Pathophysiological Relationship between Type 2 Diabetes Mellitus and Metabolic Dysfunction-Associated Steatotic Liver Disease: Novel Therapeutic Approaches
Abstract
:1. Introduction
1.1. Epidemiology
1.2. Type 2 Diabetes Mellitus (T2DM)
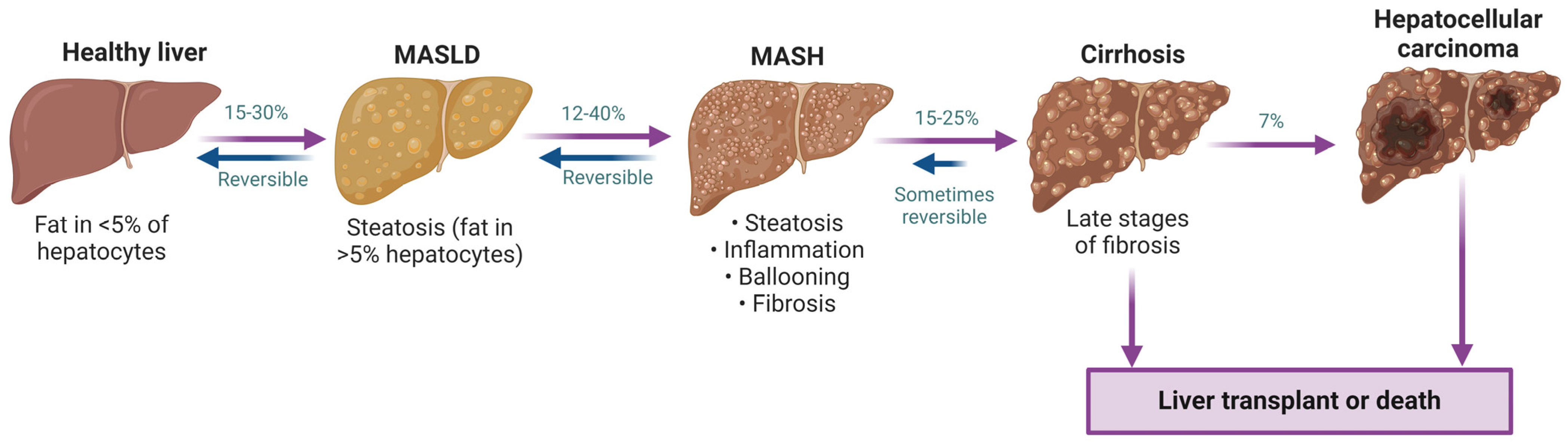
1.3. Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD)
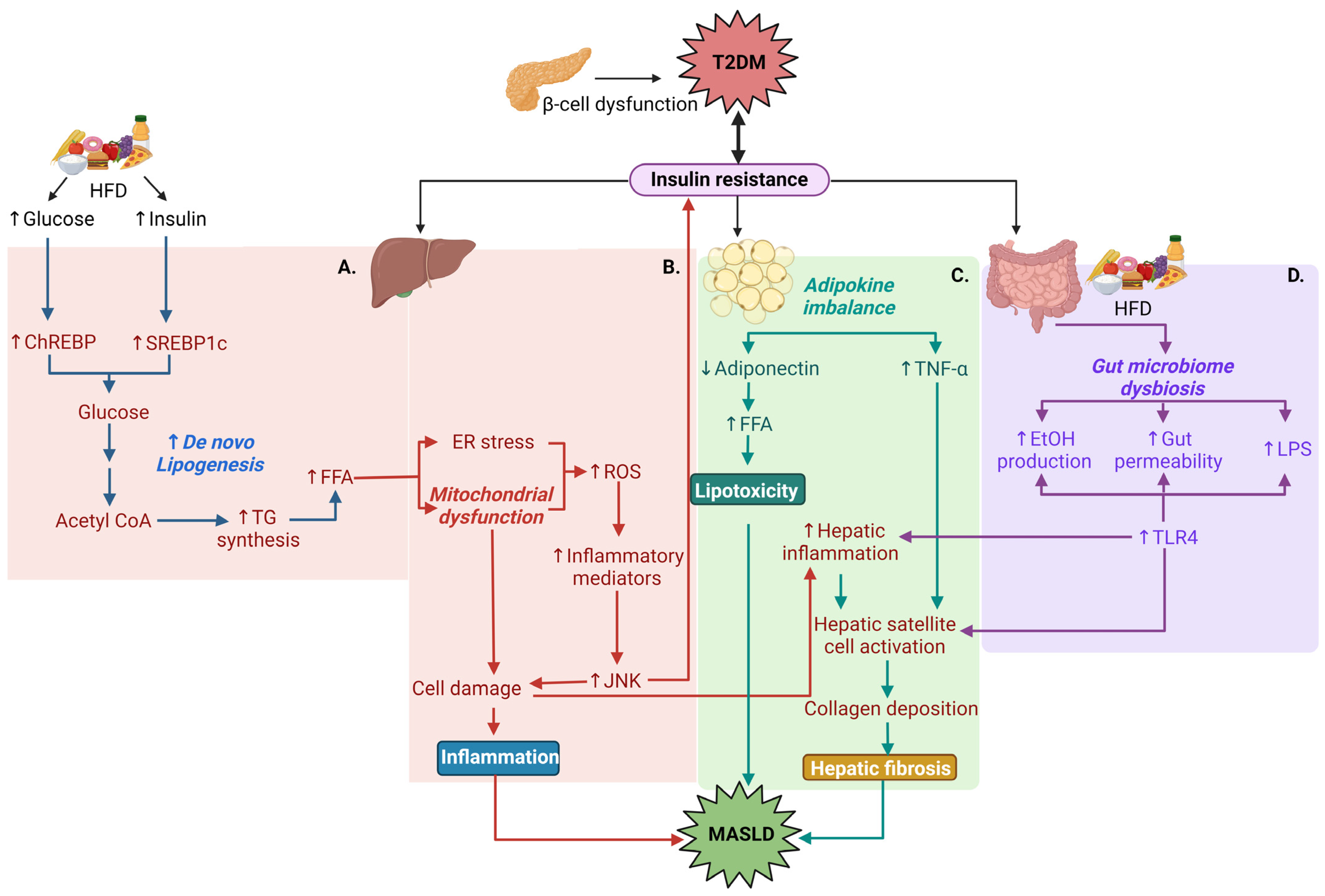
1.4. Pathophysiological Relationships between T2DM and MASLD
2. Novel Drug Approaches Common in T2DM and MASLD
2.1. GPR119 Agonists and Incretin Mimetics
2.2. Peroxisome Proliferator-Activated Receptor (PPAR) Agonists
2.3. THR Agonists
2.4. SGLT2 Inhibitors
2.5. Drugs Targeting the Mitochondria
2.5.1. Mitochondrial Pyruvate Carrier (MPC) Modulator
2.5.2. Mitochondrial Uncoupling Agents
2.6. Direct AMPK Activators
3. Treatment Approaches for T2DM
3.1. Novel Treatment Strategies for T2DM
3.1.1. SNO-CoA-Assisted Nitrosylase (SCAN) Enzyme Inhibition
3.1.2. Ketohexokinase Inhibition
3.1.3. Angiopoietin-Related Protein-3 (ANGPTL3) Inhibition
3.1.4. 11-β Hydroxysteroid Dehydrogenase-1 (11β-HSD1) Inhibition
3.1.5. Lyn Protein Tyrosine Kinase Activation
4. Current Treatment Approaches for MASLD
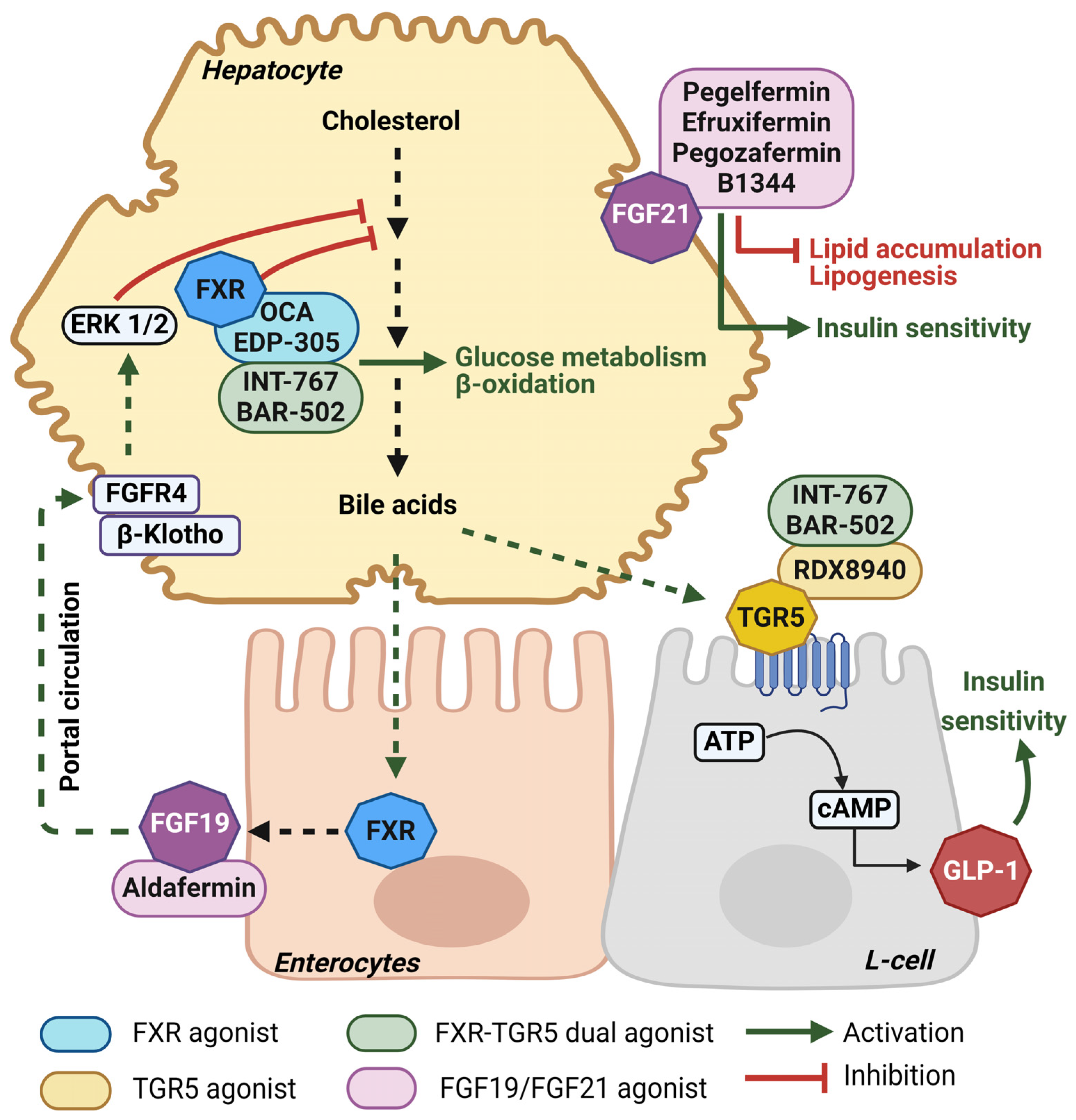
4.1. Novel Bile-Acid-Based Treatment Approaches for MASLD
4.1.1. Farnesoid X Receptor (FXR) Agonism
4.1.2. Takeda G-Protein-Coupled Receptor (TGR5) Agonism
4.1.3. Fibroblast Growth Factor (FGF) Analogs
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, R.C.W.; Tong, P.C.Y. Epidemiology of Type 2 Diabetes. In Textbook of Diabetes; Wiley: Hoboken, NJ, USA, 2024; pp. 55–74. ISBN 9781119697473. [Google Scholar]
- Miao, L.; Targher, G.; Byrne, C.D.; Cao, Y.-Y.; Zheng, M.-H. Current Status and Future Trends of the Global Burden of MASLD. Trends Endocrinol. Metab. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Ajmera, V.; Cepin, S.; Tesfai, K.; Hofflich, H.; Cadman, K.; Lopez, S.; Madamba, E.; Bettencourt, R.; Richards, L.; Behling, C.; et al. A Prospective Study on the Prevalence of NAFLD, Advanced Fibrosis, Cirrhosis and Hepatocellular Carcinoma in People with Type 2 Diabetes. J. Hepatol. 2023, 78, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Golabi, P.; Price, J.K.; Owrangi, S.; Gundu-Rao, N.; Satchi, R.; Paik, J.M. The Global Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis Among Patients With Type 2 Diabetes. Clin. Gastroenterol. Hepatol. 2024; online ahead of print. [Google Scholar] [CrossRef]
- Mantovani, A.; Byrne, C.D.; Bonora, E.; Targher, G. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-Analysis. Diabetes Care 2018, 41, 372–382. [Google Scholar] [CrossRef] [PubMed]
- Björkström, K.; Stål, P.; Hultcrantz, R.; Hagström, H. Histologic Scores for Fat and Fibrosis Associate With Development of Type 2 Diabetes in Patients with Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2017, 15, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Le, M.H.; Yeo, Y.H.; Zou, B.; Barnet, S.; Henry, L.; Cheung, R.; Nguyen, M.H. Forecasted 2040 Global Prevalence of Nonalcoholic Fatty Liver Disease Using Hierarchical Bayesian Approach. Clin. Mol. Hepatol. 2022, 28, 841–850. [Google Scholar] [CrossRef]
- Wong, V.W.-S.; Ekstedt, M.; Wong, G.L.-H.; Hagström, H. Changing Epidemiology, Global Trends and Implications for Outcomes of NAFLD. J. Hepatol. 2023, 79, 842–852. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.; Peng, X.; Li, X.; An, K.; He, H.; Fu, X.; Li, S.; An, Z. Unmasking the Enigma of Lipid Metabolism in Metabolic Dysfunction-Associated Steatotic Liver Disease: From Mechanism to the Clinic. Front. Med. 2023, 10, 1294267. [Google Scholar] [CrossRef]
- Dezső, K.; Paku, S.; Kóbori, L.; Thorgeirsson, S.S.; Nagy, P. What Makes Cirrhosis Irreversible?—Consideration on Structural Changes. Front. Med. 2022, 9, 876293. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Bedossa, P.; Guy, C.D.; Schattenberg, J.M.; Loomba, R.; Taub, R.; Labriola, D.; Moussa, S.E.; Neff, G.W.; Rinella, M.E.; et al. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. N. Engl. J. Med. 2024, 390, 497–509. [Google Scholar] [CrossRef]
