Molecular Determinants for Photodynamic Therapy Resistance and Improved Photosensitizer Delivery in Glioma
Abstract
1. Introduction
2. Molecules Implicated in Resistance to Photodynamic Therapy
2.1. Vascular Endothelial Growth Factor
2.2. Hypoxia-Inducible Factor 1α
2.3. Adenosine Triphosphate (ATP)-Binding Cassette Efflux Transporter G2
2.4. Nitric Oxide Synthase
2.5. Glutathione
2.6. Ferrochelatase
2.7. Heme Oxygenase 1
2.8. Na+/H+ Exchanger Isoform 1
2.9. Other Molecules Involved in Glioma Resistance to PDT
3. Molecular Determinants for Photodynamic Therapy Resistance and Improved Photosensitizer Delivery in Glioma
3.1. Molecules without a Clear Role in Glioma Resistance Whose Regulation Enhances the Effect of PDT in a PDT-Dependent Manner
3.1.1. Histone Deacetylases
3.1.2. Nuclear Factor kB
3.1.3. Fibroblast Growth Factors
3.1.4. Glutamate
3.1.5. Peripheral-Type Benzodiazepine Receptors
3.1.6. Matrix Metalloproteinases
3.1.7. PD-L1/PD-1
3.1.8. Transporter Associated with Antigen Processing 1
3.1.9. GLUT Glucose Transporters
3.2. Molecules without a Clear Role in Glioma Resistance Whose Regulation Enhances the Effect of PDT in a PDT-Independent Manner
3.2.1. Protein Kinase C
3.2.2. Hepatocyte Growth Factor
3.2.3. Vascular Cell Adhesion Protein 1
3.2.4. Glial Fibrillary Acidic Protein
3.2.5. Receptor-Interacting Serine/Threonine Kinases
3.3. Other Molecules Affecting the Efficacy of Glioma PDT with No Established Role in Resistance
4. Molecules Used to Improve Photosensitizer Delivery
4.1. Epidermal Growth Factor Receptor
4.2. Neuropilin-1
4.3. Integrins
4.4. Neuropeptide Y Receptors
4.5. Low-Density Lipoprotein Receptor
4.6. Other Molecules Used to Improve Photosensitizer Delivery
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schwartz, S.M. Epidemiology of Cancer. Clin. Chem. 2024, 70, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef] [PubMed]
- Vigneswaran, K.; Neill, S.; Hadjipanayis, C.G. Beyond the World Health Organization grading of infiltrating gliomas: Advances in the molecular genetics of glioma classification. Ann. Transl. Med. 2015, 3, 95. [Google Scholar] [PubMed]
- Davis, M.E. Epidemiology and Overview of Gliomas. Semin. Oncol. Nurs. 2018, 34, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.M.; Englander, Z.K.; Miller, M.L.; Bruce, J.N. Malignant Glioma. Adv. Exp. Med. Biol. 2023, 1405, 1–30. [Google Scholar] [PubMed]
- Jakola, A.S.; Myrmel, K.S.; Kloster, R.; Torp, S.H.; Lindal, S.; Unsgård, G.; Solheim, O. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA 2012, 308, 1881–1888. [Google Scholar] [CrossRef]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef]
- Lee, J.H.; Wee, C.W. Treatment of Adult Gliomas: A Current Update. Brain Neurorehabilit. 2022, 15, e24. [Google Scholar] [CrossRef]
- Yang, K.; Wu, Z.; Zhang, H.; Zhang, N.; Wu, W.; Wang, Z.; Dai, Z.; Zhang, X.; Zhang, L.; Peng, Y.; et al. Glioma targeted therapy: Insight into future of molecular approaches. Mol. Cancer 2022, 21, 39. [Google Scholar] [CrossRef]
- Bush, N.A.; Chang, S.M.; Berger, M.S. Current and future strategies for treatment of glioma. Neurosurg. Rev. 2017, 40, 1–14. [Google Scholar] [CrossRef]
- Vermandel, M.; Dupont, C.; Lecomte, F.; Leroy, H.A.; Tuleasca, C.; Mordon, S.; Hadjipanayis, C.G.; Reyns, N. Standardized intraoperative 5-ALA photodynamic therapy for newly diagnosed glioblastoma patients: A preliminary analysis of the INDYGO clinical trial. J. Neuro-Oncol. 2021, 152, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Itoo, A.M.; Paul, M.; Padaga, S.G.; Ghosh, B.; Biswas, S. Nanotherapeutic Intervention in Photodynamic Therapy for Cancer. ACS Omega 2022, 7, 45882–45909. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, S.; Zhang, L.; Xu, S.; Dai, C.; Gan, S.; Xie, G.; Feng, G.; Tang, B.Z. Cationization to boost both type I and type II ROS generation for photodynamic therapy. Biomaterials 2022, 280, 121255. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.; Reis, L.V. Photodynamic Therapy: From the Basics to the Current Progress of N-Heterocyclic-Bearing Dyes as Effective Photosensitizers. Molecules 2023, 28, 5092. [Google Scholar] [CrossRef]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef] [PubMed]
- Samuel, E.L.; Marcano, D.C.; Berka, V.; Bitner, B.R.; Wu, G.; Potter, A.; Fabian, R.H.; Pautler, R.G.; Kent, T.A.; Tsai, A.L.; et al. Highly efficient conversion of superoxide to oxygen using hydrophilic carbon clusters. Proc. Natl. Acad. Sci. USA 2015, 112, 2343–2348. [Google Scholar] [CrossRef] [PubMed]
- Fujii, J.; Soma, Y.; Matsuda, Y. Biological Action of Singlet Molecular Oxygen from the Standpoint of Cell Signaling, Injury and Death. Molecules 2023, 28, 4085. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Amin Doustvandi, M.; Mohammadnejad, F.; Kamari, F.; Gjerstorff, M.F.; Baradaran, B.; Hamblin, M.R. Photodynamic therapy for cancer: Role of natural products. Photodiagnosis Photodyn. Ther. 2019, 26, 395–404. [Google Scholar] [CrossRef]
- Aebisher, D.; Przygórzewska, A.; Myśliwiec, A.; Dynarowicz, K.; Krupka-Olek, M.; Bożek, A.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. Current Photodynamic Therapy for Glioma Treatment: An Update. Biomedicines 2024, 12, 375. [Google Scholar] [CrossRef] [PubMed]
- Weathers, S.P.; de Groot, J. VEGF Manipulation in Glioblastoma. Oncology 2015, 29, 720–727. [Google Scholar] [PubMed]
- Seyedmirzaei, H.; Shobeiri, P.; Turgut, M.; Hanaei, S.; Rezaei, N. VEGF levels in patients with glioma: A systematic review and meta-analysis. Rev. Neurosci. 2020, 32, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Onishi, M.; Ichikawa, T.; Kurozumi, K.; Date, I. Angiogenesis and invasion in glioma. Brain Tumor Pathol. 2011, 28, 13–24. [Google Scholar] [CrossRef]
- Xu, D.; Chen, X.; Peng, Y.; Chen, W.; Li, Y.; Wang, X.; Bui, B.; Chen, K.; Zhou, M.; Kawai, N.; et al. Effects of Phthalocyanine-Based Molecular Beacon-Mediated Photodynamic Therapy on U251 Cells. Nanosci. Nanotechnol. Lett. 2016, 8, 714–722. [Google Scholar] [CrossRef]
- Deininger, M.H.; Weinschenk, T.; Morgalla, M.H.; Meyermann, R.; Schluesener, H.J. Release of regulators of angiogenesis following Hypocrellin-A and -B photodynamic therapy of human brain tumor cells. Biochem. Biophys. Res. Commun. 2002, 298, 520–530. [Google Scholar] [CrossRef]
- Xu, D.; Chen, X.; Chen, K.; Peng, Y.; Li, Y.; Ke, Y.; Gan, D. Tetra-sulfonate phthalocyanine zinc-bovine serum albumin conjugate-mediated photodynamic therapy of human glioma. J. Biomater. Appl. 2014, 29, 378–385. [Google Scholar] [CrossRef]
- Xu, D.; Ke, Y.; Jiang, X.; Cai, Y.; Peng, Y.; Li, Y. In vitro photodynamic therapy on human U251 glioma cells with a novel photosensitiser ZnPcS4-BSA. Br. J. Neurosurg. 2010, 24, 660–665. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, F.; Kalkanis, S.N.; Zhang, Z.; Hong, X.; Yang, H.; Chopp, M. Post-acute response of 9L gliosarcoma to Photofrin-mediated PDT in athymic nude mice. Lasers Med. Sci. 2007, 22, 253–259. [Google Scholar] [CrossRef]
- Jiang, F.; Zhang, Z.; Katakowski, M.; Robin, A.; Faber, M.; Zhang, F.; Chopp, M. Angiogenesis induced by photodynamic therapy in normal rat brain. Photochem. Photobiol. 2004, 79, 494–498. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, F.; Zhang, Z.G.; Kalkanis, S.N.; Hong, X.; deCarvalho, A.C.; Chen, J.; Yang, H.; Robin, A.M.; Chopp, M. Low-dose photodynamic therapy increases endothelial cell proliferation and VEGF expression in nude mice brain. Lasers Med. Sci. 2005, 20, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Manuelli, V.; Pecorari, C.; Filomeni, G.; Zito, E. Regulation of redox signaling in HIF-1-dependent tumor angiogenesis. FEBS J. 2022, 289, 5413–5425. [Google Scholar] [CrossRef]
- Tzerkovsky, D.A.; Osharin, V.V.; Istomin, Y.P.; Alexandrova, E.N.; Vozmitel, M.A. Fluorescent diagnosis and photodynamic therapy for C6 glioma in combination with antiangiogenic therapy in subcutaneous and intracranial tumor models. Exp. Oncol. 2014, 36, 85–89. [Google Scholar] [PubMed]
- Jiang, F.; Zhang, X.; Kalkanis, S.N.; Zhang, Z.; Yang, H.; Katakowski, M.; Hong, X.; Zheng, X.; Zhu, Z.; Chopp, M. Combination therapy with antiangiogenic treatment and photodynamic therapy for the nude mouse bearing U87 glioblastoma. Photochem. Photobiol. 2008, 84, 128–137. [Google Scholar] [CrossRef]
- Tatar, O.; Shinoda, K.; Adam, A.; Eckert, T.; Eckardt, C.; Lucke, K.; Deuter, C.; Bartz-Schmidt, K.U.; Grisanti, S. Effect of verteporfin photodynamic therapy on endostatin and angiogenesis in human choroidal neovascular membranes. Br. J. Ophthalmol. 2007, 91, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Q.; Yue, W.; Hu, S. Effect of photodynamic therapy and endostatin on human glioma xenografts in nude mice. Photodiagnosis Photodyn. Ther. 2011, 8, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Walia, A.; Yang, J.F.; Huang, Y.H.; Rosenblatt, M.I.; Chang, J.H.; Azar, D.T. Endostatin’s emerging roles in angiogenesis, lymphangiogenesis, disease, and clinical applications. Biochim. Biophys. Acta 2015, 1850, 2422–2438. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.L.; Lin, H.C.; Chiang, W.L.; Shih, Y.H.; Chiang, P.F.; Luo, T.Y.; Cheng, C.C.; Shieh, M.J. Anti-angiogenic treatment (Bevacizumab) improves the responsiveness of photodynamic therapy in colorectal cancer. Photodiagnosis Photodyn. Ther. 2018, 23, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Wen, P.Y. Antiangiogenic Therapy of High-Grade Gliomas. Prog. Neurol. Surg. 2018, 31, 180–199. [Google Scholar]
- Larue, L.; Myrzakhmetov, B.; Ben-Mihoub, A.; Moussaron, A.; Thomas, N.; Arnoux, P.; Baros, F.; Vanderesse, R.; Acherar, S.; Frochot, C. Fighting Hypoxia to Improve PDT. Pharmaceuticals 2019, 12, 163. [Google Scholar] [CrossRef]
- Chédeville, A.L.; Madureira, P.A. The Role of Hypoxia in Glioblastoma Radiotherapy Resistance. Cancers 2021, 13, 542. [Google Scholar] [CrossRef]
- Pezzuto, A.; Carico, E. Role of HIF-1 in Cancer Progression: Novel Insights. A Review. Curr. Mol. Med. 2018, 18, 343–351. [Google Scholar] [CrossRef]
- Albert, I.; Hefti, M.; Luginbuehl, V. Physiological oxygen concentration alters glioma cell malignancy and responsiveness to photodynamic therapy in vitro. Neurol. Res. 2014, 36, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Dong, P.; Hu, J.; Yu, S.; Zhou, Y.; Shi, T.; Zhao, Y.; Wang, X.; Liu, X. A Mitochondrial Oxidative Stress Amplifier to Overcome Hypoxia Resistance for Enhanced Photodynamic Therapy. Small Methods 2021, 5, 2100581. [Google Scholar] [CrossRef] [PubMed]
- Broekgaarden, M.; Weijer, R.; van Gulik, T.M.; Hamblin, M.R.; Heger, M. Tumor cell survival pathways activated by photodynamic therapy: A molecular basis for pharmacological inhibition strategies. Cancer Metastasis Rev. 2015, 34, 643–690. [Google Scholar] [CrossRef]
- Lamberti, M.J.; Pansa, M.F.; Vera, R.E.; Fernández-Zapico, M.E.; Rumie Vittar, N.B.; Rivarola, V.A. Transcriptional activation of HIF-1 by a ROS-ERK axis underlies the resistance to photodynamic therapy. PLoS ONE 2017, 12, e0177801. [Google Scholar] [CrossRef]
