Virtual Screening and Validation of Affinity DNA Functional Ligands for IgG Fc Segment
Abstract
:1. Introduction
2. Results
2.1. Molecular Docking
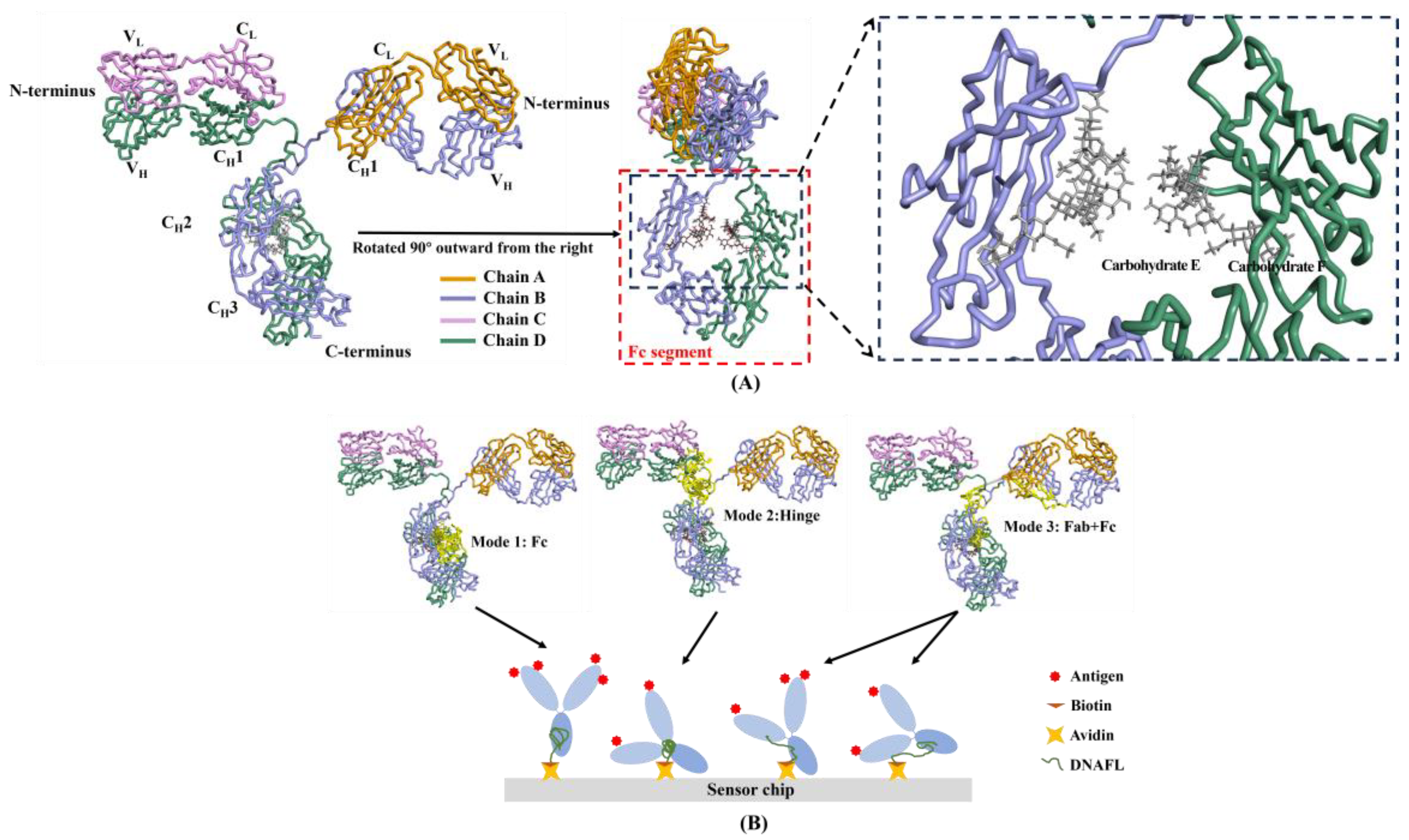
2.1.1. Binding Mode in Molecular Docking Results
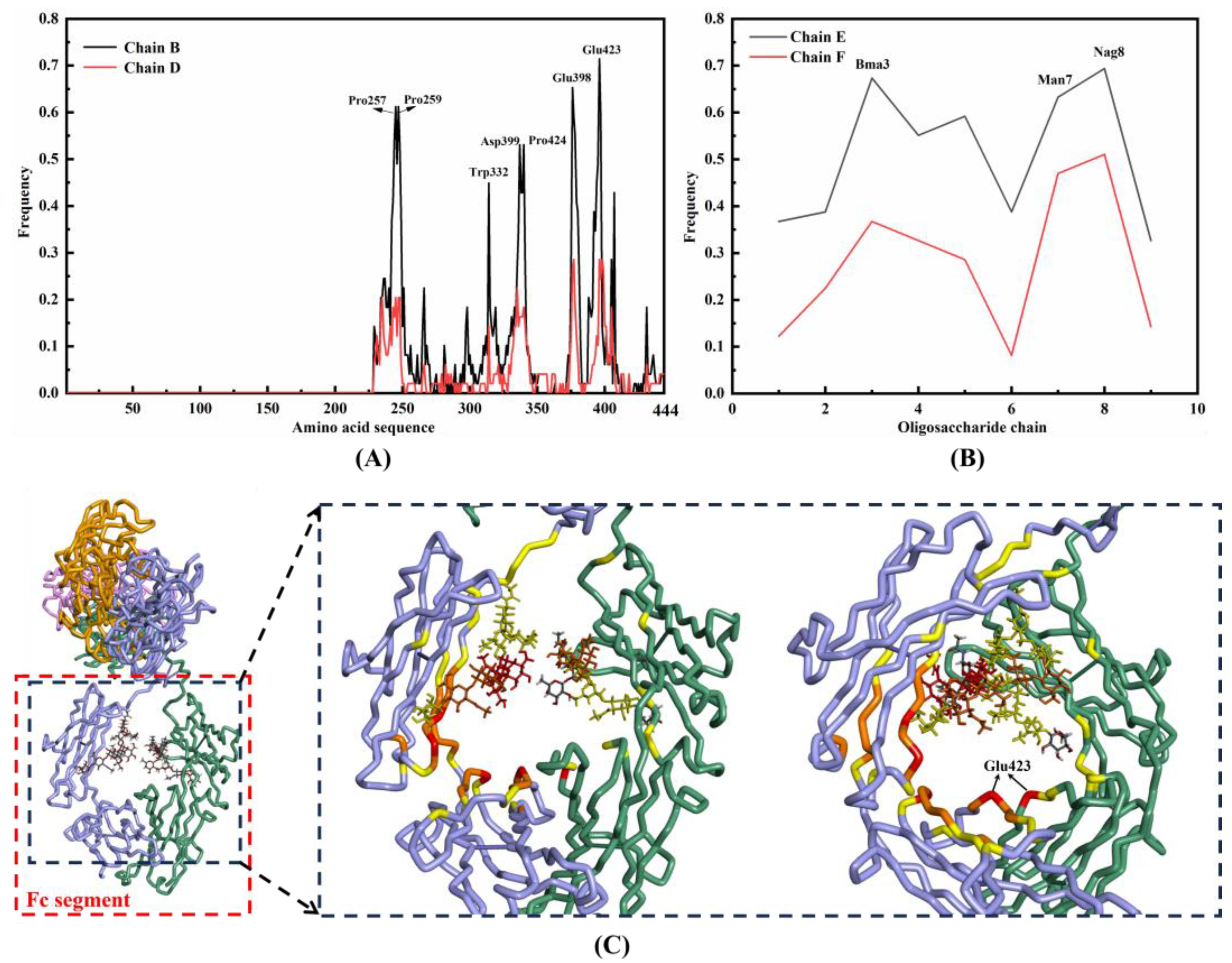
2.1.2. Binding Sites between IgG and DNAFL
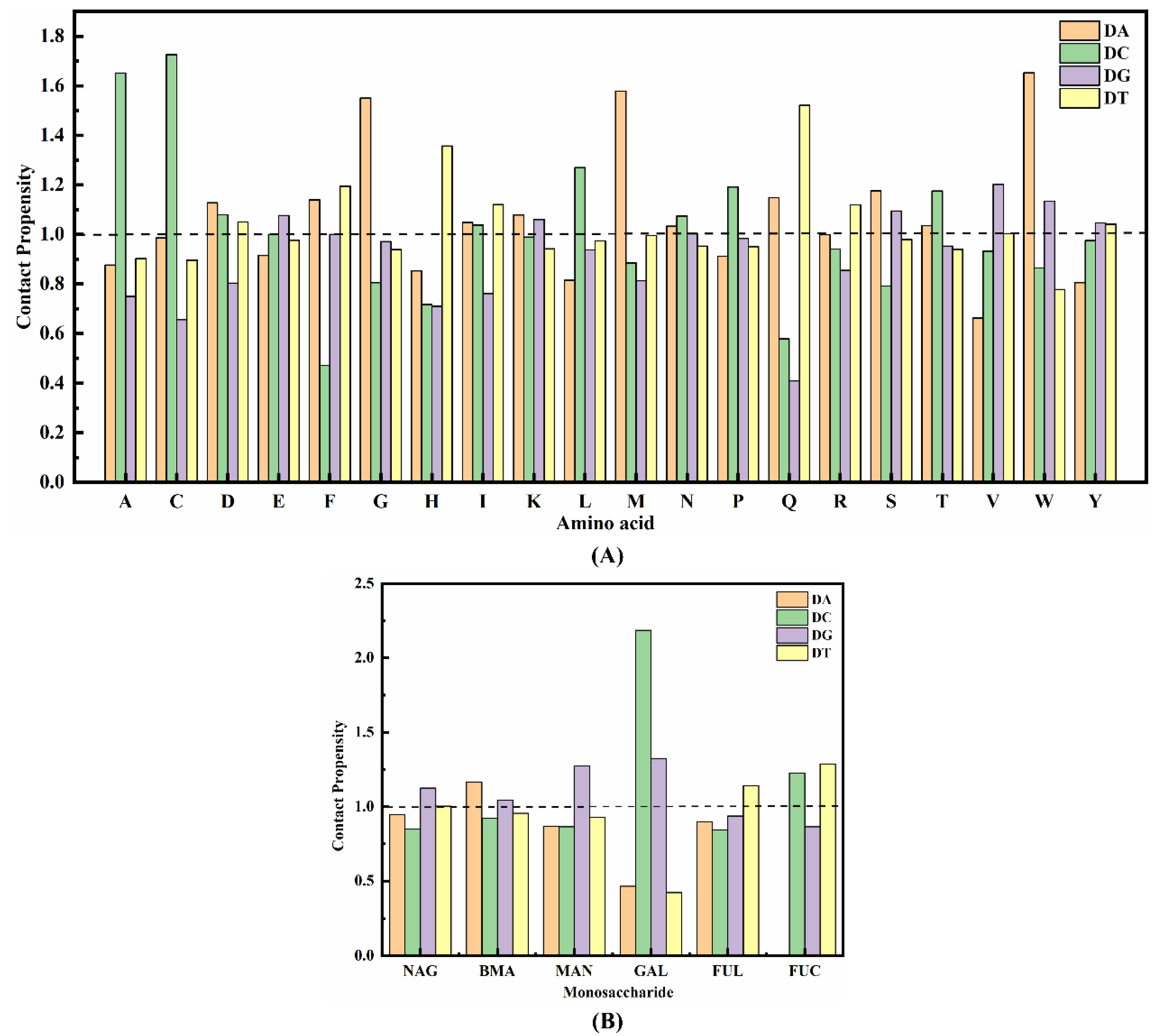
2.1.3. Contact Propensity of Protein Units to Bases
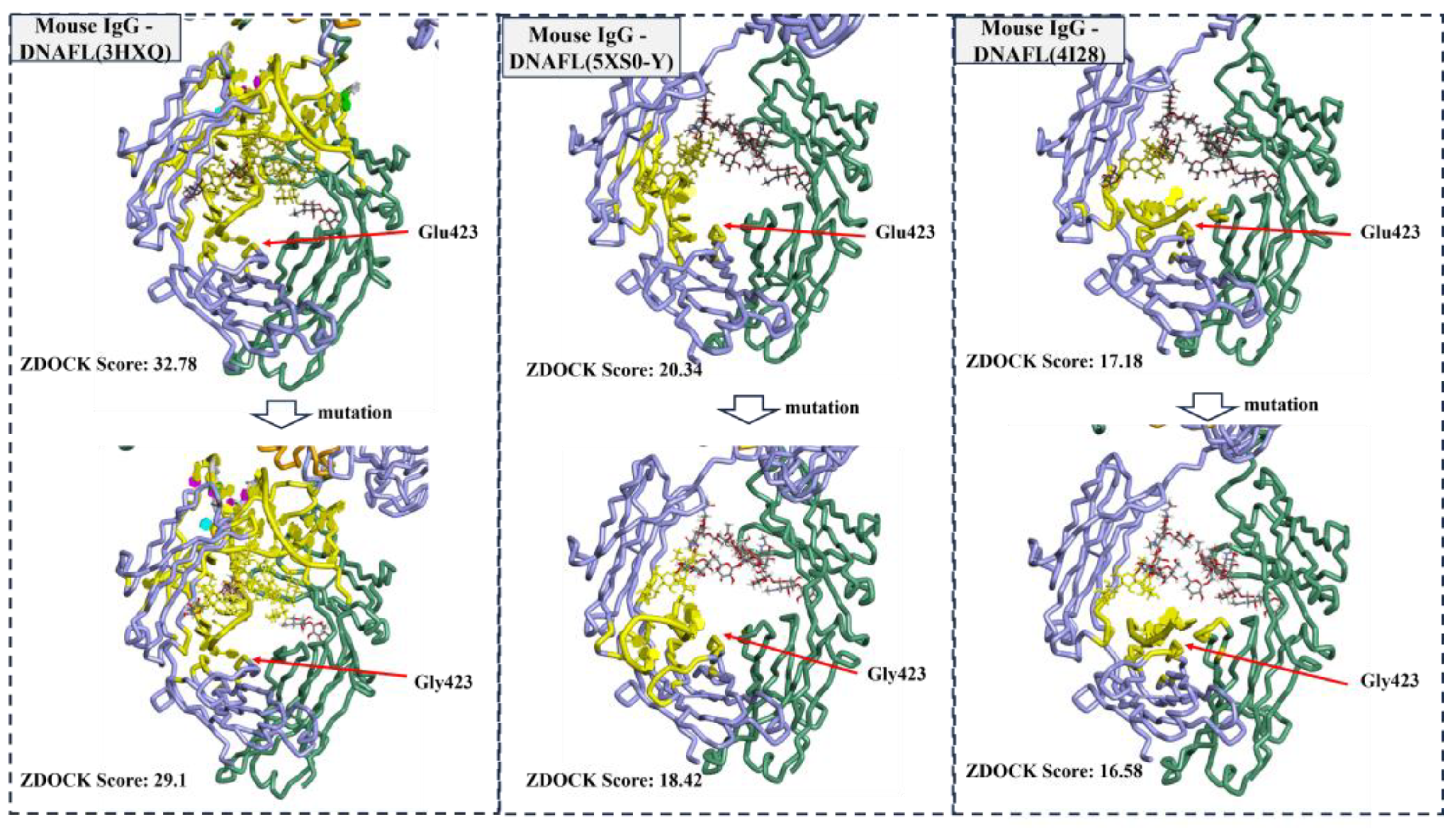
2.2. Affinity and Sites Validation
2.2.1. Affinity and Sites Validation of Virtual Screening DNAFLs
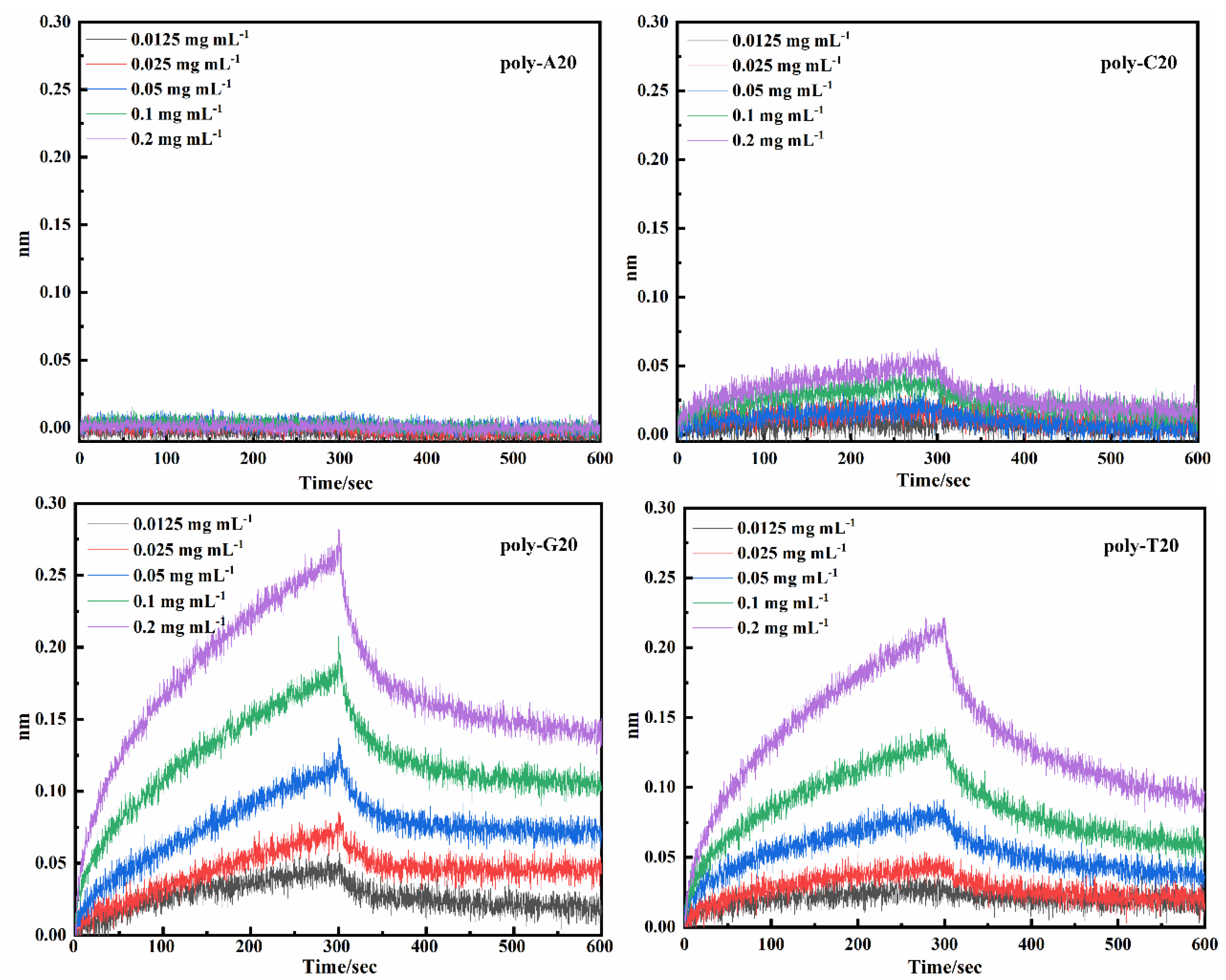
2.2.2. Affinity of Poly-Bases DNAFLs
3. Discussion
4. Materials and Methods
4.1. Reagents and Apparatus
4.2. Molecular Docking
4.2.1. Preparing the Receptor and Ligands
4.2.2. Running ZDOCK
4.2.3. Virtual Screening
4.3. Affinity of the DNAFLs to Mouse IgG
4.3.1. Affinity and Binding Sites of Virtual Screening DNAFLs to Mouse IgG
4.3.2. Affinity of Poly-Base to Mouse IgG
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kong, Y.; Zhu, Y.; Song, J.; Liu, Q.; Song, L.; Fei, X.; Li, X. A Novel Multimode Biosensor for Sensitive Detection of AFB1 in Food Based on Mxenes Nano Enzymes. Food Chem. 2023, 426, 136645. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Mu, X.; Du, B.; Tong, Z. A Review of Electrochemical Biosensor Application in the Detection of the SARS-COV-2. Micro Nano Lett. 2022, 17, 49–58. [Google Scholar] [CrossRef]
- Negahdary, M.; Angnes, L. Application of Electrochemical Biosensors for the Detection of microRNAs (miRNAs) Related to Cancer. Coord. Chem. Rev. 2022, 464, 214565. [Google Scholar] [CrossRef]
