The Use of Photodynamic Therapy in the Treatment of Endometrial Cancer—A Review of the Literature
Abstract
1. Introduction
2. Material and Methods
3. History of Photodynamic Therapy
4. Endometrium
5. Endometrial Cancer
6. Treatment
7. Components Used in Photodynamic Therapy
8. The Importance of Light in Photodynamic Therapy
- -
- High monochromaticity;
- -
- Small divergence, as a result of which high power density can be obtained;
- -
- Coherence [20].
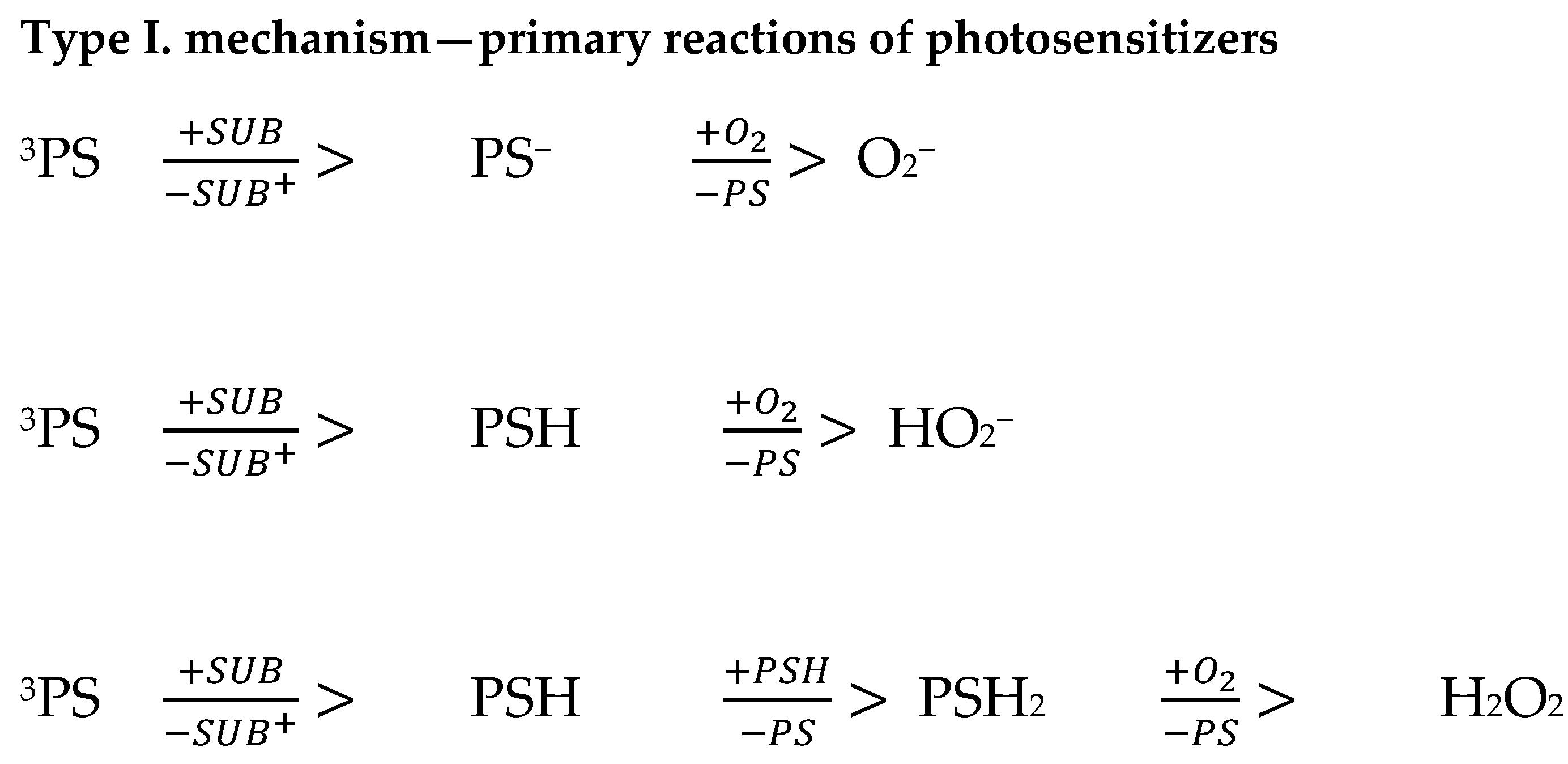
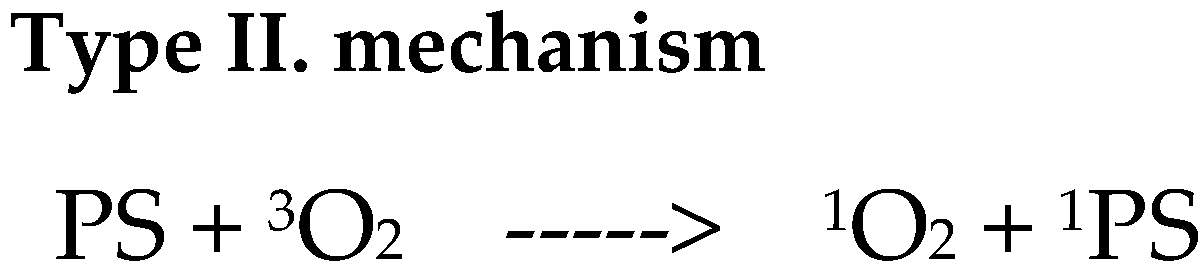
9. Mechanism of Action of Photodynamic Therapy
10. Photodynamic Therapy in the Treatment of Endometrial Cancer
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy-mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef]
- Mohanty, N.; Jalaluddin, M.D.; Kotina, S.; Routray, S.; Ingale, Y. Photodynamic Therapy: The Imminent Milieu For Treating Oral Lesions. J. Clin. Diagn. Res. 2013, 7, 1254–1257. [Google Scholar] [CrossRef]
- Czajkowska, K.; Czajkowski, M.; Matuszewski, M.; Nowicki, R.J.; Sokołowska-Wojdyło, M. Terapia fotodynamiczna w dermatologii. Farm. Współczesna 2019, 12, 15–22. [Google Scholar]
- Acroyd, R.; Kelty, C.; Brown, N.; Reed, M. The history of photodetection and photodynamic therapy. Photochem. Photobiol. 2001, 74, 656–669. [Google Scholar] [CrossRef]
- Kessel, D. Photodynamic Therapy: A Brief History. J. Clin. Med. 2019, 8, 1581. [Google Scholar] [CrossRef]
- Lee, C.N.; Hsu, R.; Chen, H.; Wong, T.W. Daylight Photodynamic Therapy: An Update. Molecules 2020, 25, 5195. [Google Scholar] [CrossRef]
- Hambl, M.R. Photodynamic Therapy for Cancer: What’s Past is Prologue. Photochem. Photobiol. 2020, 96, 506–516. [Google Scholar] [CrossRef]
- Dougherty, T.J.; Kaufman, J.E.; Goldfarb, A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978, 38, 2628–2635. [Google Scholar]
- Carvalho, M.J.; Laranjo, M.; Abrantes, A.M.; Torgal, I.; Botelho, M.F.; Oliveira, C.F. Clinical translation for endometrial cancer stem cells hypothesis. Cancer Metastasis Rev. 2015, 34, 401–416. [Google Scholar] [CrossRef]
- Lõhmussaar, K.; Boretto, M.; Clevers, H. Human-Derived Model Systems in Gynecological Cancer Research. Trends Cancer. 2020, 6, 1031–1043. [Google Scholar] [CrossRef]
- Hong, I.-S. Endometrial stem/progenitor cells: Properties, origins, and functions. Genes Dis. 2023, 10, 931–947. [Google Scholar] [CrossRef]
- Makker, V.; MacKay, H.; Ray-Coquard, I.; Levine, D.A.; Westin, S.N.; Aoki, D.; Oaknin, A. Endometrial Cancer. Nat. Rev. Dis. Prim. 2022, 7, 88. [Google Scholar] [CrossRef]
