Xanthoxylin Attenuates Lipopolysaccharide-Induced Lung Injury through Modulation of Akt/HIF-1α/NF-κB and Nrf2 Pathways
Abstract
1. Introduction
2. Results
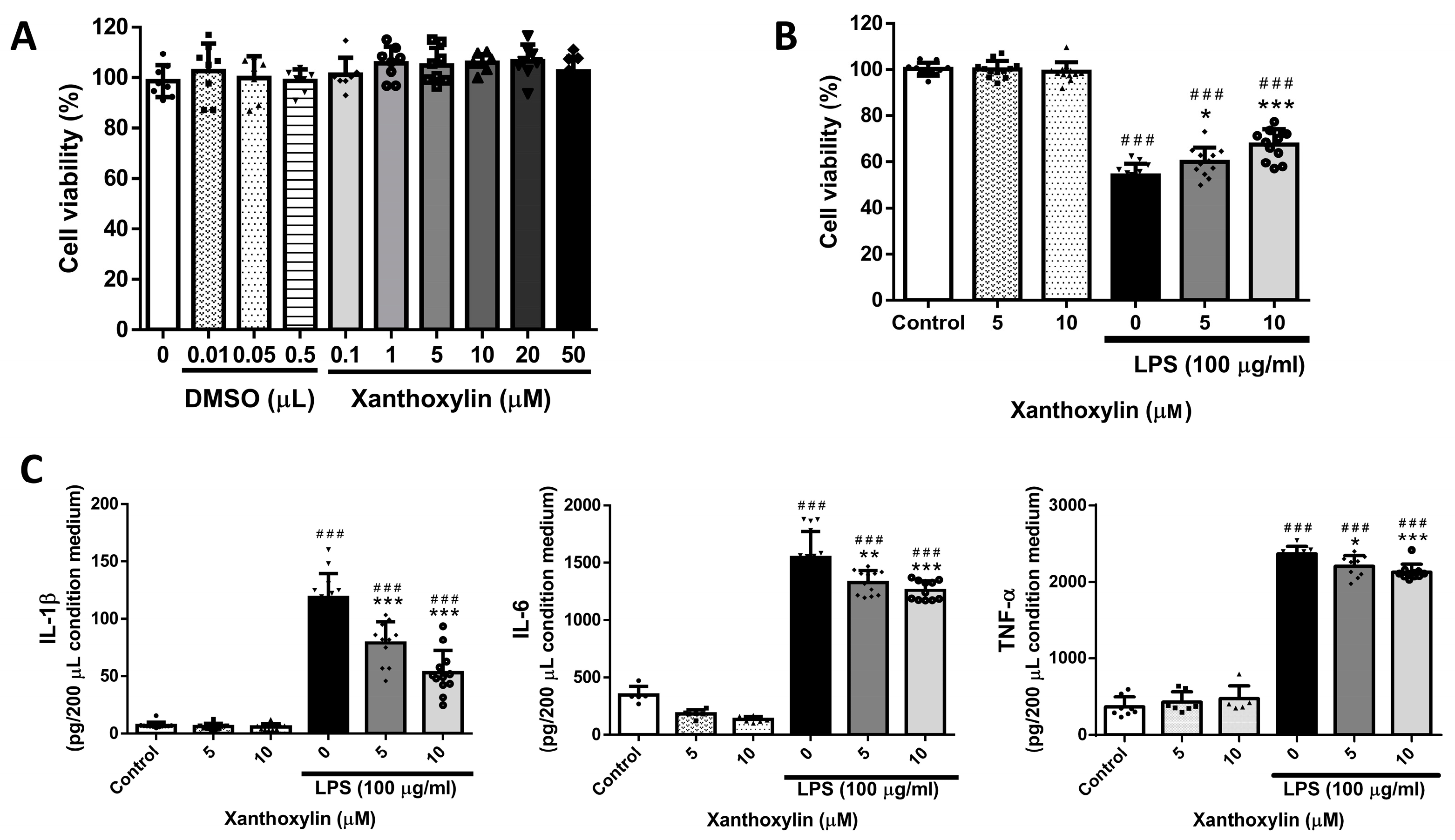
2.1. Effects of Xanthoxylin Treatment on RAW 264.7 Cells in LPS-Induced Cell Injury
2.2. Effects of Xanthoxylin Treatment on Lung Appearance in LPS-Induced ALI
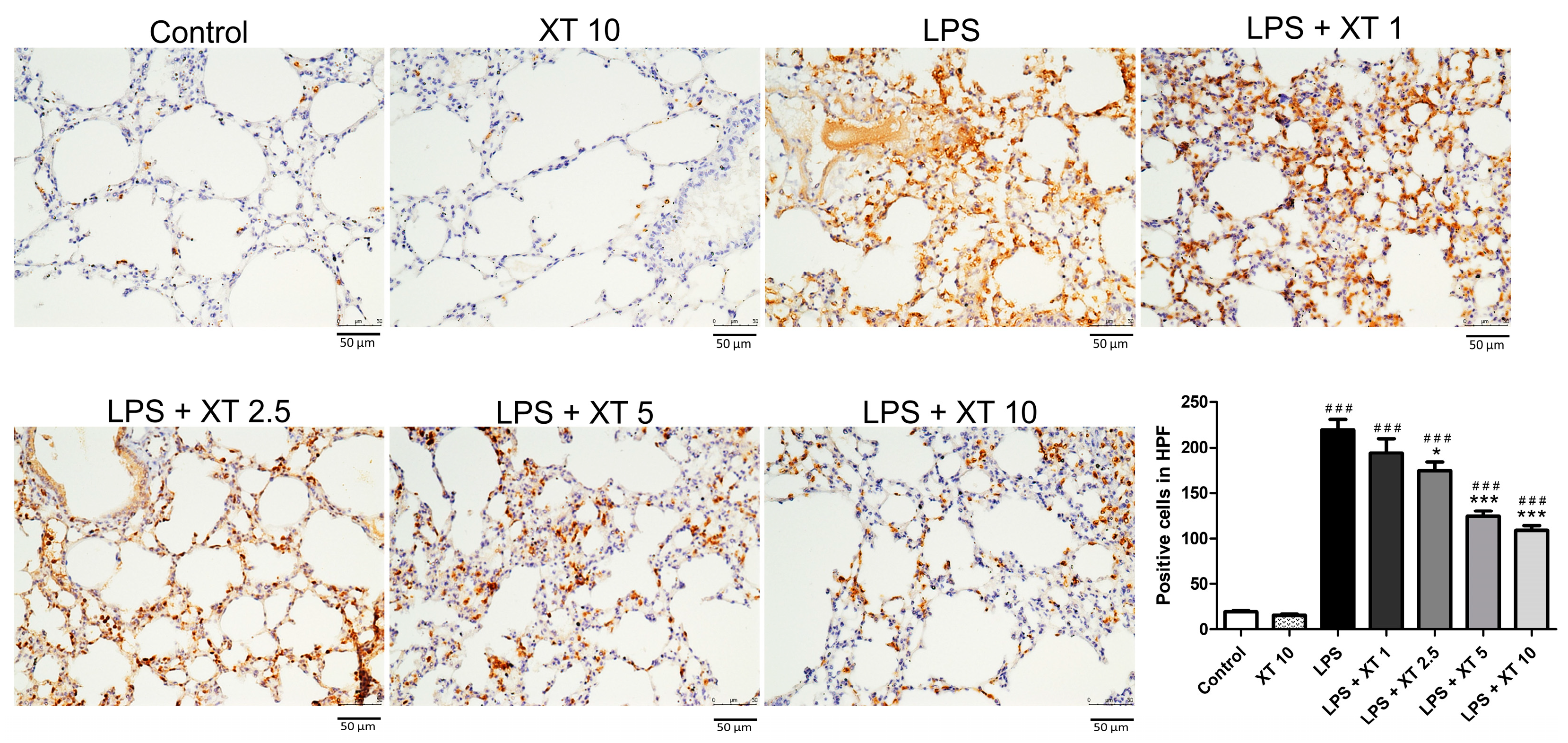
2.3. Histological Pattern Changes of Xanthoxylin Treatment on H&E and Inflammatory Cell Infiltration in LPS-Induced ALI
2.4. Effects of Xanthoxylin Treatment on Pulmonic Pro-Inflammatory Cytokines in LPS-Induced ALI
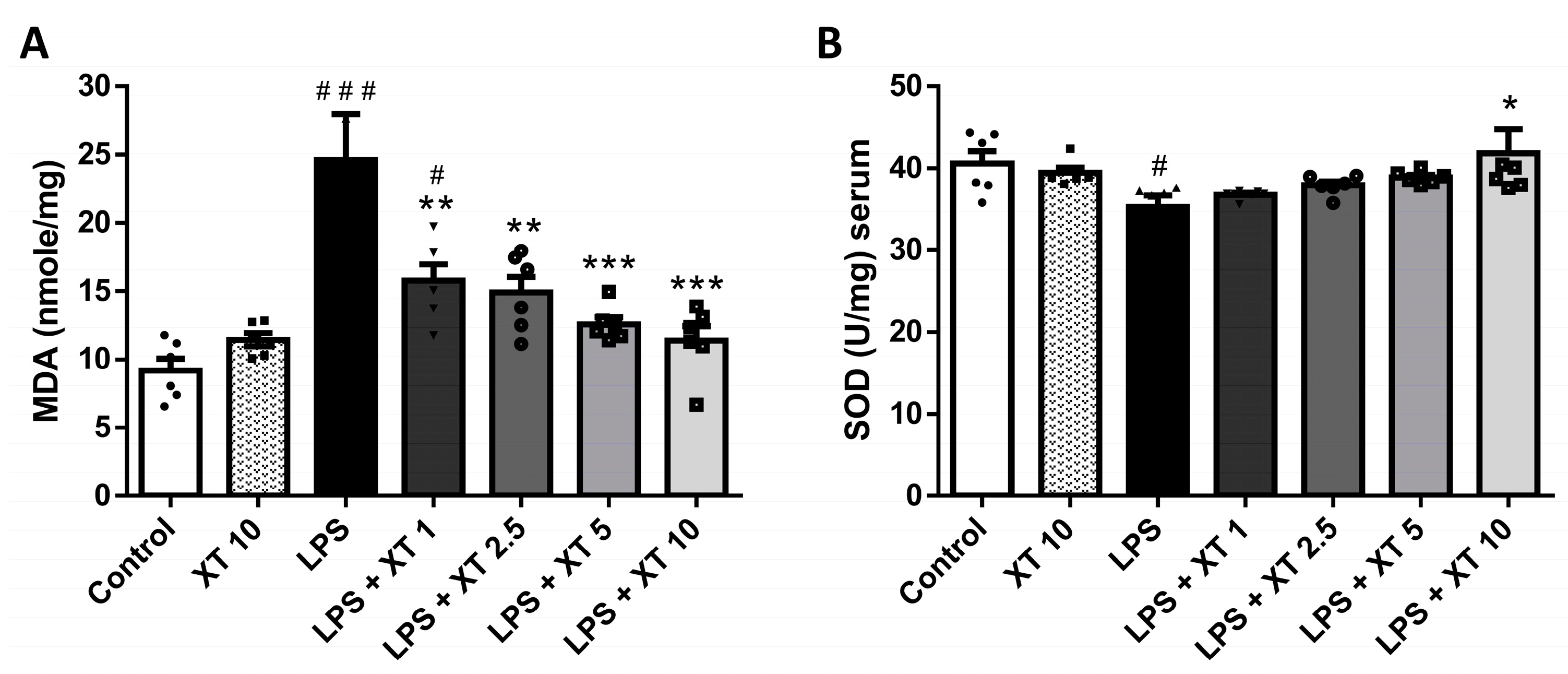
2.5. Effects of Xanthoxylin Treatment on Pulmonic Oxidative Stress in LPS-Induced ALI
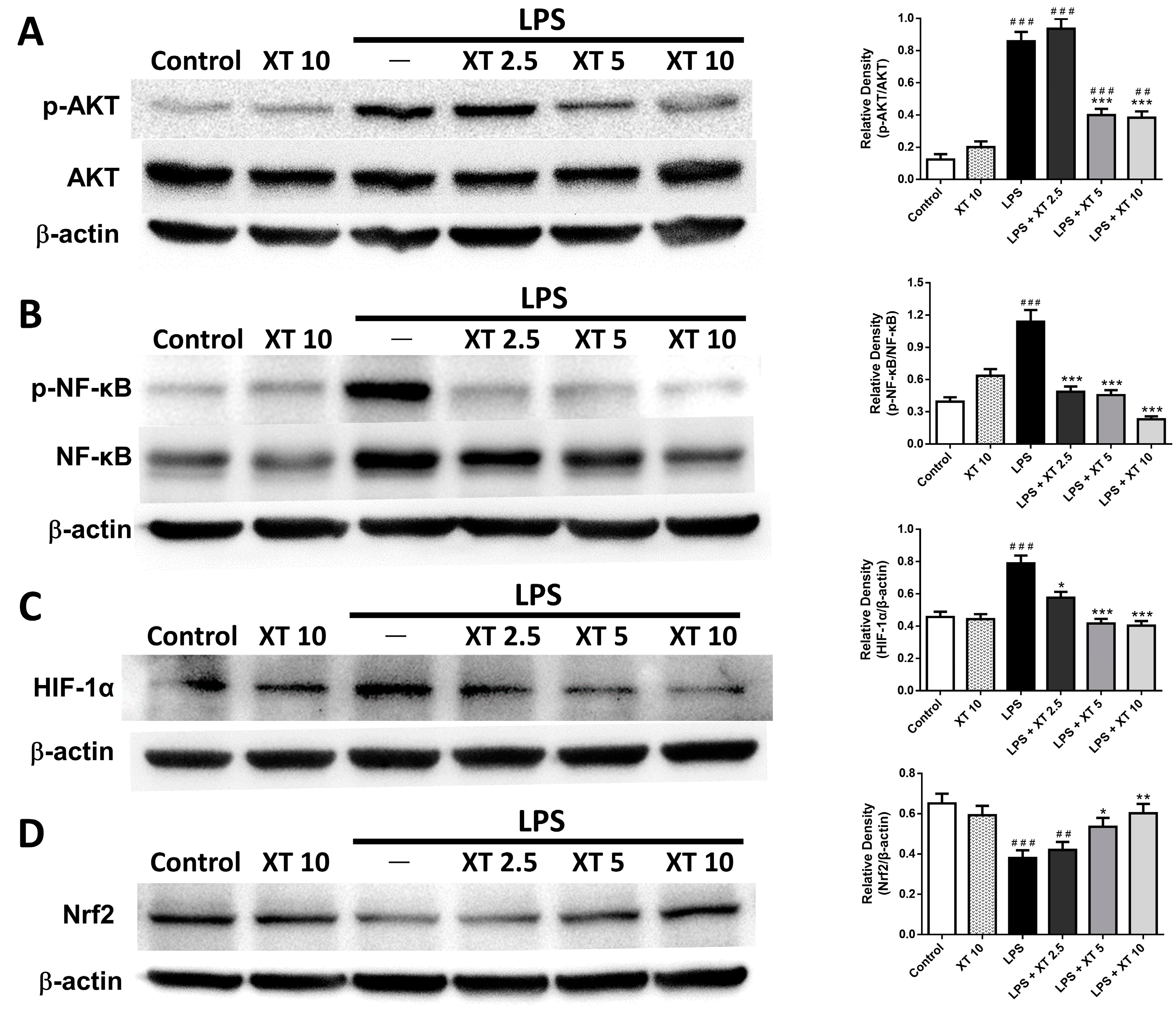
2.6. Effects of Xanthoxylin Treatment on Pneumonic Akt/HIF-1α/NF-κB and Nrf2 Expression in LPS-Induced ALI
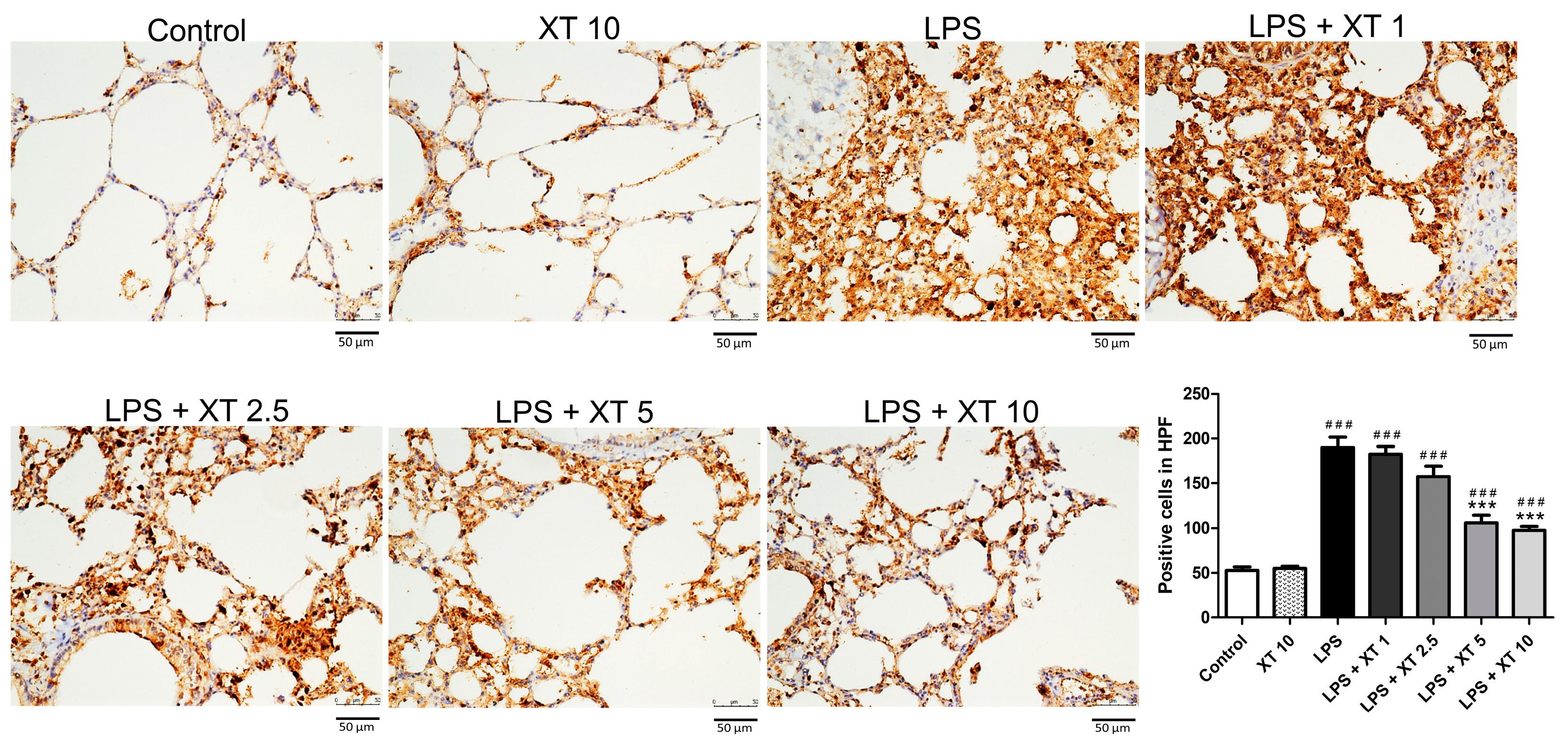
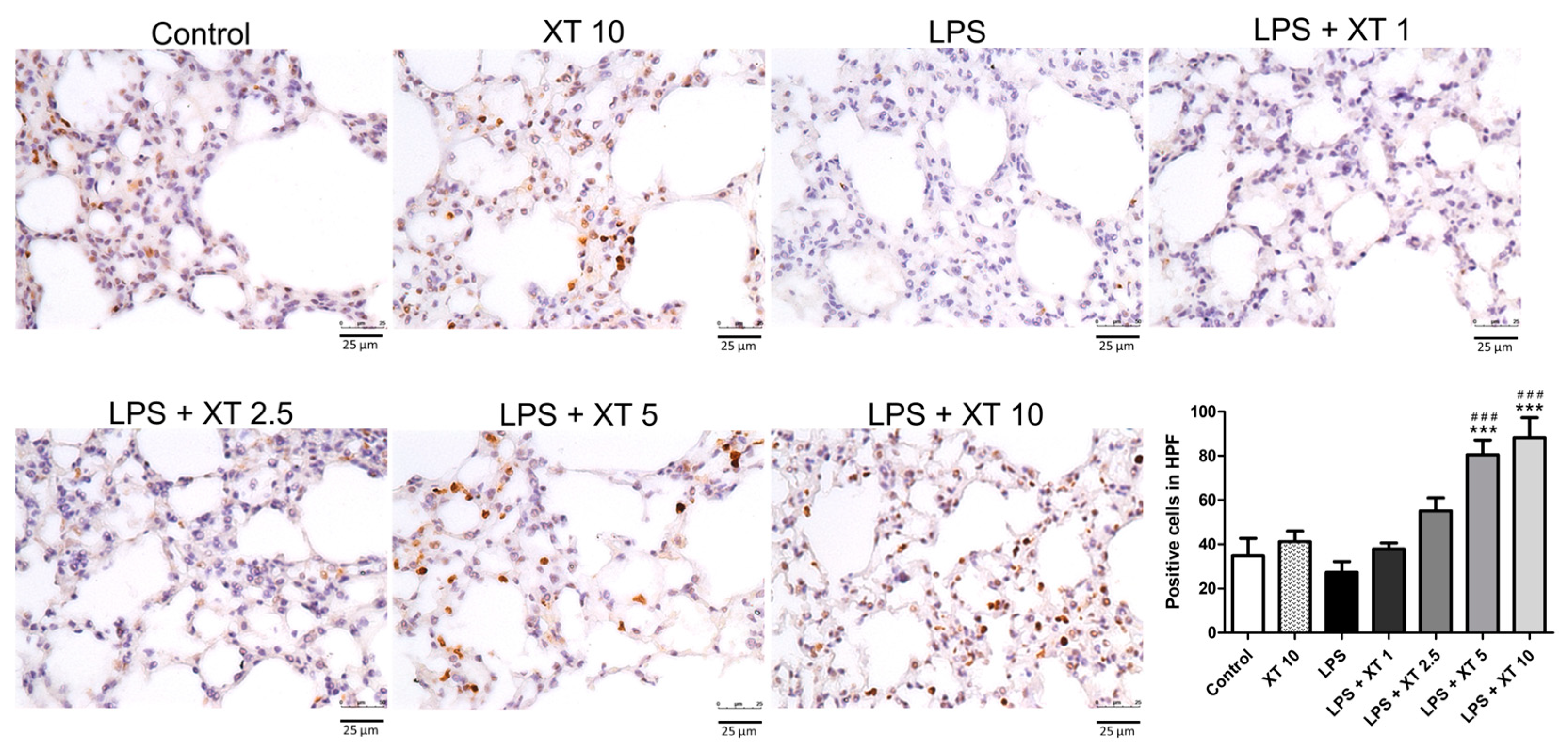
2.7. Effects of Xanthoxylin Treatment on Pneumonic Immunostained Nrf2 Antibodies in LPS-Induced ALI
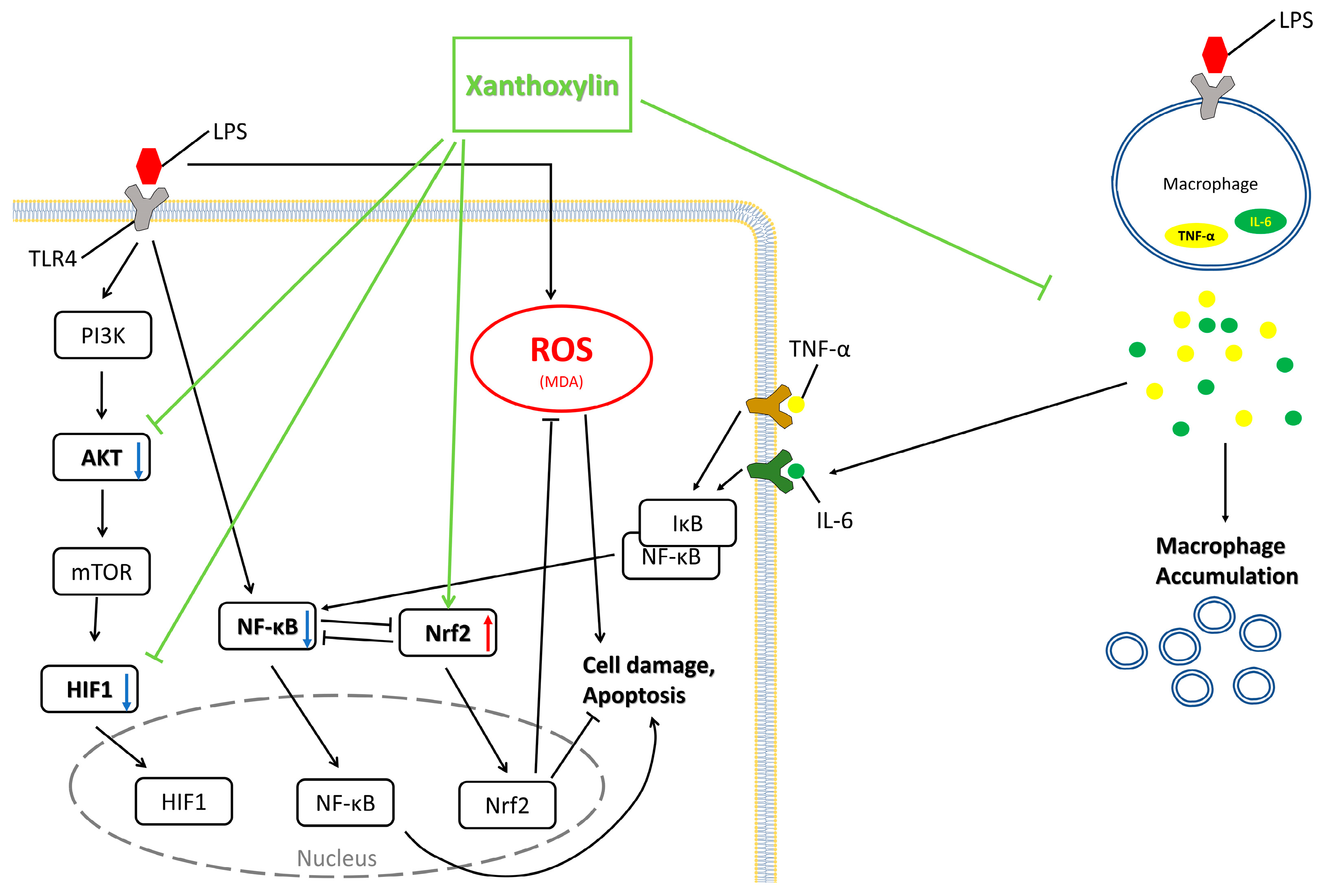
3. Discussion
4. Materials and Methods
4.1. RAW 264.7 Cells
4.2. Testing Non-Toxic Concentration of RAW 264.7 Cells
4.3. Cell Culturing Experimental Protocols
4.4. Animals
4.5. Experimental Protocols and Xanthoxylin Treatment
4.6. Histology Examination
4.7. Immunohistochemistry
4.8. Measurement of Tissue Cytokine by ELISA
4.9. Measurement of the Malondialdehyde (MDA) Levels and Superoxide Dismutase (SOD) Activity
4.10. Western Blotting
