Benefits and Challenges of Drug-Coated Balloons in Peripheral Artery Disease: From Molecular Mechanisms to Clinical Practice
Abstract
:1. Introduction
2. Molecular Basis
2.1. Paclitaxel
2.2. Sirolimus
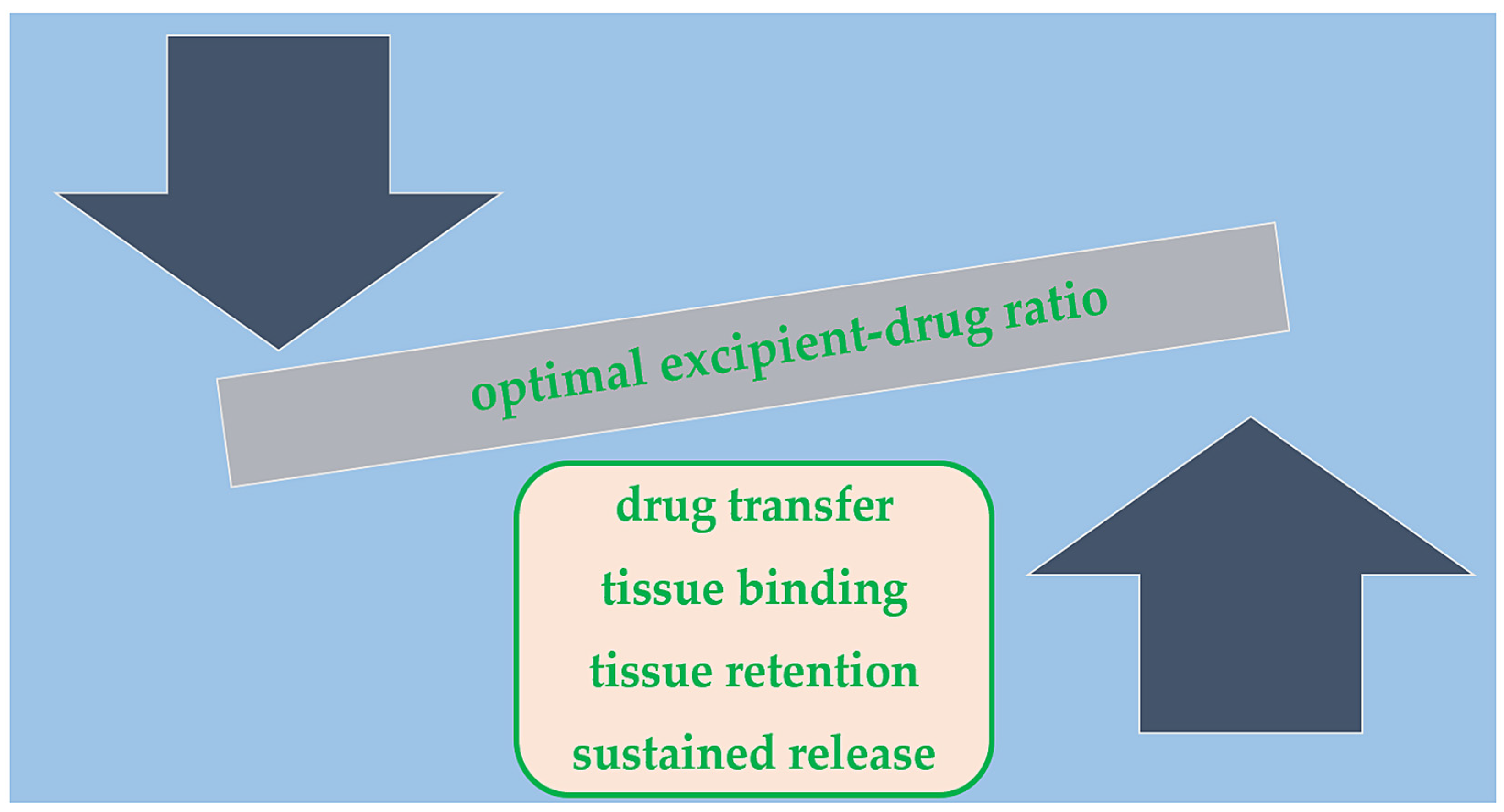
2.3. Balloon Coating/Excipient
3. Clinical Practice
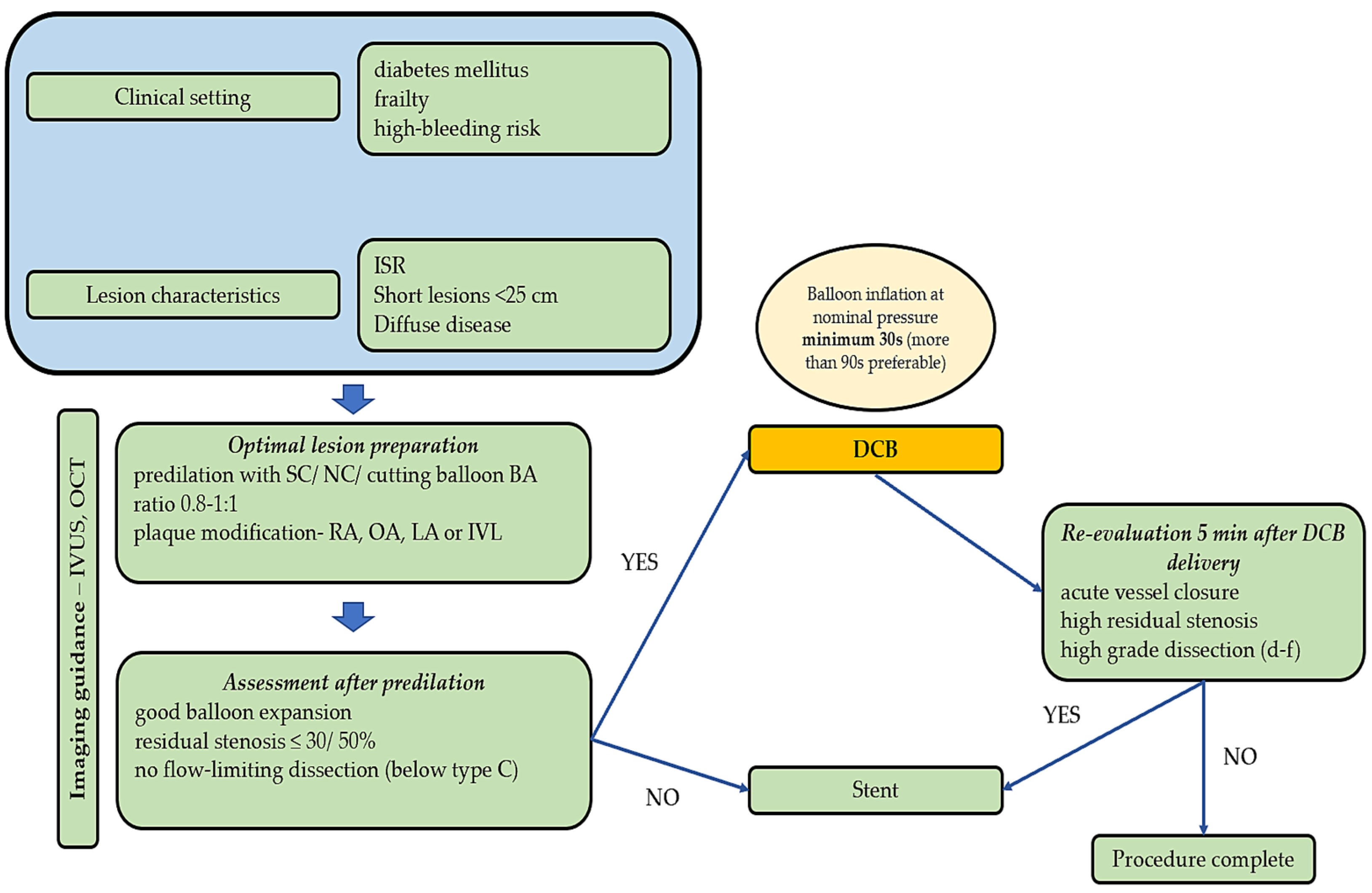
3.1. Lesion Preparation and Imaging
3.1.1. Standard, Cutting, and Scoring Balloons
3.1.2. Orbital Atherectomy
3.1.3. Rotational Atherectomy
3.1.4. Directional Atherectomy
3.1.5. Laser Atherectomy
3.1.6. Intravascular Lithotripsy
3.1.7. Imaging Modalities
3.2. Clinical Implications
3.3. Above-the-Knee Lesions
3.3.1. In-Stent Restenosis
3.3.2. De Novo Lesions
3.4. Below-the-Knee Lesions
3.4.1. De Novo Lesions
3.4.2. In-Stent Restenosis
3.5. Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horváth, L.; Németh, N.; Fehér, G.; Kívés, Z.; Endrei, D.; Boncz, I. Epidemiology of Peripheral Artery Disease: Narrative Review. Life 2022, 12, 1041. [Google Scholar] [CrossRef] [PubMed]
- Olinic, M.; Lazar, F.-L.; Onea, H.-L.; Homorodean, C.; Ober, M.; Tataru, D.; Spinu, M.; Achim, A.; Olinic, D.-M. Peripheral Artery Disease Ultrasound Assessment in Predicting the Severity of Coronary Artery Disease. Life 2024, 14, 333. [Google Scholar] [CrossRef] [PubMed]
- Martelli, E.; Enea, I.; Zamboni, M.; Federici, M.; Bracale, U.M.; Sangiorgi, G.; Martelli, A.R.; Messina, T.; Settembrini, A.M. Focus on the Most Common Paucisymptomatic Vasculopathic Population, from Diagnosis to Secondary Prevention of Complications. Diagnostics 2023, 13, 2356. [Google Scholar] [CrossRef] [PubMed]
- Tătaru, D.-A.; Olinic, M.; Homorodean, C.; Ober, M.-C.; Spînu, M.; Lazăr, F.-L.; Onea, L.; Olinic, D.-M. Correlation between Ultrasound Peak Systolic Velocity and Angiography for Grading Internal Carotid Artery Stenosis. J. Clin. Med. 2024, 13, 517. [Google Scholar] [CrossRef] [PubMed]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. ESC Scientific Document Group. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries Endorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef] [PubMed]
- Soga, Y.; Iida, O.; Seki, S.I.; Kawasaki, D.; Anzai, H.; Ando, H.; Nakama, T.; Shinozaki, N.; Kozuki, A.; Ishihara, M.; et al. TCD-17187 Japan Investigators. Twenty-Four-Month Safety and Effectiveness of TCD-17187 Drug-Coated Balloon for Treatment of Atherosclerotic Lesions in Superficial Femoral and Proximal Popliteal Artery. Cardiovasc. Intervent Radiol. 2024, 47, 730–740. [Google Scholar] [CrossRef] [PubMed]
- di Palma, G.; Sanchez-Jimenez, E.F.; Lazar, L.; Cortese, B. Should paclitaxel be considered an old generation DCB? The limus era. Rev. Cardiovasc. Med. 2021, 22, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Chaddad, R.; El-Mokdad, R.; Lazar, L.; Cortese, B. DCBs as an adjuvant tool to DES for very complex coronary lesions. Rev. Cardiovasc. Med. 2022, 23, 13. [Google Scholar] [CrossRef] [PubMed]
- Gray, W.A.; Granada, J.F. Drug-coated balloons for the prevention of vascular restenosis. Circulation 2010, 121, 2672–2680. [Google Scholar] [CrossRef]
- Maga, P.; Mikolajczyk, T.P.; Partyka, L.; Siedlinski, M.; Maga, M.; Krzanowski, M.; Malinowski, K.; Luc, K.; Nizankowski, R.; Bhatt, D.L.; et al. Involvement of CD8+ T cell subsets in early response to vascular injury in patients with peripheral artery disease in vivo. Clin. Immunol. 2018, 94, 26–33. [Google Scholar] [CrossRef]
- Ferrer, I.R.; Araki, K.; Ford, M.L. Paradoxical aspects of rapamycin immunobiology in transplantation. Am. J. Transplant. 2011, 11, 654–659. [Google Scholar] [CrossRef] [PubMed]
