SOX2, JAGGED1, β-Catenin, and Vitamin D Receptor Expression Patterns during Early Development and Innervation of the Human Inner Ear
Abstract
1. Introduction
2. Results
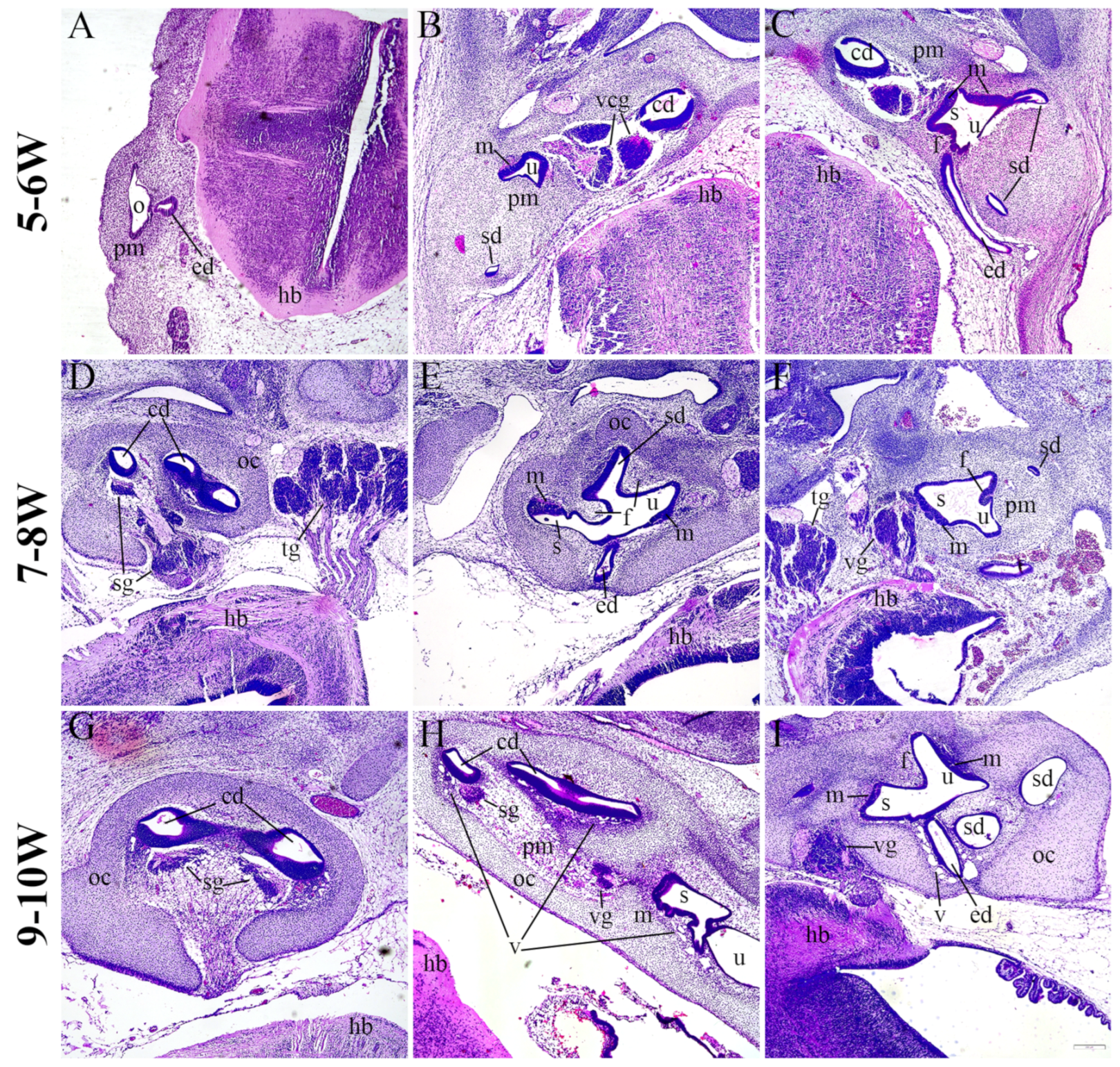
2.1. Morphology of the Developing Human Inner Ear
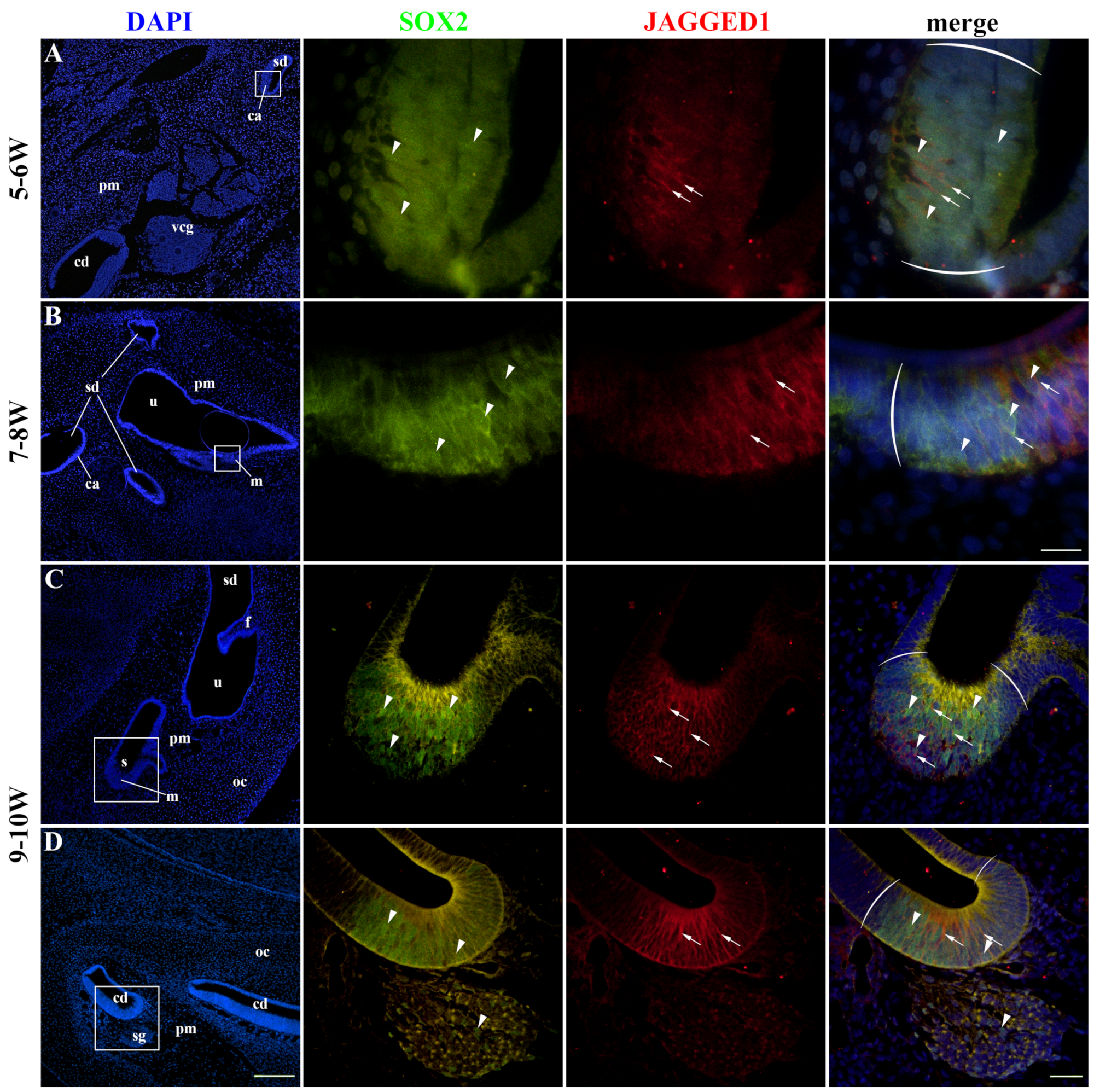
2.2. SOX2 and JAGGED1 Expression Characterizes Prosensory Domains of the Developing Human Inner Ear
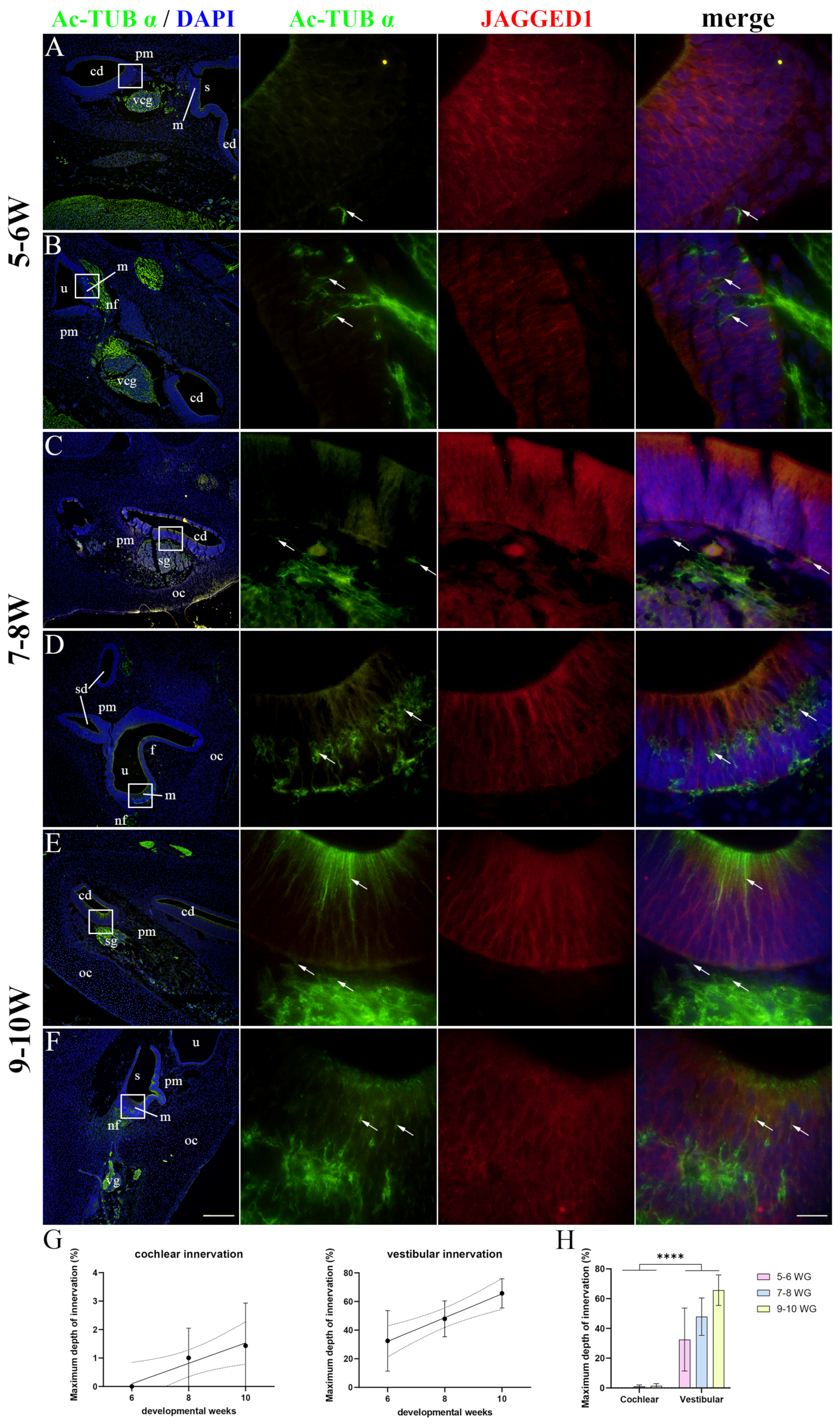
2.3. Innervation of the Developing Human Inner Ear
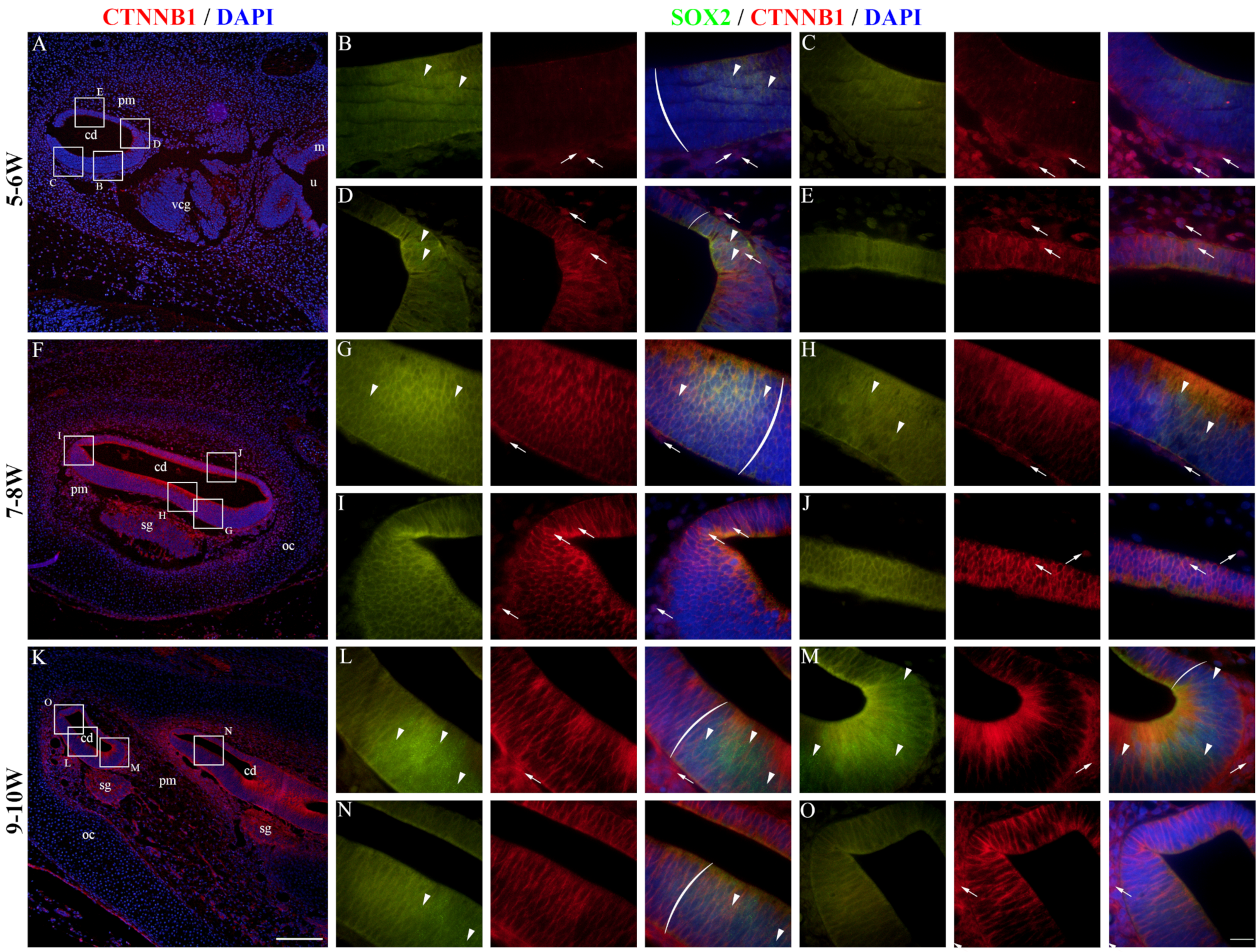
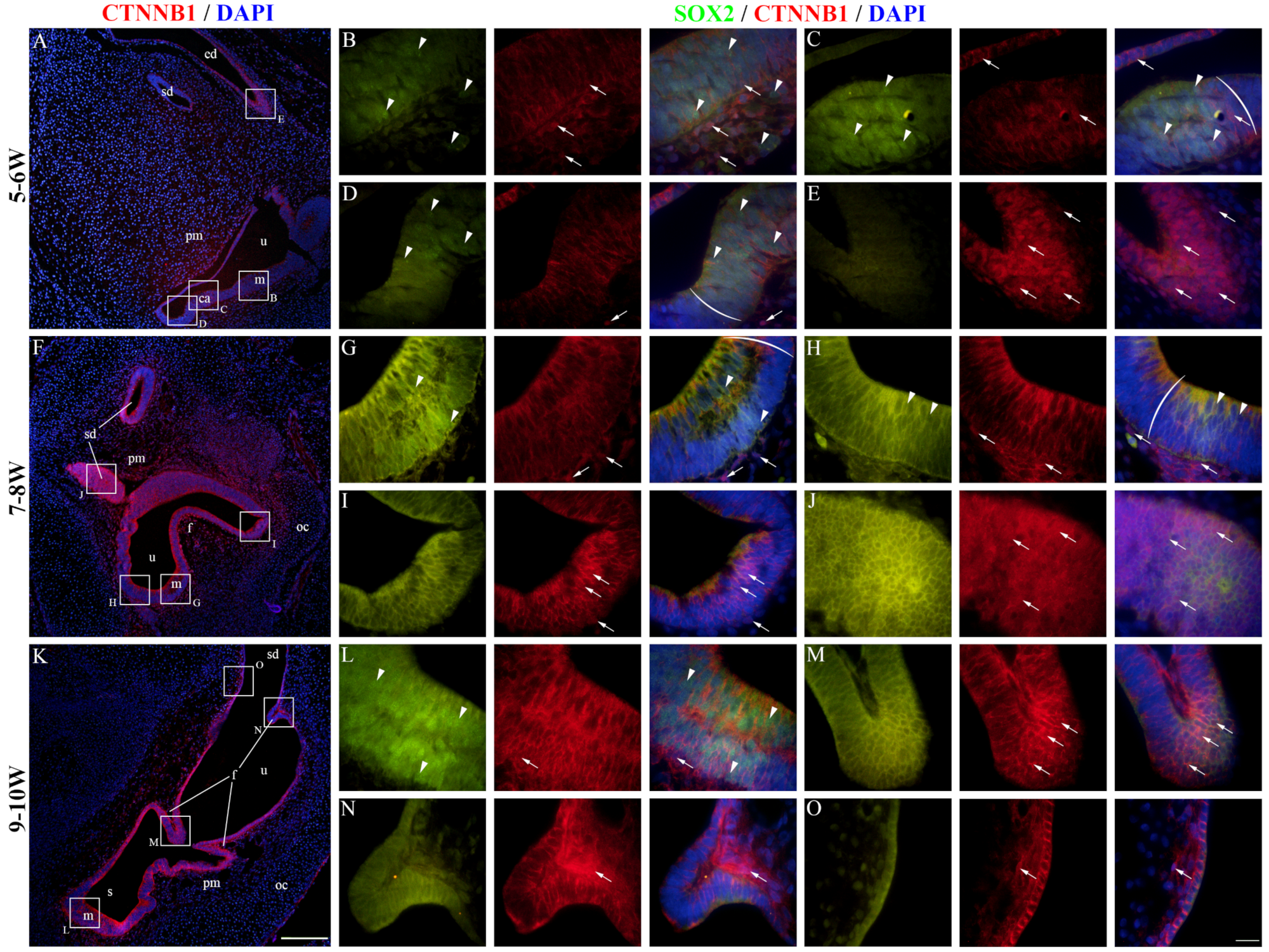
2.4. CTNNB1 Expression in the Developing Human Inner Ear
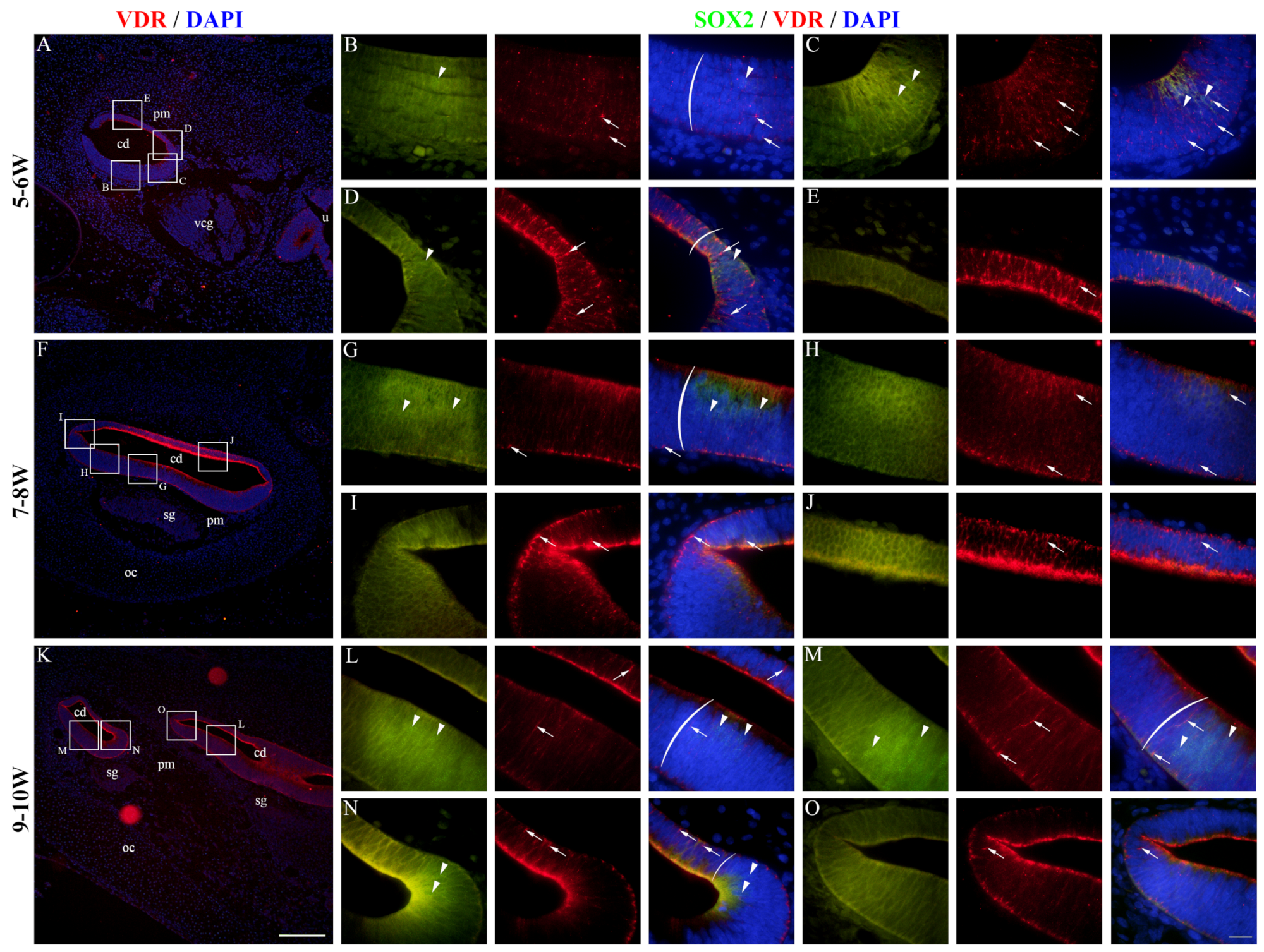
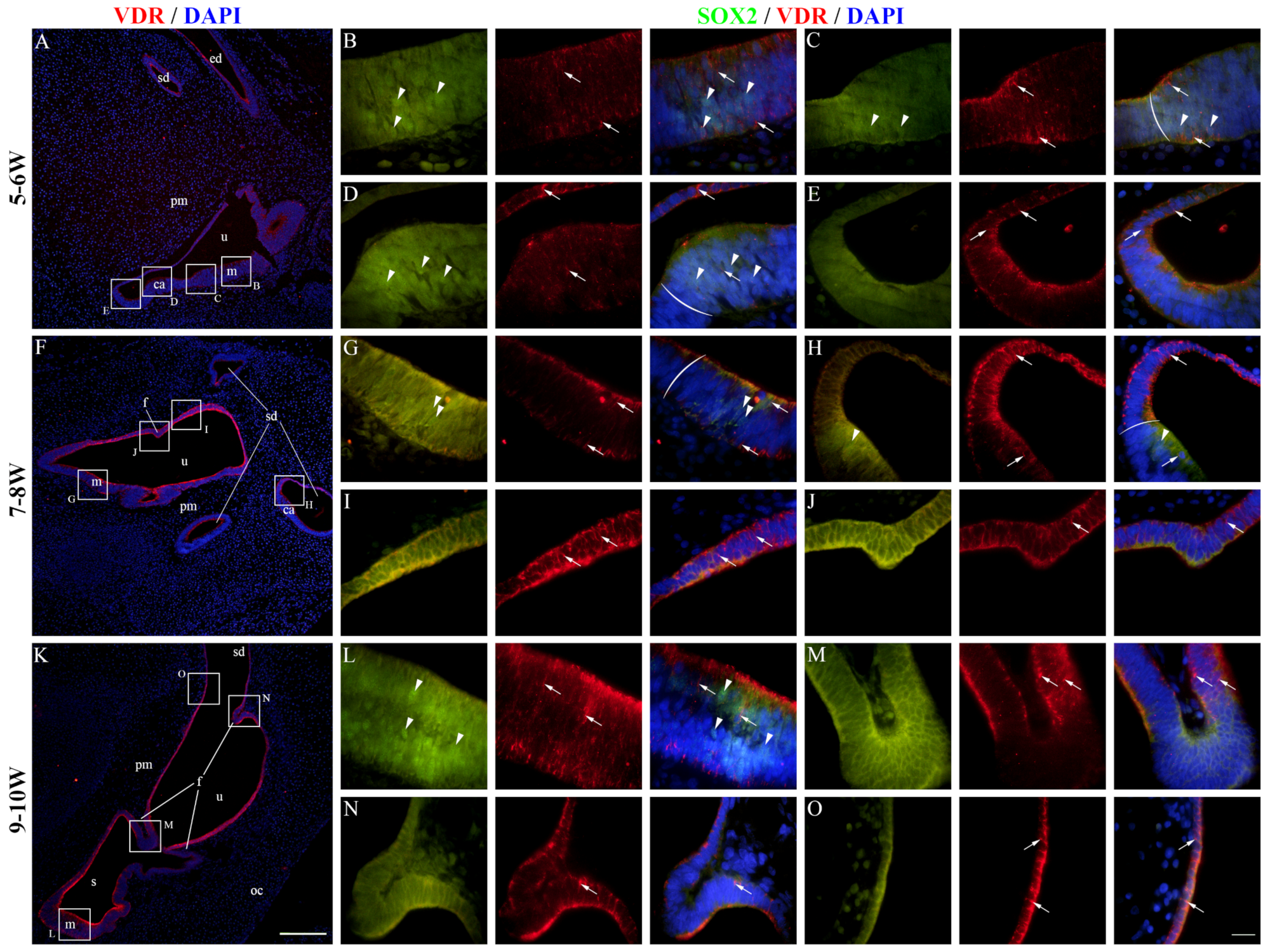
2.5. VDR Expression in the Developing Human Inner Ear
3. Discussion
4. Materials and Methods
4.1. Human Samples
4.2. Immunofluorescence Staining
4.3. Innervation Analysis
4.4. Immunofluorescence Signal Quantification
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garg, S.; Kohli, C.; Mangla, V.; Chadha, S.; Singh, M.M.; Dahiya, N. An Epidemiological Study on Burden of Hearing Loss and Its Associated Factors in Delhi, India. Ann. Otol. Rhinol. Laryngol. 2018, 127, 614–619. [Google Scholar] [CrossRef]
- Mark, A.S.; Seltzer, S.; Harnsberger, H.R. Sensorineural hearing loss: More than meets the eye? AJNR. Am. J. Neuroradiol. 1993, 14, 37–45. [Google Scholar] [PubMed]
- Korver, A.M.; Smith, R.J.; Van Camp, G.; Schleiss, M.R.; Bitner-Glindzicz, M.A.; Lustig, L.R.; Usami, S.I.; Boudewyns, A.N. Congenital hearing loss. Nat. Rev. Dis. Primers 2017, 3, 16094. [Google Scholar] [CrossRef] [PubMed]
- Bruska, M.; Ulatowska-Blaszyk, K.; Weglowski, M.; Wozniak, W.; Piotrowski, A. Differentiation of the facio-vestibulocochlear ganglionic complex in human embryos of developmental stages 13–15. Folia Morphol. 2009, 68, 167–173. [Google Scholar]