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mokra, D.; Kosutova, P. Biomarkers in acute lung injury. Respir. Physiol. Neurobiol. 2015, 209, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Zambon, M.; Vincent, J.L. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest 2008, 133, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Butt, Y.; Kurdowska, A.; Allen, T.C. Acute Lung Injury: A Clinical and Molecular Review. Arch. Pathol. Lab. Med. 2016, 140, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Long, M.E.; Mallampalli, R.K.; Horowitz, J.C. Pathogenesis of pneumonia and acute lung injury. Clin. Sci. 2022, 136, 747–769. [Google Scholar] [CrossRef] [PubMed]
- Kellner, M.; Noonepalle, S.; Lu, Q.; Srivastava, A.; Zemskov, E.; Black, S.M. ROS Signaling in the Pathogenesis of Acute Lung Injury (ALI) and Acute Respiratory Distress Syndrome (ARDS). Adv. Exp. Med. Biol. 2017, 967, 105–137. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Wang, L.; Song, Y.; Bai, C. Inhibition of inflammatory responses by ambroxol, a mucolytic agent, in a murine model of acute lung injury induced by lipopolysaccharide. Intensive Care Med. 2004, 30, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Deal, E.N.; Hollands, J.M.; Schramm, G.E.; Micek, S.T. Role of corticosteroids in the management of acute respiratory distress syndrome. Clin. Ther. 2008, 30, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, J.; Ying, S.; Chen, G.; Wu, B.; Xu, T.; Liu, Z.; Liu, X.; Huang, L.; Shan, X.; et al. Discovery of new MD2 inhibitor from chalcone derivatives with anti-inflammatory effects in LPS-induced acute lung injury. Sci. Rep. 2016, 6, 25130. [Google Scholar] [CrossRef]