- Pei, F.; Feng, S.; Zhang, Y.; Wu, Y.; Chen, C.; Sun, Y.; Xie, Z.; Hao, Q.; Cao, Y.; Tong, Z.; et al. A Photoelectrochemical Immunosensor Based on Z-Scheme CdS Composite Heterojunction for Aflatoxin B1. Biosens. Bioelectron. 2022, 214, 114500. [Google Scholar] [CrossRef] [PubMed]
- Dostalova, S.; Cerna, T.; Hynek, D.; Koudelkova, Z.; Vaculovic, T.; Kopel, P.; Hrabeta, J.; Heger, Z.; Vaculovicova, M.; Eckschlager, T.; et al. Site-Directed Conjugation of Antibodies to Apoferritin Nanocarrier for Targeted Drug Delivery to Prostate Cancer Cells. ACS Appl. Mater. Interfaces 2016, 8, 14430–14441. [Google Scholar] [CrossRef]
- Wu, Q.; Song, D.; Zhang, D.; Zhang, H.; Ding, Y.; Yu, Y.; Sun, Y. A Highly Sensitive SPR Biosensor Based on a Graphene Oxide Sheet Modified with Gold Bipyramids, and Its Application to an Immunoassay for Rabbit IgG. Microchim. Acta 2015, 182, 1739–1746. [Google Scholar] [CrossRef]
- Trilling, A.K.; Beekwilder, J.; Zuilhof, H. Antibody Orientation on Biosensor Surfaces: A Minireview. Analyst 2013, 138, 1619. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, X.; Grant, C.; Lu, J.R.; Williams, D.E.; Penfold, J. Orientation of a Monoclonal Antibody Adsorbed at the Solid/Solution Interface: A Combined Study Using Atomic Force Microscopy and Neutron Reflectivity. Langmuir 2006, 22, 6313–6320. [Google Scholar] [CrossRef]
- Kumada, Y.; Tokunaga, Y.; Imanaka, H.; Imamura, K.; Sakiyama, T.; Katoh, S.; Nakanishi, K. Screening and Characterization of Affinity Peptide Tags Specific to Polystyrene Supports for the Orientated Immobilization of Proteins. Biotechnol. Prog. 2006, 22, 401–405. [Google Scholar] [CrossRef]
- Jung, Y.; Kang, H.J.; Lee, J.M.; Jung, S.O.; Yun, W.S.; Chung, S.J.; Chung, B.H. Controlled Antibody Immobilization onto Immunoanalytical Platforms by Synthetic Peptide. Anal. Biochem. 2008, 374, 99–105. [Google Scholar] [CrossRef]
