Quantification of Photoreceptors’ Changes in a Diabetic Retinopathy Model with Two-Photon Imaging Microscopy
Abstract
1. Introduction
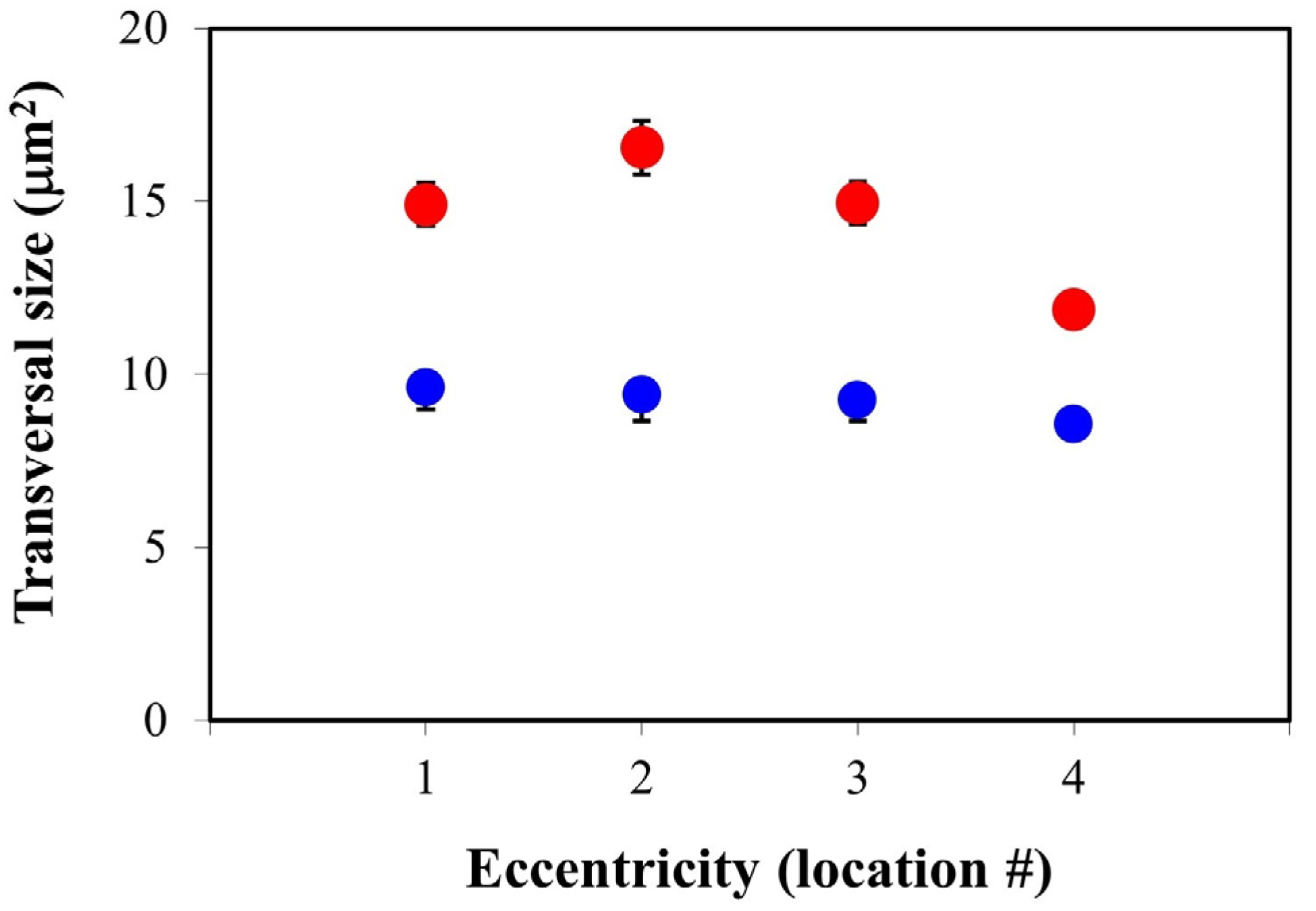
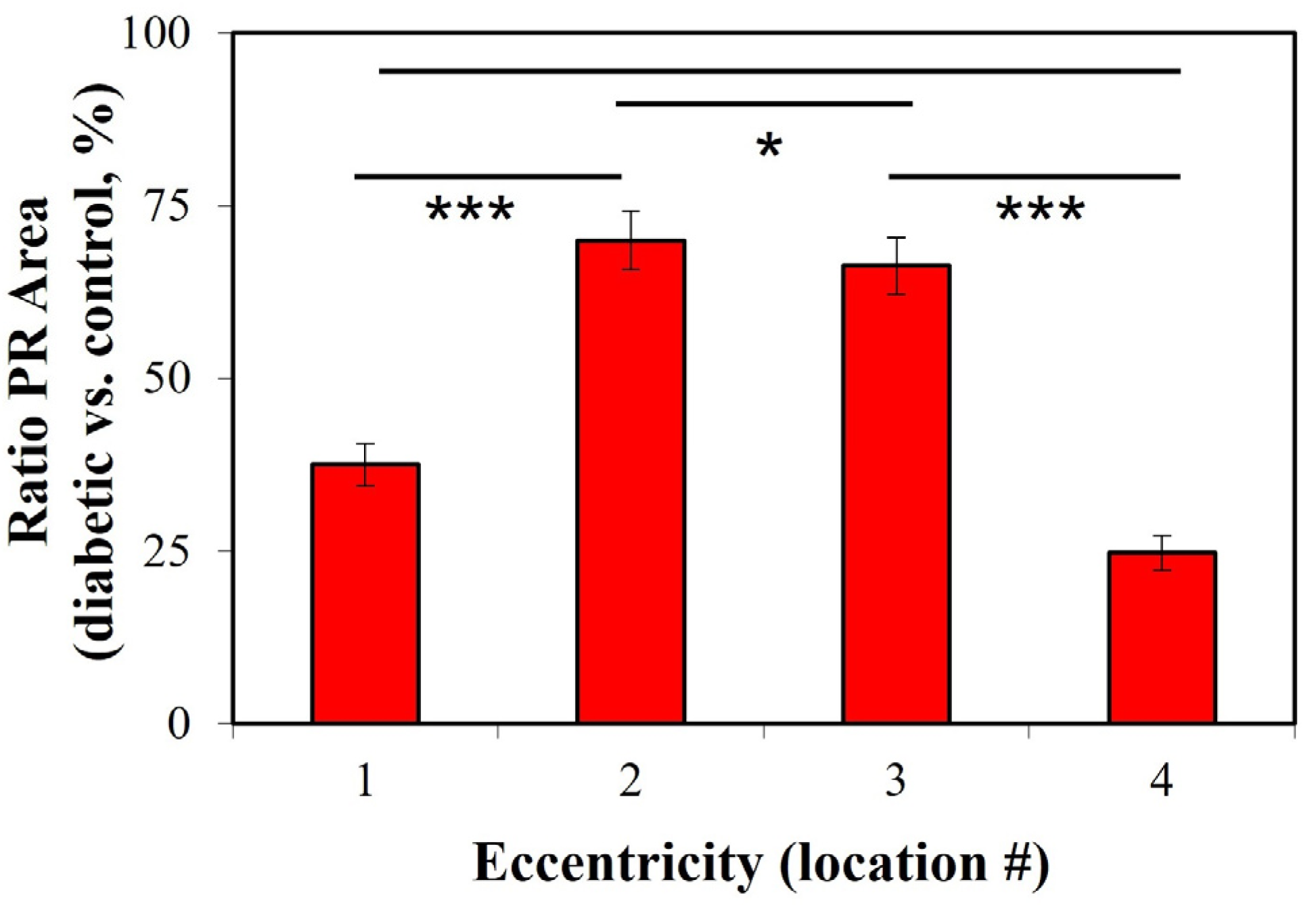
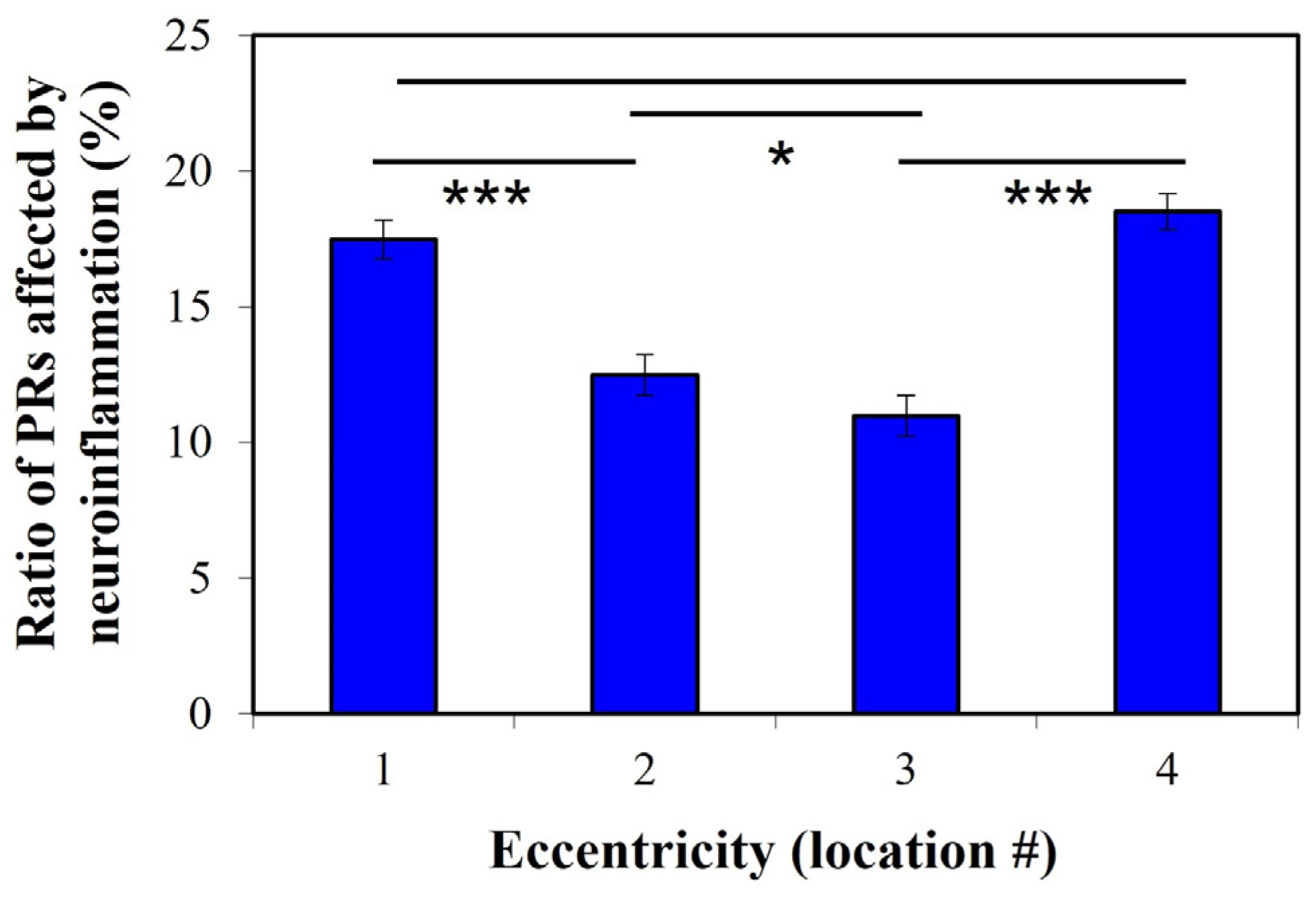
2. Results
3. Discussion
4. Material and Methods
4.1. Animal Model and Tissue Preparation
4.2. Experimental System
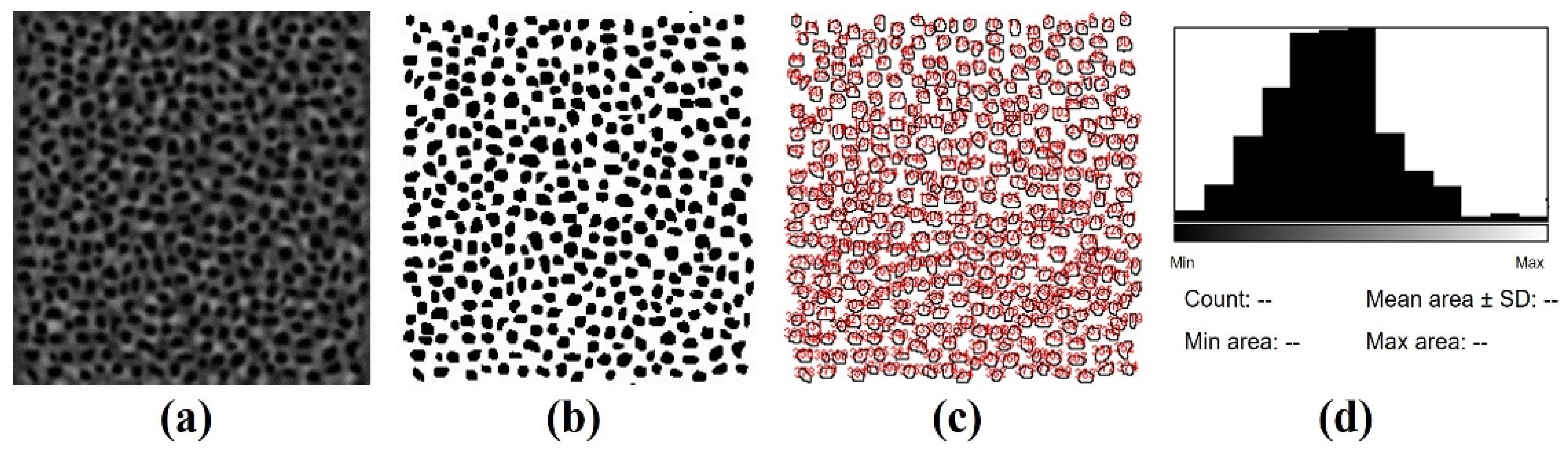
4.3. Image Acquisition, Processing and Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frank, R.N. Diabetic retinopathy. N. Engl. J. Med. 2004, 350, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Avogaro, A.; Fadini, G.P. Microvascular complications in diabetes: A growing concern for cardiologists. Int. J. Cardiol. 2019, 291, 29–35. [Google Scholar] [CrossRef]
- Miller, D.J.; Cascio, M.A.; Rosca, M.G. Diabetic retinopathy: The role of mitochondria in the neural retina and microvascular disease. Antioxidants 2020, 9, 905. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lo, A.C.Y. Diabetic retinopathy: Pathophysiology and treatments. Int. J. Mol. Sci. 2018, 19, 1816. [Google Scholar] [CrossRef] [PubMed]
- Hendrick, A.M.; Gibson, M.V.; Kulshreshtha, A. Diabetic retinopathy. Prim. Care 2015, 42, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Kropp, M.; Golubnitschaja, O.; Mazurakova, A.; Koklesova, L.; Sargheini, N.; Vo, T.K.S.; de Clerck, E.; Polivka, J., Jr.; Potuznik, P.; Polivka, J.; et al. Diabetic retinopathy as the leading cause of blindness and early predictor of cascading complications-risks and mitigation. EPMA J. 2023, 14, 21–42. [Google Scholar] [CrossRef]
- Solomon, S.D.; Chew, E.; Duh, E.J.; Sobrin, L.; Sun, J.K.; VanderBeek, B.L.; Wykoff, C.C.; Gardner, T.W. Diabetic retinopathy: A position statement by the American Diabetes Association. Diabetes Care 2017, 40, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Nian, S.; Lo, A.C.Y.; Mi, Y.; Ren, K.; Yang, D. Neurovascular unit in diabetic retinopathy: Pathophysiological roles and potential therapeutical targets. Eye Vis. 2021, 8, 15. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Singh, N.K. The role of inflammation in retinal neurodegeneration and degenerative diseases. Int. J. Mol. Sci. 2022, 23, 386. [Google Scholar] [CrossRef]