- Bertani, B.; Ruiz, N. Function and Biogenesis of Lipopolysaccharides. EcoSal Plus 2018, 8, 10–1128. [Google Scholar] [CrossRef]
- Jung, S.; Park, J.; Ko, K.S. Lipopolysaccharide-induced innate immune responses are exacerbated by Prohibitin 1 deficiency and mitigated by S-adenosylmethionine in murine macrophages. PLoS ONE 2020, 15, e0241224. [Google Scholar] [CrossRef]
- Rubenfeld, G.D.; Caldwell, E.; Peabody, E.; Weaver, J.; Martin, D.P.; Neff, M.; Stern, E.J.; Hudson, L.D. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005, 353, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.K.Y.; Fan, J. Regulation of alveolar macrophage death in acute lung inflammation. Respir. Res. 2018, 19, 50. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Li, S.; Chen, H. Macrophages in Lung Injury, Repair, and Fibrosis. Cells 2021, 10, 436. [Google Scholar] [CrossRef]
- West, M.A.; Bennet, T.; Seatter, S.C.; Clair, L.; Bellingham, J. LPS pretreatment reprograms macrophage LPS-stimulated TNF and IL-1 release without protein tyrosine kinase activation. J. Leukoc. Biol. 1997, 61, 88–95. [Google Scholar] [CrossRef]
- Jabaudon, M.; Blondonnet, R.; Ware, L.B. Biomarkers in acute respiratory distress syndrome. Curr. Opin. Crit. Care 2021, 27, 46–54. [Google Scholar] [CrossRef]
- Mazzon, E.; Cuzzocrea, S. Role of TNF-α in lung tight junction alteration in mouse model of acute lung inflammation. Respir. Res. 2007, 8, 75. [Google Scholar] [CrossRef]