- Weijer, R.; Broekgaarden, M.; van Golen, R.F.; Bulle, E.; Nieuwenhuis, E.; Jongejan, A.; Moerland, P.D.; van Kampen, A.H.; van Gulik, T.M.; Heger, M. Low-power photodynamic therapy induces survival signaling in perihilar cholangiocarcinoma cells. BMC Cancer 2015, 15, 1014. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Liu, W. Effect of low energy ALA-PDT on angiogenesis and glioma growth in brain. Zhongguo Jiguang/Chin. J. Lasers 2012, 39, 804001. [Google Scholar]
- Zheng, X.; Jiang, F.; Katakowski, M.; Zhang, X.; Jiang, H.; Zhang, Z.G.; Chopp, M. Sensitization of cerebral tissue in nude mice with photodynamic therapy induces ADAM17/TACE and promotes glioma cell invasion. Cancer Lett. 2008, 265, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Yang, G.; Shahzidi, S.; Tkacz-Stachowska, K.; Suo, Z.; Nesland, J.M.; Peng, Q. Induction of hypoxia-inducible factor-1alpha overexpression by cobalt chloride enhances cellular resistance to photodynamic therapy. Cancer Lett. 2006, 244, 182–189. [Google Scholar]
- Rodríguez, M.E.; Catrinacio, C.; Ropolo, A.; Rivarola, V.A.; Vaccaro, M.I. A novel HIF-1α/VMP1-autophagic pathway induces resistance to photodynamic therapy in colon cancer cells. Photochem. Photobiol. Sci. 2017, 16, 1631–1642. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Fu, L.H.; Li, C.; Lin, J.; Huang, P. Conquering the Hypoxia Limitation for Photodynamic Therapy. Adv. Mater. 2021, 33, e2103978. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, D.; Zhang, Z.; Wang, Y.; Zhang, Z.; Liang, Z.; Liu, F.; Chen, L. Photodynamic therapy enhances the cytotoxicity of temozolomide against glioblastoma via reprogramming anaerobic glycolysis. Photodiagnosis Photodyn. Ther. 2023, 42, 103342. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Liu, Q.; Adu-Frimpong, M.; Shi, W.; Liu, K.; Deng, T.; Yuan, H.; Weng, X.; Gao, Y.; Yu, Q.; et al. Microfluidic Generation of Near-Infrared Photothermal Vitexin/ICG Liposome with Amplified Photodynamic Therapy. AAPS PharmSciTech 2023, 24, 82. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Wang, F.; Dong, J.; Wang, N.; Tao, S.; Du, J.; Hu, S. Inhibition of hypoxia-inducible factor 1 by acriflavine renders glioblastoma sensitive for photodynamic therapy. J. Photochem. Photobiol. B 2022, 234, 112537. [Google Scholar] [CrossRef]
- Lv, Z.; Jin, L.; Gao, W.; Cao, Y.; Zhang, H.; Xue, D.; Yin, N.; Zhang, T.; Wang, Y.; Zhang, H. Novel YOF-Based Theranostic Agents with a Cascade Effect for NIR-II Fluorescence Imaging and Synergistic Starvation/Photodynamic Therapy of Orthotopic Gliomas. ACS Appl. Mater. Interfaces 2022, 14, 30523–30532. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Hu, D.; Chung, H.Y.; Sheng, Z.; Yao, S. Lipid-Polymer Bilaminar Oxygen Nanobubbles for Enhanced Photodynamic Therapy of Cancer. ACS Appl. Mater. Interfaces 2018, 10, 36805–36813. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, J.M.; Yu, H.; Deng, K.; Zhou, W.; Wang, C.X.; Zhang, Y.; Li, K.H.; Zhuo, R.X.; Huang, S.W. Fluorinated polymeric micelles to overcome hypoxia and enhance photodynamic cancer therapy. Biomater. Sci. 2018, 6, 3096–3107. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Han, Y.; Zhao, G.; Zhang, L.; Zhao, Z.; Wang, Z.; Zhao, L.; Hua, L.; Naveena, K.; Lu, J.; et al. Hypoxia-Responsive Lipid-Polymer Nanoparticle-Combined Imaging-Guided Surgery and Multitherapy Strategies for Glioma. ACS Appl. Mater. Interfaces 2020, 12, 52319–52328. [Google Scholar] [CrossRef]
- Aniogo, E.C.; George, B.P.; Abrahamse, H. Molecular Effectors of Photodynamic Therapy-Mediated Resistance to Cancer Cells. Int. J. Mol. Sci. 2021, 22, 13182. [Google Scholar] [CrossRef]
- Kukal, S.; Guin, D.; Rawat, C.; Bora, S.; Mishra, M.K.; Sharma, P.; Paul, P.R.; Kanojia, N.; Grewal, G.K.; Kukreti, S.; et al. Multidrug efflux transporter ABCG2: Expression and regulation. Cell. Mol. Life Sci. 2021, 78, 6887–6939. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.; Petrucci, C.M. The effect of ALA/PpIX PDT on putative cancer stem cells in tumor side populations. In Photodynamic Therapy: Back to the Future, Proceedings of the 12th World Congress of the International Photodynamic Association, Seattle, WA, USA, 11–15 June 2009; Kessel, D., Ed.; SPIE: Bellingham, WA, USA, 2009; Volume 7380. [Google Scholar]
- Mueller, P.; Gaber, S.A.A.; Zimmermann, W.; Wittig, R.; Stepp, H. ABCG2 influence on the efficiency of photodynamic therapy in glioblastoma cells. J. Photochem. Photobiol. B Biol. 2020, 210, 111963. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Lin, H.; Tian, S.; Bai, D.; Kong, Y.; Yu, L. The Sensitivity of Glioma Cells to Pyropheophorbide-αMethyl Ester-Mediated Photodynamic Therapy Is Enhanced by Inhibiting ABCG2. Lasers Surg. Med. 2017, 49, 719–726. [Google Scholar] [CrossRef]
- Krishnamurthy, P.; Ross, D.D.; Nakanishi, T.; Bailey-Dell, K.; Zhou, S.; Mercer, K.E.; Sarkadi, B.; Sorrentino, B.P.; Schuetz, J.D. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J. Biol. Chem. 2004, 279, 24218–24225. [Google Scholar] [CrossRef] [PubMed]
- Weidner, L.D.; Zoghbi, S.S.; Lu, S.; Shukla, S.; Ambudkar, S.V.; Pike, V.W.; Mulder, J.; Gottesman, M.M.; Innis, R.B.; Hall, M.D. The Inhibitor Ko143 Is Not Specific for ABCG2. J. Pharmacol. Exp. Ther. 2015, 354, 384–393. [Google Scholar] [CrossRef]
- Wang, W.; Tabu, K.; Hagiya, Y.; Sugiyama, Y.; Kokubu, Y.; Murota, Y.; Ogura, S.I.; Taga, T. Enhancement of 5-aminolevulinic acid-based fluorescence detection of side population-defined glioma stem cells by iron chelation. Sci. Rep. 2017, 7, 42070. [Google Scholar] [CrossRef]
- Abdel Gaber, S.A.; Müller, P.; Zimmermann, W.; Hüttenberger, D.; Wittig, R.; Abdel Kader, M.H.; Stepp, H. ABCG2-mediated suppression of chlorin e6 accumulation and photodynamic therapy efficiency in glioblastoma cell lines can be reversed by KO143. J. Photochem. Photobiol. B 2018, 178, 182–191. [Google Scholar] [CrossRef]
- Hamid, S.A.; Zimmermann, W.; Huettenberger, D.; Wittig, R.; Kader, M.A.; Stepp, H. In vitro Study for Photodynamic Therapy using Fotolon® in Glioma Treatment. In Medical Laser Applications and Laser-Tissue Interactions VII, Proceedings of the European Conference on Biomedical Optics 2015, Munich Germany, 21–25 June 2015; Lilge, L., Sroka, R., Eds.; SPIE: Bellingham, WA, USA, 2015; Volume 9542. [Google Scholar]
- Selbo, P.K.; Weyergang, A.; Eng, M.S.; Bostad, M.; Maelandsmo, G.M.; Hogset, A.; Berg, K. Strongly amphiphilic photosensitizers are not substrates of the cancer stem cell marker ABCG2 and provides specific and efficient light-triggered drug delivery of an EGFR-targeted cytotoxic drug. J. Control. Release 2012, 159, 197–203. [Google Scholar] [CrossRef]
- Sun, W.; Kajimoto, Y.; Inoue, H.; Miyatake, S.I.; Ishikawa, T.; Kuroiwa, T. Gefitinib enhances the efficacy of photodynamic therapy using 5-aminolevulinic acid in malignant brain tumor cells. Photodiagnosis Photodyn. Ther. 2013, 10, 42–50. [Google Scholar] [CrossRef]
- Mansi, M.; Howley, R.; Chandratre, S.; Chen, B. Inhibition of ABCG2 transporter by lapatinib enhances 5-aminolevulinic acid-mediated protoporphyrin IX fluorescence and photodynamic therapy response in human glioma cell lines. Biochem. Pharmacol. 2022, 200, 115031. [Google Scholar] [CrossRef]
- Byeon, S.; Hong, J.Y.; Lee, J.; Nam, D.H.; Park, S.H.; Park, J.O.; Park, Y.S.; Lim, H.Y.; Kang, W.K.; Kim, S.T. Use of Gefitinib in EGFR-Amplified Refractory Solid Tumors: An Open-Label, Single-Arm, Single-Center Prospective Pilot Study. Target Oncol. 2020, 15, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Karajannis, M.A.; Legault, G.; Fisher, M.J.; Milla, S.S.; Cohen, K.J.; Wisoff, J.H.; Harter, D.H.; Goldberg, J.D.; Hochman, T.; Merkelson, A.; et al. Phase II study of sorafenib in children with recurrent or progressive low-grade astrocytomas. Neuro-Oncol. 2014, 16, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Lee, H.K.; To, K.K.; Fok, B.S.; Wo, S.K.; Ho, C.S.; Wong, C.K.; Zuo, Z.; Chan, T.Y.; Chan, J.C.; et al. Telmisartan increases systemic exposure to rosuvastatin after single and multiple doses, and in vitro studies show telmisartan inhibits ABCG2-mediated transport of rosuvastatin. Eur. J. Clin. Pharmacol. 2016, 72, 1471–1478. [Google Scholar] [CrossRef] [PubMed]
- Miyata, H.; Takada, T.; Toyoda, Y.; Matsuo, H.; Ichida, K.; Suzuki, H. Identification of Febuxostat as a New Strong ABCG2 Inhibitor: Potential Applications and Risks in Clinical Situations. Front. Pharmacol. 2016, 7, 518. [Google Scholar] [CrossRef] [PubMed]
- Peña-Solórzano, D.; Stark, S.A.; König, B.; Sierra, C.A.; Ochoa-Puentes, C. ABCG2/BCRP: Specific and Nonspecific Modulators. Med. Res. Rev. 2017, 37, 987–1050. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, M.; Rola, R. The implications of nitric oxide metabolism in the treatment of glial tumors. Neurochem. Int. 2021, 150, 105172. [Google Scholar] [CrossRef]
- Yang, D.I.; Yin, J.H.; Mishra, S.; Mishra, R.; Hsu, C.Y. NO-mediated chemoresistance in C6 glioma cells. Ann. N. Y. Acad. Sci. 2002, 962, 8–17. [Google Scholar] [CrossRef]
- Casas, A.; Di Venosa, G.; Hasan, T.; Batlle, A. Mechanisms of resistance to photodynamic therapy. Curr. Med. Chem. 2011, 18, 2486–2515. [Google Scholar] [CrossRef]
- Zhu, L.J.; Li, F.; Zhu, D.Y. nNOS and Neurological, Neuropsychiatric Disorders: A 20-Year Story. Neurosci. Bull. 2023, 39, 1439–1453. [Google Scholar] [CrossRef]
- Kolluru, G.K.; Siamwala, J.H.; Chatterjee, S. eNOS phosphorylation in health and disease. Biochimie 2010, 92, 1186–1198. [Google Scholar] [CrossRef]
- Lechner, M.; Lirk, P.; Rieder, J. Inducible nitric oxide synthase (iNOS) in tumor biology: The two sides of the same coin. Semin. Cancer Biol. 2005, 15, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Jahani-Asl, A.; Bonni, A. iNOS: A potential therapeutic target for malignant glioma. Curr. Mol. Med. 2013, 13, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Maccallini, C.; Gallorini, M.; Cataldi, A.; Amoroso, R. Targeting iNOS As a Valuable Strategy for the Therapy of Glioma. ChemMedChem 2020, 15, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.M.; Korytowski, W.; Girotti, A.W. Upstream signaling events leading to elevated production of pro-survival nitric oxide in photodynamically-challenged glioblastoma cells. Free Radic. Biol. Med. 2019, 137, 37–45. [Google Scholar] [CrossRef]
- Fahey, J.M.; Emmer, J.V.; Korytowski, W.; Hogg, N.; Girotti, A.W. Antagonistic Effects of Endogenous Nitric Oxide in a Glioblastoma Photodynamic Therapy Model. Photochem. Photobiol. 2016, 92, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.M.; Stancill, J.S.; Smith, B.C.; Girotti, A.W. Nitric oxide antagonism to glioblastoma photodynamic therapy and mitigation thereof by BET bromodomain inhibitor JQ1. J. Biol. Chem. 2018, 293, 5345–5359. [Google Scholar] [CrossRef] [PubMed]
