The Protective Effect of Quercetin against the Cytotoxicity Induced by Fumonisin B1 in Sertoli Cells
Abstract
1. Introduction
2. Results
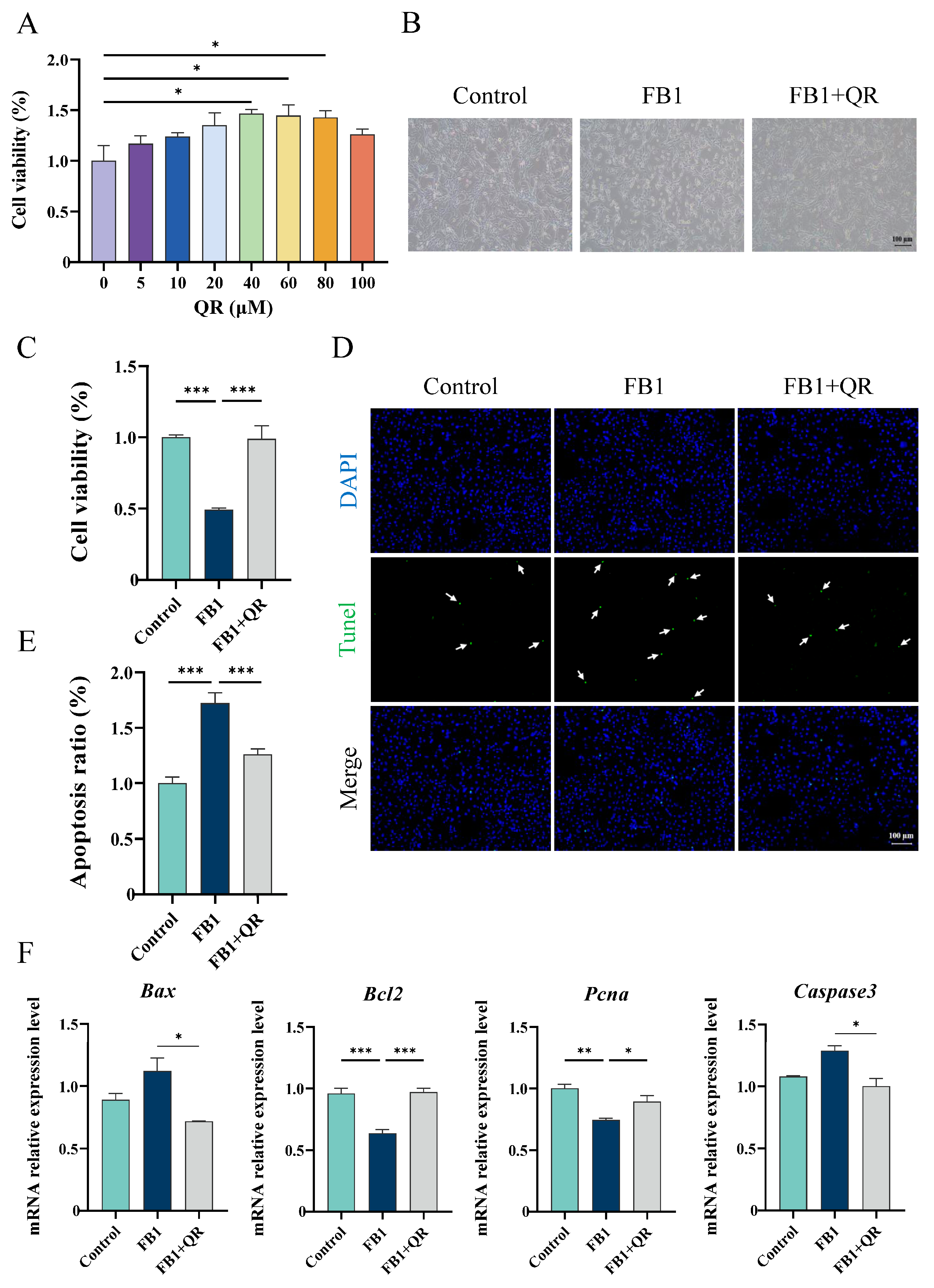
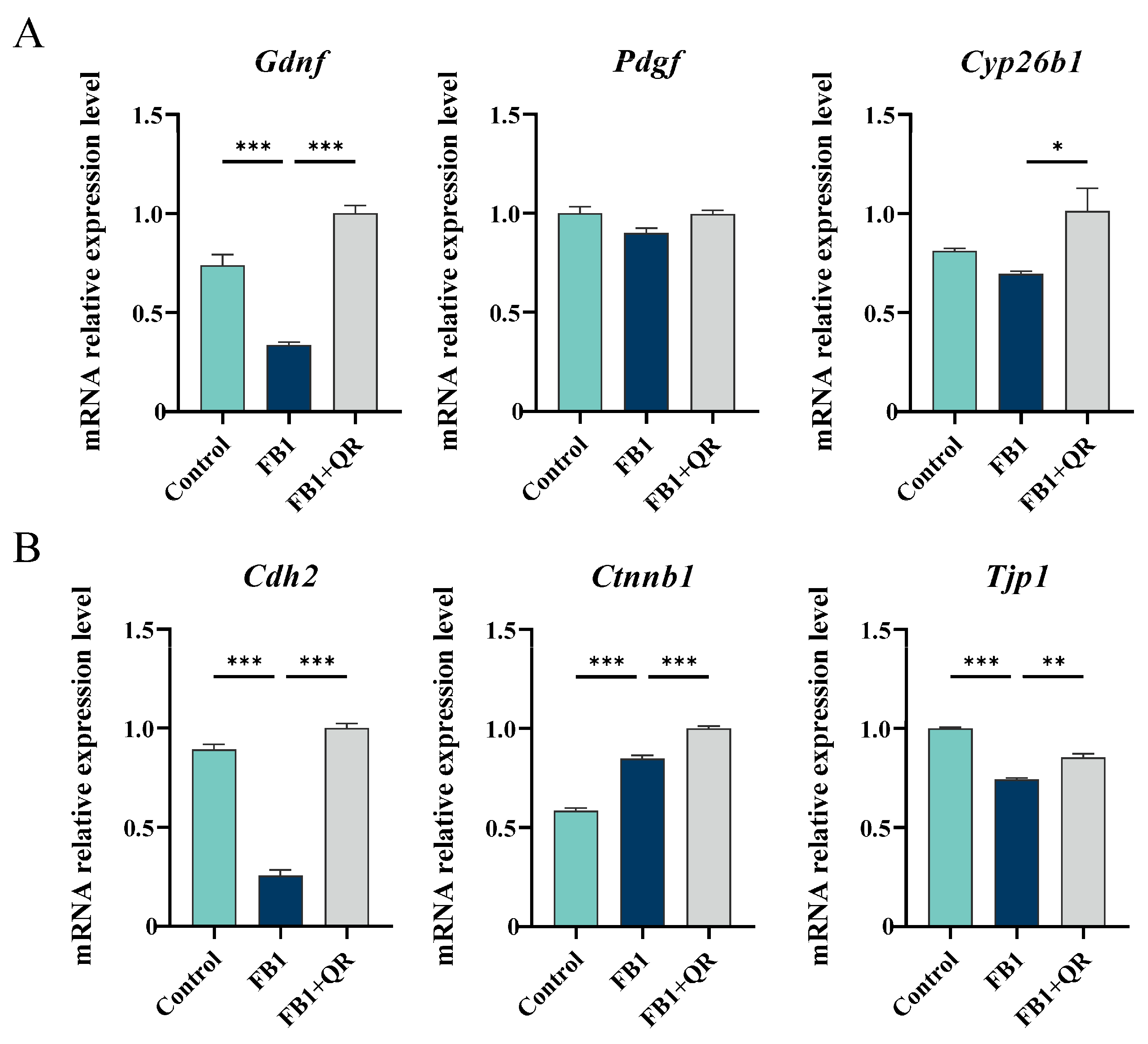
2.1. Quercetin Prevents Cellular Apoptosis and Defective Cell Functions in TM4 Cells Treated with FB1
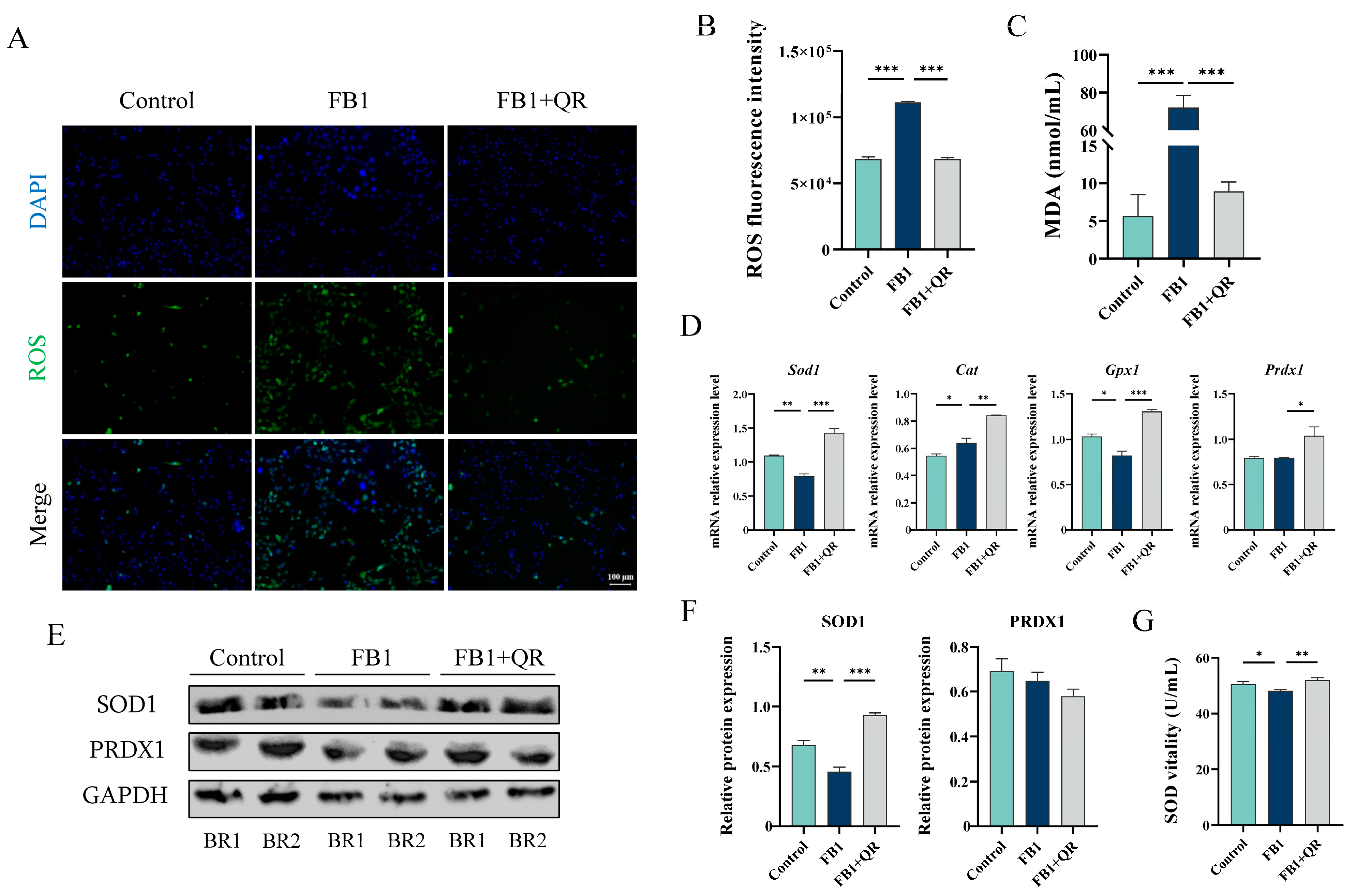
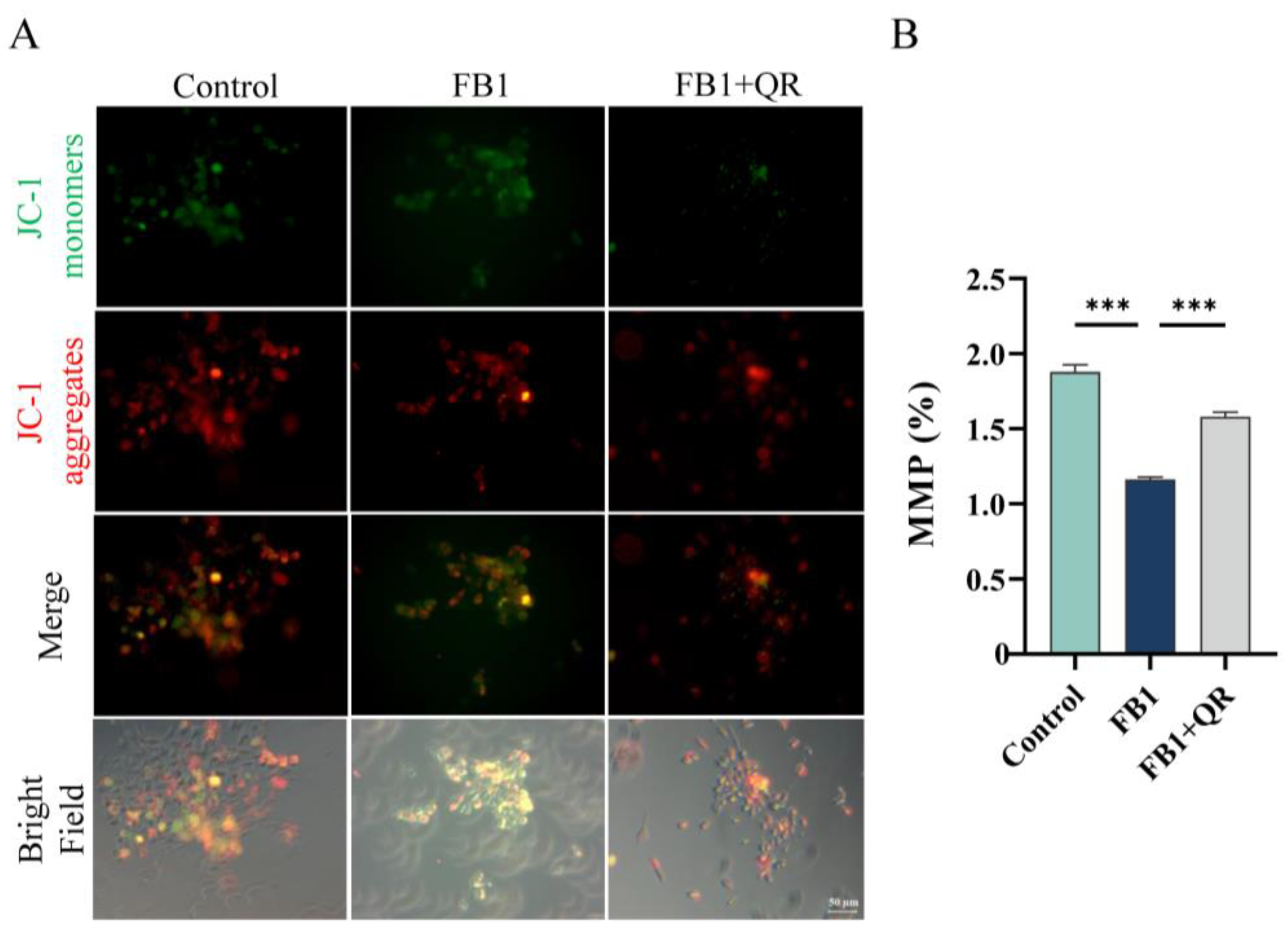
2.2. Quercetin Eliminates Excess Oxidative Stress and Restores the Mitochondrial Membrane Potential Damage in TM4 Cells Treated with FB1
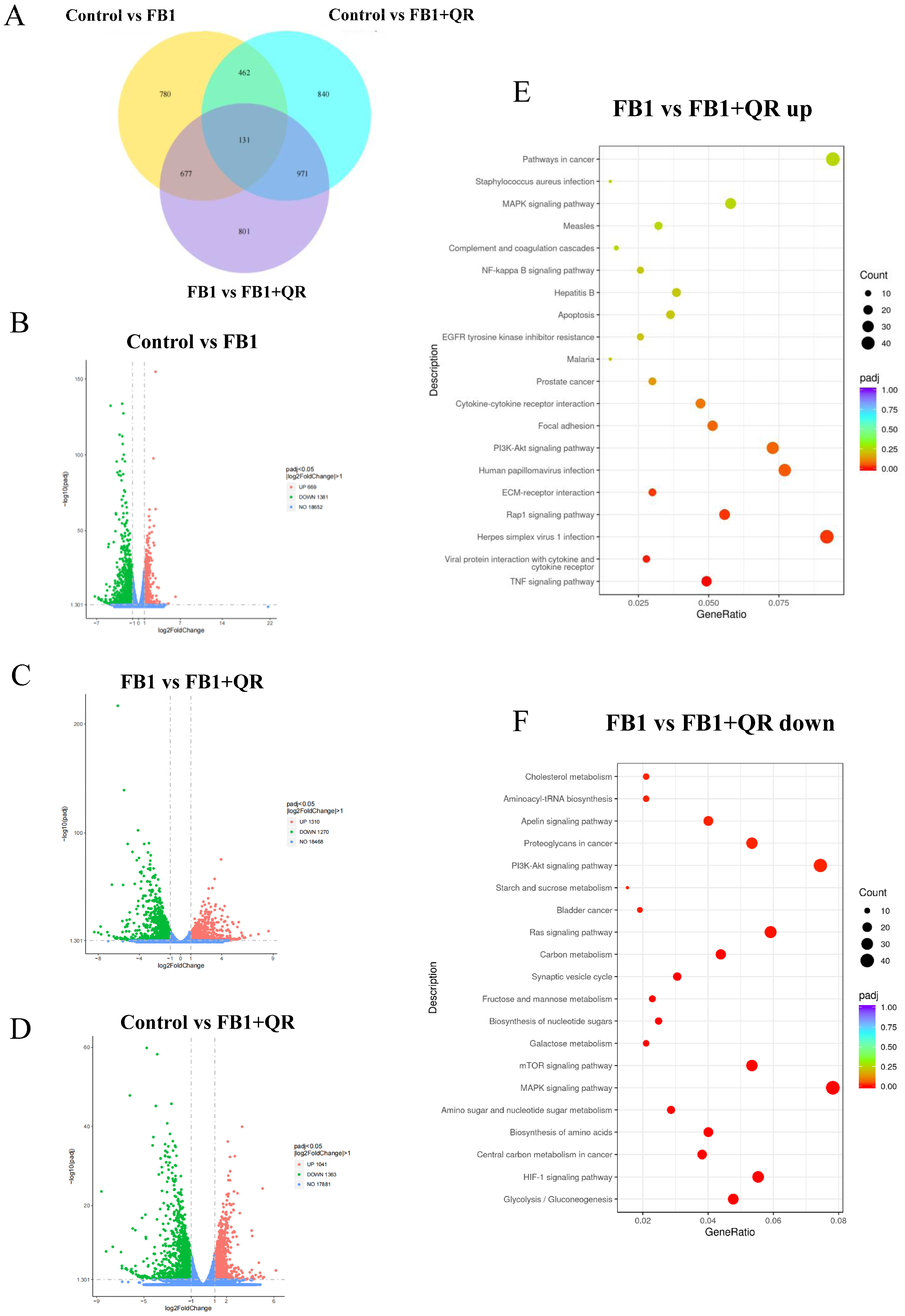
2.3. Dynamic Transcriptome Changes in TM4 Cells Treated with FB1 and Quercetin
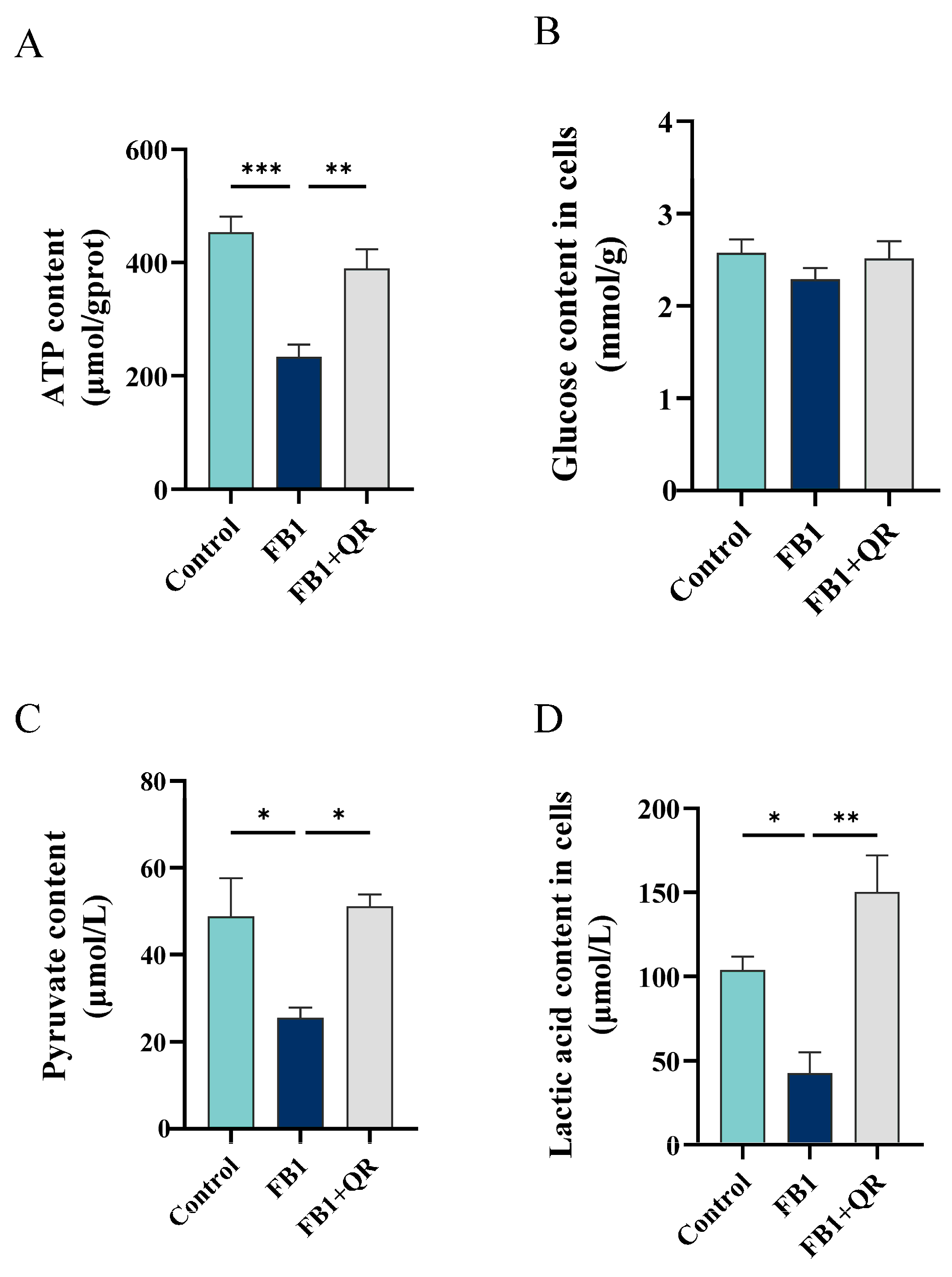
2.4. Quercetin Treatment Restored the Defective Energy Metabolism Level in FB1-Treated TM4 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatment
4.2. CCK-8 Assay
4.3. TUNEL Assay
4.4. Measurement of ROS, MDA and SOD Activity
4.5. RNA Extraction, cDNA Synthesis, and Real-Time PCR
4.6. Western Blot
4.7. MMP Detection
4.8. Measurement of ATP
4.9. Determination of Glucose, Pyruvate, and Lactic Acid Levels
4.10. Measurement of PKM and LDHA Activity
4.11. Mitochondrial Staining of Cultured Cells
4.12. RNA-Seq and Transcriptome Data Analysis
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Donnell, L.; Smith, L.B.; Rebourcet, D. Sertoli Cells as Key Drivers of Testis Function. Semin. Cell Dev. Biol. 2022, 121, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.F.; Li, Y.K.; Ren, K.; Xie, Y.J.; Yin, W.D.; Mo, Z.C. Characterization of Cholesterol Metabolism in Sertoli Cells and Spermatogenesis (Review). Mol. Med. Rep. 2018, 17, 705–713. [Google Scholar] [CrossRef]
- Griswold, M.D. The Central Role of Sertoli Cells in Spermatogenesis. Semin. Cell Dev. Biol. 1998, 9, 411–416. [Google Scholar] [CrossRef]