- Rinkwitz, S.; Bober, E.; Baker, R. Development of the vertebrate inner ear. Ann. N. Y. Acad. Sci. 2001, 942, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y. Signaling regulating inner ear development: Cell fate determination, patterning, morphogenesis, and defects. Congenit. Anom. 2015, 55, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.; Abello, G.; Petrovic, J.; Giraldez, F. Patterning and cell fate in the inner ear: A case for Notch in the chicken embryo. Dev. Growth Differ. 2013, 55, 96–112. [Google Scholar] [CrossRef]
- Petrovic, J.; Formosa-Jordan, P.; Luna-Escalante, J.C.; Abello, G.; Ibanes, M.; Neves, J.; Giraldez, F. Ligand-dependent Notch signaling strength orchestrates lateral induction and lateral inhibition in the developing inner ear. Development 2014, 141, 2313–2324. [Google Scholar] [CrossRef]
- Hosoya, M.; Fujioka, M.; Okano, H.; Ozawa, H. Mapping of Notch signaling in the developing organ of Corti in common marmosets. Front. Neuroanat. 2023, 17, 1188886. [Google Scholar] [CrossRef]
- Kiernan, A.E.; Xu, J.; Gridley, T. The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet. 2006, 2, e4. [Google Scholar] [CrossRef]
- Hosoya, M.; Fujioka, M.; Okahara, J.; Yoshimatsu, S.; Okano, H.; Ozawa, H. Early development of the cochlea of the common marmoset, a non-human primate model. Neural Dev. 2022, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, P.J.; Huarcaya Najarro, E.; Sayyid, Z.N.; Cheng, A.G. Sensory hair cell development and regeneration: Similarities and differences. Development 2015, 142, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Jin, Y.; Stanger, B.; Kiernan, A.E. Notch signaling is required for the generation of hair cells and supporting cells in the mammalian inner ear. Proc. Natl. Acad. Sci. USA 2010, 107, 15798–15803. [Google Scholar] [CrossRef] [PubMed]
- Dechesne, C.J.; Sans, A. Development of vestibular receptor surfaces in human fetuses. Am. J. Otolaryngol. 1985, 6, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Pujol, R.; Lavigne-Rebillard, M. Sensory and neural structures in the developing human cochlea. Int. J. Pediatr. Otorhinolaryngol. 1995, 32, S177–S182. [Google Scholar] [CrossRef]