- Bazak, J.; Korytowski, W.; Girotti, A.W. Bystander Effects of Nitric Oxide in Cellular Models of Anti-Tumor Photodynamic Therapy. Cancers 2019, 11, 1674. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.M.; Girotti, A.W. Nitric oxide-mediated resistance to photodynamic therapy in a human breast tumor xenograft model: Improved outcome with NOS2 inhibitors. Nitric Oxide 2017, 62, 52–61. [Google Scholar] [CrossRef]
- Merenzon, M.A.; Hincapie Arias, E.; Bhatia, S.; Shah, A.H.; Higgins, D.M.O.; Villaverde, M.; Belgorosky, D.; Eijan, A.M. Nitric oxide synthase inhibitors as potential therapeutic agents for gliomas: A systematic review. Nitric Oxide 2023, 138–139, 10–16. [Google Scholar] [CrossRef]
- Bansal, A.; Simon, M.C. Glutathione metabolism in cancer progression and treatment resistance. J. Cell Biol. 2018, 217, 2291–2298. [Google Scholar] [CrossRef]
- Zhu, Z.; Du, S.; Du, Y.; Ren, J.; Ying, G.; Yan, Z. Glutathione reductase mediates drug resistance in glioblastoma cells by regulating redox homeostasis. J. Neurochem. 2018, 144, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Edano, M.; Kanda, T.; Tarumoto, R.; Hamamoto, W.; Hasegawa, T.; Mae, Y.; Onoyama, T.; Takata, T.; Sugihara, T.; Isomoto, H. Intracellular glutathione levels affect the outcomes of verteporfin-mediated photodynamic therapy in esophageal cancer cells. Photodiagnosis Photodyn. Ther. 2022, 40, 103090. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Sun, L.; Zhang, Y.; Wang, Y.; Zheng, J. Imbalanced GSH/ROS and sequential cell death. J. Biochem. Mol. Toxicol. 2022, 36, e22942. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelopoulou, M.; Grigalavicius, M.; Raabe, T.H.; Skarpen, E.; Juzenas, P.; Peng, Q.; Berg, K.; Theodossiou, T.A. Predictive biomarkers for 5-ALA-PDT can lead to personalized treatments and overcome tumor-specific resistances. Cancer Rep. 2022, 5, e1278. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Robin, A.M.; Katakowski, M.; Tong, L.; Espiritu, M.; Singh, G.; Chopp, M. Photodynamic therapy with photofrin in combination with Buthionine Sulfoximine (BSO) of human glioma in the nude rat. Lasers Med. Sci. 2003, 18, 128–133. [Google Scholar] [CrossRef] [PubMed]
- dos Reis Oliveira, C.; Pereira, J.C.; Barros Ibiapina, A.; Roseno Martins, I.R.; de Castro e Sousa, J.M.; Ferreira, P.M.; Carneiro da Silva, F.C. Buthionine sulfoximine and chemoresistance in cancer treatments: A systematic review with meta-analysis of preclinical studies. J. Toxicol. Environ. Health 2023, 26 Pt B, 417–441. [Google Scholar] [CrossRef]
- Hwang, B.; Kim, T.I.; Kim, H.; Jeon, S.; Choi, Y.; Kim, Y. Ubiquinone-BODIPY nanoparticles for tumor redox-responsive fluorescence imaging and photodynamic activity. J. Mater. Chem. B 2021, 9, 824–831. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Liu, L.; Wei, S.; Huang, Z.; Qiu, L.; Lin, J.; Liu, H.; Ye, D. Controlling Disassembly of Paramagnetic Prodrug and Photosensitizer Nanoassemblies for On-Demand Orthotopic Glioma Theranostics. ACS Nano 2022, 16, 20607–20621. [Google Scholar] [CrossRef]
- Wu, S.-Y.; Ye, Y.-X.; Zhang, Q.; Kang, Q.-J.; Xu, Z.-M.; Ren, S.-Z.; Lin, F.; Duan, Y.-T.; Xu, H.-J.; Hu, Z.-Y.; et al. Multifunctional Protein Hybrid Nanoplatform for Synergetic Photodynamic-Chemotherapy of Malignant Carcinoma by Homologous Targeting Combined with Oxygen Transport. Adv. Sci. 2023, 10, 2203742. [Google Scholar] [CrossRef]
- Cao, X.; Li, S.; Chen, W.; Lu, H.; Ye, L.; Min, Z.; Sun, S.; Teng, C.; Yin, H.; Zhang, Q.; et al. Multifunctional Hybrid Hydrogel System Enhanced the Therapeutic Efficacy of Treatments for Postoperative Glioma. ACS Appl. Mater. Interfaces 2022, 14, 27623–27633. [Google Scholar] [CrossRef] [PubMed]
- Obi, C.D.; Bhuiyan, T.; Dailey, H.A.; Medlock, A.E. Ferrochelatase: Mapping the Intersection of Iron and Porphyrin Metabolism in the Mitochondria. Front. Cell Dev. Biol. 2022, 10, 894591. [Google Scholar] [CrossRef] [PubMed]
- Briel-Pump, A.; Beez, T.; Ebbert, L.; Remke, M.; Weinhold, S.; Sabel, M.C.; Sorg, R.V. Accumulation of protoporphyrin IX in medulloblastoma cell lines and sensitivity to subsequent photodynamic treatment. J. Photochem. Photobiol. B 2018, 189, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Ihata, T.; Nonoguchi, N.; Fujishiro, T.; Omura, N.; Kawabata, S.; Kajimoto, Y.; Wanibuchi, M. The effect of hypoxia on photodynamic therapy with 5-aminolevulinic acid in malignant gliomas. Photodiagnosis Photodyn. Ther. 2022, 40, 103056. [Google Scholar] [CrossRef] [PubMed]
- Reburn, C.; Anayo, L.; Magnussen, A.; Perry, A.; Wood, M.; Curnow, A. Experimental findings utilising a new iron chelating ALA prodrug to enhance protoporphyrin IX-induced photodynamic therapy. In Proceedings of the 17th International Photodynamic Association World Congress, Cambridge, MA, USA, 28 June–4 July 2019; Hasan, T., Ed.; SPIE: Bellingham, WA, USA, 2019; Volume 11070. [Google Scholar]
- Blake, E.; Curnow, A. The hydroxypyridinone iron chelator CP94 can enhance PpIX-induced PDT of cultured human glioma cells. Photochem. Photobiol. 2010, 86, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Blake, E.; Allen, J.; Curnow, A. An in vitro comparison of the effects of the iron-chelating agents, CP94 and dexrazoxane, on protoporphyrin IX accumulation for photodynamic therapy and/or fluorescence guided resection. Photochem. Photobiol. 2011, 87, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Teng, L.; Nakada, M.; Zhao, S.G.; Endo, Y.; Furuyama, N.; Nambu, E.; Pyko, I.V.; Hayashi, Y.; Hamada, J.I. Silencing of ferrochelatase enhances 5-aminolevulinic acid-based fluorescence and photodynamic therapy efficacy. Br. J. Cancer 2011, 104, 798–807. [Google Scholar] [CrossRef]
- Chau, L.Y. Heme oxygenase-1: Emerging target of cancer therapy. J. Biomed. Sci. 2015, 22, 22. [Google Scholar] [CrossRef]
- Nowis, D.; Legat, M.; Grzela, T.; Niderla, J.; Wilczek, E.; Wilczynski, G.M.; Głodkowska, E.; Mrówka, P.; Issat, T.; Dulak, J.; et al. Heme oxygenase-1 protects tumor cells against photodynamic therapy-mediated cytotoxicity. Oncogene 2006, 25, 3365–3374. [Google Scholar] [CrossRef]
- Kyriakis, J.M.; Avruch, J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001, 81, 807–869. [Google Scholar] [CrossRef]
- Sigaud, R.; Rösch, L.; Gatzweiler, C.; Benzel, J.; von Soosten, L.; Peterziel, H.; Selt, F.; Najafi, S.; Ayhan, S.; Gerloff, X.F.; et al. The first-in-class ERK inhibitor ulixertinib shows promising activity in mitogen-activated protein kinase (MAPK)-driven pediatric low-grade glioma models. Neuro Oncol. 2023, 25, 566–579. [Google Scholar] [CrossRef]
- Haller, V.; Nahidino, P.; Forster, M.; Laufer, S.A. An updated patent review of p38 MAP kinase inhibitors (2014–2019). Expert Opin. Ther. Pat. 2020, 30, 453–466. [Google Scholar] [CrossRef] [PubMed]
- Schelling, J.R.; Abu Jawdeh, B.G. Regulation of cell survival by Na+/H+ exchanger-1. Am. J. Physiol. Renal. Physiol. 2008, 295, F625–F632. [Google Scholar] [CrossRef]
- Guan, X.; Hasan, M.N.; Begum, G.; Kohanbash, G.; Carney, K.E.; Pigott, V.M.; Persson, A.I.; Castro, M.G.; Jia, W.; Sun, D. Blockade of Na/H exchanger stimulates glioma tumor immunogenicity and enhances combinatorial TMZ and anti-PD-1 therapy. Cell Death Dis. 2018, 9, 1010. [Google Scholar] [CrossRef]
- Zhu, W.; Carney, K.E.; Pigott, V.M.; Falgoust, L.M.; Clark, P.A.; Kuo, J.S.; Sun, D. Glioma-mediated microglial activation promotes glioma proliferation and migration: Roles of Na+/H+ exchanger isoform 1. Carcinogenesis 2016, 37, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.N.; Luo, L.; Ding, D.; Song, S.; Bhuiyan, M.I.; Liu, R.; Foley, L.M.; Guan, X.; Kohanbash, G.; Hitchens, T.K.; et al. Blocking NHE1 stimulates glioma tumor immunity by restoring OXPHOS function of myeloid cells. Theranostics 2021, 11, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Liu, J.; Du, J.; Mi, S.; Ma, S.; Ba, Y.; Ji, H.; Li, B.; Hu, S. Dihydroartemisinin prompts amplification of photodynamic therapy-induced reactive oxygen species to exhaust Na/H exchanger 1-mediated glioma cells invasion and migration. J. Photochem. Photobiol. B 2021, 219, 112192. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Chen, L.; Chi, D.; Cong, D.; Zhou, P.; Jin, J.; Ji, H.; Liang, B.; Gao, S.; Hu, S. Photodynamic therapy combined with temozolomide inhibits C6 glioma migration and invasion and promotes mitochondrial-associated apoptosis by inhibiting sodium-hydrogen exchanger isoform 1. Photodiagnosis Photodyn. Ther. 2019, 26, 405–412. [Google Scholar] [CrossRef]
- Shimoda, L.A.; Fallon, M.; Pisarcik, S.; Wang, J.; Semenza, G.L. HIF-1 regulates hypoxic induction of NHE1 expression and alkalinization of intracellular pH in pulmonary arterial myocytes. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 291, L941–L949. [Google Scholar] [CrossRef]
- Tamtaji, O.R.; Mirzaei, H.; Shamshirian, A.; Shamshirian, D.; Behnam, M.; Asemi, Z. New trends in glioma cancer therapy: Targeting Na+/H+ exchangers. J. Cell Physiol. 2019, 235, 658–665. [Google Scholar] [CrossRef]
- Haapasalo, J.; Nordfors, K.; Haapasalo, H.; Parkkila, S. The Expression of Carbonic Anhydrases II, IX and XII in Brain Tumors. Cancers 2020, 12, 1723. [Google Scholar] [CrossRef]
- Proescholdt, M.A.; Merrill, M.J.; Stoerr, E.M.; Lohmeier, A.; Pohl, F.; Brawanski, A. Function of carbonic anhydrase IX in glioblastoma multiforme. Neuro Oncol. 2012, 14, 1357–1366. [Google Scholar] [CrossRef]
- Lee, S.Y.; Luk, S.K.; Chuang, C.P.; Yip, S.P.; To, S.S.; Yung, Y.M. TP53 regulates human AlkB homologue 2 expression in glioma resistance to Photofrin-mediated photodynamic therapy. Br. J. Cancer 2010, 103, 362–369. [Google Scholar] [CrossRef]
- Park, J.; Oh, S.J.; Shim, J.K.; Ji, Y.B.; Moon, J.H.; Kim, E.H.; Huh, Y.M.; Suh, J.S.; Chang, J.H.; Lee, S.J.; et al. C5α secreted by tumor mesenchymal stem-like cells mediates resistance to 5-aminolevulinic acid-based photodynamic therapy against glioblastoma tumorspheres. J. Cancer Res. Clin. Oncol. 2023, 149, 4391–4402. [Google Scholar] [CrossRef]
- Shahmoradi Ghahe, S.; Kosicki, K.; Wojewódzka, M.; Majchrzak, B.A.; Fogtman, A.; Iwanicka-Nowicka, R.; Ciuba, A.; Koblowska, M.; Kruszewski, M.; Tudek, B.; et al. Increased DNA repair capacity augments resistance of glioblastoma cells to photodynamic therapy. DNA Repair. 2021, 104, 103136. [Google Scholar] [CrossRef]
- Rroji, O.; Kumar, A.; Karuppagounder, S.S.; Ratan, R.R. Epigenetic regulators of neuronal ferroptosis identify novel therapeutics for neurological diseases: HDACs, transglutaminases, and HIF prolyl hydroxylases. Neurobiol. Dis. 2021, 147, 105145. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Li, Y.; Li, N.; Shen, L.; Li, Z. Comprehensive analysis of histone deacetylases genes in the prognosis and immune infiltration of glioma patients. Aging 2022, 14, 4050–4068. [Google Scholar] [CrossRef]
- Li, P.T.; Tsai, Y.J.; Lee, M.J.; Chen, C.T. Increased Histone Deacetylase Activity Involved in the Suppressed Invasion of Cancer Cells Survived from ALA-Mediated Photodynamic Treatment. Int. J. Mol. Sci. 2015, 16, 23994–24010. [Google Scholar] [CrossRef]