- Caputo, T.; Gilardi, F.; Desvergne, B. From Chronic Overnutrition to Metaflammation and Insulin Resistance: Adipose Tissue and Liver Contributions. FEBS Lett. 2017, 591, 3061–3088. [Google Scholar] [CrossRef]
- Titchenell, P.M.; Chu, Q.; Monks, B.R.; Birnbaum, M.J. Hepatic Insulin Signalling Is Dispensable for Suppression of Glucose Output by Insulin in Vivo. Nat. Commun. 2015, 6, 7078. [Google Scholar] [CrossRef]
- Wan, M.; Leavens, K.F.; Hunter, R.W.; Koren, S.; von Wilamowitz-Moellendorff, A.; Lu, M.; Satapati, S.; Chu, Q.; Sakamoto, K.; Burgess, S.C.; et al. A Noncanonical, GSK3-Independent Pathway Controls Postprandial Hepatic Glycogen Deposition. Cell Metab. 2013, 18, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.I.; Shankaran, M.; Yoshino, M.; Schweitzer, G.G.; Chondronikola, M.; Beals, J.W.; Okunade, A.L.; Patterson, B.W.; Nyangau, E.; Field, T.; et al. Insulin Resistance Drives Hepatic de Novo Lipogenesis in Nonalcoholic Fatty Liver Disease. J. Clin. Invest. 2020, 130, 1453–1460. [Google Scholar] [CrossRef]
- Alexopoulos, A.; Crowley, M.J.; Wang, Y.; Moylan, C.A.; Guy, C.D.; Henao, R.; Piercy, D.L.; Seymour, K.A.; Sudan, R.; Portenier, D.D.; et al. Glycemic Control Predicts Severity of Hepatocyte Ballooning and Hepatic Fibrosis in Nonalcoholic Fatty Liver Disease. Hepatology 2021, 74, 1220–1233. [Google Scholar] [CrossRef] [PubMed]
- Acierno, C.; Caturano, A.; Pafundi, P.C.; Nevola, R.; Adinolfi, L.E.; Sasso, F.C. Nonalcoholic Fatty Liver Disease and Type 2 Diabetes: Pathophysiological Mechanisms Shared between the Two Faces of the Same Coin. Explor. Med. 2020, 1, 287–306. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Costea, C.F.; Ciocoiu, M.; Lacatusu, C.M.; Maranduca, M.A.; Ouatu, A.; Floria, M. The Intricate Relationship between Type 2 Diabetes Mellitus (T2DM), Insulin Resistance (IR), and Nonalcoholic Fatty Liver Disease (NAFLD). J. Diabetes Res. 2020, 2020, 3920196. [Google Scholar] [CrossRef] [PubMed]
- Vesković, M.; Šutulović, N.; Hrnčić, D.; Stanojlović, O.; Macut, D.; Mladenović, D. The Interconnection between Hepatic Insulin Resistance and Metabolic Dysfunction-Associated Steatotic Liver Disease—The Transition from an Adipocentric to Liver-Centric Approach. Curr. Issues Mol. Biol. 2023, 45, 9084–9102. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, D.; Wang, Z.; Sun, J.; Xu, B.; Chen, Y.; Ding, L.; Huang, X.; Lv, X.; Lu, J.; et al. Insulin Resistance Is Associated with Total Bile Acid Level in Type 2 Diabetic and Nondiabetic Population: A Cross-Sectional Study. Medicine 2016, 95, e2778. [Google Scholar] [CrossRef]
- Haeusler, R.A.; Astiarraga, B.; Camastra, S.; Accili, D.; Ferrannini, E. Human Insulin Resistance Is Associated with Increased Plasma Levels of 12α-Hydroxylated Bile Acids. Diabetes 2013, 62, 4184–4191. [Google Scholar] [CrossRef]
- Ferrell, J.M.; Chiang, J.Y.L.L. Understanding Bile Acid Signaling in Diabetes: From Pathophysiology to Therapeutic Targets. Diabetes Metab. J. 2019, 43, 257–272. [Google Scholar] [CrossRef]
- Schlein, C.; Talukdar, S.; Heine, M.; Fischer, A.W.; Krott, L.M.; Nilsson, S.K.; Brenner, M.B.; Heeren, J.; Scheja, L. FGF21 Lowers Plasma Triglycerides by Accelerating Lipoprotein Catabolism in White and Brown Adipose Tissues. Cell Metab. 2016, 23, 441–453. [Google Scholar] [CrossRef]
- Xu, J.; Lloyd, D.J.; Hale, C.; Stanislaus, S.; Chen, M.; Sivits, G.; Vonderfecht, S.; Hecht, R.; Li, Y.S.; Lindberg, R.A.; et al. Fibroblast Growth Factor 21 Reverses Hepatic Steatosis, Increases Energy Expenditure, and Improves Insulin Sensitivity in Diet-Induced Obese Mice. Diabetes 2009, 58, 250–259. [Google Scholar] [CrossRef]