- Pujol, R.; Lavigne-Rebillard, M. Development of neurosensory structures in the human cochlea. Acta Oto-Laryngol. 1992, 112, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Lim, R.; Brichta, A.M. Anatomical and physiological development of the human inner ear. Hear. Res. 2016, 338, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Carney, P.R.; Silver, J. Studies on cell migration and axon guidance in the developing distal auditory system of the mouse. J. Comp. Neurol. 1983, 215, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Farinas, I.; Jones, K.R.; Tessarollo, L.; Vigers, A.J.; Huang, E.; Kirstein, M.; de Caprona, D.C.; Coppola, V.; Backus, C.; Reichardt, L.F.; et al. Spatial shaping of cochlear innervation by temporally regulated neurotrophin expression. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 6170–6180. [Google Scholar] [CrossRef]
- Fritzsch, B. Development of inner ear afferent connections: Forming primary neurons and connecting them to the developing sensory epithelia. Brain Res. Bull. 2003, 60, 423–433. [Google Scholar] [CrossRef][Green Version]
- Locher, H.; de Groot, J.C.; van Iperen, L.; Huisman, M.A.; Frijns, J.H.; Chuva de Sousa Lopes, S.M. Distribution and development of peripheral glial cells in the human fetal cochlea. PLoS ONE 2014, 9, e88066. [Google Scholar] [CrossRef] [PubMed]
- Yoko, Y. Early formation of nerve fibers in the human otocyst. Acta Anat. 1971, 80, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T. Scanning electron microscopic observation of the foetal labyrinthine vestibule. Acta Oto-Laryngol. 1982, 93, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Fernandez, J.M.; Rivera-Pomar, J.M. A study of the development of utricular and saccular maculae in man and in rat. Am. J. Otol. 1983, 5, 44–55. [Google Scholar]
- Sans, A.; Dechesne, C. Early development of vestibular receptors in human embryos. An electron microscopic study. Acta Oto-Laryngol. Suppl. 1985, 423, 51–58. [Google Scholar] [CrossRef]
