Expression Patterns of MOTS-c in Adrenal Tumors: Results from a Preliminary Study
Abstract
1. Introduction
2. Results
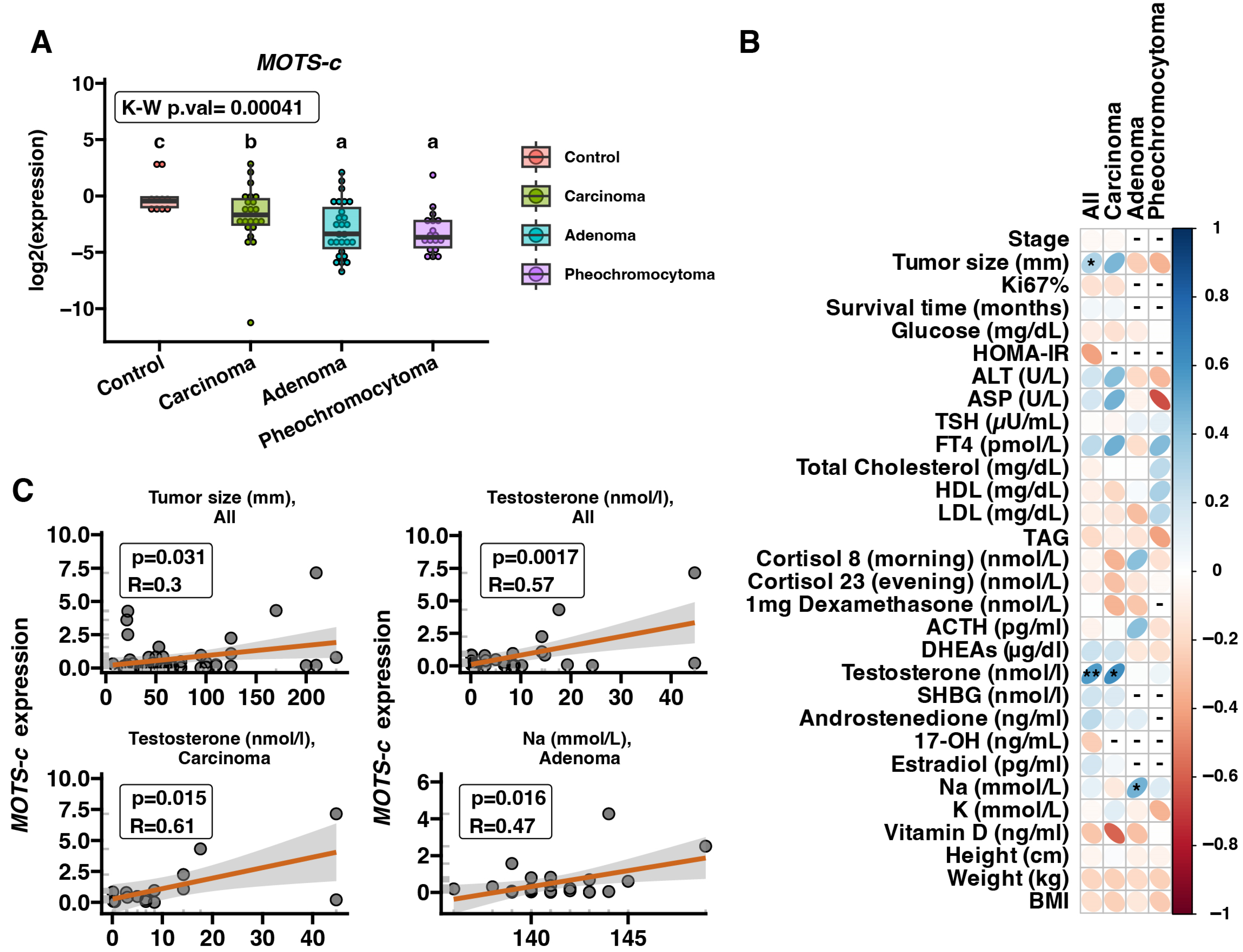
2.1. MOTS-c mRNA Expression in Adrenal Disease Spectrum Samples—qRT-PCR
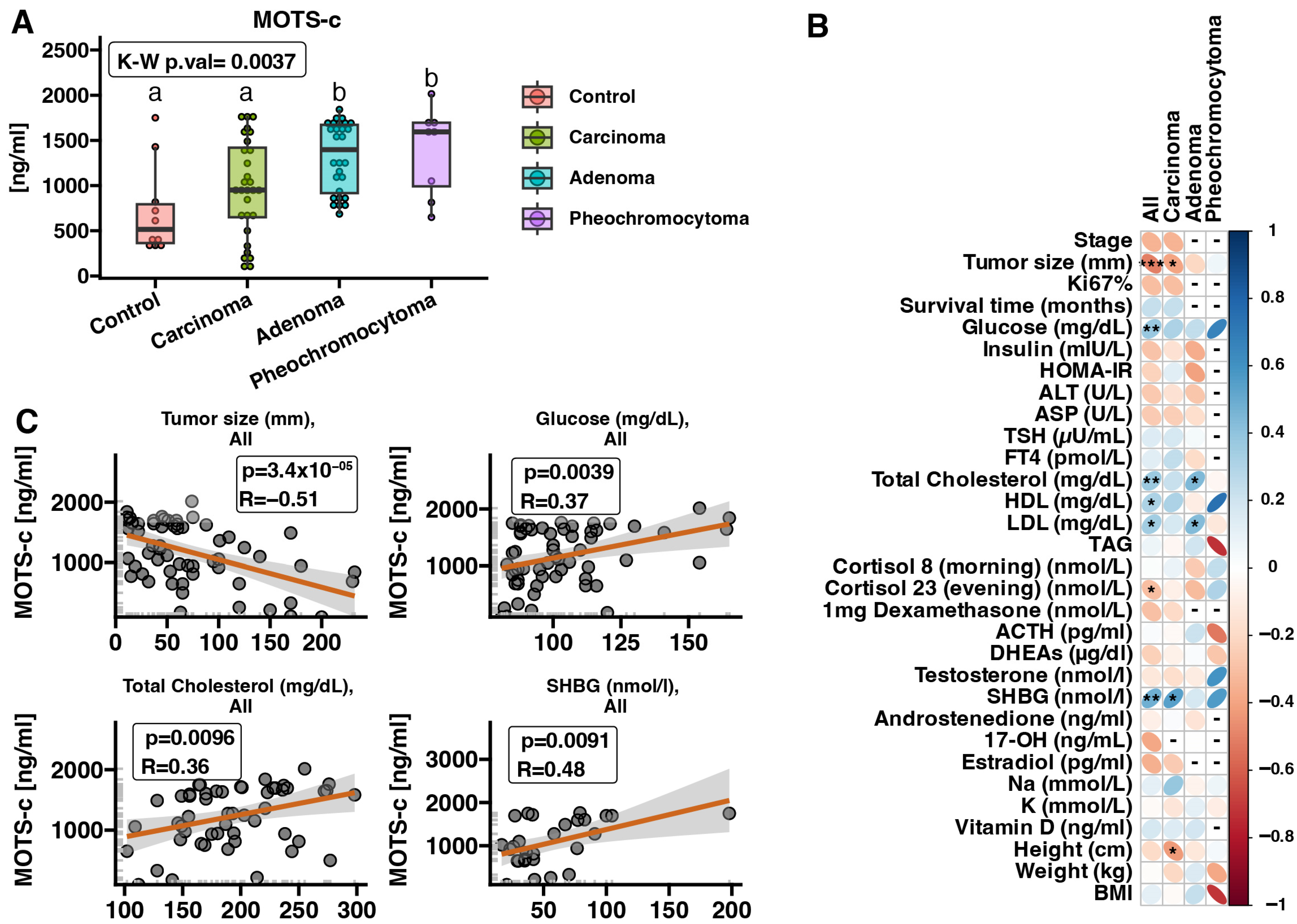
2.2. MOTS-c Protein Expression in Serum Samples—ELISA
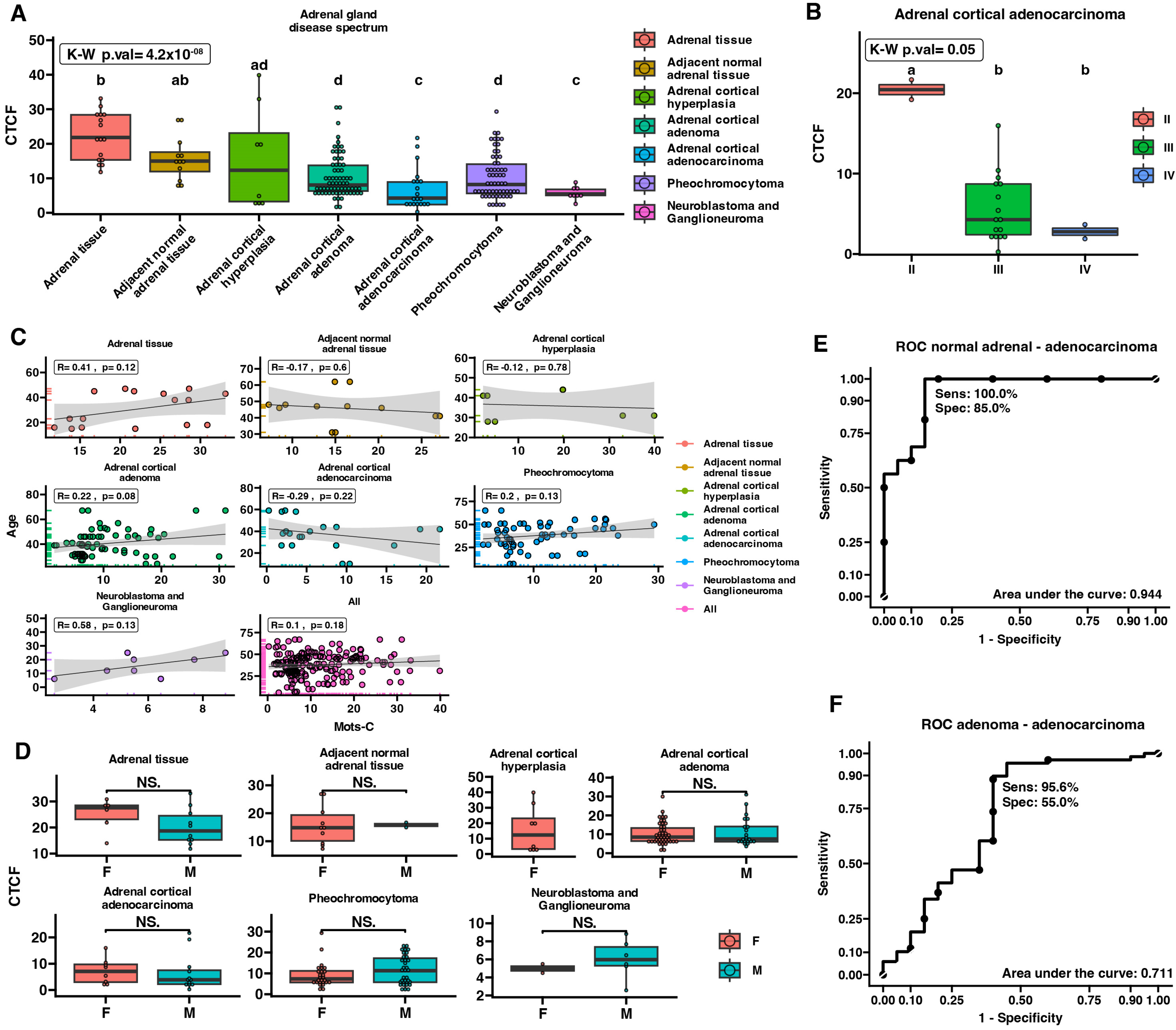
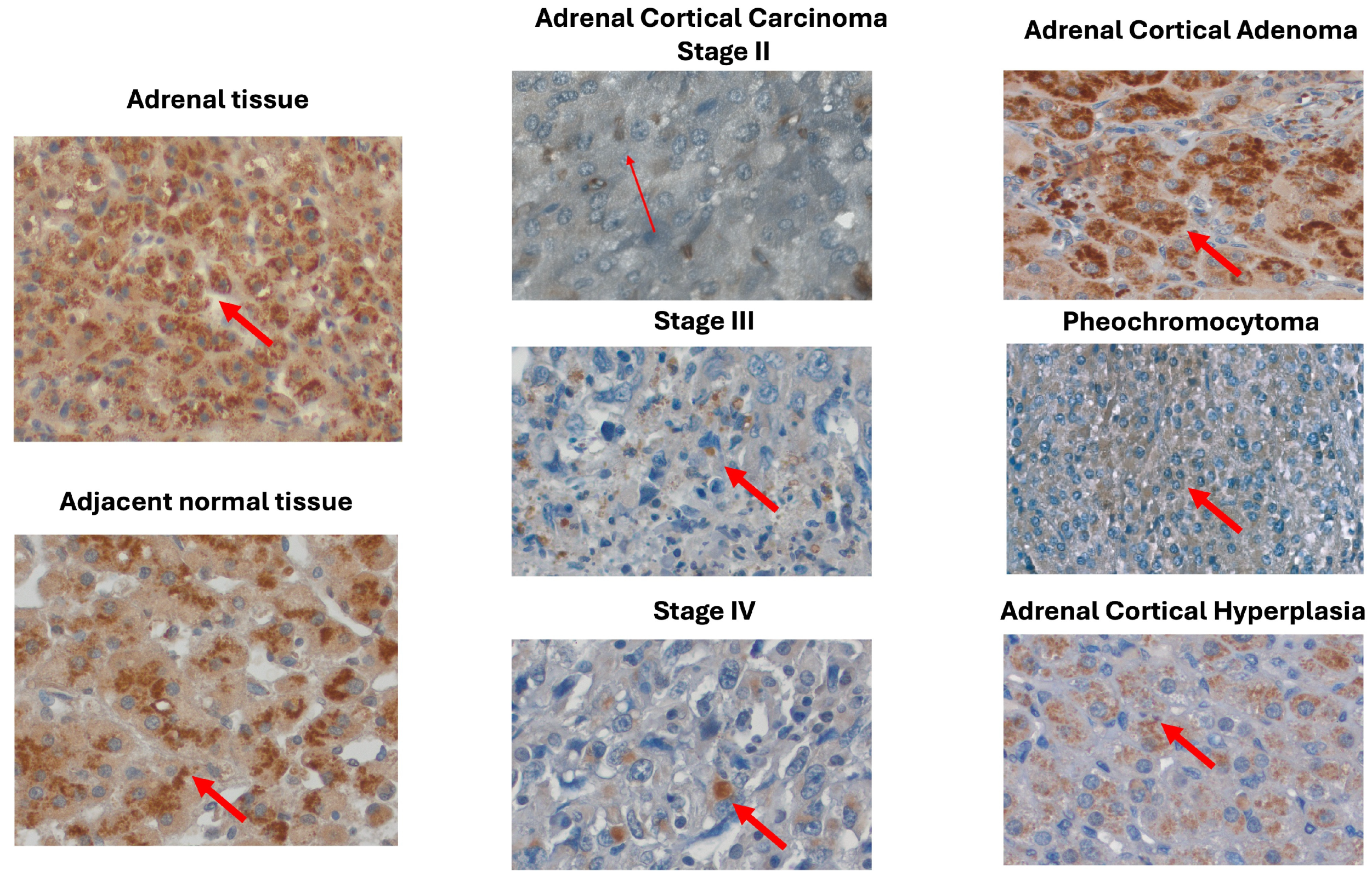
2.3. MOTS-c Protein Expression in Adrenal Disease Spectrum—Tissue Microarray Slide
3. Discussion
4. Materials and Methods
4.1. Patients’ Characteristics
4.2. RNA Extraction and Quantification of the Gene Expression
4.3. Quantifying MOTS-c Protein Levels Using ELISA
4.4. Immunohistochemical Analyses
4.5. Semiquantitative Evaluation of MOTS-c Protein Expression
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mansmann, G.; Lau, J.; Balk, E.; Rothberg, M.; Miyachi, Y.; Bornstein, S.R. The clinically inapparent adrenal mass: Update in diagnosis and management. Endocr. Rev. 2004, 25, 309–340. [Google Scholar] [CrossRef] [PubMed]
- Corssmit, E.P.M.; Dekkers, O.M. Screening in adrenal tumors. Curr. Opin. Oncol. 2019, 31, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Arlt, W.; Bancos, I.; Dralle, H.; Newell-Price, J.; Sahdev, A.; Tabarin, A.; Terzolo, M.; Tsagarakis, S.; Dekkers, O.M. Management of adrenal incidentalomas: European Society of Endocrinology Clinical Practice Guideline in collaboration with the European Network for the Study of Adrenal Tumors. Eur. J. Endocrinol. 2016, 175, G1–G34. [Google Scholar] [CrossRef] [PubMed]
- Else, T.; Kim, A.C.; Sabolch, A.; Raymond, V.M.; Kandathil, A.; Caoili, E.M.; Jolly, S.; Miller, B.S.; Giordano, T.J.; Hammer, G.D. Adrenocortical carcinoma. Endocr. Rev. 2014, 35, 282–326. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, K.Y.; Shen, W.T.; Elaraj, D.; Bentrem, D.J.; Winchester, D.J.; Kebebew, E.; Sturgeon, C. Adrenocortical carcinoma in the United States: Treatment utilization and prognostic factors. Cancer 2008, 113, 3130–3136. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, N.; Moskopp, M.L.; Constantinescu, G.; Stell, A.; Ernst, A.; Berthold, F.; Westermann, F.; Jiang, J.; Lui, L.; Nowak, E.; et al. Asymmetric Adrenals: Sexual Dimorphism of Adrenal Tumors. J. Clin. Endocrinol. Metab. 2024, 109, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Audenet, F.; Mejean, A.; Chartier-Kastler, E.; Roupret, M. Adrenal tumours are more predominant in females regardless of their histological subtype: A review. World J. Urol. 2013, 31, 1037–1043. [Google Scholar] [CrossRef]
- Allolio, B.; Fassnacht, M. Clinical review: Adrenocortical carcinoma: Clinical update. J. Clin. Endocrinol. Metab. 2006, 91, 2027–2037. [Google Scholar] [CrossRef] [PubMed]
- Fassnacht, M.; Allolio, B. Clinical management of adrenocortical carcinoma. Best. Pract. Res. Clin. Endocrinol. Metab. 2009, 23, 273–289. [Google Scholar] [CrossRef]
- Luton, J.P.; Cerdas, S.; Billaud, L.; Thomas, G.; Guilhaume, B.; Bertagna, X.; Laudat, M.H.; Louvel, A.; Chapuis, Y.; Blondeau, P.; et al. Clinical features of adrenocortical carcinoma, prognostic factors, and the effect of mitotane therapy. N. Engl. J. Med. 1990, 322, 1195–1201. [Google Scholar] [CrossRef]
- Giordano, T.J.; Thomas, D.G.; Kuick, R.; Lizyness, M.; Misek, D.E.; Smith, A.L.; Sanders, D.; Aljundi, R.T.; Gauger, P.G.; Thompson, N.W.; et al. Distinct transcriptional profiles of adrenocortical tumors uncovered by DNA microarray analysis. Am. J. Pathol. 2003, 162, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Assie, G.; Letouze, E.; Fassnacht, M.; Jouinot, A.; Luscap, W.; Barreau, O.; Omeiri, H.; Rodriguez, S.; Perlemoine, K.; Rene-Corail, F.; et al. Integrated genomic characterization of adrenocortical carcinoma. Nat. Genet. 2014, 46, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Tissier, F.; Cavard, C.; Groussin, L.; Perlemoine, K.; Fumey, G.; Hagnere, A.M.; Rene-Corail, F.; Jullian, E.; Gicquel, C.; Bertagna, X.; et al. Mutations of beta-catenin in adrenocortical tumors: Activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005, 65, 7622–7627. [Google Scholar] [CrossRef] [PubMed]