- Potthoff, M.J.; Inagaki, T.; Satapati, S.; Ding, X.; He, T.; Goetz, R.; Mohammadi, M.; Finck, B.N.; Mangelsdorf, D.J.; Kliewer, S.A.; et al. FGF21 Induces PGC-1alpha and Regulates Carbohydrate and Fatty Acid Metabolism during the Adaptive Starvation Response. Proc. Natl. Acad. Sci. USA 2009, 106, 10853–10858. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhao, Y.; Hu, Y.; Peng, J. Targeting the GPR119/Incretin Axis: A Promising New Therapy for Metabolic-Associated Fatty Liver Disease. Cell. Mol. Biol. Lett. 2021, 26, 32. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.W.; Kim, H.S.; Im, J.H.; Kim, J.W.; Jun, D.W.; Lim, S.C.; Lee, K.; Choi, J.M.; Kim, S.K.; Kang, K.W. GPR119: A Promising Target for Nonalcoholic Fatty Liver Disease. FASEB J. 2016, 30, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Hartman, M.L.; Lawitz, E.J.; Vuppalanchi, R.; Boursier, J.; Bugianesi, E.; Yoneda, M.; Behling, C.; Cummings, O.W.; Tang, Y.; et al. Tirzepatide for Metabolic Dysfunction–Associated Steatohepatitis with Liver Fibrosis. N. Engl. J. Med. 2024, 391, 299–310. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, J.; Zhang, D.; Mu, X.; Shi, Y.; Chen, S.; Wu, Z.; Li, S. Efficacy and Safety of GLP-1 Receptor Agonists in Patients With Type 2 Diabetes Mellitus and Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2021, 12, 769069. [Google Scholar] [CrossRef]
- Zhou, R.; Lin, C.; Cheng, Y.; Zhuo, X.; Li, Q.; Xu, W.; Zhao, L.; Yang, L. Liraglutide Alleviates Hepatic Steatosis and Liver Injury in T2MD Rats via a GLP-1R Dependent AMPK Pathway. Front. Pharmacol. 2021, 11, 600175. [Google Scholar] [CrossRef]
- Astapova, O.; Leff, T. Adiponectin and PPARγ: Cooperative and Interdependent Actions of Two Key Regulators of Metabolism. In Adiponectin; Litwack, G.B.T.-V.H., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 90, pp. 143–162. ISBN 0083-6729. [Google Scholar]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR Control of Metabolism and Cardiovascular Functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef]
- Lefere, S.; Puengel, T.; Hundertmark, J.; Penners, C.; Frank, A.K.; Guillot, A.; de Muynck, K.; Heymann, F.; Adarbes, V.; Defrêne, E.; et al. Differential Effects of Selective- and Pan-PPAR Agonists on Experimental Steatohepatitis and Hepatic Macrophages☆. J. Hepatol. 2020, 73, 757–770. [Google Scholar] [CrossRef]
- Gawrieh, S.; Noureddin, M.; Loo, N.; Mohseni, R.; Awasty, V.; Cusi, K.; Kowdley, K.V.; Lai, M.; Schiff, E.; Parmar, D.; et al. Saroglitazar, a PPAR-α/γ Agonist, for Treatment of NAFLD: A Randomized Controlled Double-Blind Phase 2 Trial. Hepatology 2021, 74, 1809–1824. [Google Scholar] [CrossRef]
- Siddiqui, M.S.; Parmar, D.; Shaikh, F.; Forsgren, M.; Patel, S.; Bui, A.T.; Boyett, S.; Patel, V.; Sanyal, A.J. Saroglitazar Improves Nonalcoholic Fatty Liver Disease and Metabolic Health in Liver Transplant Recipients. Liver Transplant. 2023, 29, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.A.; Singh, B.K.; Yen, P.M. Direct Effects of Thyroid Hormones on Hepatic Lipid Metabolism. Nat. Rev. Endocrinol. 2018, 14, 259–269. [Google Scholar] [CrossRef]
- Tanase, D.M.; Gosav, E.M.; Neculae, E.; Costea, C.F.; Ciocoiu, M.; Hurjui, L.L.; Tarniceriu, C.C.; Floria, M. Hypothyroidism-Induced Nonalcoholic Fatty Liver Disease (HIN): Mechanisms and Emerging Therapeutic Options. Int. J. Mol. Sci. 2020, 21, 5927. [Google Scholar] [CrossRef]
- Karim, G.; Bansal, M. Resmetirom: An Orally Administered, Small-Molecule, Liver-Directed, β-Selective THR Agonist for the Treatment of Non-Alcoholic Fatty Liver Disease and Non-Alcoholic Steatohepatitis. touchEndocrinology 2023, 19, 60–70. [Google Scholar] [CrossRef]
- Harrison, S.A.; Bashir, M.R.; Guy, C.D.; Zhou, R.; Moylan, C.A.; Frias, J.P.; Alkhouri, N.; Bansal, M.B.; Baum, S.; Neuschwander-Tetri, B.A.; et al. Resmetirom (MGL-3196) for the Treatment of Non-Alcoholic Steatohepatitis: A Multicentre, Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet 2019, 394, 2012–2024. [Google Scholar] [CrossRef]
- Tee, S.A.; Tsatlidis, V.; Razvi, S. The GLP-1 Receptor Agonist Exenatide Reduces Serum TSH by Its Effect on Body Weight in People with Type 2 Diabetes. Clin. Endocrinol. 2023, 99, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Fitchett, D.; Hantel, S.; George, J.T.; Zinman, B. Empagliflozin Is Associated with Improvements in Liver Enzymes Potentially Consistent with Reductions in Liver Fat: Results from Randomised Trials Including the EMPA-REG OUTCOME® Trial. Diabetologia 2018, 61, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Xu, X.; Guo, L.; Li, J.; Li, L. Effect of SGLT2 Inhibitors on Type 2 Diabetes Mellitus With Non-Alcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. 2021, 12, 635556. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Liu, X.; Li, T.; Fang, T.; Cheng, Y.; Han, L.; Sun, B.; Chen, L. The SGLT2 Inhibitor Empagliflozin Negatively Regulates IL-17/IL-23 Axis-Mediated Inflammatory Responses in T2DM with NAFLD via the AMPK/MTOR/Autophagy Pathway. Int. Immunopharmacol. 2021, 94, 107492. [Google Scholar] [CrossRef]