- Ni, F.D.; Hao, S.L.; Yang, W.X. Multiple Signaling Pathways in Sertoli Cells: Recent Findings in Spermatogenesis. Cell Death Dis. 2019, 10, 541. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Mruk, D.D. The blood-Testis Barrier and Its Implications for Male Contraception. Pharmacol. Rev. 2012, 64, 16–64. [Google Scholar] [CrossRef]
- Mruk, D.D.; Cheng, C.Y. The Mammalian Blood-Testis Barrier: Its Biology and Regulation. Endocr. Rev. 2015, 36, 564–591. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.; Feng, N.; Zheng, W.; Zheng, H.; Zou, H.; Yuan, Y.; Liu, X.; Liu, Z.; Gu, J.; Bian, J. Treatment with, Resveratrol, a SIRT1 Activator, Prevents Zearalenone-Induced Lactic Acid Metabolism Disorder in Rat Sertoli Cells. Molecules 2019, 24, 2474. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.F.; Martins, A.D.; Moreira, A.C.; Cheng, C.Y.; Alves, M.G. The Warburg Effect Revisited—Lesson from the Sertoli Cell. Med. Res. Rev. 2015, 35, 126–151. [Google Scholar] [CrossRef]
- Scott, P.M. Recent Research on Fumonisins: A Review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 242–248. [Google Scholar] [CrossRef]
- Wangia-Dixon, R.N.; Nishimwe, K. Molecular Toxicology and Carcinogenesis of Fumonisins: A Review. J. Environ. Sci. Health. Part C Toxicol. Carcinog. 2021, 39, 44–67. [Google Scholar] [CrossRef]
- Bertero, A.; Spicer, L.J.; Caloni, F. Fusarium Mycotoxins and In Vitro Species-Specific Approach with Porcine Intestinal and Brain In Vitro Barriers: A Review. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 121, 666–675. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.K.; Devi, S.; Lee, K.E.; Kang, S.G.; Kumar, P. Fumonisins: Impact on Agriculture, Food, and Human Health and their Management Strategies. Toxins 2019, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Q.; Wan, D.; Liu, Q.; Chen, D.; Liu, Z.; Martínez-Larrañaga, M.R.; Martínez, M.A.; Anadón, A.; Yuan, Z. Fumonisins: Oxidative Stress-Mediated Toxicity and Metabolism In Vivo and In Vitro. Arch. Toxicol. 