- Demyanenko, S.V.; Uzdensky, A.B.; Sharifulina, S.A.; Lapteva, T.O.; Polyakova, L.P. PDT-induced epigenetic changes in the mouse cerebral cortex: A protein microarray study. Biochim. Biophys. Acta 2014, 1840, 262–270. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, M.; Zhou, Y.; Guo, W.; Yi, M.; Zhang, Z.; Ding, Y.; Wang, Y. The application of histone deacetylases inhibitors in glioblastoma. J. Exp. Clin. Cancer Res. 2020, 39, 138. [Google Scholar] [CrossRef] [PubMed]
- Friday, B.B.; Anderson, S.K.; Buckner, J.; Yu, C.; Giannini, C.; Geoffroy, F.; Schwerkoske, J.; Mazurczak, M.; Gross, H.; Pajon, E.; et al. Phase II trial of vorinostat in combination with bortezomib in recurrent glioblastoma: A north central cancer treatment group study. Neuro Oncol. 2012, 14, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Sawa, H.; Murakami, H.; Ohshima, Y.; Sugino, T.; Nakajyo, T.; Kisanuki, T.; Tamura, Y.; Satone, A.; Ide, W.; Hashimoto, I.; et al. Histone deacetylase inhibitors such as sodium butyrate and trichostatin A induce apoptosis through an increase of the bcl-2-related protein Bad. Brain Tumor Pathol. 2001, 18, 109–114. [Google Scholar] [CrossRef]
- Sawa, H.; Murakami, H.; Ohshima, Y.; Murakami, M.; Yamazaki, I.; Tamura, Y.; Mima, T.; Satone, A.; Ide, W.; Hashimoto, I.; et al. Histone deacetylase inhibitors such as sodium butyrate and trichostatin A inhibit vascular endothelial growth factor (VEGF) secretion from human glioblastoma cells. Brain Tumor Pathol. 2002, 19, 77–81. [Google Scholar] [CrossRef]
- Nör, C.; Sassi, F.A.; de Farias, C.B.; Schwartsmann, G.; Abujamra, A.L.; Lenz, G.; Brunetto, A.L.; Roesler, R. The histone deacetylase inhibitor sodium butyrate promotes cell death and differentiation and reduces neurosphere formation in human medulloblastoma cells. Mol. Neurobiol. 2013, 48, 533–543. [Google Scholar] [CrossRef]
- Bueno-Carrazco, J.; Castro-Leyva, V.; García-Gomez, F.; Solís-Paredes, M.; Ramon-Gallegos, E.; Cruz-Orea, A.; Eguía-Aguilar, P.; Arenas-Huertero, F. Sodium butyrate increases the effect of the photodynamic therapy: A mechanism that involves modulation of gene expression and differentiation in astrocytoma cells. Childs Nerv. Syst. 2012, 28, 1723–1730. [Google Scholar] [CrossRef]
- Flores-Ancona, R.M.; García-Gómez, F.Y.; Jiménez-Betanzos, A.M.; Solis-Paredes, M.; Castro-Leyva, V.; Cruz-Orea, A.; Arenas-Huertero, F.; Ramón-Gallegos, E. Effects of sodium butyrate on cell death induced by photodynamic therapy in U373-MG and D54-MG astrocytoma cell lines. Photochem. Photobiol. 2009, 85, 1182–1188. [Google Scholar] [CrossRef]
- Wei, W.; Huang, C.; Zhang, J.; Chen, Q.; Liu, Z.; Ren, X.; Gan, S.; Wu, P.; Wang, D.; Tang, B.Z.; et al. HDAC6-Activatable Multifunctional Near-Infrared Probe for Glioma Cell Detection and Elimination. Anal. Chem. 2024, 96, 2406–2414. [Google Scholar] [CrossRef]
- Dolcet, X.; Llobet, D.; Pallares, J.; Matias-Guiu, X. NF-kB in development and progression of human cancer. Virchows Arch. 2005, 446, 475–482. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Q.L.; Sun, W.; Chandrasekharan, P.; Cheng, H.S.; Ying, Z.; Lakshmanan, M.; Raju, A.; Tenen, D.G.; Cheng, S.Y.; et al. Non-canonical NF-κB signalling and ETS1/2 cooperatively drive C250T mutant TERT promoter activation. Nat. Cell Biol. 2015, 17, 1327–1338. [Google Scholar] [CrossRef]
- Hu, Y.H.; Jiao, B.H.; Wang, C.Y.; Wu, J.L. Regulation of temozolomide resistance in glioma cells via the RIP2/NF-κB/MGMT pathway. CNS Neurosci. Ther. 2021, 27, 552–563. [Google Scholar] [CrossRef]
- Coupienne, I.; Bontems, S.; Dewaele, M.; Rubio, N.; Habraken, Y.; Fulda, S.; Agostinis, P.; Piette, J. NF-kappaB inhibition improves the sensitivity of human glioblastoma cells to 5-aminolevulinic acid-based photodynamic therapy. Biochem. Pharmacol. 2011, 81, 606–616. [Google Scholar] [CrossRef]
- Piette, J. Signalling pathway activation by photodynamic therapy: NF-κB at the crossroad between oncology and immunology. Photochem. Photobiol. Sci. 2015, 14, 1510–1517. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.; Banik, N.L.; Patel, S.J.; Ray, S.K. 5-Aminolevulinic acid-based photodynamic therapy suppressed survival factors and activated proteases for apoptosis in human glioblastoma U87MG cells. Neurosci. Lett. 2007, 415, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Alqawi, O.; Espiritu, M. Metronomic PDT and cell death pathways. Methods Mol. Biol. 2010, 635, 65–78. [Google Scholar] [PubMed]
- He, Y.; Duan, L.; Wu, H.; Chen, S.; Lu, T.; Li, T.; He, Y. Integrated Transcriptome Analysis Reveals the Impact of Photodynamic Therapy on Cerebrovascular Endothelial Cells. Front. Oncol. 2021, 11, 731414. [Google Scholar] [CrossRef]
- Medeiros, M.; Candido, M.F.; Valera, E.T.; Brassesco, M.S. The multifaceted NF-kB: Are there still prospects of its inhibition for clinical intervention in pediatric central nervous system tumors? Cell. Mol. Life Sci. 2021, 78, 6161–6200. [Google Scholar] [CrossRef] [PubMed]
- Kumthekar, P.; Grimm, S.; Chandler, J.; Mehta, M.; Marymont, M.; Levy, R.; Muro, K.; Helenowski, I.; McCarthy, K.; Fountas, L.; et al. A phase II trial of arsenic trioxide and temozolomide in combination with radiation therapy for patients with malignant gliomas. J. Neuro-Oncol. 2017, 133, 589–594. [Google Scholar] [CrossRef]
- Xie, Y.; Su, N.; Yang, J.; Tan, Q.; Huang, S.; Jin, M.; Ni, Z.; Zhang, B.; Zhang, D.; Luo, F.; et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target. Ther. 2020, 5, 181. [Google Scholar] [CrossRef] [PubMed]
- Klimaschewski, L.; Claus, P. Fibroblast Growth Factor Signalling in the Diseased Nervous System. Mol. Neurobiol. 2021, 58, 3884–3902. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Zhang, Z.; Lin, M.; Zhou, B.; Wang, Z. Bevacizumab has bidirectional regulatory effects on the secretion of basic fibroblast growth factor in glioma cells. Cytokine 2020, 129, 155022. [Google Scholar] [CrossRef]
- Chai, N.; Stachon, T.; Berger, T.; Li, Z.; Seitz, B.; Langenbucher, A.; Szentmáry, N. Human corneal epithelial cell and fibroblast migration and growth factor secretion after rose bengal photodynamic therapy (RB-PDT) and the effect of conditioned medium. PLoS ONE 2023, 18, e0296022. [Google Scholar] [CrossRef]
- LaMuraglia, G.M.; Adili, F.; Karp, S.J.; Statius van Eps, R.G.; Watkins, M.T. Photodynamic therapy inactivates extracellular matrix-basic fibroblast growth factor: Insights to its effect on the vascular wall. J. Vasc. Surg. 1997, 26, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Vilchez, M.L.; Rodríguez, L.B.; Palacios, R.E.; Prucca, C.G.; Caverzán, M.D.; Caputto, B.L.; Rivarola, V.A.; Milla Sanabria, L.N. Isolation and initial characterization of human glioblastoma cells resistant to photodynamic therapy. Photodiagnosis Photodyn. Ther. 2021, 33, 102097. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.; Hsiao, Y.; Teng, L.; Chen, C.; Kao, M. Comparative study on the ALA photodynamic effects of human glioma and meningioma cells. Lasers Surg. Med. Off. J. Am. Soc. Laser Med. Surg. 1999, 24, 296–305. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Banik, N.L.; Ray, S.K. Photofrin based photodynamic therapy and miR-99a transfection inhibited FGFR3 and PI3K/Akt signaling mechanisms to control growth of human glioblastoma In vitro and in vivo. PLoS ONE 2013, 8, e55652. [Google Scholar] [CrossRef]
- Repetto, M.; Crimini, E.; Giugliano, F.; Morganti, S.; Belli, C.; Curigliano, G. Selective FGFR/FGF pathway inhibitors: Inhibition strategies, clinical activities, resistance mutations, and future directions. Expert Rev. Clin. Pharmacol. 2021, 14, 1233–1252. [Google Scholar] [CrossRef]
- Tabernero, J.; Bahleda, R.; Dienstmann, R.; Infante, J.R.; Mita, A.; Italiano, A.; Calvo, E.; Moreno, V.; Adamo, B.; Gazzah, A.; et al. Phase I Dose-Escalation Study of JNJ-42756493, an Oral Pan-Fibroblast Growth Factor Receptor Inhibitor, in Patients with Advanced Solid Tumors. J. Clin. Oncol. 2015, 33, 3401–3408. [Google Scholar] [CrossRef]
- Sharma, M.; Schilero, C.; Peereboom, D.M.; Hobbs, B.P.; Elson, P.; Stevens, G.H.J.; McCrae, K.; Nixon, A.B.; Ahluwalia, M.S. Phase II study of Dovitinib in recurrent glioblastoma. J. Neuro-Oncol. 2019, 144, 359–368. [Google Scholar] [CrossRef]
- de Groot, J.; Sontheimer, H. Glutamate and the biology of gliomas. Glia 2011, 59, 1181–1189. [Google Scholar] [CrossRef]
- Pei, Z.; Lee, K.C.; Khan, A.; Erisnor, G.; Wang, H.Y. Pathway analysis of glutamate-mediated, calcium-related signaling in glioma progression. Biochem. Pharmacol. 2020, 176, 113814. [Google Scholar] [CrossRef]
- Du, P.; Hu, S.; Cheng, Y.; Li, F.; Li, M.; Li, J.; Yi, L.; Feng, H. Photodynamic therapy leads to death of C6 glioma cells partly through AMPAR. Brain Res. 2012, 1433, 153–159. [Google Scholar] [CrossRef]
- Lyons, S.A.; Chung, W.J.; Weaver, A.K.; Ogunrinu, T.; Sontheimer, H. Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res. 2007, 67, 9463–9471. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.L.; Du, P.; Hu, R.; Li, F.; Feng, H. Imbalance of Ca2+ and K+ fluxes in C6 glioma cells after PDT measured with scanning ion-selective electrode technique. Lasers Med. Sci. 2014, 29, 1261–1267. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, S.; Qi, C.; Zhao, X.; Liu, B.; Hao, F.; Zhao, Z. Inhibition of metabotropic glutamate receptor 5 facilitates hypoxia-induced glioma cell death. Brain Res. 2019, 1704, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, V.; Lecanu, L.; Brown, R.C.; Han, Z.; Yao, Z.X. Peripheral-type benzodiazepine receptor in neurosteroid biosynthesis, neuropathology and neurological disorders. Neuroscience 2006, 138, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Starosta-Rubinstein, S.; Ciliax, B.J.; Penney, J.B.; McKeever, P.; Young, A.B. Imaging of a glioma using peripheral benzodiazepine receptor ligands. Proc. Natl. Acad. Sci. USA 1987, 84, 891–895. [Google Scholar] [CrossRef]
- Takada, A.; Mitsuka, S.; Diksic, M.; Yamamoto, Y.L. Autoradiographic study of peripheral benzodiazepine receptors in animal brain tumor models and human gliomas. Eur. J. Pharmacol. Environ. Toxicol. Pharmacol. 1992, 228, 131–139. [Google Scholar] [CrossRef]
- Veenman, L.; Levin, E.; Weisinger, G.; Leschiner, S.; Spanier, I.; Snyder, S.H.; Weizman, A.; Gavish, M. Peripheral-type benzodiazepine receptor density and in vitro tumorigenicity of glioma cell lines. Biochem. Pharmacol. 2004, 68, 689–698. [Google Scholar] [CrossRef]
- Corsi, L.; Geminiani, E.; Baraldi, M. Peripheral benzodiazepine receptor (PBR) new insight in cell proliferation and cell differentiation review. Curr. Clin. Pharmacol. 2008, 3, 38–45. [Google Scholar] [CrossRef]
- Bisland, S.K.; Goebel, E.A.; Hassanali, N.S.; Johnson, C.; Wilson, B.C. Increased expression of mitochondrial benzodiazepine receptors following low-level light treatment facilitates enhanced protoporphyrin IX production in glioma-derived cells in vitro. Lasers Surg. Med. 2007, 39, 678–684. [Google Scholar] [CrossRef]