- Locher, H.; Frijns, J.H.; van Iperen, L.; de Groot, J.C.; Huisman, M.A.; Chuva de Sousa Lopes, S.M. Neurosensory development and cell fate determination in the human cochlea. Neural Dev. 2013, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Pujol, R.; Lavigne-Rebillard, M. Early stages of innervation and sensory cell differentiation in the human fetal organ of Corti. Acta Oto-Laryngol. Suppl. 1985, 423, 43–50. [Google Scholar] [CrossRef]
- Lavigne-Rebillard, M.; Pujol, R. Hair cell innervation in the fetal human cochlea. Acta Oto-Laryngol. 1988, 105, 398–402. [Google Scholar] [CrossRef]
- Wan, G.; Corfas, G.; Stone, J.S. Inner ear supporting cells: Rethinking the silent majority. Semin. Cell Dev. Biol. 2013, 24, 448–459. [Google Scholar] [CrossRef]
- Gomez-Casati, M.E.; Murtie, J.C.; Rio, C.; Stankovic, K.; Liberman, M.C.; Corfas, G. Nonneuronal cells regulate synapse formation in the vestibular sensory epithelium via erbB-dependent BDNF expression. Proc. Natl. Acad. Sci. USA 2010, 107, 17005–17010. [Google Scholar] [CrossRef]
- Pechriggl, E.J.; Bitsche, M.; Glueckert, R.; Rask-Andersen, H.; Blumer, M.J.; Schrott-Fischer, A.; Fritsch, H. Development of the innervation of the human inner ear. Dev. Neurobiol. 2015, 75, 683–702. [Google Scholar] [CrossRef] [PubMed]
- Rakowiecki, S.; Epstein, D.J. Divergent roles for Wnt/beta-catenin signaling in epithelial maintenance and breakdown during semicircular canal formation. Development 2013, 140, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Valenta, T.; Hausmann, G.; Basler, K. The many faces and functions of beta-catenin. EMBO J. 2012, 31, 2714–2736. [Google Scholar] [CrossRef]
- Bakke, D.; Sun, J. Ancient Nuclear Receptor VDR With New Functions: Microbiome and Inflammation. Inflamm. Bowel Dis. 2018, 24, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lou, Y.; Kong, J. VDR regulates energy metabolism by modulating remodeling in adipose tissue. Eur. J. Pharmacol. 2019, 865, 172761. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, K.; Tian, K.; Zhu, Q.; Liu, W.; Liu, Z.; An, X.; Tian, C.; Li, Y.; Lu, F.; et al. VDR Regulates BNP Promoting Neurite Growth and Survival of Cochlear Spiral Ganglion Neurons through cGMP-PKG Signaling Pathway. Cells 2022, 11, 3746. [Google Scholar] [CrossRef] [PubMed]