2016, 90, 81–101. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Leblanc, J.C.; Nielsen, E.; Ntzani, E.; et al. Assessment of Information as Regards the Toxicity of Fumonisins for Pigs, Poultry and Horses. EFSA J. Eur. Food Saf. Auth. 2022, 20, e07534. [Google Scholar] [CrossRef]
- Lumsangkul, C.; Chiang, H.I.; Lo, N.W.; Fan, Y.K.; Ju, J.C. Developmental Toxicity of Mycotoxin Fumonisin B₁ in Animal Embryogenesis: An Overview. Toxins 2019, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wen, J.; Tang, Y.; Shi, J.; Mu, G.; Yan, R.; Cai, J.; Long, M. Research Progress on Fumonisin B1 Contamination and Toxicity: A Review. Molecules 2021, 26, 5238. [Google Scholar] [CrossRef]
- Li, W.; Zhao, H.; Zhuang, R.; Wang, Y.; Cao, W.; He, Y.; Jiang, Y.; Rui, R.; Ju, S. Fumonisin B(1) Exposure Adversely Affects Porcine Oocyte Maturation In Vitro by Inducing Mitochondrial Dysfunction and Oxidative Stress. Theriogenology 2021, 164, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, Y.; Ju, J.Q.; Liu, J.C.; Sun, S.C. Fumonisin B1 Exposure Deteriorates Oocyte Quality by Inducing Organelle Dysfunction and DNA Damage in Mice. Ecotoxicol. Environ. Saf. 2021, 223, 112598. [Google Scholar] [CrossRef]
- Cortinovis, C.; Caloni, F.; Schreiber, N.B.; Spicer, L.J. Effects of Fumonisin B1 Alone and Combined with Deoxynivalenol or Zearalenone on Porcine Granulosa Cell Proliferation and Steroid Production. Theriogenology 2014, 81, 1042–1049. [Google Scholar] [CrossRef]
- Ouyang, H.; Zhu, H.; Li, J.; Chen, L.; Zhang, R.; Fu, Q.; Li, X.; Cao, C. Fumonisin B(1) Promotes Germ Cells Apoptosis Associated with Oxidative Stress-Related Nrf2 Signaling in Mice Testes. Chem.-Biol. Interact. 2022, 363, 110009. [Google Scholar] [CrossRef]