- Madeira, M.H.; Boia, R.; Santos, P.F.; Ambrosio, A.F.; Santiago, A.R. Contribution of microglia-mediated neuroinflammation to retinal degenerative diseases. Mediat. Inflamm. 2015, 2015, 673090. [Google Scholar] [CrossRef]
- Rashid, K.; Akhtar-Schaefer, I.; Langmann, T. Microglia in retinal degeneration. Front. Immunol. 2019, 10, 1975. [Google Scholar] [CrossRef] [PubMed]
- Wallach, D.; Kang, T.B.; Kovalenko, A. Concepts of tissue injury and cell death in inflammation: A historical perspective. Nat. Rev. Immunol. 2014, 14, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Murakami, Y.; Vavvas, D.G.; Sonoda, K.H. Necroptosis and neuroinflammation in retinal degeneration. Front. Neurosci. 2022, 16, 911430. [Google Scholar] [CrossRef] [PubMed]
- Joussen, A.M.; Poulaki, V.; Le, M.L.; Koizumi, K.; Esser, C.; Janicki, H.; Schraermeyer, U.; Kociok, N.; Fauser, S.; Kirchhof, B.; et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 2004, 18, 1450–1452. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Veenstra, A.; Palczewski, K.; Kern, T.S. Photoreceptor cells are major contributors to diabetes-induced oxidative stress and local inflammation in the retina. Proc. Natl. Acad. Sci. USA 2013, 110, 16586–16591. [Google Scholar] [CrossRef] [PubMed]
- Duh, E.J.; Sun, J.K.; Stitt, A.W. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight 2017, 2, e93751. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.S.; El-Remessy, A.B.; Matragoon, S.; Zhang, W.; Patel, Y.; Khan, S.; Al-Gayyar, M.M.; El-Shishtawy, M.M.; Liou, G.I. Retinal microglial activation and inflammation induced by amadori-glycated albumin in a rat model of diabetes. Diabetes 2011, 60, 1122–1133. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G.R.; Barber, A.J. Visual dysfunction associated with diabetic retinopathy. Curr. Diab. Rep. 2010, 10, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Rajab, H.A.; Baker, N.L.; Hunt, K.J.; Klein, R.; Cleary, P.A.; Lachin, J.; Virella, G.; Lopes-Virella, M.F. The predictive role of markers of inflammation and endothelial dysfunction on the course of diabetic retinopathy in type 1 diabetes. J. Diabetes Complicat. 2015, 29, 108–114. [Google Scholar] [CrossRef]
- Lechner, J.; O’Leary, O.E.; Stitt, A.W. The pathology associated with diabetic retinopathy. Vis. Res. 2017, 139, 7–14. [Google Scholar] [CrossRef]
- Radenkovic, M.; Stojanovic, M.; Prostran, M. Experimental diabetes induced by alloxan and streptozotocin: The current state of the art. J. Pharmacol Toxicol. Methods 2015, 78, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.N.; Reddya, N.M.; Patilb, K.R.; Nakhatec, K.T.; Ojhad, S.; Patil, C.R. Challenges and issues with streptozotocin-induced diabetes—A clinically relevant animal model to understand the diabetes pathogenesis and evaluate therapeutics. Chem. Biol. Interact. 2016, 244, 49–63. [Google Scholar] [CrossRef] [PubMed]
- Lenzen, S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008, 51, 216–226. [Google Scholar] [CrossRef]
- Patil, M.; Suryanarayana, P.; Putcha, U.; Srinivas, M.; Reddy, G. Evaluation of neonatal streptozotocin induced diabetic rat model for the development of cataract. Oxid. Med. Cell. Longev. 2014, 2014, 463264. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Stitt, A. Animal models of diabetic retinopathy. In Animal Models of Ophthalmic Diseases; Chan, C.C., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 67–83. [Google Scholar]
- Feenstra, D.J.; Yego, E.C.; Mohr, S. Modes of retinal cell death in diabetic retinopathy. J. Clin. Exp. Ophthalmol. 2013, 4, 298. [Google Scholar]