- Pinto, E.M.; Chen, X.; Easton, J.; Finkelstein, D.; Liu, Z.; Pounds, S.; Rodriguez-Galindo, C.; Lund, T.C.; Mardis, E.R.; Wilson, R.K.; et al. Genomic landscape of paediatric adrenocortical tumours. Nat. Commun. 2015, 6, 6302. [Google Scholar] [CrossRef] [PubMed]
- Gara, S.K.; Lack, J.; Zhang, L.; Harris, E.; Cam, M.; Kebebew, E. Metastatic adrenocortical carcinoma displays higher mutation rate and tumor heterogeneity than primary tumors. Nat. Commun. 2018, 9, 4172. [Google Scholar] [CrossRef] [PubMed]
- Ebbehoj, A.; Li, D.; Kaur, R.J.; Zhang, C.; Singh, S.; Li, T.; Atkinson, E.; Achenbach, S.; Khosla, S.; Arlt, W.; et al. Epidemiology of adrenal tumours in Olmsted County, Minnesota, USA: A population-based cohort study. Lancet Diabetes Endocrinol. 2020, 8, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Parikh, P.P.; Rubio, G.A.; Farra, J.C.; Lew, J.I. Nationwide review of hormonally active adrenal tumors highlights high morbidity in pheochromocytoma. J. Surg. Res. 2017, 215, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; El Kawkgi, O.M.; Henriquez, A.F.; Bancos, I. Cardiovascular risk and mortality in patients with active and treated hypercortisolism. Gland. Surg. 2020, 9, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Amar, L.; Bertherat, J.; Baudin, E.; Ajzenberg, C.; Bressac-de Paillerets, B.; Chabre, O.; Chamontin, B.; Delemer, B.; Giraud, S.; Murat, A.; et al. Genetic testing in pheochromocytoma or functional paraganglioma. J. Clin. Oncol. 2005, 23, 8812–8818. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.E.; O’Neill, J.A., Jr.; Holcomb, G.W., 3rd; Morgan, W.M., 3rd; Neblett, W.W., 3rd; Oates, J.A.; Brown, N.; Nadeau, J.; Smith, B.; Page, D.L.; et al. Clinical experience over 48 years with pheochromocytoma. Ann. Surg. 1999, 229, 755–764; discussion 764-6. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, F.A.; Charalampopoulos, A. Pheochromocytoma. Endocr. Regul. 2019, 53, 191–212. [Google Scholar] [CrossRef] [PubMed]