- Minasyan, A.; Keisala, T.; Zou, J.; Zhang, Y.; Toppila, E.; Syvala, H.; Lou, Y.R.; Kalueff, A.V.; Pyykko, I.; Tuohimaa, P. Vestibular dysfunction in vitamin D receptor mutant mice. J. Steroid Biochem. Mol. Biol. 2009, 114, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Minasyan, A.; Keisala, T.; Zhang, Y.; Wang, J.H.; Lou, Y.R.; Kalueff, A.; Pyykko, I.; Tuohimaa, P. Progressive hearing loss in mice with a mutated vitamin D receptor gene. Audiol. Neuro-Otol. 2008, 13, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Brookes, G.B. Vitamin D deficiency--a new cause of cochlear deafness. J. Laryngol. Otol. 1983, 97, 405–420. [Google Scholar] [CrossRef]
- Hamayal, M.; Khurshied, S.; Zahid, M.A.; Khurshid, N.; Shahid, W.; Ali, M.; Ahmed, H.; Nisa, M. Exploring the Significance of Vitamin D Levels as a Biomarker in Ear Diseases: A Narrative Review. Cureus 2024, 16, e54812. [Google Scholar] [CrossRef]
- Talebi, H.; Moallemi, M.; Ghorbani, M. Evaluation of Saccule Function in Patients with Vitamin D Deficiency. J. Audiol. Otol. 2019, 23, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Ghazavi, H.; Kargoshaie, A.A.; Jamshidi-Koohsari, M. Investigation of vitamin D levels in patients with Sudden Sensory-Neural Hearing Loss and its effect on treatment. Am. J. Otolaryngol. 2020, 41, 102327. [Google Scholar] [CrossRef] [PubMed]
- Szeto, B.; Valentini, C.; Lalwani, A.K. Low vitamin D status is associated with hearing loss in the elderly: A cross-sectional study. Am. J. Clin. Nutr. 2021, 113, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Sancho, J.M.; Larriba, M.J.; Munoz, A. Wnt and Vitamin D at the Crossroads in Solid Cancer. Cancers 2020, 12, 3434. [Google Scholar] [CrossRef] [PubMed]
- Jacques, B.E.; Puligilla, C.; Weichert, R.M.; Ferrer-Vaquer, A.; Hadjantonakis, A.K.; Kelley, M.W.; Dabdoub, A. A dual function for canonical Wnt/beta-catenin signaling in the developing mammalian cochlea. Development 2012, 139, 4395–4404. [Google Scholar] [CrossRef] [PubMed]