- Abcouwer, S.F.; Gardner, T.W. Diabetic retinopathy: Loss of neuroretinal adaptation to the diabetic metabolic environment. Ann. N. Y. Acad. Sci. 2014, 1311, 174–190. [Google Scholar] [CrossRef] [PubMed]
- Helmchen, F.; Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2005, 2, 932–939. [Google Scholar] [CrossRef]
- Wang, B.G.; Eitner, A.; Lindenau, J.; Halbhuber, K.J. High-resolution two-photon excitation microscopy of ocular tissues in porcine eye. Lasers Surg. Med. 2008, 40, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.W.; Li, Y.C.; Ye, T.; Strang, C.; Keyser, K.; Curcio, C.A.; Yao, X.C. Two-photon excited autofluorescence imaging of freshly isolated frog retinas. Biomed. Opt. Express 2011, 2, 1494–1503. [Google Scholar] [CrossRef]
- Bueno, J.M.; Giakoumaki, A.; Gualda, E.J.; Schaeffel, F.; Artal, P. Analysis of the chicken retina with an adaptive optics multiphoton microscope. Biomed. Opt. Express 2011, 2, 1637–1648. [Google Scholar] [CrossRef]
- Bueno, J.M.; Cruz-Castillo, R.; Avilés-Trigueros, M.; Bautista-Elivar, N. Arrangement of the photoreceptor mosaic in a diabetic rat model imaged with multiphoton microscopy. Biomed. Opt. Express 2020, 11, 4901–4914. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.J.; Masella, B.; Dubra, A.; Sharma, R.; Yin, L.; Merigan, W.H.; Palczewska, G.; Palczewski, K.; Williams, D.R. Images of photoreceptors in living primate eyes using adaptive optics two-photon ophthalmoscopy. Biomed. Opt. Express 2011, 2, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Ávila, F.J.; Gambín, A.; Artal, P.; Bueno, J.M. In vivo two-photon microscopy of the human eye. Sci. Rep. 2019, 9, 10121. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, H.; Su, S.B. Neuroinflammatory responses in diabetic retinopathy. J. Neuroinflamm. 2015, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Karlstetter, M.; Ebert, S.; Langmann, T. Microglia in the healthy and degenerating retina: Insights from novel mouse models. Immunobiology 2010, 215, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Danilova, I.; Medvedeva, S.; Shmakova, S.; Chereshneva, M.; Sarapultsev, A.; Sarapultsev, P. Pathological changes in the cellular structures of retina and choroidea in the early stages of alloxan-induced diabetes. World J. Diabetes 2018, 9, 206–257. [Google Scholar] [CrossRef] [PubMed]
