One-Step Genetic Modification by Embryonic Doral Aorta Injection of Adenoviral CRISPR/Cas9 Vector in Chicken
Abstract
:1. Introduction
2. Results
2.1. Effective Editing Sites Screening in DF1 Cells
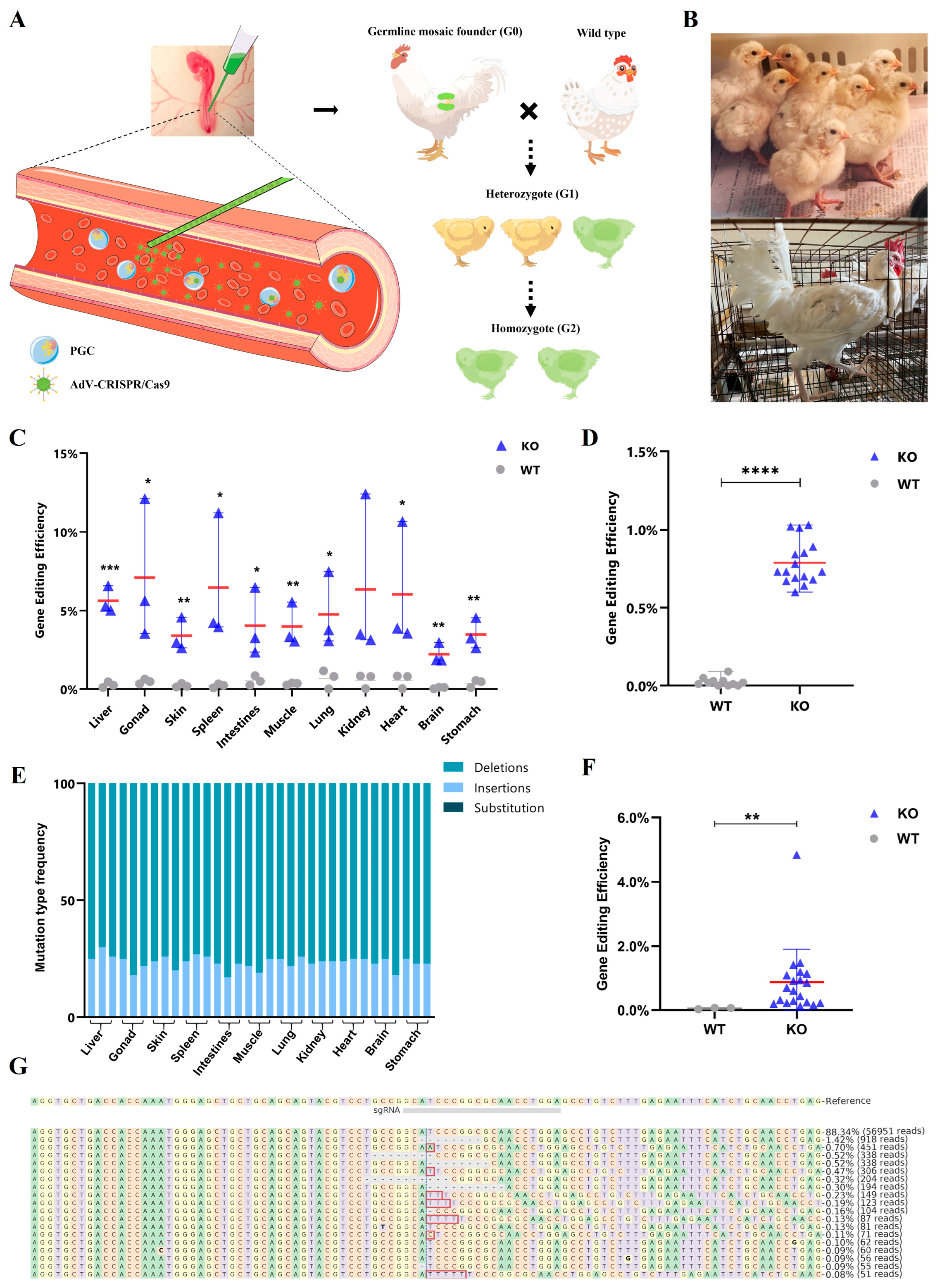
2.2. Generation of Gamete Cells Editing Chimeric Chickens
3. Discussion
4. Materials and Methods
4.1. Construction of Plasmids and Adenoviral Vectors
4.2. Cell Culture and Transfection
4.3. Microinjection of Adenoviral Vector
4.4. Animal Breeding and Generation
4.5. Analysis of Editing Efficiency
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lamas-Toranzo, I.; Guerrero-Sánchez, J.; Miralles-Bover, H.; Alegre-Cid, G.; Pericuesta, E.; Bermejo-Álvarez, P. CRISPR is knocking on barn door. Reprod. Domest. Anim. 2017, 52, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Doran, T.J.; Cooper, C.A.; Jenkins, K.A.; Tizard, M.L. Advances in genetic engineering of the avian genome: “Realising the promise”. Transgenic Res. 2016, 25, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, C.; Lillico, S.; Tait-Burkard, C. Genome editing for disease resistance in pigs and chickens. Anim. Front. 2019, 9, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Lotti, S.N.; Polkoff, K.M.; Rubessa, M.; Wheeler, M.B. Modification of the Genome of Domestic Animals. Anim. Biotechnol. 2017, 28, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Sang, H. Prospects for transgenesis in the chick. Mech. Dev. 2004, 121, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Etches, R.J.; Verrinder Gibbins, A.M. Strategies for the production of transgenic chicken. Methods Mol. Biol. 1997, 62, 433–450. [Google Scholar] [CrossRef] [PubMed]
- Park, H.J.; Park, T.S.; Kim, T.M.; Kim, J.N.; Shin, S.S.; Lim, J.M.; Han, J.Y. Establishment of an in vitro culture system for chicken preblastodermal cells. Mol. Reprod. Dev. 2006, 73, 452–461. [Google Scholar] [CrossRef]
- Lee, H.C.; Choi, H.J.; Park, T.S.; Lee, S.I.; Kim, Y.M.; Rengaraj, D.; Nagai, H.; Sheng, G.; Lim, J.M.; Han, J.Y. Cleavage events and sperm dynamics in chick intrauterine embryos. PLoS ONE 2013, 8, e80631. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.A.; Challagulla, A.; Jenkins, K.A.; Wise, T.G.; O’Neil, T.E.; Morris, K.R.; Tizard, M.L.; Doran, T.J. Generation of gene edited birds in one generation using sperm transfection assisted gene editing (STAGE). Transgenic Res. 2017, 26, 331–347. [Google Scholar] [CrossRef]
- Golkar-Narenji, A.; Dziegiel, P.; Kempisty, B.; Petitte, J.; Mozdziak, P.E.; Bryja, A. In vitro culture of reptile PGCS to preserve endangered species. Cell Biol. Int. 2023, 47, 1314–1326. [Google Scholar] [CrossRef]
- Idoko-Akoh, A.; McGrew, M.J. Generation of Genome-Edited Chicken Through Targeting of Primordial Germ Cells. In Transgenesis: Methods and Protocols; Saunders, T.L., Ed.; Springer: New York, NY, USA, 2023; pp. 419–441. [Google Scholar]