- Bryant, J.; Farmer, J.; Kessler, L.J.; Townsend, R.R.; Nathanson, K.L. Pheochromocytoma: The expanding genetic differential diagnosis. J. Natl. Cancer Inst. 2003, 95, 1196–1204. [Google Scholar] [CrossRef] [PubMed]
- Neumann, H.P.; Bausch, B.; McWhinney, S.R.; Bender, B.U.; Gimm, O.; Franke, G.; Schipper, J.; Klisch, J.; Altehoefer, C.; Zerres, K.; et al. Germ-line mutations in nonsyndromic pheochromocytoma. N. Engl. J. Med. 2002, 346, 1459–1466. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Sun, Y.; Guo, Y.; Shi, X.; Chen, X.; Feng, W.; Wu, L.L.; Zhang, J.; Yu, S.; Wang, Y.; et al. An Overview: The Diversified Role of Mitochondria in Cancer Metabolism. Int. J. Biol. Sci. 2023, 19, 897–915. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef] [PubMed]
- Midzak, A.; Papadopoulos, V. Adrenal Mitochondria and Steroidogenesis: From Individual Proteins to Functional Protein Assemblies. Front. Endocrinol. 2016, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Nunnari, J.; Suomalainen, A. Mitochondria: In sickness and in health. Cell 2012, 148, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Gorman, G.S.; Chinnery, P.F.; DiMauro, S.; Hirano, M.; Koga, Y.; McFarland, R.; Suomalainen, A.; Thorburn, D.R.; Zeviani, M.; Turnbull, D.M. Mitochondrial diseases. Nat. Rev. Dis. Primers 2016, 2, 16080. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, H. Mitochondrial quality control mechanisms as molecular targets in cardiac ischemia-reperfusion injury. Acta Pharm. Sin. B 2020, 10, 1866–1879. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, S.R.; Brown, J.W.; Carballeira, A.; Goodman, J.; Scherbaum, W.A.; Fishman, L.M. Ultrastructural dynamics of mitochondrial morphology in varying functional forms of human adrenal cortical adenoma. Horm. Metab. Res. 1996, 28, 177–182. [Google Scholar] [CrossRef]