- Reinhart, K.; Bayer, O.; Brunkhorst, F.; Meisner, M. Markers of endothelial damage in organ dysfunction and sepsis. Crit. Care Med. 2002, 30, S302–S312. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Garcia, L.; Traves, P.G.; Lopez-Fontal, R.; Herranz, S.; Higueras, M.A.; de Las Heras, B.; Hortelano, S.; Luque, A. 8,9-Dehydrohispanolone-15,16-lactol diterpene prevents LPS-triggered inflammatory responses by inhibiting endothelial activation. Biochem. J. 2016, 473, 2061–2071. [Google Scholar] [CrossRef]
- Meng, L.; Li, L.; Lu, S.; Li, K.; Su, Z.; Wang, Y.; Fan, X.; Li, X.; Zhao, G. The protective effect of dexmedetomidine on LPS-induced acute lung injury through the HMGB1-mediated TLR4/NF-κB and PI3K/Akt/mTOR pathways. Mol. Immunol. 2018, 94, 7–17. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Sun, X.; Chen, L.; He, Z. PI3K/Akt-Nrf2 and Anti-Inflammation Effect of Macrolides in Chronic Obstructive Pulmonary Disease. Curr. Drug Metab. 2019, 20, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Li, H.S.; Zhou, Y.N.; Li, L.; Li, S.F.; Long, D.; Chen, X.L.; Zhang, J.B.; Feng, L.; Li, Y.P. HIF-1α protects against oxidative stress by directly targeting mitochondria. Redox Biol. 2019, 25, 101109. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Zhou, Z.; Zheng, Y.; Chang, M.; Huang, X.; Guo, F.; Zhao, Q.; Huan, J. Suppressive Effects of GSS on Lipopolysaccharide-Induced Endothelial Cell Injury and ALI via TNF-α and IL-6. Mediators Inflamm. 2019, 2019, 4251394. [Google Scholar] [CrossRef]