- Vicari, A.P.; Luu, R.; Zhang, N.; Patel, S.; Makinen, S.R.; Hanson, D.C.; Weeratna, R.D.; Krieg, A.M. Paclitaxel reduces regulatory T cell numbers and inhibitory function and enhances the anti-tumor effects of the TLR9 agonist PF-3512676 in the mouse. Cancer Immunol. Immunother. 2009, 58, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Long, B.H.; Fairchild, C.R. Paclitaxel inhibits progression of mitotic cells to G1 phase by interference with spindle formation without affecting other microtubule functions during anaphase and telephase. Cancer Res. 1994, 54, 4355–4361. [Google Scholar] [PubMed]
- Giannakakou, P.; Robey, R.; Fojo, T.; Blagosklonny, M.V. Low concentrations of paclitaxel induce cell type-dependent p53, p21 and G1/G2 arrest instead of mitotic arrest: Molecular determinants of paclitaxel-induced cytotoxicity. Oncogene 2001, 20, 3806–3813. [Google Scholar] [CrossRef] [PubMed]
- Kolodgie, F.D.; Pacheco, E.; Yahagi, K.; Mori, H.; Ladich, E.; Virmani, R. Comparison of Particulate Embolization after Femoral Artery Treatment with IN.PACT Admiral versus Lutonix 035 Paclitaxel-Coated Balloons in Healthy Swine. J. Vasc. Interv. Radiol. 2016, 27, 1676–1685.e2. [Google Scholar] [CrossRef] [PubMed]
- Heldman, A.W.; Cheng, L.; Jenkins, G.M.; Heller, P.F.; Kim, D.W.; Ware, M.; Nater, C.; Hruban, R.H.; Rezai, B.; Abella, B.S.; et al. Paclitaxel stent coating inhibits neointimal hyperplasia at 4 weeks in a porcine model of coronary restenosis. Circulation 2001, 103, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Steiner, S.; Schmidt, A.; Zeller, T.; Tepe, G.; Thieme, M.; Maiwald, L.; Schröder, H.; Euringer, W.; Popescu, C.; Brechtel, K.; et al. Low-Dose vs High-Dose Paclitaxel-Coated Balloons for Femoropopliteal Lesions: 2-Year Results from the COMPARE Trial. JACC Cardiovasc. Interv. 2022, 15, 2093–2102. [Google Scholar] [CrossRef] [PubMed]
- Katsanos, K.; Spiliopoulos, S.; Kitrou, P.; Krokidis, M.; Karnabatidis, D. Risk of Death Following Application of Paclitaxel-Coated Balloons and Stents in the Femoro-popliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2018, 7, e011245. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Singh, K.J.; Duval, S.; Jaff, M.R.; Schneider, P.A.; Ansel, G.M.; Lyden, S.P.; Mullin, C.M.; Ioannidis, J.P.A.; Misra, S.; Tzafriri, A.R.; et al. Mortality and Paclitaxel-Coated Devices: An Individual Patient Data Meta-Analysis. Circulation 2020, 141, 1859–1869. [Google Scholar] [CrossRef]
- Secemsky, E.A.; Kundi, H.; Weinberg, I.; Jaff, M.R.; Krawisz, A.; Parikh, S.A.; Beckman, J.A.; Mustapha, J.; Rosenfield, K.; Yeh, R.W. Association of Survival With Femoropopliteal Artery Revascularization With Drug-Coated Devices. JAMA Cardiol. 2019, 4, 332–340. [Google Scholar] [CrossRef]
- Freisinger, E.; Koeppe, J.; Gerss, J.; Goerlich, D.; Malyar, N.M.; Marschall, U.; Faldum, A.; Reinecke, H. Mortality after use of paclitaxel-based devices in peripheral arteries: A real-world safety analysis. Eur. Heart J. 2020, 41, 3732–3739. [Google Scholar] [CrossRef] [PubMed]
