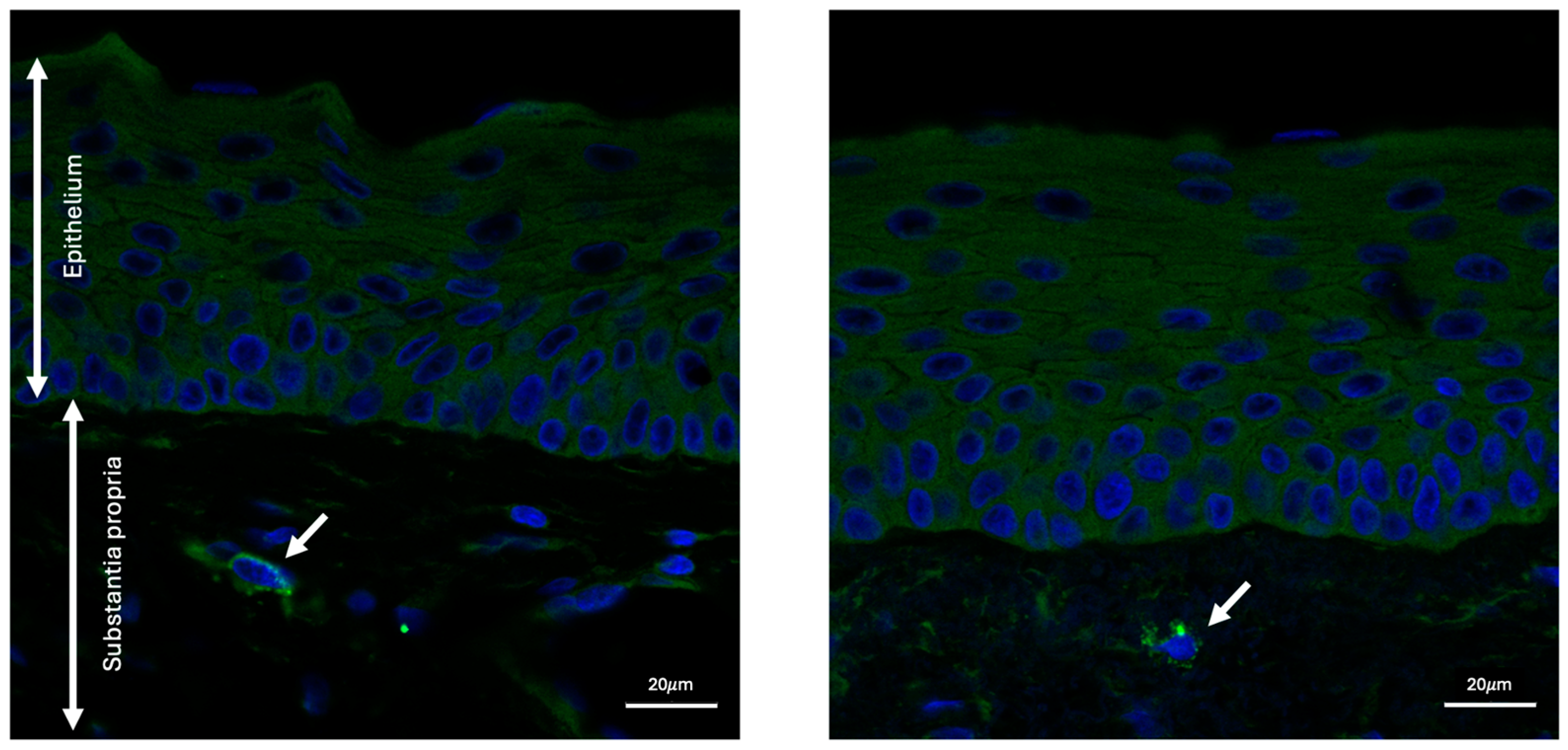
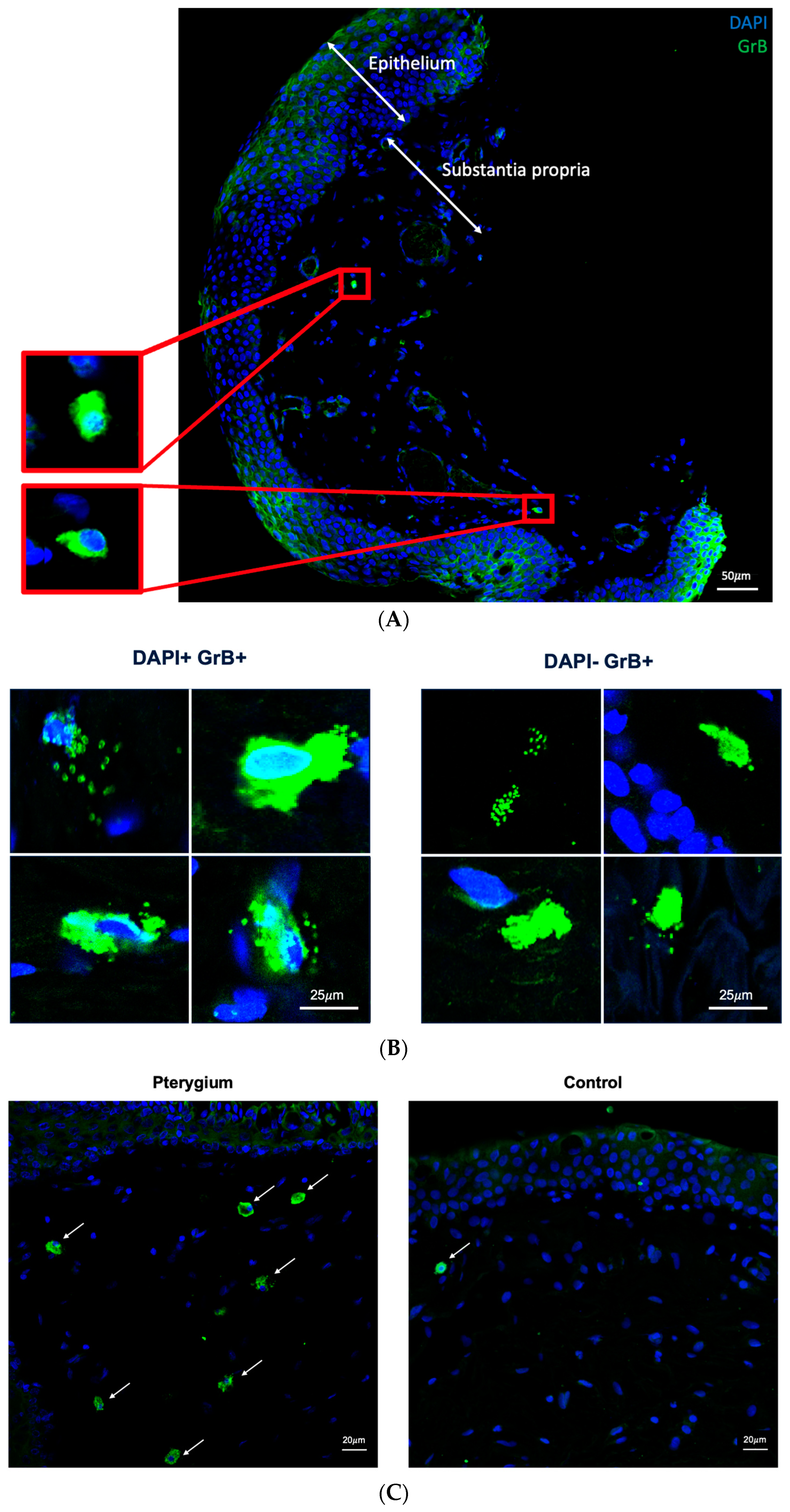
2.1. Granzyme B Expression in Human Conjunctiva
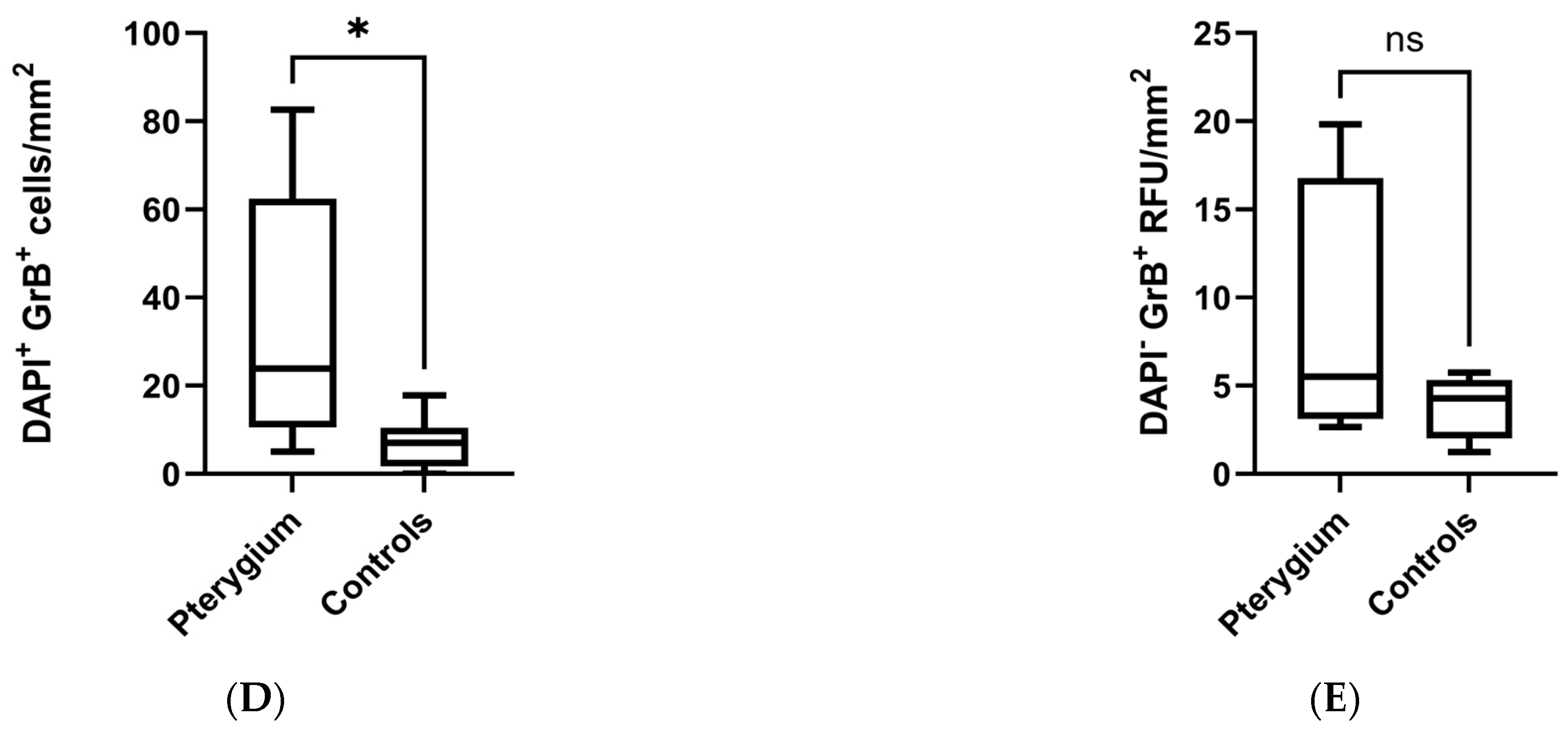
2.2. Higher Density of Granzyme B-Positive Cells in Pterygium
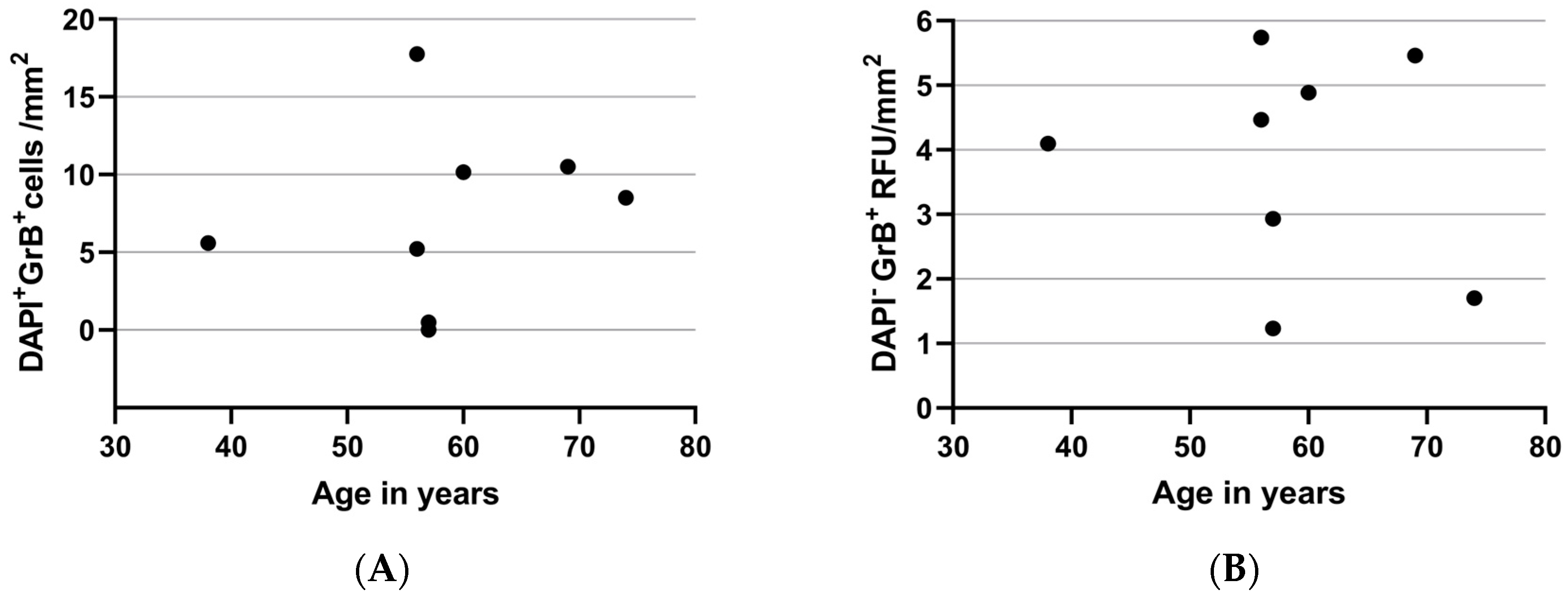
2.3. Granzyme B Expression and Age
2.4. Granzyme B and Tryptase Co-Expression in Pterygium
3. Discussion
4. Materials and Methods
4.1. Tissue Collection
4.2. Immunofluorescence
4.3. Quantification
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rezvan, F.; Khabazkhoob, M.; Hooshmand, E.; Yekta, A.; Saatchi, M.; Hashemi, H. Prevalence and Risk Factors of Pterygium: A Systematic Review and Meta-Analysis. Surv. Ophthalmol. 2018, 63, 719–735. [Google Scholar] [CrossRef] [PubMed]
- Fotouhi, A.; Hashemi, H.; Khabazkhoob, M.; Mohammad, K. Prevalence and Risk Factors of Pterygium and Pinguecula: The Tehran Eye Study. Eye 2009, 23, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Cha, X.; Wei, T.; Lin, X.; Li, X.; Li, J.; Cai, N.; Li, J.; Su, X.; Yang, Y.; et al. Prevalence of and Risk Factors for Pterygium in Rural Adult Chinese Populations of the Bai Nationality in Dali: The Yunnan Minority Eye Study. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6617–6621. [Google Scholar] [CrossRef] [PubMed]
- Martín-López, J.; Pérez-Rico, C.; Benito-Martínez, S.; Pérez-Köhler, B.; Buján, J.; Pascual, G. The Role of the Stromal Extracellular Matrix in the Development of Pterygium Pathology: An Update. J. Clin. Med. 2021, 10, 5930. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, E.C.; Rocha, E.M.; Arruda, G.V. Comparison among Adjuvant Treatments for Primary Pterygium: A Network Meta-Analysis. Br. J. Ophthalmol. 