- Kim, E.-S.; Shim, C.-K.; Lee, J.W.; Park, J.W.; Choi, K.Y. Synergistic Effect of Orientation and Lateral Spacing of Protein G on an On-Chip Immunoassay. Analyst 2012, 137, 2421. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Choi, H.K.; Lee, S.Y.; Lim, M.-W.; Chang, J.H. Enhancing Immunoassay Detection of Antigens with Multimeric Protein Gs. Biosens. Bioelectron. 2011, 28, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Bergström, G.; Mandenius, C.-F. Orientation and Capturing of Antibody Affinity Ligands: Applications to Surface Plasmon Resonance Biochips. Sens. Actuators B Chem. 2011, 158, 265–270. [Google Scholar] [CrossRef]
- Rigi, G.; Ghaedmohammadi, S.; Ahmadian, G. A Comprehensive Review on Staphylococcal Protein A (SpA): Its Production and Applications. Biotechnol. Appl. Biochem. 2019, 66, 454–464. [Google Scholar] [CrossRef]
- Shahbazi, R.; Salouti, M.; Amini, B.; Jalilvand, A.; Naderlou, E.; Amini, A.; Shams, A. Highly Selective and Sensitive Detection of Staphylococcus Aureus with Gold Nanoparticle-Based Core-Shell Nano Biosensor. Mol. Cell. Probes 2018, 41, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dou, X.; Zhang, L.; Zhao, X.; Luo, J.; Yang, M. Oriented Assembly of Surface Plasmon Resonance Biosensor through Staphylococcal Protein A for the Chlorpyrifos Detection. Anal. Bioanal. Chem. 2019, 411, 6057–6066. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, B. Sensitivity Enhanced SPR Immunosensor Based on Graphene Oxide and SPA Co-Modified Photonic Crystal Fiber. Opt. Laser Technol. 2018, 107, 210–215. [Google Scholar] [CrossRef]