- Niu, X.F.; He, L.C.; Fan, T.; Li, Y. Protecting effect of brevifolin and 8,9-single-epoxy brevifolin of Phyllanthus simplex on rat liver injury. Zhongguo Zhong Yao Za Zhi 2006, 31, 1529–1532. [Google Scholar] [PubMed]
- Lee, H.C.; Liu, F.C.; Tsai, C.N.; Chou, A.H.; Liao, C.C.; Yu, H.P. Esculetin Ameliorates Lipopolysaccharide-Induced Acute Lung Injury in Mice Via Modulation of the AKT/ERK/NF-κB and RORγt/IL-17 Pathways. Inflammation 2020, 43, 962–974. [Google Scholar] [CrossRef] [PubMed]
- Hartley, J.W.; Evans, L.H.; Green, K.Y.; Naghashfar, Z.; Macias, A.R.; Zerfas, P.M.; Ward, J.M. Expression of infectious murine leukemia viruses by RAW264.7 cells, a potential complication for studies with a widely used mouse macrophage cell line. Retrovirology 2008, 5, 1. [Google Scholar] [CrossRef]
- Shotland, A.M.; Fontenot, A.P.; McKee, A.S. Pulmonary Macrophage Cell Death in Lung Health and Disease. Am. J. Respir. Cell Mol. Biol. 2021, 64, 547–556. [Google Scholar] [CrossRef]
- Gabay, C.; Kushner, I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999, 340, 448–454. [Google Scholar] [CrossRef]
- Soni, S.; Wilson, M.R.; O'Dea, K.P.; Yoshida, M.; Katbeh, U.; Woods, S.J.; Takata, M. Alveolar macrophage-derived microvesicles mediate acute lung injury. Thorax 2016, 71, 1020–1029. [Google Scholar] [CrossRef]
- Ye, C.; Li, H.; Bao, M.; Zhuo, R.; Jiang, G.; Wang, W. Alveolar macrophage-derived exosomes modulate severity and outcome of acute lung injury. Aging 2020, 12, 6120–6128. [Google Scholar] [CrossRef]