- Baltus, G.A.; Kowalski, M.P.; Zhai, H.; Tutter, A.V.; Quinn, D.; Wall, D.; Kadam, S. Acetylation of sox2 induces its nuclear export in embryonic stem cells. Stem Cells 2009, 27, 2175–2184. [Google Scholar] [CrossRef] [PubMed]
- Saigusa, S.; Tanaka, K.; Toiyama, Y.; Yokoe, T.; Okugawa, Y.; Ioue, Y.; Miki, C.; Kusunoki, M. Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann. Surg. Oncol. 2009, 16, 3488–3498. [Google Scholar] [CrossRef] [PubMed]
- Johnson Chacko, L.; Pechriggl, E.J.; Fritsch, H.; Rask-Andersen, H.; Blumer, M.J.; Schrott-Fischer, A.; Glueckert, R. Neurosensory Differentiation and Innervation Patterning in the Human Fetal Vestibular End Organs between the Gestational Weeks 8–12. Front. Neuroanat. 2016, 10, 111. [Google Scholar] [CrossRef]
- Jansson, L.; Ebeid, M.; Shen, J.W.; Mokhtari, T.E.; Quiruz, L.A.; Ornitz, D.M.; Huh, S.H.; Cheng, A.G. beta-Catenin is required for radial cell patterning and identity in the developing mouse cochlea. Proc. Natl. Acad. Sci. USA 2019, 116, 21054–21060. [Google Scholar] [CrossRef]
- Shi, F.; Hu, L.; Jacques, B.E.; Mulvaney, J.F.; Dabdoub, A.; Edge, A.S. beta-Catenin is required for hair-cell differentiation in the cochlea. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 6470–6479. [Google Scholar] [CrossRef]
- Kim, T.S.; Nakagawa, T.; Lee, J.E.; Fujino, K.; Iguchi, F.; Endo, T.; Naito, Y.; Omori, K.; Lefebvre, P.P.; Ito, J. Induction of cell proliferation and beta-catenin expression in rat utricles in vitro. Acta Oto-Laryngol. 2004, 124, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Keino, H. Roles of beta-catenin in inner ear development in rat embryos. Anat. Embryol. 2000, 202, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xing, J.; Gong, Y.; Li, P.; Wang, B.; Xu, L. Downregulation of VDR in benign paroxysmal positional vertigo patients inhibits otolith-associated protein expression levels. Mol. Med. Rep. 2021, 24, 591. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.J. Vitamin D receptor deficiency impairs inner ear development in zebrafish. Biochem. Biophys. Res. Commun. 