- Sun, L.; Shen, F.; Qu, Y.; Liu, Z. Functional DNA as a Molecular Tool in Regulating Immunoreceptor–Ligand Interactions. JACS Au 2023, 3, 1820–1834. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.S.; Kim, K.; Yu, B.; Jon, S.; Kim, M.-S.; Yang, H. Ultrasensitive Electrochemical Detection of miRNA-21 Using a Zinc Finger Protein Specific to DNA–RNA Hybrids. Anal. Chem. 2017, 89, 2024–2031. [Google Scholar] [CrossRef]
- Wang, J.S.; Zhang, D.Y. Simulation-Guided DNA Probe Design for Consistently Ultraspecific Hybridization. Nat. Chem. 2015, 7, 545–553. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, D.; Schluesener, H.J.; Zhang, Z. Advances in SELEX and Application of Aptamers in the Central Nervous System. Biomol. Eng. 2007, 24, 583–592. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, F.; Sang, Y.; Katouzian, I.; Jafari, S.M.; Wang, X.; Li, W.; Wang, J.; Mohammadi, Z. Screening, Identification, and Application of Nucleic Acid Aptamers Applied in Food Safety Biosensing. Trends Food Sci. Technol. 2022, 123, 355–375. [Google Scholar] [CrossRef]
- Zhao, X.; Dai, X.; Zhao, S.; Cui, X.; Gong, T.; Song, Z.; Meng, H.; Zhang, X.; Yu, B. Aptamer-Based Fluorescent Sensors for the Detection of Cancer Biomarkers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 247, 119038. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, X.; Liu, X.; Ou, H.; Zhang, H.; Wang, J.; Li, Q.; Cheng, H.; Zhang, W.; Luo, Z. Discovery of Sandwich Type COVID-19 Nucleocapsid Protein DNA Aptamers. Chem. Commun. 2020, 56, 10235–10238. [Google Scholar] [CrossRef]
- Fan, J.; Yang, W. Electrochemical DNA/Aptamer Biosensors Based on SPAAC for Detection of DNA and Protein. Sens. Actuators B Chem. 2022, 353, 131100. [Google Scholar] [CrossRef]
- Elokely, K.M.; Doerksen, R.J. Docking Challenge: Protein Sampling and Molecular Docking Performance. J. Chem. Inf. Model. 2013, 53, 1934–1945. [Google Scholar] [CrossRef]