- Bain, C.C.; MacDonald, A.S. The impact of the lung environment on macrophage development, activation and function: Diversity in the face of adversity. Mucosal. Immunol. 2022, 15, 223–234. [Google Scholar] [CrossRef]
- Sazontova, T.G.; Zhukova, A.G.; Anchishkina, N.A.; Arkhipenko Iu, V. Dynamic changes in transcription factor HIF-1α, rapid response protein, and membrane structure resistance following acute hypoxia. Vestn. Ross. Akad. Med. Nauk. 2007, 2, 17–25. [Google Scholar]
- Ziello, J.E.; Jovin, I.S.; Huang, Y. Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia. Yale J. Biol. Med. 2007, 80, 51–60. [Google Scholar]
- Bardos, J.I.; Ashcroft, M. Negative and positive regulation of HIF-1: A complex network. Biochim. Biophys. Acta 2005, 1755, 107–120. [Google Scholar] [CrossRef]
- Li, M.Y.; Luo, H.J.; Wu, X.; Liu, Y.H.; Gan, Y.X.; Xu, N.; Zhang, Y.M.; Zhang, S.H.; Zhou, C.L.; Su, Z.R.; et al. Anti-Inflammatory Effects of Huangqin Decoction on Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice Through Regulation of the Gut Microbiota and Suppression of the Ras-PI3K-Akt-HIF-1α and NF-κB Pathways. Front. Pharmacol. 2019, 10, 1552. [Google Scholar] [CrossRef]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol Cell Res 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An Overview of Nrf2 Signaling Pathway and Its Role in Inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef]
- Donia, T.; Khamis, A. Management of oxidative stress and inflammation in cardiovascular diseases: Mechanisms and challenges. Environ. Sci. Pollut. Res. Int. 2021, 28, 34121–34153. [Google Scholar] [CrossRef]