- Rapizzi, E.; Ercolino, T.; Canu, L.; Giache, V.; Francalanci, M.; Pratesi, C.; Valeri, A.; Mannelli, M. Mitochondrial function and content in pheochromocytoma/paraganglioma of succinate dehydrogenase mutation carriers. Endocr. Relat. Cancer 2012, 19, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.H.; Dorsch, M.; Dujardin, P.; Silas, S.; Ueffing, K.; Holken, J.M.; Yang, D.; Winslow, M.M.; Gruner, B.M. Altered Mitochondria Functionality Defines a Metastatic Cell State in Lung Cancer and Creates an Exploitable Vulnerability. Cancer Res. 2021, 81, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Zeng, J.; Drew, B.G.; Sallam, T.; Martin-Montalvo, A.; Wan, J.; Kim, S.J.; Mehta, H.; Hevener, A.L.; de Cabo, R.; et al. The mitochondrial-derived peptide MOTS-c promotes metabolic homeostasis and reduces obesity and insulin resistance. Cell Metab. 2015, 21, 443–454. [Google Scholar] [CrossRef]
- Kim, K.H.; Son, J.M.; Benayoun, B.A.; Lee, C. The Mitochondrial-Encoded Peptide MOTS-c Translocates to the Nucleus to Regulate Nuclear Gene Expression in Response to Metabolic Stress. Cell Metab. 2018, 28, 516–524.e7. [Google Scholar] [CrossRef] [PubMed]
- Kong, B.S.; Min, S.H.; Lee, C.; Cho, Y.M. Mitochondrial-encoded MOTS-c prevents pancreatic islet destruction in autoimmune diabetes. Cell Rep. 2021, 36, 109447. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Kim, K.H.; Cohen, P. MOTS-c: A novel mitochondrial-derived peptide regulating muscle and fat metabolism. Free Radic. Biol. Med. 2016, 100, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Zhang, C.; Wu, W.; Liang, Y.; Wang, A.; Wu, S.; Zhao, Y.; Hou, L.; Ning, Q.; Luo, X. Circulating MOTS-c levels are decreased in obese male children and adolescents and associated with insulin resistance. Pediatr. Diabetes 2018. [Google Scholar] [CrossRef] [PubMed]