- Ewuola, E.O.; Egbunike, G.N. Effects of Dietary Fumonisin B1 on the Onset of Puberty, Semen Quality, Fertility Rates and Testicular Morphology in Male Rabbits. Reproduction 2010, 139, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Minervini, F.; Lacalandra, G.M.; Filannino, A.; Garbetta, A.; Nicassio, M.; Dell’aquila, M.E.; Visconti, A. Toxic Effects Induced by Mycotoxin Fumonisin B1 on Equine Spermatozoa: Assessment of Viability, Sperm Chromatin Structure Stability, ROS Production and Motility. Toxicol. Vitr. Int. J. Publ. Assoc. BIBRA 2010, 24, 2072–2078. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxidative Med. Cell. Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Y.; Du, X.; Liu, Z.; Zhu, C.; Mao, W.; Liu, G.; Jiang, Q. Anti-Aging Effects of Quercetin in Cladocera Simocephalus vetulus Using Proteomics. ACS Omega 2023, 8, 17609–17619. [Google Scholar] [CrossRef]
- Hou, D.D.; Zhang, W.; Gao, Y.L.; Sun, Y.Z.; Wang, H.X.; Qi, R.Q.; Chen, H.D.; Gao, X.H. Anti-Inflammatory Effects of Quercetin in a Mouse Model of MC903-Induced Atopic Dermatitis. Int. Immunopharmacol. 2019, 74, 105676. [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Pant, A.B.; Farombi, E.O. Dietary Antioxidant, Quercetin, Protects Sertoli-Germ Cell Coculture from Atrazine-Induced Oxidative Damage. J. Biochem. Mol. Toxicol. 2012, 26, 477–485. [Google Scholar] [CrossRef]
- Zeinvand-Lorestani, M.; Karimi, S.; Khorsandi, L. Quercetin Ameliorates Cytotoxic Effects of Zinc Oxide Nanoparticles on Sertoli Cells by Enhancing Autophagy and Suppressing Oxidative Stress. Andrologia 2021, 53, e13988. [Google Scholar] [CrossRef]
- Sheik Abdul, N.; Marnewick, J.L. Fumonisin B(1) -Induced Mitochondrial Toxicity and Hepatoprotective Potential of Rooibos: An Update. J. Appl. Toxicol. JAT 2020, 40, 1602–1613. [Google Scholar] [CrossRef]
- Zhao, X.; Gao, J.; Song, Y.; Zhang, J.; Han, Q. Determination of Fumonisin B(1) by Aptamer-Based Fluorescence Resonance Energy Transfer. Sensors 2022, 22, 8598. [Google Scholar] [CrossRef]