2018, 102, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Aidenloo, N.S.; Motarjemizadeh, Q.; Heidarpanah, M. Risk Factors for Pterygium Recurrence after Limbal-Conjunctival Autografting: A Retrospective, Single-Centre Investigation. Jpn. J. Ophthalmol. 2018, 62, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Janson, B.J.; Sikder, S. Surgical Management of Pterygium. Ocul. Surf. 2014, 12, 112–119. [Google Scholar] [CrossRef]
- Tekelioglu, Y.; Turk, A.; Avunduk, A.M.; Yulug, E. Flow Cytometrical Analysis of Adhesion Molecules, T-Lymphocyte Subpopulations and Inflammatory Markers in Pterygium. Ophthalmologica 2006, 220, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Awdeh, R.M.; DeStafeno, J.J.; Blackmon, D.M.; Cummings, T.J.; Kim, T. The Presence of T-Lymphocyte Subpopulations (CD4 and CD8) in Pterygia: Evaluation of the Inflammatory Response. Adv. Ther. 2008, 25, 479–487. [Google Scholar] [CrossRef]
- Beden, Ü.; Irkeç, M.; Orhan, D.; Orhan, M. The Roles of T-Lymphocyte Subpopulations (CD4 and CD8), Intercellular Adhesion Molecule-1 (ICAM-1), HLA-DR Receptor, and Mast Cells in Etiopathogenesis of Pterygium. Ocul. Immunol. Inflamm. 2003, 11, 115–122. [Google Scholar] [CrossRef]
- Obasanmi, G.; Zeglinski, M.R.; Hardie, E.; Wilhelm, A.-C.; Turner, C.T.; Hiroyasu, S.; Boivin, W.A.; Tian, Y.; Zhao, H.; To, E.; et al. Granzyme B Contributes to Choroidal Neovascularization and Age-Related Macular Degeneration Through Proteolysis of Thrombospondin-1. Lab. Investig. 2023, 103, 100123. [Google Scholar] [CrossRef] [PubMed]