- Kang, G.M.; Min, S.H.; Lee, C.H.; Kim, J.Y.; Lim, H.S.; Choi, M.J.; Jung, S.B.; Park, J.W.; Kim, S.; Park, C.B.; et al. Mitohormesis in Hypothalamic POMC Neurons Mediates Regular Exercise-Induced High-Turnover Metabolism. Cell Metab. 2021, 33, 334–349.e6. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhang, Q.; Shan, Y.; Ye, F.; Zhang, X.; Cheng, J.; Wang, X.; Zhao, Y.; Dan, G.; Chen, M.; et al. The Mitochondrial-Derived Peptide (MOTS-c) Interacted with Nrf2 to Defend the Antioxidant System to Protect Dopaminergic Neurons Against Rotenone Exposure. Mol. Neurobiol. 2023, 60, 5915–5930. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wei, M.; Zhai, Y.; Li, Q.; Ye, Z.; Wang, L.; Luo, W.; Chen, J.; Lu, Z. MOTS-c peptide regulates adipose homeostasis to prevent ovariectomy-induced metabolic dysfunction. J. Mol. Med. 2019, 97, 473–485. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, R.F.; Woodhead, J.S.T.; Hedges, C.P.; Zeng, N.; Wan, J.; Kumagai, H.; Lee, C.; Cohen, P.; Cameron-Smith, D.; Mitchell, C.J.; et al. Increased expression of the mitochondrial derived peptide, MOTS-c, in skeletal muscle of healthy aging men is associated with myofiber composition. Aging 2020, 12, 5244–5258. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Torres, A.; Reagan, A.L.; Howard, L.E.; Wiggins, E.; Vidal, A.C.; Wan, J.; Miller, B.; Freedland, S.J.; Cohen, P. Racial differences in circulating mitochondria-derived peptides may contribute to prostate cancer health disparities. Prostate 2022, 82, 1248–1257. [Google Scholar] [CrossRef] [PubMed]
- Cuyas, E.; Verdura, S.; Martin-Castillo, B.; Menendez, J.A.; METTEN Study Group. Circulating levels of MOTS-c in patients with breast cancer treated with metformin. Aging 2022, 15, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Dieli-Conwright, C.M.; Sami, N.; Norris, M.K.; Wan, J.; Kumagai, H.; Kim, S.J.; Cohen, P. Effect of aerobic and resistance exercise on the mitochondrial peptide MOTS-c in Hispanic and Non-Hispanic White breast cancer survivors. Sci. Rep. 2021, 11, 16916. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas. Available online: https://www.cancer.gov/ccg/research/genome-sequencing/tcga (accessed on 26 June 2024).