- Xu, H.; Wang, L.; Sun, J.; Wang, L.; Guo, H.; Ye, Y.; Sun, X. Microbial Detoxification of Mycotoxins in Food and Feed. Crit. Rev. Food Sci. Nutr. 2022, 62, 4951–4969. [Google Scholar] [CrossRef]
- Alhelaisi, A.; Alrezaki, A.; Nahdi, S.; Aldahmash, W.; Alwasel, S.; Harrath, A.H. Early-Life Exposure to the Mycotoxin Fumonisin B1 and Developmental Programming of the Ovary of the Offspring: The Possible Role of Autophagy in Fertility Recovery. Toxics 2023, 11, 980. [Google Scholar] [CrossRef] [PubMed]
- Albonico, M.; Schütz, L.F.; Caloni, F.; Cortinovis, C.; Spicer, L.J. Toxicological Effects of Fumonisin B1 Alone and in Combination with Other Fusariotoxins on Bovine Granulosa Cells. Toxicon Off. J. Int. Soc. Toxinology 2016, 118, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Lei, H.; Zhou, L.; Tang, M.; Liu, Q.; Long, F.; Li, Q.; Su, J. Effect of Fumonisin B1 on Proliferation and Apoptosis of Intestinal Porcine Epithelial Cells. Toxins 2022, 14, 471. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Liu, W.; Zhao, Y.; Zang, J.; Gao, H. Fumonisin B1 Exposure Induces Apoptosis of Human Kidney Tubular Epithelial Cells through Regulating PTEN/PI3K/AKT Signaling Pathway via Disrupting Lipid Raft Formation. Toxicon Off. J. Int. Soc. Toxinology 2021, 204, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.L.; Liu, S.J.; Hu, S.Q.; Chen, Y.C.; Guo, J. Probing the Potential Mechanism of Quercetin and Kaempferol against Heat Stress-Induced Sertoli Cell Injury: Through Integrating Network Pharmacology and Experimental Validation. Int. J. Mol. Sci. 2022, 23, 11163. [Google Scholar] [CrossRef] [PubMed]
- Miedlich, S.U.; Zalutskaya, A.; Zhu, E.D.; Demay, M.B. Phosphate-Induced Apoptosis of Hypertrophic Chondrocytes Is Associated with a Decrease in Mitochondrial Membrane Potential and is Dependent upon Erk1/2 Phosphorylation. J. Biol. Chem. 2010, 285, 18270–18275. [Google Scholar] [CrossRef] [PubMed]