- Choy, J.C.; McDonald, P.C.; Suarez, A.C.; Hung, V.H.Y.; Wilson, J.E.; McManus, B.M.; Granville, D.J. Granzyme B in Atherosclerosis and Transplant Vascular Disease: Association with Cell Death and Atherosclerotic Disease Severity. Mod. Pathol. 2003, 16, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Campos, T.M.; Novais, F.O.; Saldanha, M.; Costa, R.; Lordelo, M.; Celestino, D.; Sampaio, C.; Tavares, N.; Arruda, S.; Machado, P.; et al. Granzyme B Produced by Natural Killer Cells Enhances Inflammatory Response and Contributes to the Immunopathology of Cutaneous Leishmaniasis. J. Infect. Dis. 2020, 221, 973–982. [Google Scholar] [CrossRef]
- Parkinson, L.G.; Toro, A.; Zhao, H.; Brown, K.; Tebbutt, S.J.; Granville, D.J. Granzyme B Mediates Both Direct and Indirect Cleavage of Extracellular Matrix in Skin after Chronic Low-dose Ultraviolet Light Irradiation. Aging Cell 2015, 14, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Pigeon, H.; Jean, C.; Charruyer, A.; Haure, M.-J.; Titeux, M.; Tonasso, L.; Quillet-Mary, A.; Baudouin, C.; Charveron, M.; Laurent, G. Human Keratinocytes Acquire Cellular Cytotoxicity under UV-B Irradiation. J. Biol. Chem. 2006, 281, 13525–13532. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, J.A.; Tian, Y.; Cui, J.Z.; Zeglinski, M.R.; Hiroyasu, S.; Turner, C.T.; Granville, D.J. Retinal Distribution and Extracellular Activity of Granzyme B: A Serine Protease That Degrades Retinal Pigment Epithelial Tight Junctions and Extracellular Matrix Proteins. Front. Immunol. 2020, 11, 574. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, E.; Bisevac, J.; Hyttinen, J.M.T.; Piippo, N.; Hytti, M.; Kaarniranta, K.; Petrovski, G.; Kauppinen, A. UV-B-Induced Inflammasome Activation Can Be Prevented by Cis-Urocanic Acid in Human Corneal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2020, 61, 7. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, N.; Kumar, R.K.; Coroneo, M.T.; Wakefield, D. UVB-Mediated Induction of Interleukin-6 and -8 in Pterygia and Cultured Human Pterygium Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3430–3437. [Google Scholar]
- Shibata, N.; Ishida, H.; Kiyokawa, E.; Singh, D.P.; Sasaki, H.; Kubo, E. Relative Gene Expression Analysis of Human Pterygium Tissues and UV Radiation-Evoked Gene Expression Patterns in Corneal and Conjunctival Cells. Exp. Eye Res. 2020, 199, 108194. [Google Scholar] [CrossRef]
- Hiroyasu, S.; Zeglinski, M.R.; Zhao, H.; Pawluk, M.A.; Turner, C.T.; Kasprick, A.; Tateishi, C.; Nishie, W.; Burleigh, A.; Lennox, P.A.; et al. Granzyme B Inhibition Reduces Disease Severity in Autoimmune Blistering Diseases. Nat. Commun. 2021, 12, 302. [Google Scholar] [CrossRef]
- Fisher, G.J. The Pathophysiology of Photoaging of the Skin. Cutis 2005, 75, 5–8; discussion 8–9. [Google Scholar] [PubMed]
- Ribatti, D.; Nico, B.; Maxia, C.; Longo, V.; Murtas, D.; Mangieri, D.; Perra, M.T.; De Giorgis, M.; Piras, F.; Crivellato, E.; et al. Neovascularization and Mast Cells with Tryptase Activity Increase Simultaneously in Human Pterygium. J. Cell Mol. Med. 2007, 11, 585–589. [Google Scholar] [CrossRef]
- Powers, M.R.; Qu, Z.; O’Brien, B.; Wilson, D.J.; Thompson, J.E.; Rosenbaum, J.T. Immunolocalization of bFGF in Pterygia: Association with Mast Cells. Cornea 1997, 16, 545–549. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, T.; Murakami, A.; Okisaka, S.; Ebihara, N. Pterygium and mast cells--mast cell number, phenotype, and localization of stem cell factor. Nippon. Ganka Gakkai Zasshi 1997, 101, 662–668. [Google Scholar] [PubMed]