- Scheidereit, C. Signal transduction. Docking IkappaB kinases. Nature 1998, 395, 225–226. [Google Scholar] [CrossRef]
- Gureev, A.P.; Popov, V.N. Nrf2/ARE Pathway as a Therapeutic Target for the Treatment of Parkinson Diseases. Neurochem. Res. 2019, 44, 2273–2279. [Google Scholar] [CrossRef]
- Lawrence, T.; Natoli, G. Transcriptional regulation of macrophage polarization: Enabling diversity with identity. Nat. Rev. Immunol. 2011, 11, 750–761. [Google Scholar] [CrossRef]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Huang, C.Y.; Deng, J.S.; Huang, W.C.; Jiang, W.P.; Huang, G.J. Attenuation of Lipopolysaccharide-Induced Acute Lung Injury by Hispolon in Mice, Through Regulating the TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 Pathways, and Suppressing Oxidative Stress-Mediated ER Stress-Induced Apoptosis and Autophagy. Nutrients 2020, 12, 1742. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-κB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Karar, J.; Maity, A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front. Mol. Neurosci. 2011, 4, 51. [Google Scholar] [CrossRef]
- Masoud, G.N.; Li, W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B 2015, 5, 378–389. [Google Scholar] [CrossRef]
- Helou, D.G.; Martin, S.F.; Pallardy, M.; Chollet-Martin, S.; Kerdine-Romer, S. Nrf2 Involvement in Chemical-Induced Skin Innate Immunity. Front. Immunol. 2019, 10, 1004. [Google Scholar] [CrossRef]