- Rehfeldt, S.C.H.; Laufer, S.; Goettert, M.I. A Highly Selective In Vitro JNK3 Inhibitor, FMU200, Restores Mitochondrial Membrane Potential and Reduces Oxidative Stress and Apoptosis in SH-SY5Y Cells. Int. J. Mol. Sci. 2021, 22, 3701. [Google Scholar] [CrossRef] [PubMed]
- Akan, Z.; Garip, A.I. Antioxidants May Protect Cancer Cells from Apoptosis Signals and Enhance Cell Viability. Asian Pac. J. Cancer Prev. APJCP 2013, 14, 4611–4614. [Google Scholar] [CrossRef]
- Jana, N.; Břetislav, G.; Pavel, S.; Pavla, U. Potential of the Flavonoid Quercetin to Prevent and Treat Cancer—Current Status of Research. Klin. Onkol. Cas. Ceske A Slov. Onkol. Spol. 2018, 31, 184–190. [Google Scholar] [CrossRef]
- Yu, S.; Zou, L.; Zhao, J.; Zhu, Y. Resveratrol Alleviates Fumonisin-Induced Intestinal Cytotoxicity by Modulating Apoptosis, tight Junction, and Inflammation in IPEC-J2 Porcine Intestinal Epithelial Cells. Environ. Toxicol. 2024, 39, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Yuan, Q.; Wang, T.; Zhan, Y.; Yang, L.; Fan, Y.; Lei, H.; Su, J. Fumonisin B(1) Inhibits Cell Proliferation and Decreases Barrier Function of Swine Umbilical Vein Endothelial Cells. Toxins 2021, 13, 863. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ma, T.; Zhang, Y.; Zhang, H.; Yin, S.; Zheng, W.; Wang, L.; Wang, Z.; Khan, M.; Sheikh, S.W.; et al. Specific Deletion of Cdh2 in Sertoli Cells Leads to Altered Meiotic Progression and Subfertility of Mice. Biol. Reprod. 2015, 92, 79. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhang, H.; Liu, X.; Wen, F.; Li, B.; Bie, Z.; Qiu, H.; Hu, J. Enhanced Autophagy Reversed Aflatoxin B1-Induced Decrease in Lactate Secretion of Dairy Goat Sertoli Cells. Ecotoxicol. Environ. Saf. 2023, 259, 115063. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.J.S.; Harlan, C.J.; Fuentes, D.T.; Bankson, J.A. A Simulation of the Effects of Diffusion on Hyperpolarized [1-(13)C]-Pyruvate Signal Evolution. IEEE Trans. Bio-Med. Eng. 2023, 70, 2905–2913. [Google Scholar] [CrossRef] [PubMed]