- Kessel, D.; Antolovich, M.; Smith, K.M. The Role of the Peripheral Benzodiazepine Receptor in the Apoptotic Response to Photodynamic Therapy. Photochem. Photobiol. 2001, 74, 346–349. [Google Scholar] [CrossRef]
- Sarissky, M.; Lavicka, J.; Kocanová, S.; Sulla, I.; Mirossay, A.; Miskovsky, P.; Gajdos, M.; Mojzis, J.; Mirossay, L. Diazepam enhances hypericin-induced photocytotoxicity and apoptosis in human glioblastoma cells. Neoplasma 2005, 52, 352–359. [Google Scholar]
- Chen, J.; Ouyang, Y.; Cao, L.; Zhu, W.; Zhou, Y.; Zhou, Y.; Zhang, H.; Yang, X.; Mao, L.; Lin, S.; et al. Diazepam inhibits proliferation of human glioblastoma cells through triggering a G0/G1 cell cycle arrest. J. Neurosurg. Anesthesiol. 2013, 25, 285–291. [Google Scholar] [CrossRef]
- Lavicka, J.; Sarisský, M.; Mirossay, A.; Sulla, I.; Mojzis, J.; Mirossay, L. Diazepam enhances etoposide-induced cytotoxicity in U-87 MG human glioma cell line. Fundam. Clin. Pharmacol. 2001, 15, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Drljača, J.; Popović, A.; Bulajić, D.; Stilinović, N.; Vidičević Novaković, S.; Sekulić, S.; Milenković, I.; Ninković, S.; Ljubković, M.; Čapo, I. Diazepam diminishes temozolomide efficacy in the treatment of U87 glioblastoma cell line. CNS Neurosci. Ther. 2022, 28, 1447–1457. [Google Scholar] [CrossRef]
- de Almeida, L.G.N.; Thode, H.; Eslambolchi, Y.; Chopra, S.; Young, D.; Gill, S.; Devel, L.; Dufour, A. Matrix Metalloproteinases: From Molecular Mechanisms to Physiology, Pathophysiology, and Pharmacology. Pharmacol. Rev. 2022, 74, 712–768. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, X.; Sun, S.; Zhang, X.; Yang, W.; Zhang, J.; Zhang, X.; Jiang, Z. Increased expression of MMP-2 and MMP-9 indicates poor prognosis in glioma recurrence. Biomed. Pharmacother. 2019, 118, 109369. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, J.; Tu, Y.; Mao, X.; He, S.; Fu, G.; Zong, J.; Zhang, Y. Co-expression of MMP-14 and MMP-19 predicts poor survival in human glioma. Clin. Transl. Oncol. 2013, 15, 139–145. [Google Scholar] [CrossRef]
- Au, C.M.; Luk, S.K.; Jackson, C.J.; Ng, H.K.; Yow, C.M.N.; To, S.S.T. Differential effects of photofrin, 5-aminolevulinic acid and calphostin C on glioma cells. J. Photochem. Photobiol. B Biol. 2006, 85, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Chu, E.S.M.; Wong, T.K.S.; Yow, C.M.N. Photodynamic effect in medulloblastoma: Downregulation of matrix metalloproteinases and human telomerase reverse transcriptase expressions. Photochem. Photobiol. Sci. 2008, 7, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Etminan, N.; Peters, C.; Ficnar, J.; Anlasik, S.; Bünemann, E.; Slotty, P.J.; Hänggi, D.; Steiger, H.J.; Sorg, R.V.; Stummer, W. Modulation of migratory activity and invasiveness of human glioma spheroids following 5-aminolevulinic acid-based photodynamic treatment. Laboratory investigation. J. Neurosurg. 2011, 115, 281–288. [Google Scholar] [CrossRef]
- Li, J.; Tian, Y.; Zheng, T. A multifunctional nanoprobe for real-time SERS monitoring of invasion ability affected by photodynamic therapy. Chem. Commun. 2022, 58, 6542–6545. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lin, H.; Chen, Q.; Yu, L.; Bai, D. MPPa-PDT suppresses breast tumor migration/invasion by inhibiting Akt-NF-κB-dependent MMP-9 expression via ROS. BMC Cancer 2019, 19, 1159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shen, B.; Swinarska, J.T.; Li, W.; Xiao, K.; He, P. 9-Hydroxypheophorbide α-mediated photodynamic therapy induces matrix metalloproteinase-2 (MMP-2) and MMP-9 down-regulation in Hep-2 cells via ROS-mediated suppression of the ERK pathway. Photodiagnosis Photodyn. Ther. 2014, 11, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Li, B.O.; Meng, C.; Zhang, X.; Cong, D.; Gao, X.; Gao, W.; Ju, D.; Hu, S. Effect of photodynamic therapy combined with torasemide on the expression of matrix metalloproteinase 2 and sodium-potassium-chloride cotransporter 1 in rat peritumoral edema and glioma. Oncol. Lett. 2016, 11, 2084–2090. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kim, S.Y.; Hyun, J.W.; Min, S.W.; Kim, D.H.; Kim, H.S. Glycitein inhibits glioma cell invasion through down-regulation of MMP-3 and MMP-9 gene expression. Chem. Biol. Interact. 2010, 185, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, X.; Sun, L.; Bi, X.; He, H.; Chen, L.; Pang, J. MicroRNA 34a inhibits cell growth and migration in human glioma cells via MMP 9. Mol. Med. Rep. 2019, 20, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar] [PubMed]
- Xue, S.; Hu, M.; Iyer, V.; Yu, J. Blocking the PD-1/PD-L1 pathway in glioma: A potential new treatment strategy. J. Hematol. Oncol. 2017, 10, 81. [Google Scholar] [CrossRef]
- Gurung, P.; Lim, J.; Shrestha, R.; Kim, Y.W. Chlorin e6-associated photodynamic therapy enhances abscopal antitumor effects via inhibition of PD-1/PD-L1 immune checkpoint. Sci. Rep. 2023, 13, 4647. [Google Scholar] [CrossRef]
- Lobo, C.S.; Mendes, M.I.; Pereira, D.A.; Gomes-da-Silva, L.C.; Arnaut, L.G. Photodynamic therapy changes tumour immunogenicity and promotes immune-checkpoint blockade response, particularly when combined with micromechanical priming. Sci. Rep. 2023, 13, 11667. [Google Scholar] [CrossRef]
- Anand, S.; Shen, A.; Cheng, C.E.; Chen, J.; Powers, J.; Rayman, P.; Diaz, M.; Hasan, T.; Maytin, E.V. Combination of vitamin D and photodynamic therapy enhances immune responses in murine models of squamous cell skin cancer. Photodiagnosis Photodyn. Ther. 2024, 45, 103983. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Tait, S.W.G. Targeting immunogenic cell death in cancer. Mol. Oncol. 2020, 14, 2994–3006. [Google Scholar] [CrossRef] [PubMed]
- Nabrinsky, E.; Macklis, J.; Bitran, J. A Review of the Abscopal Effect in the Era of Immunotherapy. Cureus 2022, 14, e29620. [Google Scholar] [CrossRef]
- Aebisher, D.; Woźnicki, P.; Bartusik-Aebisher, D. Photodynamic Therapy and Adaptive Immunity Induced by Reactive Oxygen Species: Recent Reports. Cancers 2024, 16, 967. [Google Scholar] [CrossRef]
- Turubanova, V.D.; Balalaeva, I.V.; Mishchenko, T.A.; Catanzaro, E.; Alzeibak, R.; Peskova, N.N.; Efimova, I.; Bachert, C.; Mitroshina, E.V.; Krysko, O.; et al. Immunogenic cell death induced by a new photodynamic therapy based on photosens and photodithazine. J. Immunother. Cancer 2019, 7, 350. [Google Scholar] [CrossRef] [PubMed]
- Shibata, S.; Shinozaki, N.; Suganami, A.; Ikegami, S.; Kinoshita, Y.; Hasegawa, R.; Kentaro, H.; Okamoto, Y.; Aoki, I.; Tamura, Y.; et al. Photo-immune therapy with liposomally formulated phospholipid-conjugated indocyanine green induces specific antitumor responses with heat shock protein-70 expression in a glioblastoma model. Oncotarget 2019, 10, 175–183. [Google Scholar] [CrossRef]
- Pangal, D.J.; Yarovinsky, B.; Cardinal, T.; Cote, D.J.; Ruzevick, J.; Attenello, F.J.; Chang, E.L.; Ye, J.; Neman, J.; Chow, F.; et al. The abscopal effect: Systematic review in patients with brain and spine metastases. Neuro-Oncol. Adv. 2022, 4, vdac132. [Google Scholar] [CrossRef]
- Lin, R.A.; Lin, J.K.; Lin, S.Y. Mechanisms of immunogenic cell death and immune checkpoint blockade therapy. Kaohsiung J. Med. Sci. 2021, 37, 448–458. [Google Scholar] [CrossRef]
- Xu, J.; Yu, S.; Wang, X.; Qian, Y.; Wu, W.; Zhang, S.; Zheng, B.; Wei, G.; Gao, S.; Cao, Z.; et al. High Affinity of Chlorin e6 to Immunoglobulin G for Intraoperative Fluorescence Image-Guided Cancer Photodynamic and Checkpoint Blockade Therapy. ACS Nano 2019, 13, 10242–10260. [Google Scholar] [CrossRef]
- Yin, N.; Wang, Y.; Liu, Y.; Niu, R.; Zhang, S.; Cao, Y.; Lv, Z.; Song, S.; Liu, X.; Zhang, H. A Cholesterol Metabolic Regulated Hydrogen-Bonded Organic Framework (HOF)-Based Biotuner for Antibody Non-Dependent Immunotherapy Tailored for Glioblastoma. Adv. Mater. 2023, 35, e2303567. [Google Scholar] [CrossRef]
- Park, S.J.; Namkoong, H.; Doh, J.; Choi, J.C.; Yang, B.G.; Park, Y.; Chul Sung, Y. Negative role of inducible PD-1 on survival of activated dendritic cells. J. Leukoc. Biol. 2014, 95, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.; Hahn, M.; Michel, J.; Sankowski, R.; Kilian, M.; Kehl, N.; Günter, M.; Bunse, T.; Pusch, S.; von Deimling, A.; et al. Dysfunctional dendritic cells limit antigen-specific T cell response in glioma. Neuro Oncol. 2023, 25, 263–276. [Google Scholar] [CrossRef] [PubMed]
- Etminan, N.; Peters, C.; Lakbir, D.; Bünemann, E.; Börger, V.; Sabel, M.C.; Hänggi, D.; Steiger, H.J.; Stummer, W.; Sorg, R.V. Heat-shock protein 70-dependent dendritic cell activation by 5-aminolevulinic acid-mediated photodynamic treatment of human glioblastoma spheroids in vitro. Br. J. Cancer 2011, 105, 961–969. [Google Scholar] [CrossRef]
- Rothe, F.; Patties, I.; Kortmann, R.D.; Glasow, A. Immunomodulatory Effects by Photodynamic Treatment of Glioblastoma Cells In Vitro. Molecules 2022, 27, 3384. [Google Scholar] [CrossRef]
- Vedunova, M.; Turubanova, V.; Vershinina, O.; Savyuk, M.; Efimova, I.; Mishchenko, T.; Raedt, R.; Vral, A.; Vanhove, C.; Korsakova, D.; et al. DC vaccines loaded with glioma cells killed by photodynamic therapy induce Th17 anti-tumor immunity and provide a four-gene signature for glioma prognosis. Cell Death Dis. 2022, 13, 1062. [Google Scholar] [CrossRef]
- Redkin, T.S.; Sleptsova, E.E.; Turubanova, V.D.; Saviuk, M.O.; Lermontova, S.A.; Klapshina, L.G.; Peskova, N.N.; Balalaeva, I.V.; Krysko, O.; Mishchenko, T.A.; et al. Dendritic Cells Pulsed with Tumor Lysates Induced by Tetracyanotetra(aryl)porphyrazines-Based Photodynamic Therapy Effectively Trigger Anti-Tumor Immunity in an Orthotopic Mouse Glioma Model. Pharmaceutics 2023, 15, 2430. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, L.; Jia, M.; Liao, Q.; Peng, G.; Luo, G.; Zhou, Y. Dendritic cell vaccines improve the glioma microenvironment: Influence, challenges, and future directions. Cancer Med. 2023, 12, 7207–7221. [Google Scholar] [CrossRef]
- Tu, Z.; Li, K.; Ji, Q.; Huang, Y.; Lv, S.; Li, J.; Wu, L.; Huang, K.; Zhu, X. Pan-cancer analysis: Predictive role of TAP1 in cancer prognosis and response to immunotherapy. BMC Cancer 2023, 23, 133. [Google Scholar] [CrossRef]
- Satoh, E.; Mabuchi, T.; Satoh, H.; Asahara, T.; Nukui, H.; Naganuma, H. Reduced expression of the transporter associated with antigen processing 1 molecule in malignant glioma cells, and its restoration by interferon-gamma and -beta. J. Neurosurg. 2006, 104, 264–271. [Google Scholar] [CrossRef]
- Žilionytė, K.; Bagdzevičiūtė, U.; Mlynska, A.; Urbštaitė, E.; Paberalė, E.; Dobrovolskienė, N.; Krasko, J.A.; Pašukonienė, V. Correction to: Functional antigen processing and presentation mechanism as a prerequisite factor of response to treatment with dendritic cell vaccines and anti-PD-1 in preclinical murine LLC1 and GL261 tumor models. Cancer Immunol. Immunother. 2022, 71, 2701. [Google Scholar] [CrossRef]
- Yang, W.; Li, Y.; Gao, R.; Xiu, Z.; Sun, T. MHC class I dysfunction of glioma stem cells escapes from CTL-mediated immune response via activation of Wnt/beta-catenin signaling pathway. Oncogene 2020, 39, 1098–1111. [Google Scholar] [CrossRef] [PubMed]