- Celebi, A.R.C.; Ozbey, C.; Mirza, G.E. The Role of Mast Cells in Vascularized Recurrent Pterygium. Arq. Bras. Oftalmol. 2014, 77, 285–287. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Q.; Lee, S.B.; Gunja-Smith, Z.; Liu, Y.; Solomon, A.; Meller, D.; Tseng, S.C. Overexpression of Collagenase (MMP-1) and Stromelysin (MMP-3) by Pterygium Head Fibroblasts. Arch. Ophthalmol. 2001, 119, 71–80. [Google Scholar] [PubMed]
- Yang, S.-F.; Lin, C.-Y.; Yang, P.-Y.; Chao, S.-C.; Ye, Y.-Z.; Hu, D.-N. Increased Expression of Gelatinase (MMP-2 and MMP-9) in Pterygia and Pterygium Fibroblasts with Disease Progression and Activation of Protein Kinase, C. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4588–4596. [Google Scholar] [CrossRef]
- Masitas, C.; Peng, Z.; Wang, M.; Konai, M.M.; Avila-Cobian, L.F.; Lemieux, L.; Hovanesian, J.; Grady, J.E.; Mobashery, S.; Chang, M. Matrix Metalloproteinase-14 as an Instigator of Fibrosis in Human Pterygium and Its Pharmacological Intervention. ACS Pharmacol. Transl. Sci. 2022, 5, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Jung, J.-C.; Gum, S.I.; Park, S.-B.; Ma, J.Y.; Kim, Y.I.; Lee, K.W.; Park, Y.J. Inhibition of Pterygium Fibroblast Migration and Outgrowth by Bevacizumab and Cyclosporine A Involves Down-Regulation of Matrix Metalloproteinases-3 and -13. PLoS ONE 2017, 12, e0169675. [Google Scholar] [CrossRef]
- Dufour, A.; Overall, C.M. Missing the Target: Matrix Metalloproteinase Antitargets in Inflammation and Cancer. Trends Pharmacol. Sci. 2013, 34, 233–242. [Google Scholar] [CrossRef]