- Böhme, T.; Noory, E.; Beschorner, U.; Jacques, B.; Bürgelin, K.; Macharzina, R.; Gebauer, E.; Cheung, F.; Lechner, P.; Nührenberg, T.; et al. Evaluation of Mortality Following Paclitaxel Drug-Coated Balloon Angioplasty of Femoropopliteal Lesions in the Real World. JACC Cardiovasc. Interv. 2020, 13, 2052–2061. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Sawada, T.; Uzu, K.; Takaya, T.; Kawai, H.; Yasaka, Y. Possible mechanism of late lumen enlargement after treatment for de novo coronary lesions with drug-coated balloon. Int. J. Cardiol. 2020, 321, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Bisdas, T.; Beropoulis, E.; Argyriou, A.; Torsello, G.; Stavroulakis, K. 1-Year All-Comers Analysis of the Eluvia Drug-Eluting Stent for Long Femoropopliteal Lesions After Suboptimal Angioplasty. JACC Cardiovasc. Interv. 2018, 11, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, J.Y.; Wei, M.; Yuan, M.J.; Lu, Z.G. Coronary Artery Aneurysm Formation after Drug-Coated Balloon Treatment. Chin. Med. J. 2018, 131, 2249–2251. [Google Scholar] [CrossRef] [PubMed]
- Diamantopoulos, A.; Gupta, Y.; Zayed, H.; Katsanos, K. Paclitaxel-coated balloons and aneurysm formation in peripheral vessels. J. Vasc. Surg. 2015, 62, 1320–1322. [Google Scholar] [CrossRef] [PubMed]
- Scheller, B.; Gemeinhardt, O.; Kleber, F.X. Late lumen enlargement after treatment of de-novo lesions with drug coated balloon catheters—Glagov effect or plaque regression? Int. J. Cardiol. 2021, 329, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Eisen, H.J. Mechanistic Target of Rapamycin (mTOR) Inhibitors. Handb. Exp. Pharmacol. 2022, 272, 53–72. [Google Scholar] [CrossRef]
- Ali, R.M.; Abdul Kader, M.A.S.K.; Wan Ahmad, W.A.; Ong, T.K.; Liew, H.B.; Omar, A.F.; Mahmood Zuhdi, A.S.; Nuruddin, A.A.; Schnorr, B.; Scheller, B. Treatment of Coronary Drug-Eluting Stent Restenosis by a Sirolimus- or Paclitaxel-Coated Balloon. JACC Cardiovasc. Interv. 2019, 12, 558–566. [Google Scholar] [CrossRef]
- Wessely, R.; Blaich, B.; Belaiba, R.S.; Merl, S.; Görlach, A.; Kastrati, A.; Schömig, A. Comparative characterization of cellular and molecular anti-restenotic profiles of paclitaxel and sirolimus. Implications for local drug delivery. Thromb. Haemost. 2007, 97, 1003–1012. [Google Scholar]
- Choke, E.; Tang, T.Y.; Peh, E.; Damodharan, K.; Cheng, S.C.; Tay, J.S.; Finn, A.V. MagicTouch PTA Sirolimus Coated Balloon for Femoropopliteal and Below the Knee Disease: Results From XTOSI Pilot Study Up To 12 Months. J. Endovasc. Ther. 2022, 29, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Böhme, T.; Noory, E.; Beschorner, U.; Macharzina, R.; Zeller, T. The SELUTION SLR™ drug-eluting balloon system for the treatment of symptomatic femoropopliteal lesions. Future Cardiol. 2021, 17, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Zeller, T.; Brechtel, K.; Meyer, D.R.; Noory, E.; Beschorner, U.; Albrecht, T. Six-Month Outcomes From the First-in-Human, Single-Arm SELUTION Sustained-Limus-Release Drug-Eluting Balloon Trial in Femoropopliteal Lesions. J. Endovasc. Ther. 2020, 27, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.; Caiazzo, G.; Di Palma, G.; De Rosa, S. Comparison Between Sirolimus- and Paclitaxel-Coated Balloon for Revascularization of Coronary Arteries: The SIRPAC (SIRolimus-PAClitaxel) Study. Cardiovasc Revasc Med. 2021, 28, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Xiao, X.; Guo, D.; Mo, L.; Bu, C.; Ye, W.; Den, Q.; Liu, S.; Yang, X. Protective effects of Paeoniflorin against AOPP-induced oxidative injury in HUVECs by blocking the ROS-HIF-1α/VEGF pathway. Phytomedicine 2017, 34, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zeng, Y.; Zhu, X.; Miao, L.; Liang, X.; Duan, J.; Li, H.; Tian, X.; Pang, L.; Wei, Y.; et al. Significant difference between sirolimus and paclitaxel nanoparticles in anti-proliferation effect in normoxia and hypoxia: The basis of better selection of atherosclerosis treatment. Bioact. Mater. 2020, 6, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Cortese, B.; Berti, S.; Biondi-Zoccai, G.; Colombo, A.; Limbruno, U.; Bedogni, F.; Cremonesi, A.; Silva, P.L.; Sgueglia, G.A. Italian Society of Interventional Cardiology. Drug-coated balloon treatment of coronary artery disease: A position paper of the Italian Society of Interventional Cardiology. Catheter. Cardiovasc. Interv. 2014, 83, 427–435. [Google Scholar] [CrossRef]