- Yasinjan, F.; Xing, Y.; Geng, H.; Guo, R.; Yang, L.; Liu, Z.; Wang, H. Immunotherapy: A promising approach for glioma treatment. Front. Immunol. 2023, 14, 1255611. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Y.; Li, J.L.; Xu, X.K.; Zheng, M.G.; Wen, C.C.; Li, F.C. HMME-based PDT restores expression and function of transporter associated with antigen processing 1 (TAP1) and surface presentation of MHC class I antigen in human glioma. J. Neuro-Oncol. 2011, 105, 199–210. [Google Scholar] [CrossRef]
- Chen, L.Q.; Cheung, L.S.; Feng, L.; Tanner, W.; Frommer, W.B. Transport of sugars. Annu. Rev. Biochem. 2015, 84, 865–894. [Google Scholar] [CrossRef] [PubMed]
- Nabavizadeh, A.; Zhao, C.; Martinez, D.; Familiar, A.; Koptyra, M.; Ippolito, J.; Rubin, J.; Storm, P.B.; Resnick, A.C.; Santi, M. TMET-37. Sodium-Dependent Glucose Transporter 2 (SGLT2) is overexpressed in the majority of atrts and a subset of pediatric patients with high-grade glioma.; a potential imaging and therapeutic target. Neuro Oncol. 2022, 24, vii270. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.M.; Tian, R.F.; Liu, W.P.; Fei, Z.; Long, Q.F.; Wang, X.A.; Zhang, X. The expression and significance of HIF-1alpha and GLUT-3 in glioma. Brain Res. 2009, 1304, 149–154. [Google Scholar] [CrossRef]
- Jensen, R.L.; Chkheidze, R. The Role of Glucose Transporter-1 (GLUT-1) in Malignant Gliomas. In Tumors of the Central Nervous System, Volume 1. Tumors of the Central Nervous System; Hayat, M., Ed.; Springer: Dordrecht, The Netherlands, 2011; Volume 1. [Google Scholar]
- Han, W.; Shi, J.; Cao, J.; Dong, B.; Guan, W. Emerging Roles and Therapeutic Interventions of Aerobic Glycolysis in Glioma. Onco Targets Ther. 2020, 13, 6937–6955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, Z.; Blessington, D.; Li, H.; Busch, T.M.; Madrak, V.; Miles, J.; Chance, B.; Glickson, J.D.; Zheng, G. Pyropheophorbide 2-deoxyglucosamide: A new photosensitizer targeting glucose transporters. Bioconjug Chem. 2003, 14, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, K.; Huang, B.; Lin, Z.; Cai, Z. Role of Glucose Transporters in Drug Membrane Transport. Curr. Drug Metab. 2020, 21, 947–958. [Google Scholar] [CrossRef]
- Tanaka, M.; Kataoka, H.; Yano, S.; Ohi, H.; Moriwaki, K.; Akashi, H.; Taguchi, T.; Hayashi, N.; Hamano, S.; Mori, Y.; et al. Antitumor effects in gastrointestinal stromal tumors using photodynamic therapy with a novel glucose-conjugated chlorin. Mol. Cancer Ther. 2014, 13, 767–775. [Google Scholar] [CrossRef]
- Nishie, H.; Kataoka, H.; Yano, S.; Kikuchi, J.I.; Hayashi, N.; Narumi, A.; Nomoto, A.; Kubota, E.; Joh, T. A next-generation bifunctional photosensitizer with improved water-solubility for photodynamic therapy and diagnosis. Oncotarget 2016, 7, 74259–74268. [Google Scholar] [CrossRef] [PubMed]
- do Carmo, A.; Balça-Silva, J.; Matias, D.; Lopes, M.C. PKC signaling in glioblastoma. Cancer Biol. Ther. 2013, 14, 287–294. [Google Scholar] [CrossRef]
- Couldwell, W.T.; Uhm, J.H.; Antel, J.P.; Yong, V.W. Enhanced protein kinase C activity correlates with the growth rate of malignant gliomas in vitro. Neurosurgery 1991, 29, 880–886; discussion 886–887. [Google Scholar] [CrossRef] [PubMed]
- Couldwell, W.T.; Antel, J.P.; Yong, V.W. Protein kinase C activity correlates with the growth rate of malignant gliomas: Part II. Effects of glioma mitogens and modulators of protein kinase C. Neurosurgery 1992, 31, 717–724; discussion 724. [Google Scholar] [CrossRef] [PubMed]
- Wild, P.J.; Krieg, R.C.; Seidl, J.; Stoehr, R.; Reher, K.; Hofmann, C.; Louhelainen, J.; Rosenthal, A.; Hartmann, A.; Pilarsky, C.; et al. RNA expression profiling of normal and tumor cells following photodynamic therapy with 5-aminolevulinic acid-induced protoporphyrin IX in vitro. Mol. Cancer Ther. 2005, 4, 516–528. [Google Scholar] [CrossRef] [PubMed]
- Huntosova, V.; Stroffekova, K. Hypericin in the Dark: Foe or Ally in Photodynamic Therapy? Cancers 2016, 8, 93. [Google Scholar] [CrossRef]
- Takahashi, I.; Nakanishi, S.; Kobayashi, E.; Nakano, H.; Suzuki, K.; Tamaoki, T. Hypericin and pseudohypericin specifically inhibit protein kinase C: Possible relation to their antiretroviral activity. Biochem. Biophys. Res. Commun. 1989, 165, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Uzdensky, A.; Kristiansen, B.; Moan, J.; Juzeniene, A. Dynamics of signaling, cytoskeleton and cell cycle regulation proteins in glioblastoma cells after sub-lethal photodynamic treatment: Antibody microarray study. Biochim. Biophys. Acta 2012, 1820, 795–803. [Google Scholar] [CrossRef]
- Dzurová, L.; Petrovajova, D.; Nadova, Z.; Huntosova, V.; Miskovsky, P.; Stroffekova, K. The role of anti-apoptotic protein kinase Cα in response to hypericin photodynamic therapy in U-87 MG cells. Photodiagnosis Photodyn. Ther. 2014, 11, 213–226. [Google Scholar] [CrossRef]
- Pevna, V.; Wagnières, G.; Huntosova, V. Autophagy and Apoptosis Induced in U87 MG Glioblastoma Cells by Hypericin-Mediated Photodynamic Therapy Can Be Photobiomodulated with 808 nm Light. Biomedicines 2021, 9, 1703. [Google Scholar] [CrossRef]
- Jiang, F.; Cho, K.K.; Mikkelse, T.; Tong, L.; Lew, Y.S.; Hochbaum, N.; Shargorodsky, J.; Chop, M. Tamoxifen increases photodynamic therapeutic response of U87 and U25ln human glioma cells. J. Neuro-Oncol. 2002, 56, 51–58. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, R.; Yang, S.H.; Yuan, F. Chemotherapeutic effect of tamoxifen on temozolomide-resistant gliomas. Anticancer Drugs 2015, 26, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; DiBiase, S.; Meisenberg, B.; Flannery, T.; Patel, A.; Dhople, A.; Cheston, S.; Amin, P. Phase I clinical trial assessing temozolomide and tamoxifen with concomitant radiotherapy for treatment of high-grade glioma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Misuth, M.; Horvath, D.; Miskovsky, P.; Huntosova, V. Synergism between PKCδ regulators hypericin and rottlerin enhances apoptosis in U87 MG glioma cells after light stimulation. Photodiagnosis Photodyn. Ther. 2017, 18, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.C.; Kim, S.H.; Jeong, M.W.; Baek, N.I.; Kim, K.T. Effect of rottlerin, a PKC-delta inhibitor, on TLR-4-dependent activation of murine microglia. Biochem. Biophys. Res. Commun. 2005, 337, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Song, K.S.; Kim, J.S.; Yun, E.J.; Kim, Y.R.; Seo, K.S.; Park, J.H.; Jung, Y.J.; Park, J.I.; Kweon, G.R.; Yoon, W.H.; et al. Rottlerin induces autophagy and apoptotic cell death through a PKC-delta-independent pathway in HT1080 human fibrosarcoma cells: The protective role of autophagy in apoptosis. Autophagy 2008, 4, 650–658. [Google Scholar] [CrossRef]
- Lim, J.H.; Park, J.W.; Choi, K.S.; Park, Y.B.; Kwon, T.K. Rottlerin induces apoptosis via death receptor 5 (DR5) upregulation through CHOP-dependent and PKC delta-independent mechanism in human malignant tumor cells. Carcinogenesis 2009, 30, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Abounader, R.; Laterra, J. Scatter factor/hepatocyte growth factor in brain tumor growth and angiogenesis. Neuro Oncol. 2005, 7, 436–451. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.F.; Wang, X.B.; Tian, X.Y.; Li, Y.; Li, B.; Huang, Q.; Zhang, M.; Li, Z. Tumor-derived hepatocyte growth factor is associated with poor prognosis of patients with glioma and influences the chemosensitivity of glioma cell line to cisplatin in vitro. World J. Surg. Oncol. 2012, 10, 128. [Google Scholar] [CrossRef]
- Vogel, S.; Peters, C.; Etminan, N.; Börger, V.; Schimanski, A.; Sabel, M.C.; Sorg, R.V. Migration of mesenchymal stem cells towards glioblastoma cells depends on hepatocyte-growth factor and is enhanced by aminolaevulinic acid-mediated photodynamic treatment. Biochem. Biophys. Res. Commun. 2013, 431, 428–432. [Google Scholar] [CrossRef]
- Matsumoto, K.; Umitsu, M.; De Silva, D.M.; Roy, A.; Bottaro, D.P. Hepatocyte growth factor/MET in cancer progression and biomarker discovery. Cancer Sci. 2017, 108, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.H.; Kim, Y.K.; Kim, M.R.; Jang, J.H.; Lee, S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef] [PubMed]
- Mäenpää, A.; Kovanen, P.E.; Paetau, A.; Jäáskeläinen, J.; Timonen, T. Lymphocyte adhesion molecule ligands and extracellular matrix proteins in gliomas and normal brain: Expression of VCAM-1 in gliomas. Acta Neuropathol. 1997, 94, 216–225. [Google Scholar] [CrossRef]
- Cheng, V.W.T.; de Pennington, N.; Zakaria, R.; Larkin, J.R.; Serres, S.; Sarkar, M.; Kirkman, M.A.; Bristow, C.; Croal, P.; Plaha, P.; et al. VCAM-1-targeted MRI Improves Detection of the Tumor-brain Interface. Clin. Cancer Res. 2022, 28, 2385–2396. [Google Scholar] [CrossRef]
- Zhan, Q.; Yue, W.; Hu, S. The inhibitory effect of photodynamic therapy and of an anti-VCAM-1 monoclonal antibody on the in vivo growth of C6 glioma xenografts. Braz. J. Med. Biol. Res. 2011, 44, 489–496. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Volanti, C.; Gloire, G.; Vanderplasschen, A.; Jacobs, N.; Habraken, Y.; Piette, J. Downregulation of ICAM-1 and VCAM-1 expression in endothelial cells treated by photodynamic therapy. Oncogene 2004, 23, 8649–8658. [Google Scholar] [CrossRef]
- Kawczyk-Krupka, A.; Czuba, Z.P.; Kwiatek, B.; Kwiatek, S.; Krupka, M.; Sieroń, K. The effect of ALA-PDT under normoxia and cobalt chloride (CoCl2)-induced hypoxia on adhesion molecules (ICAM-1, VCAM-1) secretion by colorectal cancer cells. Photodiagnosis Photodyn. Ther. 2017, 19, 103–115. [Google Scholar] [CrossRef]
- Middeldorp, J.; Hol, E.M. GFAP in health and disease. Prog. Neurobiol. 2011, 93, 421–443. [Google Scholar] [CrossRef]
- van Asperen, J.V.; Robe, P.A.J.T.; Hol, E.M. GFAP Alternative Splicing and the Relevance for Disease—A Focus on Diffuse Gliomas. ASN Neuro 2022, 14, 17590914221102065. [Google Scholar] [CrossRef]
- van Bodegraven, E.J.; van Asperen, J.V.; Robe, P.A.J.; Hol, E.M. Importance of GFAP isoform-specific analyses in astrocytoma. Glia 2019, 67, 1417–1433. [Google Scholar] [CrossRef]
- Uceda-Castro, R.; van Asperen, J.V.; Vennin, C.; Sluijs, J.A.; van Bodegraven, E.J.; Margarido, A.S.; Robe, P.A.J.; van Rheenen, J.; Hol, E.M. GFAP splice variants fine-tune glioma cell invasion and tumour dynamics by modulating migration persistence. Sci. Rep. 2022, 12, 424. [Google Scholar] [CrossRef] [PubMed]
- Namatame, H.; Akimoto, J.; Matsumura, H.; Haraoka, J.; Aizawa, K. Photodynamic therapy of C6-implanted glioma cells in the rat brain employing second-generation photosensitizer talaporfin sodium. Photodiagnosis Photodyn. 2008, 5, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.; Sakai, N.; Yamada, H.; Niikawa, S.; Ohno, T.; Tanaka, T.; Mori, H. Proliferative assessment of GFAP-positive and GFAP-negative glioma cells by nucleolar organizer region staining. Surg. Neurol. 1991, 36, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.; Hirayama, H.; Sakai, N.; Yamada, H.; Tanaka, T.; Mori, H. Correlation between nucleolar organizer region staining and Ki-67 immunostaining in human gliomas. Surg. Neurol. 1990, 33, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Harmelin, A.; Zuckerman, A.; Nyska, A. Correlation of Ag-NOR protein measurements with prognosis in canine transmissible venereal tumour. J. Comp. Pathol. 1995, 112, 429–433. [Google Scholar] [CrossRef]