- Enzsöly, A.; Szabó, A.; Kántor, O.; Dávid, C.; Szalay, P.; Szabó, K.; Szél, A.; Nemeth, J.; Lukáts, A. Pathologic alterations of the outer retina in streptozotocin-induced diabetes. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3686–3699. [Google Scholar] [CrossRef] [PubMed]
- Thananjeyan, A.; Ullah, F.; Gyengesi, E.; Muench, G.; Cameron, M. The effect of chronic neuroinflammation on retinal morphology and the impact of curcumin treatment. Investig. Ophthalmol. Vis. Sci. 2021, 62, 3043. [Google Scholar]
- Tonade, D.; Liu, H.; Kern, T.S. Photoreceptor cells produce inflammatory mediators that contribute to endothelial cell death in diabetes. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4264–4271. [Google Scholar] [CrossRef]
- Lövestam-Adrian, M.; Agardh, C.D.; Torffvit, O.; Agardh, E. Diabetic retinopathy, visual acuity, and medical risk indi-cators: A continuous 10-year follow-up study in Type 1 diabetic patients under routine care. J. Diabetes Complicat. 2001, 15, 287–294. [Google Scholar] [CrossRef]
- Pramanik, S.; Chowdhury, S.; Ganguly, U.; Banerjee, A.; Bhattacharya, B.; Mondal, L.K. Visual contrast sensitivity could be an early marker of diabetic retinopathy. Heliyon 2020, 6, e05336. [Google Scholar] [CrossRef] [PubMed]
- Glassman, A.R.; Elmasry, M.A.; Baskin, D.A.; Brigell, M.; Chong, V.; Davis, Q.; Lesmes, L.; Levin, L.A.; Maddess, T.; Taylor, L.J.; et al. Visual function measurements in eyes with diabetic retinopathy: An expert opinion on available measures. Ophthalmol. Sci. 2024, 4, 100519. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.M.; Chakravarthy, U.; Das, R.; Graham, K.W.; Naskas, T.T.; Perais, J.; Kee, F.; Peto, T.; Hogg, R.E. Identifying the severity of diabetic retinopathy by visual function measures using both traditional statistical methods and interpretable machine learning: A cross-sectional study. Diabetologia 2023, 66, 2250–2260. [Google Scholar] [CrossRef]