- Kelsch, B.; Scheller, B.; Biedermann, M.; Clever, Y.P.; Schaffner, S.; Mahnkopf, D.; Speck, U.; Cremers, B. Dose response to Paclitaxel-coated balloon catheters in the porcine coronary overstretch and stent implantation model. Invest. Radiol. 2011, 46, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Tesfamariam, B. Local arterial wall drug delivery using balloon catheter system. J. Control Release 2016, 238, 149–156. [Google Scholar] [CrossRef]
- Granada, J.F.; Stenoien, M.; Buszman, P.P.; Tellez, A.; Langanki, D.; Kaluza, G.L.; Leon, M.B.; Gray, W.; Jaff, M.R.; Schwartz, R.S. Mechanisms of tissue uptake and retention of paclitaxel-coated balloons: Impact on neointimal proliferation and healing. Open Heart 2014, 6, e000117. [Google Scholar] [CrossRef]
- Cao, Z.; Li, J.; Fang, Z.; Feierkaiti, Y.; Zheng, X.; Jiang, X. The factors influencing the efficiency of drug-coated balloons. Front. Cardiovasc. Med. 2022, 9, 947776. [Google Scholar] [CrossRef]
- Dake, M.D.; Ansel, G.M.; Jaff, M.R.; Ohki, T.; Saxon, R.R.; Smouse, H.B.; Machan, L.S.; Snyder, S.A.; O’Leary, E.E.; Ragheb, A.O.; et al. Durable Clinical Effectiveness With Paclitaxel-Eluting Stents in the Femoropopliteal Artery: 5-Year Results of the Zilver PTX Randomized Trial. Circulation 2016, 133, 1472–1483; discussion 1483. [Google Scholar] [CrossRef] [PubMed]
- Tummala, S.; Amin, A.; Mehta, A. Infrapopliteal Artery Occlusive Disease: An Overview of Vessel Preparation and Treatment Options. J. Clin. Med. 2020, 9, 3321. [Google Scholar] [CrossRef] [PubMed]
- Safian, R.D.; Niazi, K.; Runyon, J.P.; Dulas, D.; Weinstock, B.; Ramaiah, V.; Heuser, R. OASIS Investigators Orbital atherectomy for infrapopliteal disease: Device concept and outcome data for the OASIS trial. Catheter. Cardiovasc. Interv. 2009, 73, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Zeller, T.; Giannopoulos, S.; Brodmann, M.; Werner, M.; Andrassy, M.; Schmidt, A.; Blessing, E.; Tepe, G.; Armstrong, E.J. Orbital Atherectomy Prior to Drug-Coated Balloon Angioplasty in Calcified Infrapopliteal Lesions: A Randomized, Multicenter Pilot Study. J. Endovasc. Ther. 2022, 29, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Mustapha, J.; Beasley, R.; Chopra, P.; Das, T.; Adams, G.L. Impact of lesion location on procedural and acute angiographic outcomes in patients with critical limb ischemia treated for peripheral artery disease with orbital atherectomy: A CONFIRM registries subanalysis. Catheter. Cardiovasc. Interv. 2016, 87, 440–445. [Google Scholar] [CrossRef]
- Dawood, M.; Elwany, M.; Abdelgawad, H.; Sanhoury, M.; Zaki, M.; Elsharkawy, E.; Nawar, M. Coronary calcifications, the Achilles heel in coronary interventions. Postepy Kardiol. Interwencyjnej 2024, 20, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Dorros, G.; Iyer, S.; Zaitoun, R.; Lewin, R.; Cooley, R.; Olson, K. Acute angiographic and clinical outcome of high speed percutaneous rotational atherectomy (Rotablator). Cathet Cardiovasc. Diagn. 1991, 22, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Zeller, T.; Krankenberg, H.; Steinkamp, H.; Rastan, A.; Sixt, S.; Schmidt, A.; Sievert, H.; Minar, E.; Bosiers, M.; Peeters, P.; et al. One-year outcome of percutaneous rotational atherectomy with aspiration in infrainguinal peripheral arterial occlusive disease: The multicenter pathway PVD trial. J. Endovasc. Ther. 2009, 16, 653–662. [Google Scholar] [CrossRef]