- Arden, K.C.; Pathak, S.; Frankel, L.S.; Zander, A. Ag-NOR staining in human chromosomes: Differential staining in normal and leukemic bone-marrow samples. Int. J. Cancer 1985, 36, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, K.; Chan, F.K. RIP3: A molecular switch for necrosis and inflammation. Genes. Dev. 2013, 27, 1640–1649. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, T.; Lei, T.; Zhang, D.; Du, S.; Girani, L.; Qi, D.; Lin, C.; Tong, R.; Wang, Y. RIP1/RIP3-regulated necroptosis as a target for multifaceted disease therapy (Review). Int. J. Mol. Med. 2019, 44, 771–786. [Google Scholar] [CrossRef]
- Wang, P.; Zheng, S.Y.; Jiang, R.L.; Wu, H.D.; Li, Y.A.; Lu, J.L.; Xiong, Y.; Han, B.; Lin, L. Necroptosis signaling and mitochondrial dysfunction cross-talking facilitate cell death mediated by chelerythrine in glioma. Free Radic. Biol. Med. 2023, 202, 76–96. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, W.; Zhang, Y.; Fu, X.; Ping, K.; Zhao, J.; Lei, Y.; Mou, Y.; Wang, S. Synthesis and biological evaluation of celastrol derivatives as potential anti-glioma agents by activating RIP1/RIP3/MLKL pathway to induce necroptosis. Eur. J. Med. Chem. 2022, 229, 114070. [Google Scholar] [CrossRef]
- Zhou, J.; Li, G.; Han, G.; Feng, S.; Liu, Y.; Chen, J.; Liu, C.; Zhao, L.; Jin, F. Emodin induced necroptosis in the glioma cell line U251 via the TNF-α/RIP1/RIP3 pathway. Investig. New Drugs 2020, 38, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; McDonald, D.G.; Dixon-Mah, Y.N.; Jacqmin, D.J.; Samant, V.N.; Vandergrift, W.A., 3rd; Lindhorst, S.M.; Cachia, D.; Varma, A.K.; Vanek, K.N.; et al. RIP1 and RIP3 complex regulates radiation-induced programmed necrosis in glioblastoma. Tumour Biol. 2016, 37, 7525–7534. [Google Scholar] [CrossRef] [PubMed]
- Coupienne, I.; Fettweis, G.; Rubio, N.; Agostinis, P.; Piette, J. 5-ALA-PDT induces RIP3-dependent necrosis in glioblastoma. Photochem. Photobiol. Sci. 2011, 10, 1868–1878. [Google Scholar] [CrossRef] [PubMed]
- Fettweis, G.; Di Valentin, E.; L’homme, L.; Lassence, C.; Dequiedt, F.; Fillet, M.; Coupienne, I.; Piette, J. RIP3 antagonizes a TSC2-mediated pro-survival pathway in glioblastoma cell death. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Fan, X.-Y.; Wang, A.-D.; Xia, Y.-Z.; Fu, W.-R.; Liu, J.-Y.; Jiang, F.-L.; Liu, Y. LDHA Suppression Altering Metabolism Inhibits Tumor Progress by an Organic Arsenical. Int. J. Mol. Sci. 2019, 20, 6239. [Google Scholar] [CrossRef] [PubMed]
- Tonissen, K.F.; Di Trapani, G. Thioredoxin system inhibitors as mediators of apoptosis for cancer therapy. Mol. Nutr. Food Res. 2009, 53, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.P.; Bai, L.R.; Lv, Y.F.; Zhou, Y.; Ding, C.H.; Yang, S.M.; Zhang, F.; Huang, J.L. A novel role of Cx43-composed GJIC in PDT phototoxicity: An implication of Cx43 for the enhancement of PDT efficacy. Int. J. Biol. Sci. 2019, 15, 598–609. [Google Scholar] [CrossRef] [PubMed]
- An, Y.W.; Liu, H.Q.; Zhou, Z.Q.; Wang, J.C.; Jiang, G.Y.; Li, Z.W.; Wang, F.; Jin, H.T. Sinoporphyrin sodium is a promising sensitizer for photodynamic and sonodynamic therapy in glioma. Oncol. Rep. 2020, 44, 1596–1604. [Google Scholar] [CrossRef]
- Li, F.; Cheng, Y.; Lu, J.; Hu, R.; Wan, Q.; Feng, H. Photodynamic therapy boosts anti-glioma immunity in mice: A dependence on the activities of T cells and complement C3. J. Cell. Biochem. 2011, 112, 3035–3043. [Google Scholar] [CrossRef]
- Saadeh, F.S.; Mahfouz, R.; Assi, H.I. EGFR as a clinical marker in glioblastomas and other gliomas. Int. J. Biol. Markers 2018, 33, 22–32. [Google Scholar] [CrossRef]
- Brennan, C.W.; Verhaak, R.G.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The somatic genomic landscape of glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef] [PubMed]
- Oprita, A.; Baloi, S.C.; Staicu, G.A.; Alexandru, O.; Tache, D.E.; Danoiu, S.; Micu, E.S.; Sevastre, A.S. Updated Insights on EGFR Signaling Pathways in Glioma. Int. J. Mol. Sci. 2021, 22, 587. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.E.; Weyergang, A.; Edwards, V.T.; Berg, K.; Brech, A.; Weisheit, S.; Høgset, A.; Selbo, P.K. Development of resistance to photodynamic therapy (PDT) in human breast cancer cells is photosensitizer-dependent: Possible mechanisms and approaches for overcoming PDT-resistance. Biochem. Pharmacol. 2017, 144, 63–77. [Google Scholar] [CrossRef]
- Fanuel-Barret, D.; Patrice, T.; Foultier, M.T.; Vonarx-Coinsmann, V.; Robillard, N.; Lajat, Y. Influence of epidermal growth factor on photodynamic therapy of glioblastoma cells in vitro. Res. Exp. Med. 1997, 197, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.W.; Hung, M.C.; Hsieh, C.Y.; Tung, E.C.; Wang, Y.H.; Tsai, J.C.; Lee, J.M. The effects of Photofrin-mediated photodynamic therapy on the modulation of EGFR in esophageal squamous cell carcinoma cells. Lasers Med. Sci. 2013, 28, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.; Ji, H.T.; Chiang, P.C.; Chou, R.H.; Chang, W.S.; Chen, C.T. ALA-PDT results in phenotypic changes and decreased cellular invasion in surviving cancer cells. Lasers Surg. Med. 2009, 41, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Ulfo, L.; Costantini, P.E.; Di Giosia, M.; Danielli, A.; Calvaresi, M. EGFR-Targeted Photodynamic Therapy. Pharmaceutics 2022, 14, 241. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.J.; Niu, C.J.; Lai, B.; Chen, Y.; Kuta, V.; Lilge, L.D. Modulation of PPIX synthesis and accumulation in various normal and glioma cell lines by modification of the cellular signaling and temperature. Lasers Surg. Med. 2013, 45, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.; Obaid, G.; Niu, C.; Foltz, W.; Goldstein, A.; Hasan, T.; Lilge, L. Liposomal Lapatinib in Combination with Low-Dose Photodynamic Therapy for the Treatment of Glioma. J. Clin. Med. 2019, 8, 2214. [Google Scholar] [CrossRef]
- Abourehab, M.A.S.; Alqahtani, A.M.; Youssif, B.G.M.; Gouda, A.M. Globally Approved EGFR Inhibitors: Insights into Their Syntheses, Target Kinases, Biological Activities, Receptor Interactions, and Metabolism. Molecules 2021, 26, 6677. [Google Scholar] [CrossRef]
- Rodriguez, S.M.B.; Kamel, A.; Ciubotaru, G.V.; Onose, G.; Sevastre, A.S.; Sfredel, V.; Danoiu, S.; Dricu, A.; Tataranu, L.G. An Overview of EGFR Mechanisms and Their Implications in Targeted Therapies for Glioblastoma. Int. J. Mol. Sci. 2023, 24, 11110. [Google Scholar] [CrossRef] [PubMed]
- Jamali, Z.; Khoobi, M.; Hejazi, S.M.; Eivazi, N.; Abdolahpour, S.; Imanparast, F.; Moradi-Sardareh, H.; Paknejad, M. Evaluation of targeted curcumin (CUR) loaded PLGA nanoparticles for in vitro photodynamic therapy on human glioblastoma cell line. Photodiagnosis Photodyn. Ther. 2018, 23, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Meyers, J.D.; Cheng, Y.; Broome, A.M.; Agnes, R.S.; Schluchter, M.D.; Margevicius, S.; Wang, X.; Kenney, M.E.; Burda, C.; Basilion, J.P. Peptide-Targeted Gold Nanoparticles for Photodynamic Therapy of Brain Cancer. Part. Part. Syst. Charact. 2015, 32, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Chen, L.; Sun, K.; Khan, A.A.; Yan, J.; Liu, H.; Lu, A.; Gu, N. Neuropilin-1 (NRP-1)/GIPC1 pathway mediates glioma progression. Tumor Biol. 2016, 37, 13777–13788. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Miao, W.; Tang, X.; Zhang, H.; Wang, S.; Luo, F.; Yan, J. The expression and significance of neuropilin-1 (NRP-1) on glioma cell lines and glioma tissues. J. Biomed. Nanotechnol. 2013, 9, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Miao, W.; Tang, X.; Zhang, H.; Wang, S.; Luo, F.; Yan, J. Inhibitory effect of neuropilin-1 monoclonal antibody (NRP-1 MAb) on glioma tumor in mice. J. Biomed. Nanotechnol. 2013, 9, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Caponegro, M.D.; Moffitt, R.A.; Tsirka, S.E. Expression of neuropilin-1 is linked to glioma associated microglia and macrophages and correlates with unfavorable prognosis in high grade gliomas. Oncotarget 2018, 9, 35655–35665. [Google Scholar] [CrossRef]
- Thomas, N.; Tirand, L.; Chatelut, E.; Plénat, F.; Frochot, C.; Dodeller, M.; Guillemin, F.; Barberi-Heyob, M. Tissue distribution and pharmacokinetics of an ATWLPPR-conjugated chlorin-type photosensitizer targeting neuropilin-1 in glioma-bearing nude mice. Photochem. Photobiol. Sci. 2008, 7, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Tirand, L.; Frochot, C.; Vanderesse, R.; Thomas, N.; Trinquet, E.; Pinel, S.; Viriot, M.L.; Guillemin, F.; Barberi-Heyob, M. A peptide competing with VEGF165 binding on neuropilin-1 mediates targeting of a chlorin-type photosensitizer and potentiates its photodynamic activity in human endothelial cells. J. Control. Release 2006, 111, 153–164. [Google Scholar] [CrossRef]
- Bechet, D.; Auger, F.; Couleaud, P.; Marty, E.; Ravasi, L.; Durieux, N.; Bonnet, C.; Plénat, F.; Frochot, C.; Mordon, S.; et al. Multifunctional ultrasmall nanoplatforms for vascular-targeted interstitial photodynamic therapy of brain tumors guided by real-time MRI. Nanomedicine 2015, 11, 657–670. [Google Scholar] [CrossRef]
- Gries, M.; Thomas, N.; Daouk, J.; Rocchi, P.; Choulier, L.; Jubréaux, J.; Pierson, J.; Reinhard, A.; Jouan-Hureaux, V.; Chateau, A.; et al. Multiscale Selectivity and in vivo Biodistribution of NRP-1-Targeted Theranostic AGuIX Nanoparticles for PDT of Glioblastoma. Int. J. Nanomed. 2020, 15, 8739–8758. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Colombeau, L.; Gries, M.; Peterlini, T.; Mathieu, C.; Thomas, N.; Boura, C.; Frochot, C.; Vanderesse, R.; Lux, F.; et al. Ultrasmall AGuIX theranostic nanoparticles for vascular-targeted interstitial photodynamic therapy of glioblastoma. Int. J. Nanomed. 2017, 12, 7075–7088. [Google Scholar] [CrossRef] [PubMed]
- Tirand, L.; Bastogne, T.; Bechet, D.; Linder, M.; Thomas, N.; Frochot, C.; Guillemin, F.; Barberi-Heyob, M. Response surface methodology: An extensive potential to optimize in vivo photodynamic therapy conditions. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Bechet, D.; Becuwe, P.; Tirand, L.; Vanderesse, R.; Frochot, C.; Guillemin, F.; Barberi-Heyob, M. Peptide-conjugated chlorin-type photosensitizer binds neuropilin-1 in vitro and in vivo. J. Photochem. Photobiol. B Biol. 2009, 96, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhao, X.; Fu, T.; Li, K.; He, Y.; Luo, Z.; Dai, L.; Zeng, R.; Cai, K. An iRGD-conjugated prodrug micelle with blood-brain-barrier penetrability for anti-glioma therapy. Biomaterials 2020, 230, 119666. [Google Scholar] [CrossRef]