- Shen, Y.; Cheng, L.; Xu, M.; Wang, W.; Wan, Z.; Xiong, H.; Guo, W.; Cai, M.; Xu, F. SGLT2 Inhibitor Empagliflozin Downregulates MiRNA-34a-5p and Targets GREM2 to Inactivate Hepatic Stellate Cells and Ameliorate Non-Alcoholic Fatty Liver Disease-Associated Fibrosis. Metabolism 2023, 146, 155657. [Google Scholar] [CrossRef]
- Arai, T.; Atsukawa, M.; Tsubota, A.; Mikami, S.; Haruki, U.; Yoshikata, K.; Ono, H.; Kawano, T.; Yoshida, Y.; Tanabe, T.; et al. Antifibrotic Effect and Long-term Outcome of SGLT2 Inhibitors in Patients with NAFLD Complicated by Diabetes Mellitus. Hepatol. Commun. 2022, 6, 3073–3082. [Google Scholar] [CrossRef] [PubMed]
- Akuta, N.; Kawamura, Y.; Fujiyama, S.; Saito, S.; Muraishi, N.; Sezaki, H.; Hosaka, T.; Kobayashi, M.; Kobayashi, M.; Arase, Y.; et al. Favorable Impact of Long-term SGLT2 Inhibitor for NAFLD Complicated by Diabetes Mellitus: A 5-year Follow-up Study. Hepatol. Commun. 2022, 6, 2286–2297. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, R.; Cespiati, A.; Mantovani, A.; Maffi, G.; Del Zanna, E.; Francione, P.; Cinque, F.; Villani, R.; Maffeis, C.; Passigato, N.; et al. SGLT2 Inhibitors Improve Hepatic Fibrosis Assessed by Fibroscan in NAFLD Patients with Type 2 Diabetes: A Five-Year Follow-up Study. Dig. Liver Dis. 2023, 55, S42. [Google Scholar] [CrossRef]
- Buchanan, J.L.; Taylor, E.B. Mitochondrial Pyruvate Carrier Function in Health and Disease across the Lifespan. Biomolecules 2020, 10, 1162. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Alkhouri, N.; Davison, B.A.; Sanyal, A.; Edwards, C.; Colca, J.R.; Lee, B.H.; Loomba, R.; Cusi, K.; Kolterman, O.; et al. Insulin Sensitizer MSDC-0602K in Non-Alcoholic Steatohepatitis: A Randomized, Double-Blind, Placebo-Controlled Phase IIb Study. J. Hepatol. 2020, 72, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Goedeke, L.; Shulman, G.I. Therapeutic Potential of Mitochondrial Uncouplers for the Treatment of Metabolic Associated Fatty Liver Disease and NASH. Mol. Metab. 2021, 46, 101178. [Google Scholar] [CrossRef]
- Noureddin, M.; Khan, S.; Portell, F.; Jorkasky, D.; Dennis, J.; Khan, O.; Johansson, L.; Johansson, E.; Sanyal, A.J. Safety and Efficacy of Once-Daily HU6 versus Placebo in People with Non-Alcoholic Fatty Liver Disease and High BMI: A Randomised, Double-Blind, Placebo-Controlled, Phase 2a Trial. Lancet Gastroenterol. Hepatol. 2023, 8, 1094–1105. [Google Scholar] [CrossRef]
- Cusi, K.; Alkhouri, N.; Harrison, S.A.; Fouqueray, P.; Moller, D.E.; Hallakou-Bozec, S.; Bolze, S.; Grouin, J.-M.; Jeannin Megnien, S.; Dubourg, J.; et al. Efficacy and Safety of PXL770, a Direct AMP Kinase Activator, for the Treatment of Non-Alcoholic Fatty Liver Disease (STAMP-NAFLD): A Randomised, Double-Blind, Placebo-Controlled, Phase 2a Study. Lancet Gastroenterol. Hepatol. 2021, 6, 889–902. [Google Scholar] [CrossRef]
- Sinnett, S.E.; Brenman, J.E. Past Strategies and Future Directions for Identifying AMP-Activated Protein Kinase (AMPK) Modulators. Pharmacol. Ther. 2014, 143, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Hausladen, A.; Wang, Y.-J.; Zhang, G.-F.; Stomberski, C.; Brunengraber, H.; Hess, D.T.; Stamler, J.S. Identification of S-Nitroso-CoA Reductases That Regulate Protein S-Nitrosylation. Proc. Natl. Acad. Sci. USA 2014, 111, 18572–18577. [Google Scholar] [CrossRef]