- Sixt, S.; Scheinert, D.; Rastan, A.; Krankenberg, H.; Steinkamp, H.; Schmidt, A.; Sievert, H.; Minar, E.; Bosiers, M.; Peeters, P.; et al. One-year outcome after percutaneous rotational and aspiration atherectomy in infrainguinal arteries in patient with and without type 2 diabetes mellitus. Ann. Vasc. Surg. 2011, 25, 520–529. [Google Scholar] [CrossRef]
- Ramaiah, V.; Gammon, R.; Kiesz, S.; Cardenas, J.; Runyon, J.P.; Fail, P.; Walker, C.; Allie, D.E.; Chamberlin, J.; Solis, M.; et al. Midterm outcomes from the TALON Registry: Treating peripherals with SilverHawk: Outcomes collection. J. Endovasc. Ther. 2006, 13, 592–602. [Google Scholar] [CrossRef]
- Zeller, T.; Sixt, S.; Schwarzwälder, U.; Schwarz, T.; Frank, U.; Bürgelin, K.; Pochert, V.; Müller, C.; Noory, E.; Krankenberg, H.; et al. Two-year results after directional atherectomy of infrapopliteal arteries with the SilverHawk device. J. Endovasc. Ther. 2007, 14, 232–240. [Google Scholar] [CrossRef]
- Zeller, T.; Langhoff, R.; Rocha-Singh, K.J.; Jaff, M.R.; Blessing, E.; Amann-Vesti, B.; Krzanowski, M.; Peeters, P.; Scheinert, D.; Torsello, G.; et al. Directional Atherectomy Followed by a Paclitaxel-Coated Balloon to Inhibit Restenosis and Maintain Vessel Patency: Twelve-Month Results of the DEFINITIVE AR Study. Circ. Cardiovasc. Interv. 2017, 10, e004848. [Google Scholar] [CrossRef] [PubMed]
- Gray, B.H.; Laird, J.R.; Ansel, G.M.; Shuck, J.W. Complex endovascular treatment for critical limb ischemia in poor surgical candidates: A pilot study. J. Endovasc. Ther. 2002, 9, 599–604. [Google Scholar] [CrossRef] [PubMed]
- Ducasse, E.; Sapoval, M.; Brunet, J.; Commeau, P.; Goueffic, Y.; Sabatier, J.; Steinmetz, E.; Lermusiaux, P.; Rosset, E.; Caradu, C. Outcomes and Comparative Analysis of the Initial Results of Standard Balloon Angioplasty Versus Drug-Coated Balloons Alone Versus in Association With Laser-Excimer Atherectomy in the Treatment of Femoropopliteal Artery In-Stent Restenosis (INTACT). J. Endovasc. Ther. 2024, 24, 15266028241248333. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ju, S.; Liu, H.; Chen, B.; Jiang, J.; Shi, Y.; Ma, T.; Lin, C.; Fang, G.; Guo, D.; et al. Outcomes of Excimer Laser Ablation Combined with Drug-coated Balloon in Atherosclerotic Lesions of the Popliteal Artery. Ann. Vasc. Surg. 2024, 104, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Brodmann, M.; Holden, A.; Zeller, T. Safety and feasibility of intravascular lithotripsy for treatment of below-the-knee arterial stenoses. J. Endovasc. Ther. 2018, 25, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Tepe, G.; Brodmann, M.; Werner, M.; Bachinsky, W.; Holden, A.; Zeller, T. Disrupt PAD III Investigators. Intravascular lithotripsy for peripheral artery calcification: 30-day outcomes from the randomized Disrupt PAD III trial. JACC Cardiovasc. Interv. 2021, 14, 1352–1361. [Google Scholar] [CrossRef]
- Shammas, N.W.; Mangalmurti, S.; Bernardo, N.L.; Mehrle, A.; Adams, G.; Bertolet, B.; Stavroulakis, K.; Soukas, P.A. Intravascular Lithotripsy for Treatment of Severely Calcified Common Femoral Artery Disease: Results From the Disrupt PAD III Observational Study. J. Endovasc. Ther. 2024, 15, 15266028241255622. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.K.; Lee, S.; Ahn, C.; Lee, S.; Lee, Y.; Kim, B.; Hong, M.; Jang, Y.; Kim, T.; Park, H.; et al. Intravascular ultrasound-guided drug-coated balloon angioplasty for femoropopliteal artery disease: A clinical trial. Eur. Heart J. 2024, ehae372. [Google Scholar] [CrossRef] [PubMed]