- Caballero, J. The Latest Automated Docking Technologies for Novel Drug Discovery. Expert Opin. Drug Discov. 2021, 16, 625–645. [Google Scholar] [CrossRef]
- Gilson, M.K.; Zhou, H.-X. Calculation of Protein-Ligand Binding Affinities. Annu. Rev. Biophys. 2007, 36, 21–42. [Google Scholar] [CrossRef]
- Aderinwale, T.; Christoffer, C.W.; Sarkar, D.; Alnabati, E.; Kihara, D. Computational Structure Modeling for Diverse Categories of Macromolecular Interactions. Curr. Opin. Struct. Biol. 2020, 64, 1–8. [Google Scholar] [CrossRef]
- Monticelli, L.; Tieleman, D.P. Force Fields for Classical Molecular Dynamics. In Biomolecular Simulations: Methods and Protocols; Monticelli, L., Salonen, E., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 197–213. ISBN 978-1-62703-017-5. [Google Scholar]
- Cross, J.B.; Thompson, D.C.; Rai, B.K.; Baber, J.C.; Fan, K.Y.; Hu, Y.; Humblet, C. Comparison of Several Molecular Docking Programs: Pose Prediction and Virtual Screening Accuracy. J. Chem. Inf. Model. 2009, 49, 1455–1474. [Google Scholar] [CrossRef]
- Huang, S.-Y.; Grinter, S.Z.; Zou, X. Scoring Functions and Their Evaluation Methods for Protein–Ligand Docking: Recent Advances and Future Directions. Phys. Chem. Chem. Phys. 2010, 12, 12899. [Google Scholar] [CrossRef]
- Prieto-Martínez, F.D.; Arciniega, M.; Medina-Franco, J.L.; Prieto-Martínez, F.D.; Arciniega, M.; Medina-Franco, J.L. Molecular Docking: Current Advances and Challenges. TIP Rev. Espec. En Cienc. Químico-Biológicas 2018, 21. [Google Scholar] [CrossRef]
- Feng, Y.; Yan, Y.; He, J.; Tao, H.; Wu, Q.; Huang, S.-Y. Docking and Scoring for Nucleic Acid–Ligand Interactions: Principles and Current Status. Drug Discov. Today 2022, 27, 838–847. [Google Scholar] [CrossRef]