- Lee, J.; Ma, J.; Lee, K. Direct delivery of adenoviral CRISPR/Cas9 vector into the blastoderm for generation of targeted gene knockout in quail. Proc. Natl. Acad. Sci. USA 2019, 116, 13288–13292. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, M.; Woodcock, M.; Doddamani, D.; Hu, T.; Taylor, L.; Hawken, R.J.; McGrew, M.J. Direct allele introgression into pure chicken breeds using Sire Dam Surrogate (SDS) mating. Nat. Commun. 2021, 12, 659. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; He, C.; Wang, Z.; Li, Y.; Li, S.; Tao, L.; Chen, J.; Li, D.; Yang, F.; Li, N.; et al. A novel deletion in KRT75L4 mediates the frizzle trait in a Chinese indigenous chicken. Genet. Sel. Evol. 2018, 50, 68. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn 1992, 195, 231–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, P.; Yu, F.; Yan, L.; Yuan, F.; Zhang, W.; Wang, T.; Wan, Z.; Shao, Q.; Li, Z. Transgenic quail production by microinjection of lentiviral vector into the early embryo blood vessels. PLoS ONE 2012, 7, e50817. [Google Scholar] [CrossRef] [PubMed]
- Tyack, S.G.; Jenkins, K.A.; O’Neil, T.E.; Wise, T.G.; Morris, K.R.; Bruce, M.P.; McLeod, S.; Wade, A.J.; McKay, J.; Moore, R.J.; et al. A new method for producing transgenic birds via direct in vivo transfection of primordial germ cells. Transgenic Res. 2013, 22, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, K.Y.; Jung, K.M.; Park, K.J.; Lee, K.O.; Suh, J.Y.; Yao, Y.; Nair, V.; Han, J.Y. Precise gene editing of chicken Na+/H+ exchange type 1 (chNHE1) confers resistance to avian leukosis virus subgroup J (ALV-J). Dev. Comp. Immunol. 2017, 77, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.D.; Lee, J.H.; Song, S.; Kim, S.W.; Han, J.S.; Shin, S.P.; Park, B.C.; Park, T.S. Generation of myostatin-knockout chickens mediated by D10A-Cas9 nickase. Faseb J. 2020, 34, 5688–5696. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, T.; Barry, M.A.; Ikeda, Y. Lentiviral vectors: Basic to translational. Biochem. J. 2012, 443, 603–618. [Google Scholar] [CrossRef]
- Nayerossadat, N.; Maedeh, T.; Ali, P.A. Viral and nonviral delivery systems for gene delivery. Adv. Biomed. Res. 2012, 1, 27. [Google Scholar] [CrossRef]
- Shin, S.; Choi, Y.M.; Han, J.Y.; Lee, K. Inhibition of lipolysis in the novel transgenic quail model overexpressing G0/G1 switch gene 2 in the adipose tissue during feed restriction. PLoS ONE 2014, 9, e100905. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.R.; Shin, S.; Choi, Y.M.; Kim, E.; Han, J.Y.; Lee, K. Overexpression of G0/G1 Switch Gene 2 in Adipose Tissue of Transgenic Quail Inhibits Lipolysis Associated with Egg Laying. Int. J. Mol. Sci. 2016, 17, 384. [Google Scholar] [CrossRef] [PubMed]