- Soga, Y.; Takahara, M.; Iida, O.; Tomoi, Y.; Kawasaki, D.; Tanaka, A.; Yamauchi, Y.; Tobita, K.; Kozuki, A.; Fujihara, M.; et al. Vessel Patency and Associated Factors of Drug-Coated Balloon for Femoropopliteal Lesion. J. Am. Heart Assoc. 2023, 12, e025677. [Google Scholar] [CrossRef]
- Horie, K.; Tanaka, A.; Taguri, M.; Inoue, N. Impact of Baseline and Postprocedural Intravascular Ultrasound Findings on 1-Year Primary Patency After Drug-Coated Balloon Treatment of Femoropopliteal Lesions. J. Endovasc. Ther. 2022, 29, 66–75. [Google Scholar] [CrossRef]
- Stavroulakis, K.; Bisdas, T.; Torsello, G.; Argyriou, A.; Bollenberg, L.; Schwindt, A. Optical coherence tomography guided directional atherectomy with antirestenotic therapy for femoropopliteal arterial disease. J. Cardiovasc. Surg. 2019, 60, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Tomoi, Y.; Soga, Y.; Imada, K.; Sakai, N.; Katsuki, T.; Ando, K. Impact of a Less Than 50% Residual Stenosis Following Vessel Preparation in Femoropopliteal Drug-Coated Balloon Angioplasty. J. Endovasc. Ther. 2024, 9, 15266028231223086. [Google Scholar] [CrossRef] [PubMed]
- Kinstner, C.M.; Lammer, J.; Willfort-Ehringer, A.; Matzek, W.; Gschwandtner, M.; Javor, D.; Funovics, M.; Schoder, M.; Koppensteiner, R.; Loewe, C.; et al. Paclitaxel-Eluting Balloon Versus Standard Balloon Angioplasty in In-Stent Restenosis of the Superficial Femoral and Proximal Popliteal Artery: 1-Year Results of the PACUBA Trial. JACC Cardiovasc. Interv. 2016, 9, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Tepe, G.; Zeller, T.; Albrecht, T.; Heller, S.; Schwarzwälder, U.; Beregi, J.P.; Claussen, C.D.; Oldenburg, A.; Scheller, B.; Speck, U. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N. Engl. J. Med. 2008, 358, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Krankenberg, H.; Tübler, T.; Ingwersen, M.; Schlüter, M.; Scheinert, D.; Blessing, E.; Sixt, S.; Kieback, A.; Beschorner, U.; Zeller, T. Drug-Coated Balloon Versus Standard Balloon for Superficial Femoral Artery In-Stent Restenosis: The Randomized Femoral Artery In-Stent Restenosis (FAIR) Trial. Circulation 2015, 132, 2230–2236. [Google Scholar] [CrossRef] [PubMed]
- Grotti, S.; Liistro, F.; Angioli, P.; Ducci, K.; Falsini, G.; Porto, I.; Ricci, L.; Ventoruzzo, G.; Turini, F.; Bellandi, G.; et al. Paclitaxel-Eluting Balloon vs Standard Angioplasty to Reduce Restenosis in Diabetic Patients With In-Stent Restenosis of the Superficial Femoral and Proximal Popliteal Arteries: Three-Year Results of the DEBATE-ISR Study. J. Endovasc. Ther. 2016, 23, 52–57. [Google Scholar] [CrossRef]
- Stabile, E.; Virga, V.; Salemme, L.; Cioppa, A.; Ambrosini, V.; Sorropago, G.; Tesorio, T.; Cota, L.; Popusoi, G.; Pucciarelli, A.; et al. Drug-eluting balloon for treatment of superficial femoral artery in-stent restenosis. J. Am. Coll. Cardiol. 2012, 60, 1739–1742. [Google Scholar] [CrossRef] [PubMed]
- Werk, M.; Albrecht, T.; Meyer, D.R.; Ahmed, M.N.; Behne, A.; Dietz, U.; Eschenbach, G.; Hartmann, H.; Lange, C.; Schnorr, B.; et al. Paclitaxel-coated balloons reduce restenosis after femoro-popliteal angioplasty: Evidence from the randomized PACIFIER trial. Circ. Cardiovasc. Interv. 2012, 5, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Rosenfield, K.; Jaff, M.R.; White, C.J.; Rocha-Singh, K.; Mena-Hurtado, C.; Metzger, D.C.; Brodmann, M.; Pilger, E.; Zeller, T.; Krishnan, P.; et al. Trial of a Paclitaxel-Coated Balloon for Femoropopliteal Artery Disease. N. Engl. J. Med. 2015, 373, 145–153. [Google Scholar] [CrossRef]