- Zhou, H.-L.; Grimmett, Z.W.; Venetos, N.M.; Stomberski, C.T.; Qian, Z.; McLaughlin, P.J.; Bansal, P.K.; Zhang, R.; Reynolds, J.D.; Premont, R.T.; et al. An Enzyme That Selectively S-Nitrosylates Proteins to Regulate Insulin Signaling. Cell 2023, 186, 5812–5825.e21. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Helsley, R.N.; Fadhul, T.; Willoughby, J.L.S.; Noetzli, L.; Tu, H.-C.; Solheim, M.H.; Fujisaka, S.; Pan, H.; Dreyfuss, J.M.; et al. Fructose Induced KHK-C Can Increase ER Stress Independent of Its Effect on Lipogenesis to Drive Liver Disease in Diet-Induced and Genetic Models of NAFLD. Metabolism 2023, 145, 155591. [Google Scholar] [CrossRef]
- Lee, H.-J.; Cha, J.-Y. Recent Insights into the Role of ChREBP in Intestinal Fructose Absorption and Metabolism. BMB Rep. 2018, 51, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.R.; Lyle, S.-A.; Khavandi, K.; Qiu, R.; Whitlock, M.; Esler, W.P.; Kim, A.M. A Phase 2a, Randomized, Double-Blind, Placebo-Controlled, Three-Arm, Parallel-Group Study to Assess the Efficacy, Safety, Tolerability and Pharmacodynamics of PF-06835919 in Patients with Non-Alcoholic Fatty Liver Disease and Type 2 Diabetes. Diabetes Obes. Metab. 2023, 25, 992–1001. [Google Scholar] [CrossRef]
- Kazierad, D.J.; Chidsey, K.; Somayaji, V.R.; Bergman, A.J.; Birnbaum, M.J.; Calle, R.A. Inhibition of Ketohexokinase in Adults with NAFLD Reduces Liver Fat and Inflammatory Markers: A Randomized Phase 2 Trial. Med 2021, 2, 800–813.e3. [Google Scholar] [CrossRef]
- Abu-Farha, M.; Al-Khairi, I.; Cherian, P.; Chandy, B.; Sriraman, D.; Alhubail, A.; Al-Refaei, F.; AlTerki, A.; Abubaker, J. Increased ANGPTL3, 4 and ANGPTL8/Betatrophin Expression Levels in Obesity and T2D. Lipids Health Dis. 2016, 15, 181. [Google Scholar] [CrossRef] [PubMed]
- Christopoulou, E.; Elisaf, M.; Filippatos, T. Effects of Angiopoietin-Like 3 on Triglyceride Regulation, Glucose Homeostasis, and Diabetes. Dis. Markers 2019, 2019, 6578327. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, D.; Karwatowska-Prokopczuk, E.; Baum, S.J.; Hurh, E.; Kingsbury, J.; Bartlett, V.J.; Figueroa, A.L.; Piscitelli, P.; Singleton, W.; Witztum, J.L.; et al. Vupanorsen, an N-Acetyl Galactosamine-Conjugated Antisense Drug to ANGPTL3 MRNA, Lowers Triglycerides and Atherogenic Lipoproteins in Patients with Diabetes, Hepatic Steatosis, and Hypertriglyceridaemia. Eur. Heart J. 2020, 41, 3936–3945. [Google Scholar] [CrossRef]
- Almeida, C.; Monteiro, C.; Silvestre, S. Inhibitors of 11β-Hydroxysteroid Dehydrogenase Type 1 as Potential Drugs for Type 2 Diabetes Mellitus—A Systematic Review of Clinical and In Vivo Preclinical Studies. Sci. Pharm. 2021, 89, 5. [Google Scholar] [CrossRef]
- Kupczyk, D.; Bilski, R.; Kozakiewicz, M.; Studzińska, R.; Kędziora-Kornatowska, K.; Kosmalski, T.; Pedrycz-Wieczorska, A.; Głowacka, M. 11β-HSD as a New Target in Pharmacotherapy of Metabolic Diseases. Int. J. Mol. Sci. 2022, 23, 8984. [Google Scholar] [CrossRef]
- Rosenstock, J.; Banarer, S.; Fonseca, V.A.; Inzucchi, S.E.; Sun, W.; Yao, W.; Hollis, G.; Flores, R.; Levy, R.; Williams, W.V.; et al. The 11-β-Hydroxysteroid Dehydrogenase Type 1 Inhibitor INCB13739 Improves Hyperglycemia in Patients With Type 2 Diabetes Inadequately Controlled by Metformin Monotherapy. Diabetes Care 2010, 33, 1516–1522. [Google Scholar] [CrossRef] [PubMed]
- Ochman, A.R.; Lipinski, C.A.; Handler, J.A.; Reaume, A.G.; Saporito, M.S. The Lyn Kinase Activator MLR-1023 Is a Novel Insulin Receptor Potentiator That Elicits a Rapid-Onset and Durable Improvement in Glucose Homeostasis in Animal Models of Type 2 Diabetes. J. Pharmacol. Exp. Ther. 2012, 342, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-K.; Kim, S.G.; Watkins, E.; Moon, M.K.; Rhee, S.Y.; Frias, J.P.; Chung, C.H.; Lee, S.-H.; Block, B.; Cha, B.S.; et al. A Novel Non-PPARgamma Insulin Sensitizer: MLR-1023 Clinicalproof-of-Concept in Type 2 Diabetes Mellitus. J. Diabetes Complicat. 2020, 34, 107555. [Google Scholar] [CrossRef] [PubMed]
- Marino, L.; Kim, A.; Ni, B.; Celi, F.S. Thyroid Hormone Action and Liver Disease, a Complex Interplay. Hepatology, 2023; online ahead of print. [Google Scholar] [CrossRef]
- Cusi, K.; Isaacs, S.; Barb, D.; Basu, R.; Caprio, S.; Garvey, W.T.; Kashyap, S.; Mechanick, J.I.; Mouzaki, M.; Nadolsky, K.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline for the Diagnosis and Management of Nonalcoholic Fatty Liver Disease in Primary Care and Endocrinology Clinical Settings: Co-Sponsored by the American Association for the Study of Liver Diseases (AASLD). Endocr. Pract. 2022, 28, 528–562. [Google Scholar] [CrossRef] [PubMed]