2016, 478, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Zmijewski, M.A.; Carlberg, C. Vitamin D receptor(s): In the nucleus but also at membranes? Exp. Dermatol. 2020, 29, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Larriba, M.J.; Ordonez-Moran, P.; Chicote, I.; Martin-Fernandez, G.; Puig, I.; Munoz, A.; Palmer, H.G. Vitamin D receptor deficiency enhances Wnt/beta-catenin signaling and tumor burden in colon cancer. PLoS ONE 2011, 6, e23524. [Google Scholar] [CrossRef]
- Muralidhar, S.; Filia, A.; Nsengimana, J.; Pozniak, J.; O’Shea, S.J.; Diaz, J.M.; Harland, M.; Randerson-Moor, J.A.; Reichrath, J.; Laye, J.P.; et al. Vitamin D-VDR Signaling Inhibits Wnt/beta-Catenin-Mediated Melanoma Progression and Promotes Antitumor Immunity. Cancer Res. 2019, 79, 5986–5998. [Google Scholar] [CrossRef] [PubMed]
- Tafra, R.; Brakus, S.M.; Vukojevic, K.; Kablar, B.; Colovic, Z.; Saraga-Babic, M. Interplay of proliferation and proapoptotic and antiapoptotic factors is revealed in the early human inner ear development. Otol. Neurotol. 2014, 35, 695–703. [Google Scholar] [CrossRef]
- Vukovic, D.; Ogorevc, M.; Tripkovic, I.; Puizina-Ivic, N.; Saraga-Babic, M.; Mardesic, S. The Distribution of Innervation and Immune Cell Infiltration Is Different in Genital and Extragenital Variants of Lichen Sclerosus. Biomolecules 2022, 12, 1767. [Google Scholar] [CrossRef]
- Pavic, B.; Ogorevc, M.; Boric, K.; Vukovic, D.; Saraga-Babic, M.; Mardesic, S. Connexin 37, 40, 43 and Pannexin 1 Expression in the Gastric Mucosa of Patients with Systemic Sclerosis. Biomedicines 2023, 11, 2487. [Google Scholar] [CrossRef]

| Antibodies | Host | Code No. | Dilution | Source | |
|---|---|---|---|---|---|
| Primary | Anti-Acetylated-alpha-tubulin | Mouse | 12152 | 1:500 | Cell Signaling Technology (CST), (Danvers, MA, USA) |
| Anti-Beta-catenin | Mouse | 2677 | 1:200 | Cell Signaling Technology (CST), (Danvers, MA, USA) | |
| Anti-Vitamin D Receptor | Mouse | sc-13133 | 1:50 | Santa Cruz Biotechnology, Dallas, TX, USA | |
| Anti-JAGGED1 | Goat | AF1277 | 1:100 | R&D Systems, Minneapolis, MN, USA | |