- Turner, C.T.; Bolsoni, J.; Zeglinski, M.R.; Zhao, H.; Ponomarev, T.; Richardson, K.; Hiroyasu, S.; Schmid, E.; Papp, A.; Granville, D.J. Granzyme B Mediates Impaired Healing of Pressure Injuries in Aged Skin. NPJ Aging Mech. Dis. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Hiebert, P.R.; Boivin, W.A.; Abraham, T.; Pazooki, S.; Zhao, H.; Granville, D.J. Granzyme B Contributes to Extracellular Matrix Remodeling and Skin Aging in Apolipoprotein E Knockout Mice. Exp. Gerontol. 2011, 46, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Dubchak, E.; Obasanmi, G.; Zeglinski, M.R.; Granville, D.J.; Yeung, S.N.; Matsubara, J.A. Potential Role of Extracellular Granzyme B in Wet Age-Related Macular Degeneration and Fuchs Endothelial Corneal Dystrophy. Front. Pharmacol. 2022, 13, 980742. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Samad, I.; Chakravarthy, H.; Matsubara, J.; Granville, D.J.; Yeung, S.N. Granzyme B Expression in Conjunctiva of Patients with Pterygium. Int. J. Mol. Sci. 2024, 25, 8679. https://doi.org/10.3390/ijms25168679
Choi Y, Samad I, Chakravarthy H, Matsubara J, Granville DJ, Yeung SN. Granzyme B Expression in Conjunctiva of Patients with Pterygium. International Journal of Molecular Sciences. 2024; 25(16):8679. https://doi.org/10.3390/ijms25168679
Chicago/Turabian StyleChoi, Yoojin, Isa Samad, Harshini Chakravarthy, Joanne Matsubara, David J. Granville, and Sonia N. Yeung. 2024. "Granzyme B Expression in Conjunctiva of Patients with Pterygium" International Journal of Molecular Sciences 25, no. 16: 8679. https://doi.org/10.3390/ijms25168679