- Woodfint, R.M.; Chen, P.R.; Ahn, J.; Suh, Y.; Hwang, S.; Lee, S.S.; Lee, K. Identification of the MUC2 Promoter as a Strong Promoter for Intestinal Gene Expression through Generation of Transgenic Quail Expressing GFP in Gut Epithelial Cells. Int. J. Mol. Sci. 2017, 18, 196. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Q.; Wu, H.Y.; Tian, J.; Li, N.; Hu, X.X. Targeting lentiviral vectors to primordial germ cells (PGCs): An efficient strategy for generating transgenic chickens. Zool. Res. 2020, 41, 281–291. [Google Scholar] [CrossRef]
- Naseri, D.; Dormiani, K.; Hajian, M.; Jafarpour, F.; Forouzanfar, M.; Karimi, N.; Nasr-Esfahani, M.H. Improving germline transmission efficiency in chimeric chickens using a multi-stage injection approach. PLoS ONE 2021, 16, e0247471. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Bishop, E.S.; Zhang, R.; Yu, X.; Farina, E.M.; Yan, S.; Zhao, C.; Zheng, Z.; Shu, Y.; Wu, X.; et al. Adenovirus-Mediated Gene Delivery: Potential Applications for Gene and Cell-Based Therapies in the New Era of Personalized Medicine. Genes. Dis. 2017, 4, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Crystal, R.G. Adenovirus: The first effective in vivo gene delivery vector. Hum. Gene Ther. 2014, 25, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Athanasopoulos, T.; Munye, M.M.; Yáñez-Muñoz, R.J. Nonintegrating Gene Therapy Vectors. Hematol. Oncol. Clin. N. Am. 2017, 31, 753–770. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Han, C.X.; Zhou, H.; Ding, J.M.; Xu, Z.; Yang, L.Y.; He, C.; Akinyemi, F.; Zheng, Y.M.; Qin, C.; et al. Effective MSTN Gene Knockout by AdV-Delivered CRISPR/Cas9 in Postnatal Chick Leg Muscle. Int. J. Mol. Sci. 2020, 21, 2584. [Google Scholar] [CrossRef]
- Lee, J.; Kim, D.H.; Lee, K. Research Note: Injection of adenoviral CRISPR/Cas9 system targeting melanophilin gene into different sites of embryos induced regional feather color changes in posthatch quail. Poult. Sci. 2023, 102, 103087. [Google Scholar] [CrossRef]
- Bijlani, S.; Pang, K.M.; Sivanandam, V.; Singh, A.; Chatterjee, S. The Role of Recombinant AAV in Precise Genome Editing. Front. Genome Ed. 2021, 3, 799722. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.D.; Byrne, B.J.; Corti, M. Evolving Horizons: Adenovirus Vectors’ Timeless Influence on Cancer, Gene Therapy and Vaccines. Viruses 2023, 15, 2378. [Google Scholar] [CrossRef] [PubMed]
- Bett, A.J.; Prevec, L.; Graham, F.L. Packaging capacity and stability of human adenovirus type 5 vectors. J. Virol. 1993, 67, 5911–5921. [Google Scholar] [CrossRef] [PubMed]