- Neuschwander-Tetri, B.A.; Loomba, R.; Sanyal, A.J.; Lavine, J.E.; Van Natta, M.L.; Abdelmalek, M.F.; Chalasani, N.; Dasarathy, S.; Diehl, A.M.; Hameed, B.; et al. Farnesoid X Nuclear Receptor Ligand Obeticholic Acid for Non-Cirrhotic, Non-Alcoholic Steatohepatitis (FLINT): A Multicentre, Randomised, Placebo-Controlled Trial. Lancet 2015, 385, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Mudaliar, S.; Henry, R.R.; Sanyal, A.J.; Morrow, L.; Marschall, H.U.; Kipnes, M.; Adorini, L.; Sciacca, C.I.; Clopton, P.; Castelloe, E.; et al. Efficacy and Safety of the Farnesoid x Receptor Agonist Obeticholic Acid in Patients with Type 2 Diabetes and Nonalcoholic Fatty Liver Disease. Gastroenterology 2013, 145, 574–582.e1. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Ratziu, V.; Loomba, R.; Anstee, Q.M.; Kowdley, K.V.; Rinella, M.E.; Sheikh, M.Y.; Trotter, J.F.; Knapple, W.; Lawitz, E.J.; et al. Results from a New Efficacy and Safety Analysis of the REGENERATE Trial of Obeticholic Acid for Treatment of Pre-Cirrhotic Fibrosis Due to Non-Alcoholic Steatohepatitis. J. Hepatol. 2023, 79, 1110–1120. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Rinella, M.E.; Neuschwander-Tetri, B.A.; Lawitz, E.; Denham, D.; Kayali, Z.; Sheikh, A.; Kowdley, K.V.; Desta, T.; Elkhashab, M.; et al. EDP-305 in Patients with NASH: A Phase II Double-Blind Placebo-Controlled Dose-Ranging Study. J. Hepatol. 2022, 76, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhang, H.; Xiao, D.; Wei, H.; Chen, Y. Farnesoid X Receptor (FXR): Structures and Ligands. Comput. Struct. Biotechnol. J. 2021, 19, 2148–2159. [Google Scholar] [CrossRef]
- van Nierop, F.S.; Scheltema, M.J.; Eggink, H.M.; Pols, T.W.; Sonne, D.P.; Knop, F.K.; Soeters, M.R. Clinical Relevance of the Bile Acid Receptor TGR5 in Metabolism. Lancet Diabetes Endocrinol. 2017, 5, 224–233. [Google Scholar] [CrossRef]
- Thomas, C.; Gioiello, A.; Noriega, L.; Strehle, A.; Oury, J.; Rizzo, G.; Macchiarulo, A.; Yamamoto, H.; Mataki, C.; Pruzanski, M.; et al. TGR5-Mediated Bile Acid Sensing Controls Glucose Homeostasis. Cell Metab. 2009, 10, 167–177. [Google Scholar] [CrossRef]
- Carino, A.; Cipriani, S.; Marchianò, S.; Biagioli, M.; Scarpelli, P.; Zampella, A.; Monti, M.C.; Fiorucci, S. Gpbar1 Agonism Promotes a Pgc-1α-Dependent Browning of White Adipose Tissue and Energy Expenditure and Reverses Diet-Induced Steatohepatitis in Mice. Sci. Rep. 2017, 7, 13689. [Google Scholar] [CrossRef] [PubMed]
- Carino, A.; Marchianò, S.; Biagioli, M.; Bucci, M.; Vellecco, V.; Brancaleone, V.; Fiorucci, C.; Zampella, A.; Monti, M.C.; Distrutti, E.; et al. Agonism for the Bile Acid Receptor GPBAR1 Reverses Liver and Vascular Damage in a Mouse Model of Steatohepatitis. FASEB J. 2019, 33, 2809–2822. [Google Scholar] [CrossRef]
- Finn, P.D.; Rodriguez, D.; Kohler, J.; Jiang, Z.; Wan, S.; Blanco, E.; King, A.J.; Chen, T.; Bell, N.; Dragoli, D.; et al. Intestinal TGR5 Agonism Improves Hepatic Steatosis and Insulin Sensitivity in Western Diet-Fed Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 316, G412–G424. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Gerspach, A.C.; Steinert, R.E.; Keller, S.; Malarski, A.; Schulte, F.H.; Beglinger, C. Effects of Chenodeoxycholic Acid on the Secretion of Gut Peptides and Fibroblast Growth Factors in Healthy Humans. J. Clin. Endocrinol. Metab. 2013, 98, 3351–3358. [Google Scholar] [CrossRef]
- Roth, J.D.; Feigh, M.; Veidal, S.S.; Fensholdt, L.K.; Rigbolt, K.T.; Hansen, H.H.; Chen, L.C.; Petitjean, M.; Friley, W.; Vrang, N.; et al. INT-767 Improves Histopathological Features in a Diet-Induced Ob/Ob Mouse Model of Biopsy-Confirmed Non-Alcoholic Roth JD, Feigh M, Veidal SS, Fensholdt LK, Rigbolt KT, Hansen HH, Chen LC, Petitjean M, Friley W, Vrang N, Jelsing J, Young M. INT-767 Improv. World J. Gastroenterol. 2018, 24, 195–210. [Google Scholar] [CrossRef]