- Fan, J.; Fu, A.; Zhang, L. Progress in Molecular Docking. Quant. Biol. 2019, 7, 83–89. [Google Scholar] [CrossRef]
- Chen, R.; Weng, Z. Docking Unbound Proteins Using Shape Complementarity, Desolvation, and Electrostatics. Proteins Struct. Funct. Bioinform. 2002, 47, 281–294. [Google Scholar] [CrossRef]
- Pierce, B.G.; Wiehe, K.; Hwang, H.; Kim, B.-H.; Vreven, T.; Weng, Z. ZDOCK Server: Interactive Docking Prediction of Protein–Protein Complexes and Symmetric Multimers. Bioinformatics 2014, 30, 1771–1773. [Google Scholar] [CrossRef]
- Pagadala, N.S.; Syed, K.; Tuszynski, J. Software for Molecular Docking: A Review. Biophys. Rev. 2017, 9, 91–102. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Sales, M.G.F.; Pereira, C.M. Electrochemistry Combined-Surface Plasmon Resonance Biosensors: A Review. TrAC Trends Anal. Chem. 2022, 157, 116766. [Google Scholar] [CrossRef]
- Ullah, S.F.; Moreira, G.; Datta, S.P.A.; McLamore, E.; Vanegas, D. An Experimental Framework for Developing Point-of-Need Biosensors: Connecting Bio-Layer Interferometry and Electrochemical Impedance Spectroscopy. Biosensors 2022, 12, 938. [Google Scholar] [CrossRef]
- Jug, A.; Bratkovič, T.; Ilaš, J. Biolayer Interferometry and Its Applications in Drug Discovery and Development. TrAC Trends Anal. Chem. 2024, 176, 117741. [Google Scholar] [CrossRef]
- Biolayer Interferometry for DNA-Protein Interactions|PLOS ONE. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0263322 (accessed on 24 July 2024).
- Rapid and Sensitive Detection of SARS-CoV-2 Antibodies by Biolayer Interferometry|Scientific Reports. Available online: https://www.nature.com/articles/s41598-020-78895-x?utm_source=xmol&utm_medium=affiliate&utm_content=meta&utm_campaign=DDCN_1_GL01_metadata_scirep (accessed on 25 July 2024).