- Israelsen, W.J.; Vander Heiden, M.G. Pyruvate Kinase: Function, Regulation and Role in Cancer. Semin. Cell Dev. Biol. 2015, 43, 43–51. [Google Scholar] [CrossRef]
- Rihan, M.; Sharma, S.S. Role of Pyruvate Kinase M2 (PKM2) in Cardiovascular Diseases. J. Cardiovasc. Transl. Res. 2023, 16, 382–402. [Google Scholar] [CrossRef]
- Galardo, M.N.; Gorga, A.; Merlo, J.P.; Regueira, M.; Pellizzari, E.H.; Cigorraga, S.B.; Riera, M.F.; Meroni, S.B. Participation of HIFs in the Regulation of Sertoli Cell Lactate Production. Biochimie 2017, 132, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Boussouar, F.; Benahmed, M. Lactate and Energy Metabolism in Male Germ Cells. Trends Endocrinol. Metab. TEM 2004, 15, 345–350. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Kang, S.; Gnanaprakasam, J.R.; Wang, R. The Lactate Dehydrogenase (LDH) Isoenzyme Spectrum Enables Optimally Controlling T Cell Glycolysis and Differentiation. Sci. Adv. 2023, 9, eadd9554. [Google Scholar] [CrossRef]
- Guo, Q.; Zhu, Z.; Wang, J.; Huang, W.; Zhang, C.; Zeng, J.; Zhao, H.; Qi, T.; Zhou, W.; Zhang, T.; et al. Preparation, Stability and Commutability of Candidate Reference Materials for Lactate Dehydrogenase (LDH). Clin. Biochem. 2021, 91, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Huang, S.; Yin, X.; Zan, Y.; Guo, Y.; Han, L. Quercetin Suppresses the Mobility of Breast Cancer by Suppressing Glycolysis through Akt-mTOR Pathway Mediated Autophagy Induction. Life Sci. 2018, 208, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.L.; Liu, S.; Li, N.; Ye, L.F.; Zha, M.; Li, C.Y.; Zhao, Y.; Pu, Q.; Bao, J.J.; Chen, X.J.; et al. Quercetin Antagonizes Glucose Fluctuation Induced Renal Injury by Inhibiting Aerobic Glycolysis via HIF-1α/miR-210/ISCU/FeS Pathway. Front. Med. 2021, 8, 656086. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ma, J.; Mo, L.; Xiong, Y.; Xiong, X.; Lan, D.; Fu, W.; Yin, S. Molecular Cloning and Characterization of STC1 Gene and its Functional Analyses in Yak (Bos grunniens) Cumulus Granulosa Cells. Theriogenology 2023, 208, 185–193. [Google Scholar] [CrossRef]
- Gao, S.; Yang, L.; Yang, X.; Xiong, Y.; Fu, W.; Li, J.; Yin, S. Sirtuin 7 is Essential for the Survival and Synthesis of Oestrogen in Yak (Bos grunniens) Cumulus Granulosa Cells. Reprod. Domest. Anim. Zuchthyg. 2023, 58, 323–332. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Huang, R.; Zhang, H.; Liu, D.; Dong, X.; Xiong, Y.; Xiong, X.; Lan, D.; Fu, W.; He, H.; et al. The Protective Effect of Quercetin against the Cytotoxicity Induced by Fumonisin B1 in Sertoli Cells. Int. J. Mol. Sci. 2024, 25, 8764. https://doi.org/10.3390/ijms25168764
Ma J, Huang R, Zhang H, Liu D, Dong X, Xiong Y, Xiong X, Lan D, Fu W, He H, et al. The Protective Effect of Quercetin against the Cytotoxicity Induced by Fumonisin B1 in Sertoli Cells. International Journal of Molecular Sciences. 2024; 25(16):8764. https://doi.org/10.3390/ijms25168764
Chicago/Turabian StyleMa, Jun, Ruixue Huang, Huai Zhang, Dongju Liu, Xiaodong Dong, Yan Xiong, Xianrong Xiong, Daoliang Lan, Wei Fu, Honghong He, and et al. 2024. "The Protective Effect of Quercetin against the Cytotoxicity Induced by Fumonisin B1 in Sertoli Cells" International Journal of Molecular Sciences 25, no. 16: 8764. https://doi.org/10.3390/ijms25168764
APA StyleMa, J., Huang, R., Zhang, H., Liu, D., Dong, X., Xiong, Y., Xiong, X., Lan, D., Fu, W., He, H., Li, J., & Yin, S. (2024). The Protective Effect of Quercetin against the Cytotoxicity Induced by Fumonisin B1 in Sertoli Cells. International Journal of Molecular Sciences, 25(16), 8764. https://doi.org/10.3390/ijms25168764