- Prabhakar, N.R.; Peng, Y.J.; Nanduri, J. Hypoxia-inducible factors and obstructive sleep apnea. J. Clin. Investig. 2020, 130, 5042–5051. [Google Scholar] [CrossRef]
- Matute-Bello, G.; Downey, G.; Moore, B.B.; Groshong, S.D.; Matthay, M.A.; Slutsky, A.S.; Kuebler, W.M. An Official American Thoracic Society Workshop Report: Features and Measurements of Experimental Acute Lung Injury in Animals. Am. J. Respir. Cell Mol. Biol. 2011, 44, 725–738. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.-C.; Yang, Y.-H.; Liao, C.-C.; Lee, H.-C. Xanthoxylin Attenuates Lipopolysaccharide-Induced Lung Injury through Modulation of Akt/HIF-1α/NF-κB and Nrf2 Pathways. Int. J. Mol. Sci. 2024, 25, 8742. https://doi.org/10.3390/ijms25168742
Liu F-C, Yang Y-H, Liao C-C, Lee H-C. Xanthoxylin Attenuates Lipopolysaccharide-Induced Lung Injury through Modulation of Akt/HIF-1α/NF-κB and Nrf2 Pathways. International Journal of Molecular Sciences. 2024; 25(16):8742. https://doi.org/10.3390/ijms25168742
Chicago/Turabian StyleLiu, Fu-Chao, Yuan-Han Yang, Chia-Chih Liao, and Hung-Chen Lee. 2024. "Xanthoxylin Attenuates Lipopolysaccharide-Induced Lung Injury through Modulation of Akt/HIF-1α/NF-κB and Nrf2 Pathways" International Journal of Molecular Sciences 25, no. 16: 8742. https://doi.org/10.3390/ijms25168742
APA StyleLiu, F.-C., Yang, Y.-H., Liao, C.-C., & Lee, H.-C. (2024). Xanthoxylin Attenuates Lipopolysaccharide-Induced Lung Injury through Modulation of Akt/HIF-1α/NF-κB and Nrf2 Pathways. International Journal of Molecular Sciences, 25(16), 8742. https://doi.org/10.3390/ijms25168742