- Anfuso, B.; Tiribelli, C.; Adorini, L.; Rosso, N. Obeticholic Acid and INT-767 Modulate Collagen Deposition in a NASH in Vitro Model. Sci. Rep. 2020, 10, 1699. [Google Scholar] [CrossRef]
- Carino, A.; Cipriani, S.; Marchiano, S.; Biagioli, M.; Santorelli, C.; Donini, A.; Zampella, A.; Monti, M.C.; Fiorucci, S. BAR502, a Dual FXR and GPBAR1 Agonist, Promotes Browning of White Adipose Tissue and Reverses Liver Steatosis and Fibrosis. Sci. Rep. 2017, 7, 42801. [Google Scholar] [CrossRef]
- Marchianò, S.; Biagioli, M.; Morretta, E.; Di Giorgio, C.; Roselli, R.; Bordoni, M.; Bellini, R.; Urbani, G.; Massa, C.; Monti, M.C.; et al. Combinatorial Therapy with BAR502 and UDCA Resets FXR and GPBAR1 Signaling and Reverses Liver Histopathology in a Model of NASH. Sci. Rep. 2023, 13, 1602. [Google Scholar] [CrossRef]
- Talukdar, S.; Kharitonenkov, A. FGF19 and FGF21: In NASH We Trust. Mol. Metab. 2021, 46, 101152. [Google Scholar] [CrossRef]
- Harrison, S.A.; Neff, G.; Guy, C.D.; Bashir, M.R.; Paredes, A.H.; Frias, J.P.; Younes, Z.; Trotter, J.F.; Gunn, N.T.; Moussa, S.E.; et al. Efficacy and Safety of Aldafermin, an Engineered FGF19 Analog, in a Randomized, Double-Blind, Placebo-Controlled Trial of Patients With Nonalcoholic Steatohepatitis. Gastroenterology 2021, 160, 219–231.e1. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Abdelmalek, M.F.; Neff, G.; Gunn, N.; Guy, C.D.; Alkhouri, N.; Bashir, M.R.; Freilich, B.; Kohli, A.; Khazanchi, A.; et al. Aldafermin in Patients with Non-Alcoholic Steatohepatitis (ALPINE 2/3): A Randomised, Double-Blind, Placebo-Controlled, Phase 2b Trial. Lancet Gastroenterol. Hepatol. 2022, 7, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.A.; Minnich, A.; Sanyal, A.J.; Loomba, R.; Du, S.; Schwarz, J.; Ehman, R.L.; Karsdal, M.; Leeming, D.J.; Cizza, G.; et al. Effect of Pegbelfermin on NASH and Fibrosis-Related Biomarkers and Correlation with Histological Response in the FALCON 1 Trial. JHEP Rep. 2023, 5, 100661. [Google Scholar] [CrossRef] [PubMed]
- Abdelmalek, M.F.; Sanyal, A.J.; Nakajima, A.; Neuschwander-Tetri, B.A.; Goodman, Z.D.; Lawitz, E.J.; Harrison, S.A.; Jacobson, I.M.; Imajo, K.; Gunn, N.; et al. Pegbelfermin in Patients With Nonalcoholic Steatohepatitis and Compensated Cirrhosis (FALCON 2): A Randomized Phase 2b Study. Clin. Gastroenterol. Hepatol. 2024, 22, 113–123.e9. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Li, J.; Ji, S.; Ma, F.; Wang, G.; Xue, Y.; Liu, Z.; Gao, J.; Han, J.; Tai, P.; et al. The Effects of B1344, a Novel Fibroblast Growth Factor 21 Analog, on Nonalcoholic Steatohepatitis in Nonhuman Primates. Diabetes 2020, 69, 1611–1623. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Ruane, P.J.; Freilich, B.L.; Neff, G.; Patil, R.; Behling, C.A.; Hu, C.; Fong, E.; de Temple, B.; Tillman, E.J.; et al. Efruxifermin in Non-Alcoholic Steatohepatitis: A Randomized, Double-Blind, Placebo-Controlled, Phase 2a Trial. Nat. Med. 2021, 27, 1262–1271. [Google Scholar] [CrossRef]
- Watanabe, M.; Houten, S.M.; Wang, L.; Moschetta, A.; Mangelsdorf, D.J.; Heyman, R.A.; Moore, D.D.; Auwerx, J. Bile Acids Lower Triglyceride Levels via a Pathway Involving FXR, SHP, and SREBP-1c. J. Clin. Investig. 2004, 113, 1408–1418. [Google Scholar] [CrossRef] [PubMed]
- Caron, S.; Huaman Samanez, C.; Dehondt, H.; Ploton, M.; Briand, O.; Lien, F.; Dorchies, E.; Dumont, J.; Postic, C.; Cariou, B.; et al. Farnesoid X Receptor Inhibits the Transcriptional Activity of Carbohydrate Response Element Binding Protein in Human Hepatocytes. Mol. Cell. Biol. 2013, 33, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lee, F.Y.; Barrera, G.; Lee, H.; Vales, C.; Gonzalez, F.J.; Willson, T.M.; Edwards, P.A. Activation of the Nuclear Receptor FXR Improves Hyperglycemia and Hyperlipidemia in Diabetic Mice. Proc. Natl. Acad. Sci. USA 2006, 103, 1006–1011. [Google Scholar] [CrossRef]
- Kim, C.-W.; Addy, C.; Kusunoki, J.; Anderson, N.N.; Deja, S.; Fu, X.; Burgess, S.C.; Li, C.; Ruddy, M.; Chakravarthy, M.; et al. Acetyl CoA Carboxylase Inhibition Reduces Hepatic Steatosis but Elevates Plasma Triglycerides in Mice and Humans: A Bedside to Bench Investigation. Cell Metab. 2017, 26, 394–406.e6. [Google Scholar] [CrossRef]