- Reynolds, J.C.; Lai, R.W.; Woodhead, J.S.T.; Joly, J.H.; Mitchell, C.J.; Cameron-Smith, D.; Lu, R.; Cohen, P.; Graham, N.A.; Benayoun, B.A.; et al. MOTS-c is an exercise-induced mitochondrial-encoded regulator of age-dependent physical decline and muscle homeostasis. Nat. Commun. 2021, 12, 470. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Miller, B.; Kumagai, H.; Silverstein, A.R.; Flores, M.; Yen, K. Mitochondrial-derived peptides in aging and age-related diseases. Geroscience 2021, 43, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Sanchez, C.E.; Gomez-Sanchez, E.P. Cholesterol Availability and Adrenal Steroidogenesis. Endocrinology 2024, 165, bqae032. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L. Role of mitochondria in steroidogenesis. Endocr. Dev. 2011, 20, 1–19. [Google Scholar] [PubMed]
- Quinkler, M.; Born-Frontsberg, E.; Fourkiotis, V.G. Comorbidities in primary aldosteronism. Horm. Metab. Res. 2010, 42, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Hanslik, G.; Wallaschofski, H.; Dietz, A.; Riester, A.; Reincke, M.; Allolio, B.; Lang, K.; Quack, I.; Rump, L.C.; Willenberg, H.S.; et al. Increased prevalence of diabetes mellitus and the metabolic syndrome in patients with primary aldosteronism of the German Conn’s Registry. Eur. J. Endocrinol. 2015, 173, 665–675. [Google Scholar] [CrossRef] [PubMed]
- La Batide-Alanore, A.; Chatellier, G.; Plouin, P.F. Diabetes as a marker of pheochromocytoma in hypertensive patients. J. Hypertens. 2003, 21, 1703–1707. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Pan, Y.; He, J.; Zhong, H.; Wu, Y.; Ji, C.; Liu, L.; Cui, X. The mitochondrial-derived peptide MOTS-c relieves hyperglycemia and insulin resistance in gestational diabetes mellitus. Pharmacol. Res. 2022, 175, 105987. [Google Scholar] [CrossRef] [PubMed]
- Kirik, A.; Dogru, T.; Yanik, B.; Sen, H.; Eroglu, M.; Baykan, O.; Bozyel, E.A.; Ergene, A.; Selcuk, E.; Tasci, I.; et al. The relationship of circulating MOTS-c level with liver fibrosis and metabolic components in patients with metabolic dysfunction-associated fatty liver disease. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 8074–8080. [Google Scholar] [PubMed]
- Ramanjaneya, M.; Bettahi, I.; Jerobin, J.; Chandra, P.; Abi Khalil, C.; Skarulis, M.; Atkin, S.L.; Abou-Samra, A.B. Mitochondrial-Derived Peptides Are Down Regulated in Diabetes Subjects. Front. Endocrinol. 2019, 10, 331. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-Response Analysis Using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [PubMed]
- Blatkiewicz, M.; Kaminski, K.; Szyszka, M.; Al-Shakarchi, Z.; Olechnowicz, A.; Stelcer, E.; Komarowska, H.; Tyczewska, M.; Klimont, A.; Karczewski, M.; et al. The Enhanced Expression of ZWILCH Predicts Poor Survival of Adrenocortical Carcinoma Patients. Biomedicines 2023, 11, 1233. [Google Scholar] [CrossRef] [PubMed]
- Komarowska, H.; Malinska, A.; Komekbai, Z.; Brominska, B.; Bednarek-Rajewska, K.; Ruchala, M.; Rucinski, M. Immunohistochemical analysis of ghrelin expression in various types of adrenal tumors. Folia Histochem. Cytobiol. 2021, 59, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Blatkiewicz, M.; Szyszka, M.; Olechnowicz, A.; Kaminski, K.; Jopek, K.; Komarowska, H.; Tyczewska, M.; Klimont, A.; Wierzbicki, T.; Karczewski, M.; et al. Impaired Expression of Humanin during Adrenocortical Carcinoma. Int. J. Mol. Sci. 2024, 25, 1038. [Google Scholar] [CrossRef] [PubMed]
- The Open Lab Book. Available online: https://theolb.readthedocs.io/en/latest/imaging/measuring-cell-fluorescence-using-imagej.html (accessed on 16 November 2023).
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.C.; Muller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- ggprism: A ‘ggplot2’ Extension Inspired by ‘GraphPad Prism’. Available online: https://csdaw.github.io/ggprism/authors.html#citation (accessed on 26 June 2024).