- Brown, M.M.; Brown, G.C.; Sharma, S.; Landy, J.; Bakal, J. Quality of life with visual acuity loss from diabetic retinopathy and age-related macular degeneration. Arch Ophthalmol. 2002, 120, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.; Klein, B.E.; Moss, S.E. Visual impairment in diabetes. Ophthalmology 1984, 91, 1–9. [Google Scholar] [CrossRef]
- Aung, M.H.; Kim, M.K.; Olson, D.E.; Thule, P.M.; Pardue, M.T. Early visual deficits in streptozotocin-induced diabetic long evans rats. Invest. Ophthalmol. Vis. Sci. 2013, 54, 1370–1377. [Google Scholar] [CrossRef]
- Prusky, G.T.; Alam, N.M.; Beekman, S.; Douglas, R.M. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4611–4616. [Google Scholar] [CrossRef]
- Curcio, C.A.; Sloan, K.R. Packing geometry of human cone photoreceptors—Variation with eccentricity and evidence for local anisotropy. Vis. Neurosci. 1992, 9, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Norouzian, D.; Mehrabi, M.R.; Jamshidi, S.; Farhangi, A.; Verdi, A.A.; Mofidian, S.M.; Rad, B.L. Induction of diabetes by streptozotocin in rats. Indian J. Clin. Biochem. 2007, 22, 60–64. [Google Scholar] [CrossRef]
- Salinas-Navarro, M.; Mayor-Torroglosa, S.; Jiménez-López, M.; Avilés-Trigueros, M.; Holmes, T.M.; Lund, R.D.; Villegas-Pérez, M.P.; Vidal-Sanz, M. A computerized analysis of the entire retinal ganglion cell population and its spatial distribution in adult rats. Vision Res. 2009, 49, 115–126. [Google Scholar] [CrossRef]