- Li, J.; Schantz, A.; Schwegler, M.; Shankar, G. Detection of Low-Affinity Anti-Drug Antibodies and Improved Drug Tolerance in Immunogenicity Testing by Octet® Biolayer Interferometry. J. Pharm. Biomed. Anal. 2011, 54, 286–294. [Google Scholar] [CrossRef]
- Concepcion, J.; Witte, K.; Wartchow, C.; Choo, S.; Yao, D.; Persson, H.; Wei, J.; Li, P.; Heidecker, B.; Ma, W.; et al. Label-Free Detection of Biomolecular Interactions Using BioLayer Interferometry for Kinetic Characterization. Comb. Chem. High Throughput Screen. 2009, 12, 791–800. [Google Scholar] [CrossRef]
- Harris, L.J.; Larson, S.B.; Hasel, K.W.; McPherson, A. Refined Structure of an Intact IgG2a Monoclonal Antibody. Biochemistry 1997, 36, 1581–1597. [Google Scholar] [CrossRef]
- Kono, H.; Sarai, A. Structure-Based Prediction of DNA Target Sites by Regulatory Proteins. Proteins: Struct. Funct. Bioinform. 1999, 35, 114–131. [Google Scholar] [CrossRef]
- Harini, K.; Kihara, D.; Michael Gromiha, M. PDA-Pred: Predicting the Binding Affinity of Protein-DNA Complexes Using Machine Learning Techniques and Structural Features. Methods 2023, 213, 10–17. [Google Scholar] [CrossRef]
- Copeland, R.A. The Drug–Target Residence Time Model: A 10-Year Retrospective. Nat. Rev. Drug Discov. 2016, 15, 87–95. [Google Scholar] [CrossRef]
- Meysman, P.; Zhou, C.; Cule, B.; Goethals, B.; Laukens, K. Mining the Entire Protein DataBank for Frequent Spatially Cohesive Amino Acid Patterns. BioData Min. 2015, 8, 4. [Google Scholar] [CrossRef]

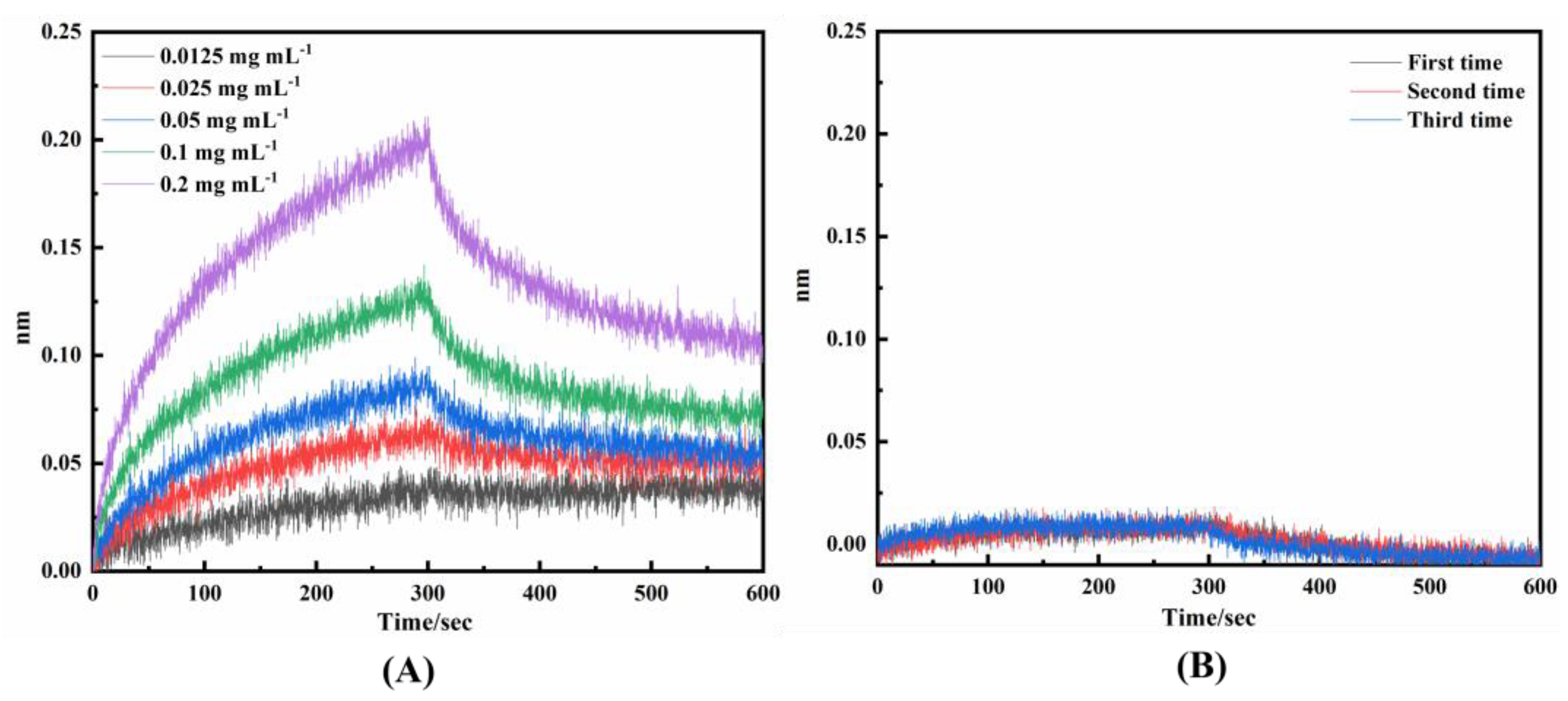
| PDB Code | Kon (1/Ms) | Kdis (1/s) | KD (M) | R2 | |
|---|---|---|---|---|---|
| 1 | 5XRZ | 3.76 × 103 | 3.17 × 10−3 | 8.43 × 10−7 | 0.9402 |
| 2 | 6GN7 | 9.52 × 103 | 2.29 × 10−3 | 2.41 × 10−7 | 0.9294 |
| 3 | 4GNX | 1.81 × 103 | 3.80 × 10−3 | 2.10 × 10−6 | 0.9622 |
| 4 | 7JSG | 3.11 × 103 | 3.48 × 10−3 | 1.12 × 10−6 | 0.9692 |
| 5 | 2C62 | 1.85 × 103 | 6.00 × 10−3 | 3.24 × 10−6 | 0.9251 |
| 6 | 7XHD | 6.29 × 103 | 3.28 × 10−3 | 5.21 × 10−7 | 0.9229 |
| 7 | 6SKO | 4.26 × 103 | 4.54 × 10−3 | 1.07 × 10−6 | 0.9636 |
| 8 | 1RDE | 2.91 × 103 | 3.18 × 10−3 | 1.09 × 10−6 | 0.9661 |
| 9 | 2CCZ | 4.42 × 103 | 4.02 × 10−3 | 9.09 × 10−7 | 0.9614 |
| 10 | 6CRM | 5.14 × 103 | 5.70 × 10−3 | 1.11 × 10−6 | 0.9257 |
| 11 | 1PH1-D | 6.97 × 103 | 6.14 × 10−3 | 8.81 × 10−7 | 0.8856 |
| 12 | 1OTC | 8.24 × 103 | 3.41 × 10−3 | 4.14 × 10−7 | 0.9031 |
| 13 | 4JS5 | 3.34 × 103 | 5.78 × 10−3 | 1.73 × 10−6 | 0.9447 |
| 14 | 3UGO | 5.38 × 103 | 4.27 × 10−3 | 7.93 × 10−7 | 0.8999 |