- Takada, Y.; Ye, X.; Simon, S. The integrins. Genome Biol. 2007, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wu, Q.; Dong, Z.; Liu, K. Integrins in cancer: Emerging mechanisms and therapeutic opportunities. Pharmacol. Ther. 2023, 247, 108458. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, V.P.; Moura Neto, V.; Mentlein, R. Glioma infiltration and extracellular matrix: Key players and modulators. Glia 2018, 66, 1542–1565. [Google Scholar] [CrossRef] [PubMed]
- Di Venosa, G.; Perotti, C.; Batlle, A.; Casas, A. The role of cytoskeleton and adhesion proteins in the resistance to photodynamic therapy. Possible therapeutic interventions. Photochem. Photobiol. Sci. 2015, 14, 1451–1464. [Google Scholar] [CrossRef]
- Pacheco Soares, C.; Maftou Costa, M.; da Cunha Menezes Costa, C.G.; de Siqueira Silva, A.C.; Moraes, K.C. Evaluation of photodynamic therapy in adhesion protein expression. Oncol. Lett. 2014, 8, 714–718. [Google Scholar] [CrossRef]
- Slack, R.J.; Macdonald, S.J.F.; Roper, J.A.; Jenkins, R.G.; Hatley, R.J.D. Emerging therapeutic opportunities for integrin inhibitors. Nat. Rev. Drug Discov. 2022, 21, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zhou, F.; Zhang, D.; Chen, Q.; Xing, D. A graphene oxide based smart drug delivery system for tumor mitochondria-targeting photodynamic therapy. Nanoscale 2016, 8, 3530–3538. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, M.; Lu, C.; Pons, J.; Maheswaran, I.; Ciborowski, P.; Zhang, L.; Cheema, A.; Kitlinska, J. Neuropeptide Y receptor interactions regulate its mitogenic activity. Neuropeptides 2019, 73, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Körner, M.; Reubi, J.C. Neuropeptide Y receptors in primary human brain tumors: Overexpression in high-grade tumors. J. Neuropathol. Exp. Neurol. 2008, 67, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, X.; Wang, P.; Yuan, B.; Pan, Y.; Hu, X.; Lu, L.; Wu, A.; Li, J. A D-Y Shaped Neuropeptide Y Mimetic Peptide-Dye Self-Assembly with Maximal Emission Beyond 1300 nm and Glioma Mitochondrial Activity Modulation. Small 2024, 20, e2308621. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Du, Y.; Jiang, Z.; Tian, Y.; Qiu, N.; Wang, Y.; Lqbal, M.Z.; Hu, M.; Zou, R.; Luo, L.; et al. Y1 receptor ligand-based nanomicelle as a novel nanoprobe for glioma-targeted imaging and therapy. Nanoscale 2018, 10, 5845–5851. [Google Scholar] [CrossRef]
- He, X.; Luo, Y.; Li, Y.; Pan, Y.; Kwok, R.T.K.; He, L.; Duan, X.; Zhang, P.; Wu, A.; Tang, B.Z.; et al. D-type neuropeptide decorated AIEgen/RENP hybrid nanoprobes with light-driven ROS generation ability for NIR-II fluorescence imaging-guided through-skull photodynamic therapy of gliomas. Aggregate 2023, 5, e396. [Google Scholar] [CrossRef]
- Maletínská, L.; Blakely, E.A.; Bjornstad, K.A.; Deen, D.F.; Knoff, L.J.; Forte, T.M. Human glioblastoma cell lines: Levels of low-density lipoprotein receptor and low-density lipoprotein receptor-related protein. Cancer Res. 2000, 60, 2300–2303. [Google Scholar] [PubMed]
- Han, L.; Jiang, C. Evolution of blood-brain barrier in brain diseases and related systemic nanoscale brain-targeting drug delivery strategies. Acta Pharm. Sin. B 2021, 11, 2306–2325. [Google Scholar] [CrossRef]
- Pawar, S.; Koneru, T.; McCord, E.; Tatiparti, K.; Sau, S.; Iyer, A.K. LDL receptors and their role in targeted therapy for glioma: A review. Drug Discov. Today 2021, 26, 1212–1225. [Google Scholar] [CrossRef]
- Buzova, D.; Huntosova, V.; Kasak, P.; Petrovajova, D.; Joniova, J.; Dzurova, L.; Nadova, Z.; Sureau, F.; Miskovsky, P.; Jancura, D. Towards increased selectivity of drug delivery to cancer cells: Development of a LDL-based nanodelivery system for hydrophobic photosensitizers. In Biosensing and Nanomedicine V, Proceedings of the SPIE NANOSCIENCE + ENGINEERING, San Diego, CA, USA, 12–16 August 2012; Mohseni, H., Agahi, M., Razeghi, M., Eds.; SPIE: Bellingham, WA, USA, 2012; Volume 8460, p. 8460. [Google Scholar]
- Huntosova, V.; Alvarez, L.; Bryndzova, L.; Nadova, Z.; Jancura, D.; Buriankova, L.; Bonneau, S.; Brault, D.; Miskovsky, P.; Sureau, F. Interaction dynamics of hypericin with low-density lipoproteins and U87-MG cells. Int. J. Pharm. 2010, 389, 32–40. [Google Scholar] [CrossRef]
- Luiza Andreazza, N.; Vevert-Bizet, C.; Bourg-Heckly, G.; Sureau, F.; José Salvador, M.; Bonneau, S. Berberine as a photosensitizing agent for antitumoral photodynamic therapy: Insights into its association to low density lipoproteins. Int. J. Pharm. 2016, 510, 240–249. [Google Scholar] [CrossRef]
- Huntosova, V.; Nadova, Z.; Dzurova, L.; Jakusova, V.; Sureau, F.; Miskovsky, P. Cell death response of U87 glioma cells on hypericin photoactivation is mediated by dynamics of hypericin subcellular distribution and its aggregation in cellular organelles. Photochem. Photobiol. Sci. 2012, 11, 1428–1436. [Google Scholar] [CrossRef]
- Song, L.; Li, H.; Sunar, U.; Chen, J.; Corbin, I.; Yodh, A.G.; Zheng, G. Naphthalocyanine-reconstituted LDL nanoparticles for in vivo cancer imaging and treatment. Int. J. Nanomed. 2007, 2, 767–774. [Google Scholar]
- Acker, G.; Palumbo, A.; Neri, D.; Vajkoczy, P.; Czabanka, M. F8-SIP mediated targeted photodynamic therapy leads to microvascular dysfunction and reduced glioma growth. J. Neuro-Oncol. 2016, 129, 33–38. [Google Scholar] [CrossRef]
- De Groof, T.W.M.; Mashayekhi, V.; Fan, T.S.; Bergkamp, N.D.; Sastre Toraño, J.; van Senten, J.R.; Heukers, R.; Smit, M.J.; Oliveira, S. Nanobody-Targeted Photodynamic Therapy Selectively Kills Viral GPCR-Expressing Glioblastoma Cells. Mol. Pharm. 2019, 16, 3145–3156. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, H.; Liu, Y.; Wen, Y.; Wei, C.; Yu, Q.; Liu, J. Transferrin/aptamer conjugated mesoporous ruthenium nanosystem for redox-controlled and targeted chemo-photodynamic therapy of glioma. Acta Biomater. 2018, 82, 143–157. [Google Scholar] [CrossRef]
- Akhlynina, T.V.; Jans, D.A.; Statsyuk, N.V.; Balashova, I.Y.; Toth, G.; Pavo, I.; Rosenkranz, A.A.; Naroditsky, B.S.; Sobolev, A.S. Adenoviruses synergize with nuclear localization signals to enhance nuclear delivery and photodynamic action of internalizable conjugates containing chlorin e6. Int. J. Cancer 1999, 81, 734–740. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, P.; Huang, Z.; Chen, X.; Yan, X.; Zhai, J.; Ma, Y. Evaluation of curcumin-mediated photodynamic therapy on the reverse of multidrug resistance in tumor cells. RSC Adv. 2020, 10, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Martins, W.K.; Belotto, R.; Silva, M.N.; Grasso, D.; Suriani, M.D.; Lavor, T.S.; Itri, R.; Baptista, M.S.; Tsubone, T.M. Autophagy Regulation and Photodynamic Therapy: Insights to Improve Outcomes of Cancer Treatment. Front. Oncol. 2021, 10, 610472. [Google Scholar] [CrossRef] [PubMed]
- Corthay, A.; Bakacs, T.; Thangavelu, G.; Anderson, C.C. Tackling cancer cell dormancy: Insights from immune models, and transplantation. Semin. Cancer Biol. 2022, 78, 5–16. [Google Scholar] [CrossRef]
- Bunse, L.; Bunse, T.; Krämer, C.; Chih, Y.C.; Platten, M. Clinical and Translational Advances in Glioma Immunotherapy. Neurotherapeutics 2022, 19, 1799–1817. [Google Scholar] [CrossRef] [PubMed]
- Wood, M.D.; Halfpenny, A.M.; Moore, S.R. Applications of molecular neuro-oncology—A review of diffuse glioma integrated diagnosis and emerging molecular entities. Diagn. Pathol. 2019, 14, 29. [Google Scholar] [CrossRef]
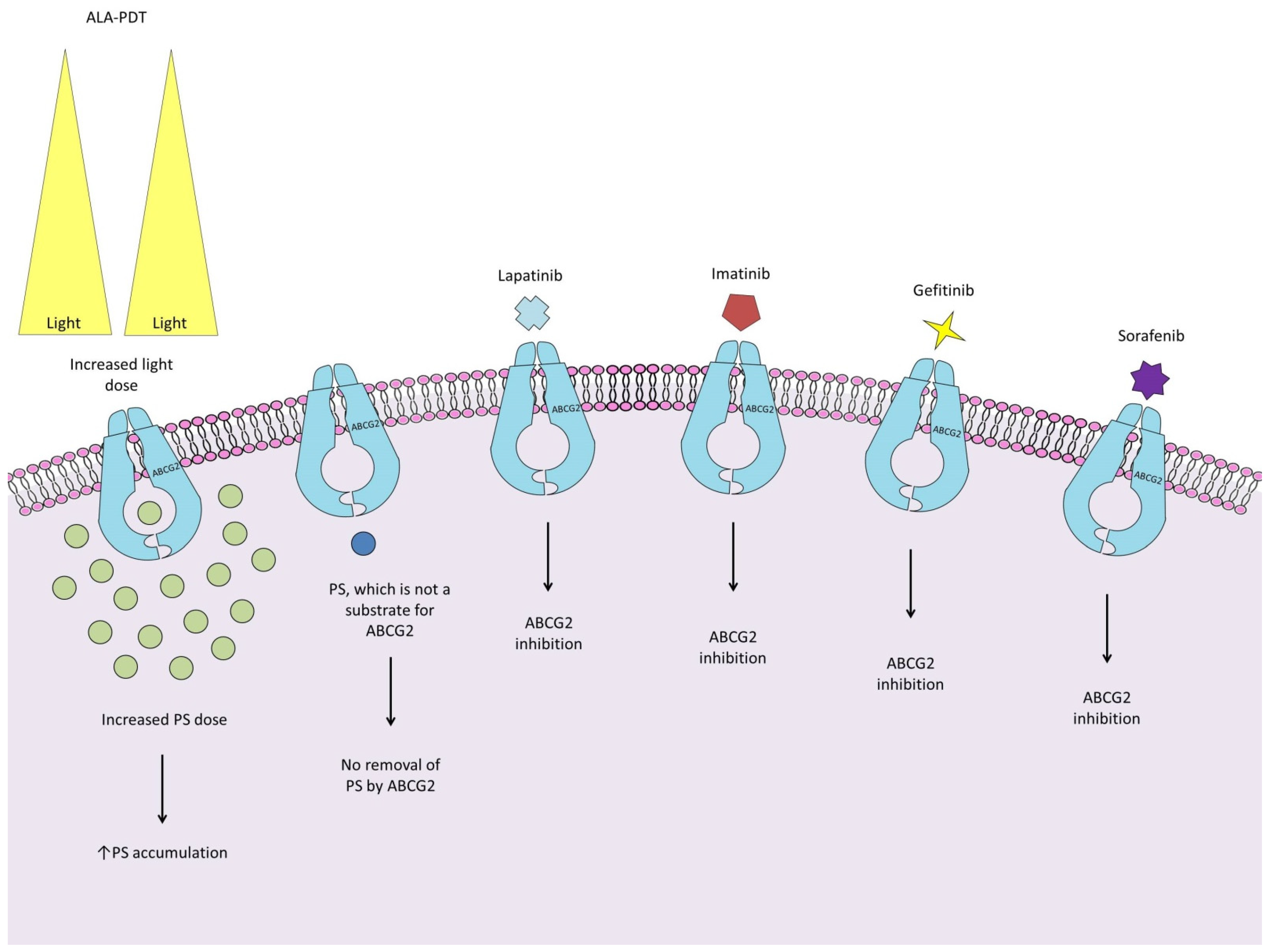
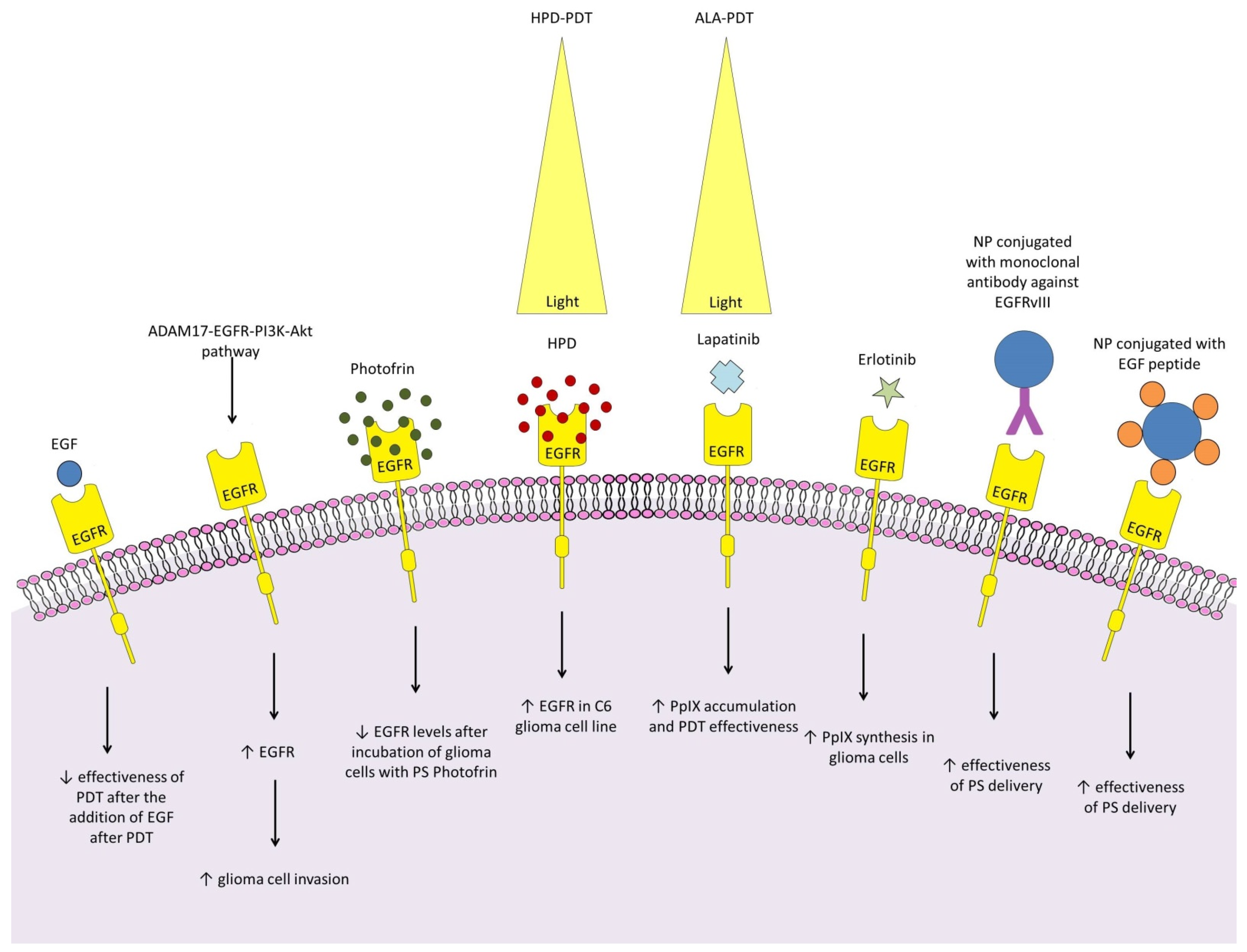
| Neurotransmitter | NS Levels | Receptor Expression | Impact on the Effectiveness of PDT | Tested Therapeutic Approach |
|---|---|---|---|---|
| NO | Increase [87,88,89] | No data available | Increased resistance, survival, and migration of glioma cells after PDT [86,87,88,89] | Association of PDT with iNOS inhibitors reduces NO levels and glioma cell growth and invasion after PDT [88] |
| Glutamate | Increase [163] | Increase in AMPAR expression [163,165] | Increase in apoptosis of glioma cells [163,165] | No data available |
| GABA | No data available | Increase in PBR expression [172] | Increased production of PpIX and phototoxic effect against glioma cells (via increase in PBR) [172] | Association of PDT with diazepam enhances apoptosis of glioma cells [174] |
| Neuropeptide Y | No data available | No data available | No data available | NP coupling with NPY improves targeting and PS delivery in the glioma [311] |
| Molecular Target | Targeting Method | Article |
|---|---|---|
| EGFR | Monoclonal antibody against EGFRvIII | Jamali et al. [286] |
| EGFR | EGF peptide | Meyers, J.D. et al. [287] |
| Neuropilin-1 | ATWLPPR heptapeptide targeting NRP-1 | Thomas et al. [292] Tirand et al. [293] Bechet et al. [294] Tirand et al. [297] Thomas et al. [298] |
| Neuropilin-1 | KDKPPR peptide targeting NRP-1 | Gries et al. [295] Thomas et al. [296] |
| Neuropilin-1 Integrin αvβ3 Integrin αvβ5 | RGD internalizing peptide targeting NRP1, INT αvβ3, and αvβ5 | Lu et al. [299] |
| Integrin αvβ3 | Modified monoclonal antibody against INT αvβ3 | Wei et al. [306] |
| Neuropeptide Y receptor 1 | Neuropeptide Y type D | He et al. [311] |
| Low-density lipoprotein receptor | Low-density lipoprotein | Andreazza et al. [317] Huntosova et al. [318] |
| Extra domain A of fibronectin (ED A) | Ligand targeting epitope ED A small immunoprotein antibody F8 | Acker et al. [320] |
| US28 protein | Nanoprotein binding discontinuous epitope US28 with high affinity | De Groof et al. [321] |
| Nucleolin | Single-stranded DNA aptamer AS1411 with high affinity for nucleolin | Zhu et al. [322] |
| Transferrin receptor | Transferrin | Zhu et al. [322] |
| Cell nucleus | T-ag antigen variants of SV40 virus | Akhlynina et al. [323] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aebisher, D.; Woźnicki, P.; Czarnecka-Czapczyńska, M.; Dynarowicz, K.; Szliszka, E.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. Molecular Determinants for Photodynamic Therapy Resistance and Improved Photosensitizer Delivery in Glioma. Int. J. Mol. Sci. 2024, 25, 8708. https://doi.org/10.3390/ijms25168708
Aebisher D, Woźnicki P, Czarnecka-Czapczyńska M, Dynarowicz K, Szliszka E, Kawczyk-Krupka A, Bartusik-Aebisher D. Molecular Determinants for Photodynamic Therapy Resistance and Improved Photosensitizer Delivery in Glioma. International Journal of Molecular Sciences. 2024; 25(16):8708. https://doi.org/10.3390/ijms25168708
Chicago/Turabian StyleAebisher, David, Paweł Woźnicki, Magdalena Czarnecka-Czapczyńska, Klaudia Dynarowicz, Ewelina Szliszka, Aleksandra Kawczyk-Krupka, and Dorota Bartusik-Aebisher. 2024. "Molecular Determinants for Photodynamic Therapy Resistance and Improved Photosensitizer Delivery in Glioma" International Journal of Molecular Sciences 25, no. 16: 8708. https://doi.org/10.3390/ijms25168708
APA StyleAebisher, D., Woźnicki, P., Czarnecka-Czapczyńska, M., Dynarowicz, K., Szliszka, E., Kawczyk-Krupka, A., & Bartusik-Aebisher, D. (2024). Molecular Determinants for Photodynamic Therapy Resistance and Improved Photosensitizer Delivery in Glioma. International Journal of Molecular Sciences, 25(16), 8708. https://doi.org/10.3390/ijms25168708