- An Introduction to corrplot Package. Available online: https://cran.r-project.org/web/packages/corrplot/vignettes/corrplot-intro.html (accessed on 26 June 2024).
| Characteristic Mean (min–max) | ACC | ACA | PCC |
|---|---|---|---|
| Sex (male/female) | 9/13 | 4/23 | 9/9 |
| Age (years) | 54 (27–82) | 62.3 (30–86) | 58.1 (31–82) |
| Tumor size (mm) | 130.5 (57–230) | 39.1 (7–67) | 44.7 (19–75) |
| Glucose (mg/dL) | 136.9 (83–466) | 102.3 (73–175) | 118.8 (81–185) |
| Total cholesterol (mg/dL) | 154.4 (109–203) | 198.8 (146–266) | 217.4 (130–298) |
| HDL (mg/dL) | 42.8 (31–62) | 67.2 (39–119) | 68.9 (37–121) |
| Testosterone (nmol/L) | 10.3 (0.2–44.7) | 4.8 (0.1–24.3) | 8.0 (0.8–19.3) |
| NA (mmol/L) | 140.5 (137–145) | 141.3 (136–149) | 141.1 (138–144) |
| K (mmol/L) | 4.4 (3.1–5.3) | 4.2 (2.4–5.2) | 4.6 (4–5.4) |
| Height (cm) | 167.1 (156–180) | 163.4 (149–175) | 171 (158–188) |
| Weight (kg) | 71.7 (56–89) | 74.2 (52–104) | 73.4 (51–107) |
| BMI | 25.7 (17.9–31.2) | 27.1 (17.9–37.6) | 24.4 (18.8–31.1) |
| Characteristic Mean (min–max) | ACC | ACA | PCC |
|---|---|---|---|
| Sex (male/female) | 9/20 | 8/20 | 8/0 |
| Age (years) | 53 (25–74) | 61 (27–81) | 45 (33–65) |
| Tumor size (mm) | 116.6 (45–232) | 28.3 (11–53) | 57.9 (37–75) |
| Glucose (mg/dL) | 98.6 (81–154) | 103.8 (85–165) | 120.1 (98–154) |
| Total cholesterol (mg/dL) | 180.3 (102–277) | 190.5 (131–274) | 240.5 (170–298) |
| HDL (mg/dL) | 50.4 (21–82) | 57.7 (32–79) | 61.2 (48–85) |
| Testosterone (nmol/L) | 9.1 (0.2–52) | 4.5 (0.1–19.4) | 18.7 (10.2–26.6) |
| Na (mmol/L) | 141.7 (137–146) | 141.7 (136–150) | 140 (135–143) |
| K (mmol/L) | 4.5 (3.3–5.6) | 4.5 (3.8–5.3) | 4.7 (4.3–5) |
| Height (cm) | 167.4 (156–183) | 167.24 (146–198) | 175.7 (162–196) |
| Weight (kg) | 76.8 (54–133) | 82 (55–150) | 68.5 (54–85) |
| BMI | 27.4 (17.9–41) | 29.4 (20.5–55) | 22.1 (19–24.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kamiński, K.; Blatkiewicz, M.; Szyszka, M.; Olechnowicz, A.; Komarowska, H.; Klimont, A.; Wierzbicki, T.; Karczewski, M.; Ruchała, M.; Rucinski, M. Expression Patterns of MOTS-c in Adrenal Tumors: Results from a Preliminary Study. Int. J. Mol. Sci. 2024, 25, 8721. https://doi.org/10.3390/ijms25168721
Kamiński K, Blatkiewicz M, Szyszka M, Olechnowicz A, Komarowska H, Klimont A, Wierzbicki T, Karczewski M, Ruchała M, Rucinski M. Expression Patterns of MOTS-c in Adrenal Tumors: Results from a Preliminary Study. International Journal of Molecular Sciences. 2024; 25(16):8721. https://doi.org/10.3390/ijms25168721
Chicago/Turabian StyleKamiński, Kacper, Małgorzata Blatkiewicz, Marta Szyszka, Anna Olechnowicz, Hanna Komarowska, Anna Klimont, Tomasz Wierzbicki, Marek Karczewski, Marek Ruchała, and Marcin Rucinski. 2024. "Expression Patterns of MOTS-c in Adrenal Tumors: Results from a Preliminary Study" International Journal of Molecular Sciences 25, no. 16: 8721. https://doi.org/10.3390/ijms25168721
APA StyleKamiński, K., Blatkiewicz, M., Szyszka, M., Olechnowicz, A., Komarowska, H., Klimont, A., Wierzbicki, T., Karczewski, M., Ruchała, M., & Rucinski, M. (2024). Expression Patterns of MOTS-c in Adrenal Tumors: Results from a Preliminary Study. International Journal of Molecular Sciences, 25(16), 8721. https://doi.org/10.3390/ijms25168721