- Reid, T.; Warren, R.; Kirn, D. Intravascular adenoviral agents in cancer patients: Lessons from clinical trials. Cancer Gene Ther. 2002, 9, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Kanduc, D. Translational regulation of human papillomavirus type 16 E7 mRNA by the peptide SEQIKA, shared by rabbit alpha(1)-globin and human cytokeratin 7. J. Virol. 2002, 76, 7040–7048. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Hobbs, R.P.; Coulombe, P.A. The expanding significance of keratin intermediate filaments in normal and diseased epithelia. Curr. Opin. Cell Biol. 2013, 25, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Wallace, L.; Roberts-Thompson, L.; Reichelt, J. Deletion of K1/K10 does not impair epidermal stratification but affects desmosomal structure and nuclear integrity. J. Cell Sci. 2012, 125 Pt 7, 1750–1758. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraj, P.; Kröger, C.; Reuter, U.; Windoffer, R.; Leube, R.E.; Magin, T.M. Keratins regulate protein biosynthesis through localization of GLUT1 and -3 upstream of AMP kinase and Raptor. J. Cell Biol. 2009, 187, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kellner, J.; Lee, C.H.; Coulombe, P.A. Interaction between the keratin cytoskeleton and eEF1Bgamma affects protein synthesis in epithelial cells. Nat. Struct. Mol. Biol. 2007, 14, 982–983. [Google Scholar] [CrossRef]
- Toivola, D.M.; Ku, N.-O.; Resurreccion, E.Z.; Nelson, D.R.; Wright, T.L.; Omary, M.B. Keratin 8 and 18 hyperphosphorylation is a marker of progression of human liver disease. Hepatology 2004, 40, 459–466. [Google Scholar] [CrossRef]
- Ran, F.A.; Hsu, P.D.; Wright, J.; Agarwala, V.; Scott, D.A.; Zhang, F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013, 8, 2281–2308. [Google Scholar] [CrossRef] [PubMed]
- Labun, K.; Montague, T.G.; Krause, M.; Torres Cleuren, Y.N.; Tjeldnes, H.; Valen, E. CHOPCHOP v3: Expanding the CRISPR web toolbox beyond genome editing. Nucleic Acids Res. 2019, 47, W171–W174. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, C.; Jiang, S.; Xu, K.; Zhu, J.; Wang, L.; Yang, W.; Xiao, F.; Yang, K.; Huang, Q.; Meng, H. One-Step Genetic Modification by Embryonic Doral Aorta Injection of Adenoviral CRISPR/Cas9 Vector in Chicken. Int. J. Mol. Sci. 2024, 25, 8692. https://doi.org/10.3390/ijms25168692
Qin C, Jiang S, Xu K, Zhu J, Wang L, Yang W, Xiao F, Yang K, Huang Q, Meng H. One-Step Genetic Modification by Embryonic Doral Aorta Injection of Adenoviral CRISPR/Cas9 Vector in Chicken. International Journal of Molecular Sciences. 2024; 25(16):8692. https://doi.org/10.3390/ijms25168692
Chicago/Turabian StyleQin, Chao, Shengyao Jiang, Ke Xu, Jianshen Zhu, Liyuan Wang, Wenhao Yang, Fuquan Xiao, Kaixuan Yang, Qizhong Huang, and He Meng. 2024. "One-Step Genetic Modification by Embryonic Doral Aorta Injection of Adenoviral CRISPR/Cas9 Vector in Chicken" International Journal of Molecular Sciences 25, no. 16: 8692. https://doi.org/10.3390/ijms25168692
APA StyleQin, C., Jiang, S., Xu, K., Zhu, J., Wang, L., Yang, W., Xiao, F., Yang, K., Huang, Q., & Meng, H. (2024). One-Step Genetic Modification by Embryonic Doral Aorta Injection of Adenoviral CRISPR/Cas9 Vector in Chicken. International Journal of Molecular Sciences, 25(16), 8692. https://doi.org/10.3390/ijms25168692