- Gualda, E.J.; Bueno, J.M.; Artal, P. Wavefront optimized nonlinear microscopy of ex vivo human retinas. J. Biomed. Opt. 2010, 15, 026007. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, Y.; Batten, M.L.; Piston, D.W.; Baehr, W.; Palczewski, K. Noninvasive two-photon imaging reveals retinyl ester storage structures in the eye. J. Cell. Biol. 2004, 164, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, Y. Lodowski, K.H.; Koutalos, Y. Two-photon microscopy: Shedding light on the chemistry of vision. Biochemistry 2007, 46, 9674–9684. [Google Scholar] [CrossRef] [PubMed]
- Reymond, L. Spatial visual acuity of the eagle Aquila audax: A behavioural, optical and anatomical investigation. Vision Res. 1985, 25, 1477–1491. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, A.; Hallett, P.E.; Parker, J.A. Aspheric curvatures, refractive indices and chromatic aberration for the rat eye. Vision Res. 1984, 23, 1351–1363. [Google Scholar] [CrossRef]
- Yellott, J.I., Jr. Spectral analysis of spatial sampling by photoreceptors: Topological disorder prevents aliasing. Vision Res. 1982, 22, 1205–1210. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bautista-Elivar, N.; Avilés-Trigueros, M.; Bueno, J.M. Quantification of Photoreceptors’ Changes in a Diabetic Retinopathy Model with Two-Photon Imaging Microscopy. Int. J. Mol. Sci. 2024, 25, 8756. https://doi.org/10.3390/ijms25168756
Bautista-Elivar N, Avilés-Trigueros M, Bueno JM. Quantification of Photoreceptors’ Changes in a Diabetic Retinopathy Model with Two-Photon Imaging Microscopy. International Journal of Molecular Sciences. 2024; 25(16):8756. https://doi.org/10.3390/ijms25168756
Chicago/Turabian StyleBautista-Elivar, Nazario, Marcelino Avilés-Trigueros, and Juan M. Bueno. 2024. "Quantification of Photoreceptors’ Changes in a Diabetic Retinopathy Model with Two-Photon Imaging Microscopy" International Journal of Molecular Sciences 25, no. 16: 8756. https://doi.org/10.3390/ijms25168756
APA StyleBautista-Elivar, N., Avilés-Trigueros, M., & Bueno, J. M. (2024). Quantification of Photoreceptors’ Changes in a Diabetic Retinopathy Model with Two-Photon Imaging Microscopy. International Journal of Molecular Sciences, 25(16), 8756. https://doi.org/10.3390/ijms25168756