| Anti-SOX2 | Rabbit | 3579 | 1:400 | Cell Signaling Technology (CST), (Danvers, MA, USA) | |
| Secondary | Alexa Fluor®488 Anti-Mouse lgG | Donkey | 715-545-150 | 1:400 | Jackson Immuno Research Laboratories, Inc., Baltimore, PA, USA |
| Alexa Fluor®488 Anti-Rabbit lgG | Donkey | 711-545-152 | 1:400 | Jackson Immuno Research Laboratories, Inc., Baltimore, PA, USA | |
| Rhodamine Red™-X Anti-Mouse IgG | Donkey | 715-295-151 | 1:400 | Jackson Immuno Research Laboratories, Inc., Baltimore, PA, USA | |
| Rhodamine Red™-X Anti-Goat IgG | Donkey | 705-295-003 | 1:400 | Jackson Immuno Research Laboratories, Inc., Baltimore, PA, USA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikulić, P.; Ogorevc, M.; Petričević, M.; Kaličanin, D.; Tafra, R.; Saraga-Babić, M.; Mardešić, S. SOX2, JAGGED1, β-Catenin, and Vitamin D Receptor Expression Patterns during Early Development and Innervation of the Human Inner Ear. Int. J. Mol. Sci. 2024, 25, 8719. https://doi.org/10.3390/ijms25168719
Mikulić P, Ogorevc M, Petričević M, Kaličanin D, Tafra R, Saraga-Babić M, Mardešić S. SOX2, JAGGED1, β-Catenin, and Vitamin D Receptor Expression Patterns during Early Development and Innervation of the Human Inner Ear. International Journal of Molecular Sciences. 2024; 25(16):8719. https://doi.org/10.3390/ijms25168719
Chicago/Turabian StyleMikulić, Petra, Marin Ogorevc, Marin Petričević, Dean Kaličanin, Robert Tafra, Mirna Saraga-Babić, and Snježana Mardešić. 2024. "SOX2, JAGGED1, β-Catenin, and Vitamin D Receptor Expression Patterns during Early Development and Innervation of the Human Inner Ear" International Journal of Molecular Sciences 25, no. 16: 8719. https://doi.org/10.3390/ijms25168719
APA StyleMikulić, P., Ogorevc, M., Petričević, M., Kaličanin, D., Tafra, R., Saraga-Babić, M., & Mardešić, S. (2024). SOX2, JAGGED1, β-Catenin, and Vitamin D Receptor Expression Patterns during Early Development and Innervation of the Human Inner Ear. International Journal of Molecular Sciences, 25(16), 8719. https://doi.org/10.3390/ijms25168719