| 15 | 2A0I | 6.39 × 103 | 5.57 × 10−3 | 8.72 × 10−7 | 0.9134 |
| 16 | 2KN7 | 5.06 × 103 | 6.08 × 10−3 | 1.20 × 10−6 | 0.94 |
| 17 | 4OU6 | 5.06 × 103 | 4.79 × 10−3 | 9.47 × 10−7 | 0.9497 |
| 18 | 7JSI | 2.58 × 103 | 4.73 × 10−3 | 1.83 × 10−6 | 0.9672 |
| 19 | 5FGP | 2.74 × 103 | 4.13 × 10−3 | 1.51 × 10−6 | 0.9522 |
| 20 | 6S3M | 6.93 × 103 | 8.06 × 10−3 | 1.16 × 10−6 | 0.9262 |
| 21 | SPA−IgG | 4.80 × 104 | 7.16 × 10−4 | 1.49 × 10−8 | 0.959 |
| PDB Code | Kon (1/Ms) | Kdis (1/s) | KD (M) | R2 | |
|---|---|---|---|---|---|
| 1 | poly-A10 | No response | |||
| 2 | poly-A15 | No response | |||
| 3 | poly-A20 | No response | |||
| 4 | poly-A25 | No response | |||
| 5 | poly-A30 | No response | |||
| 6 | poly-C10 | No response | |||
| 7 | poly-C15 | 4.27 × 103 | 5.81 × 10−3 | 1.36 × 10−6 | 0.9117 |
| 8 | poly-C20 | 7.42 × 103 | 5.65 × 10−3 | 7.61 × 10−7 | 0.8997 |
| 9 | poly-C25 | No response | |||
| 10 | poly-C30 | No response | |||
| 11 | poly-G10 | 6.87 × 103 | 2.02 × 10−3 | 2.95 × 10−7 | 0.9301 |
| 12 | poly-G15 | 8.42 × 103 | 2.30 × 10−3 | 2.73 × 10−7 | 0.9676 |
| 13 | poly-G20 | 7.85 × 103 | 2.45 × 10−3 | 3.12 × 10−7 | 0.9583 |
| 14 | poly-G25 | 7.24 × 103 | 5.01 × 10−3 | 6.92 × 10−7 | 0.8785 |
| 15 | poly-G30 | 4.39 × 103 | 2.45 × 10−3 | 5.57 × 10−7 | 0.8844 |
| 16 | poly-T10 | 3.79 × 103 | 5.66 × 10−3 | 1.49 × 10−6 | 0.9438 |
| 17 | poly-T15 | 4.42 × 103 | 4.02 × 10−3 | 9.09 × 10−7 | 0.9614 |
| 18 | poly-T20 | 4.50 × 103 | 3.74 × 10−3 | 8.30 × 10−7 | 0.9628 |
| 19 | poly-T25 | 4.18 × 103 | 4.20 × 10−3 | 1.01 × 10−6 | 0.9661 |
| 20 | poly-T30 | 2.50 × 103 | 3.57 × 10−3 | 1.43 × 10−6 | 0.9448 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Q.; Liu, Z.; Xu, X.; Wang, J.; Du, B.; Zhang, P.; Liu, B.; Mu, X.; Tong, Z. Virtual Screening and Validation of Affinity DNA Functional Ligands for IgG Fc Segment. Int. J. Mol. Sci. 2024, 25, 8681. https://doi.org/10.3390/ijms25168681
Yang Q, Liu Z, Xu X, Wang J, Du B, Zhang P, Liu B, Mu X, Tong Z. Virtual Screening and Validation of Affinity DNA Functional Ligands for IgG Fc Segment. International Journal of Molecular Sciences. 2024; 25(16):8681. https://doi.org/10.3390/ijms25168681
Chicago/Turabian StyleYang, Qianyu, Zhiwei Liu, Xinrui Xu, Jiang Wang, Bin Du, Pengjie Zhang, Bing Liu, Xihui Mu, and Zhaoyang Tong. 2024. "Virtual Screening and Validation of Affinity DNA Functional Ligands for IgG Fc Segment" International Journal of Molecular Sciences 25, no. 16: 8681. https://doi.org/10.3390/ijms25168681