- Scheinert, D.; Schulte, K.L.; Zeller, T.; Lammer, J.; Tepe, G. Paclitaxel-releasing balloon in femoropopliteal lesions using a BTHC excipient: Twelve-month results from the BIOLUX P-I randomized trial. J. Endovasc. Ther. 2015, 22, 14–21. [Google Scholar] [CrossRef]
- Tepe, G.; Laird, J.R.; Schneider, P.; Brodmann, M.; Krishnan, P.; Micari, A.; Metzger, C.; Scheinert, D.; Zeller, T.; Cohen, D.J.; et al. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation 2015, 131, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Laird, J.R.; Schneider, P.A.; Tepe, G.; Brodmann, M.; Zeller, T.; Metzger, C.; Krishnan, P.; Scheinert, D.; Micari, A.; Cohen, D.J.; et al. Durability of Treatment Effect Using a Drug-Coated Balloon for Femoropopliteal Lesions: 24-Month Results of IN.PACT SFA. J. Am. Coll. Cardiol. 2015, 66, 2329–2338. [Google Scholar] [CrossRef] [PubMed]
- Sallustro, M.; Peluso, A.; Turchino, D.; Maione, I.; Vita, F.; Martelli, E.; Serra, R.; Bracale, U.M. Results of New Dual-Drug Coated Balloon Angioplasty versus POBA for Femoropopliteal Lesions. Ann. Vasc. Surg. 2023, 89, 52–59. [Google Scholar] [CrossRef]
- Liistro, F.; Angioli, P.; Porto, I.; Ducci, K.; Falsini, G.; Ventoruzzo, G.; Ricci, L.; Scatena, A.; Grotti, S.; Bolognese, L. Drug-Eluting Balloon Versus Drug-Eluting Stent for Complex Femoropopliteal Arterial Lesions: The DRASTICO Study. J. Am. Coll. Cardiol. 2019, 74, 205–215. [Google Scholar] [CrossRef]
- Zeller, T.; Beschorner, U.; Pilger, E.; Bosiers, M.; Deloose, K.; Peeters, P.; Scheinert, D.; Schulte, K.L.; Rastan, A.; Brodmann, M. Paclitaxel-Coated Balloon in Infrapopliteal Arteries: 12-Month Results From the BIOLUX P-II Randomized Trial (BIOTRONIK’S-First in Man study of the Passeo-18 LUX drug releasing PTA Balloon Catheter vs. the uncoated Passeo-18 PTA balloon catheter in subjects requiring revascularization of infrapopliteal arteries). JACC Cardiovasc. Interv. 2015, 8, 1614–1622. [Google Scholar] [CrossRef] [PubMed]
- Zeller, T.; Baumgartner, I.; Scheinert, D.; Brodmann, M.; Bosiers, M.; Micari, A.; Peeters, P.; Vermassen, F.; Landini, M.; Snead, D.B.; et al. Drug-eluting balloon versus standard balloon angioplasty for infrapopliteal arterial revascularization in critical limb ischemia: 12-month results from the IN.PACT DEEP randomized trial. J. Am. Coll. Cardiol. 2014, 64, 1568–1576. [Google Scholar] [CrossRef]
- Mustapha, J.A.; Brodmann, M.; Geraghty, P.J.; Saab, F.; Settlage, R.A.; Jaff, M.R.; Lutonix BTK Study Investigators. Drug-Coated vs Uncoated Percutaneous Transluminal Angioplasty in Infrapopliteal Arteries: Six-Month Results of the Lutonix BTK Trial. J. Invasive Cardiol. 2019, 31, 205–211. [Google Scholar]
- Katsanos, K.; Spiliopoulos, S.; Kitrou, P.; Krokidis, M.; Paraskevopoulos, I.; Karnabatidis, D. Risk of Death and Amputation with Use of Paclitaxel-Coated Balloons in the Infrapopliteal Arteries for Treatment of Critical Limb Ischemia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Vasc. Interv. Radiol. 2020, 31, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Varcoe, R.L.; Paravastu, S.C.; Thomas, S.D.; Bennett, M.H. The use of drug-eluting stents in infrapopliteal arteries: An updated systematic review and meta-analysis of randomized trials. Int. Angiol. 2019, 38, 121–135. [Google Scholar] [CrossRef]