- Ratziu, V.; de Guevara, L.; Safadi, R.; Poordad, F.; Fuster, F.; Flores-Figueroa, J.; Arrese, M.; Fracanzani, A.L.; Ben Bashat, D.; Lackner, K.; et al. Aramchol in Patients with Nonalcoholic Steatohepatitis: A Randomized, Double-Blind, Placebo-Controlled Phase 2b Trial. Nat. Med. 2021, 27, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.B.; Carvajal-Gonzalez, S.; Purkal, J.; Zhu, T.; Crowley, C.; Perez, S.; Chidsey, K.; Kim, A.M.; Goodwin, B. Targeting Diacylglycerol Acyltransferase 2 for the Treatment of Nonalcoholic Steatohepatitis. Sci. Transl. Med. 2019, 11, eaav9701. [Google Scholar] [CrossRef] [PubMed]
- Sanyal, A.J.; Chalasani, N.; Kowdley, K.V.; McCullough, A.; Diehl, A.M.; Bass, N.M.; Neuschwander-Tetri, B.A.; Lavine, J.E.; Tonascia, J.; Unalp, A.; et al. Pioglitazone, Vitamin E, or Placebo for Nonalcoholic Steatohepatitis. N. Engl. J. Med. 2010, 362, 1675–1685. [Google Scholar] [CrossRef]
- Mantovani, A.; Petracca, G.; Beatrice, G.; Tilg, H.; Byrne, C.D.; Targher, G. Non-Alcoholic Fatty Liver Disease and Risk of Incident Diabetes Mellitus: An Updated Meta-Analysis of 501 022 Adult Individuals. Gut 2021, 70, 962–969. [Google Scholar] [CrossRef]

| Category | Class | Examples |
|---|---|---|
| Insulin | Rapid-acting (3–5 h) | Lispro, Aspart |
| Rapid-acting inhaled (3 h) | Human inhaled insulin | |
| Short-acting (5–8 h) | Regular human insulin | |
| Intermediate-acting (14–24 h) | Neutral protamine hagedorn (NPH) | |
| Long-acting (~24 h) | Glargine (U100), Detemir (U100) | |
| Ultra-long acting (>36 h) | Glargine (U300), Degludec | |
| Insulin secretagogue | Sulfonylurea | Glipizide, Glyburide, Glimepiride |
| Meglitinide | Repaglinide, Nateglinide | |
| Glucagon-like peptide-1 (GLP-1) receptor agonist | Semaglutide, Exenatide, Liraglutide, Dulaglutide | |
| Dipeptidyl peptidase-4 (DPP-4) inhibitor | Sitagliptin, Lingagliptin | |
| Oral hypoglycemic agents | Biguanides | Metformin |
| Thiazolidinediones (TZD) | Rosiglitazone, Pioglitazone | |
| Sodium–glucose cotransporter 2 (SGLT2) inhibitors | Empagliflozin, Dapagliflozin, Canagliflozin | |
| Alpha-glucosidase inhibitors | Acarbose, Miglitol |
| Class | Example | Off-Label Indication for MASLD | Trial |
|---|---|---|---|
| Biguanide | Metformin | Reduced serum aminotransferases in patients with MASH | NCT00063232 |
| GLP-1 agonist | Liraglutide | Resolution of MASH with no worsening of fibrosis | NCT01237119 |
| SGLT2 inhibitor | Empagliflozin | Reduced steatosis in patients with T2DM and MASLD | NCT02686476 |
| Thiazolidinedione | Pioglitazone | Reduced steatosis in patients with MASH and without T2DM | NCT00063622 |
| Anti-lipidemic | Ezetimibe | Improved insulin resistance in patients with MASLD | N/A |
| Class | Example | Trial |
|---|---|---|
| Acetyl-CoA Carboxylase Inhibitor | Fircostat | NCT02856555 |
| NCT03987074 | ||
| NCT04971785 | ||
| NCT03449446 | ||
| PF-05221304 | NCT03248882 | |
| NCT03776175 | ||
| Fatty Acid Synthase Inhibitor | Denifanstat | NCT04906421 |
| Stearoyl-CoA Desaturase Inhibitor | ION224 | NCT04932512 |
| PF-06865571 | NCT03513588 | |
| Galectin-3 Inhibitor | Belapectin | NCT04365868 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferdous, S.-E.; Ferrell, J.M. Pathophysiological Relationship between Type 2 Diabetes Mellitus and Metabolic Dysfunction-Associated Steatotic Liver Disease: Novel Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 8731. https://doi.org/10.3390/ijms25168731
Ferdous S-E, Ferrell JM. Pathophysiological Relationship between Type 2 Diabetes Mellitus and Metabolic Dysfunction-Associated Steatotic Liver Disease: Novel Therapeutic Approaches. International Journal of Molecular Sciences. 2024; 25(16):8731. https://doi.org/10.3390/ijms25168731
Chicago/Turabian StyleFerdous, Shifat-E, and Jessica M. Ferrell. 2024. "Pathophysiological Relationship between Type 2 Diabetes Mellitus and Metabolic Dysfunction-Associated Steatotic Liver Disease: Novel Therapeutic Approaches" International Journal of Molecular Sciences 25, no. 16: 8731. https://doi.org/10.3390/ijms25168731