- Li, J.; Varcoe, R.; Manzi, M.; Kum, S.; Iida, O.; Schmidt, A.; Shishehbor, M.H. Below-the-Knee Endovascular Revascularization: A Position Statement. JACC Cardiovasc. Interv. 2024, 17, 589–607. [Google Scholar] [CrossRef]
- Rastan, A.; Böhme, T.; Zeller, T. Percutaneous intervention versus surgery in the treatment of common femoral artery lesions: Study protocol for the prospective, multi-center, randomized PESTO-CFA trial. Trials 2024, 25, 370. [Google Scholar] [CrossRef] [PubMed]
- Adam, D.J.; Beard, J.D.; Cleveland, T.; Bell, J.; Bradbury, A.W.; Forbes, J.F.; Fowkes, F.G.; Gillepsie, I.; Ruckley, C.V.; Raab, G.; et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): Multicentre, randomised controlled trial. Lancet 2005, 366, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Farber, A.; Menard, M.T.; Conte, M.S.; Kaufman, J.A.; Powell, R.J.; Choudhry, N.K.; Hamza, T.H.; Assmann, S.F.; Creager, M.A.; Cziraky, M.J.; et al. Surgery or Endovascular Therapy for Chronic Limb-Threatening Ischemia. N. Engl. J. Med. 2022, 387, 2305–2316. [Google Scholar] [CrossRef] [PubMed]
| Paclitaxel | Sirolimus | |
|---|---|---|
| Biochemical Properties | ||
| Cytostatic | YES | YES |
| Cytotoxic | YES | NO |
| Apoptotic | YES | NO |
| Antiproliferative | YES | YES |
| Lipophilic | YES | NO |
| Suppress neutrophilic leukocyte activation | NO | YES |
| Highly effective during hypoxic | NO | YES |
| Effects in normoxic conditions | YES | YES |
| Broader therapeutic window | NO | YES |
| Drug-Coated Balloon Characteristics | ||
| Similar coating method | YES | NO |
| Late vessel remodeling | YES | NO |
| Product | Company | Drug Dose (µg/mm2) | Excipient |
|---|---|---|---|
| PACLITAXEL-DCB | |||
| IN.PACT Admiral | Medtronic | 3.5 | Urea |
| Lutonix | CR Bard | 2.0 | Polysorbate and sorbitol |
| Stellarex | Philips | 2.0 | Polyethylene glycol |
| SeQuent Please | B. Braun | 3.0 | Resveratrol |
| LEGFLOW | Cardionovum (Bonn, Germany) | 3.0 | Shelloic acid |
| Ranger | Boston Scientific | 2.0 | Citrate ester |
| Passeo-18 Lux | Biotronik | 3.0 | Butyryl-tri-hexyl citrate |
| Luminor | iVascular (Barcelona, Spain) | 3.0 | Organic ester |
| Surveil | SurModics (Eden Prairie, MN, USA) | 3.2 | Proprietary photo-link |
| SIROLIMUS-DCB | |||
| Magic Touch PTA | Concept Medical | 1.27 | Phospholipid-based |
| SELUTION | MedAlliance | 1.0 | PLGA and phospholipid micro reservoir |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tataru, D.-A.; Lazar, F.-L.; Onea, H.-L.; Homorodean, C.; Ober, M.-C.; Olinic, M.; Spinu, M.; Olinic, D.-M. Benefits and Challenges of Drug-Coated Balloons in Peripheral Artery Disease: From Molecular Mechanisms to Clinical Practice. Int. J. Mol. Sci. 2024, 25, 8749. https://doi.org/10.3390/ijms25168749
Tataru D-A, Lazar F-L, Onea H-L, Homorodean C, Ober M-C, Olinic M, Spinu M, Olinic D-M. Benefits and Challenges of Drug-Coated Balloons in Peripheral Artery Disease: From Molecular Mechanisms to Clinical Practice. International Journal of Molecular Sciences. 2024; 25(16):8749. https://doi.org/10.3390/ijms25168749
Chicago/Turabian StyleTataru, Dan-Alexandru, Florin-Leontin Lazar, Horea-Laurentiu Onea, Calin Homorodean, Mihai-Claudiu Ober, Maria Olinic, Mihail Spinu, and Dan-Mircea Olinic. 2024. "Benefits and Challenges of Drug-Coated Balloons in Peripheral Artery Disease: From Molecular Mechanisms to Clinical Practice" International Journal of Molecular Sciences 25, no. 16: 8749. https://doi.org/10.3390/ijms25168749