- Concin, N.; Creutzberg, C.L.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.A.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP Guidelines for the management of patients with endometrial carcinoma. Virchows Arch. 2021, 478, 153–190. [Google Scholar] [CrossRef]
- Klimaszyk, K.; Nielsen, H.S.; Wender-Ozegowska, E.; Kedzia, M. Chronic endometritis—Is it time to clarify diagnostic criteria? Ginekol Pol. 2023, 94, 152–157. [Google Scholar] [CrossRef]
- Moreno, I.; Cicinelli, E.; Garcia-Grau, I.; Gonzalez-Monfort, M.; Bau, D.; Vilella, F.; De Ziegler, D.; Resta, L.; Valbuena, D.; Simon, C. The diagnosis of chronic endometritis in infertile asymptomatic women: A comparative study of histology, microbial cultures, hysteroscopy, and molecular microbiology. Am. J. Obstet. Gynecol. 2018, 218, 602.e1–602.e16. [Google Scholar] [CrossRef]
- Son, J.; Carr, C.; Yao, M.; Radeva, M.; Priyadarshini, A.; Marquard, J.; Michener, C.M.; AlHilli, M. Endometrial cancer in young women: Prognostic factors and treatment outcomes in women aged ≤40 years. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2020, 30, 631–639. [Google Scholar] [CrossRef]
- Colombo, N.; Creutzberg, C.; Amant, F.; Bosse, T.; González-Martín, A.; Ledermann, J.; Marth, C.; Nout, R.; Querleu, D.; Mirza, M.R.; et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-up. Int. J. Gynecol. Cancer 2016, 26, 2–30. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Wojtunik-Kulesza, K.A.; Oniszczuk, T.; Kasprzak, K. The potential of photodynamic therapy (PDT)-Experimental investigations and clinical use. Biomed. Pharmacother. 2016, 83, 912–929. [Google Scholar] [CrossRef]
- Bednarkiewicz, A.; Stręk, W. Odziaływanie światła laserowego z tkanką. In Diagnostyka i Terapia Fotodynamiczna; Wydawnictwo Medyczne Urban & Partner: Wrocław, Poland, 2004; pp. 33–87. [Google Scholar]
- Lane, N. Nowe światło dla medycyny. Świat Nauk. 2003, 2, 58–65. [Google Scholar]
- Shinmori, H.; Kodaira, F.; Matsugo, S.; Kawabata, S.; Osuka, A. Photosensiztizing properties of diazaporphyrin derivatives for singlet oxygen generation. Chem. Lett. 2005, 34, 322–323. [Google Scholar] [CrossRef]
- Luksiene, Z. Photodynamic therapy: Mechanism of action and ways to improve the efficiency of treatment. Medicina 2003, 39, 1137–1150. [Google Scholar]
- Graczykowa, A. Biochemiczne i biofizyczne podstawy fotodynamicznej metody wykrywania i leczenia nowotworów. In Fotodynamiczna Metoda Rozpoznawania i Leczenia Nowotworów; Dom Wydawniczy Bellona: Warszawa, Poland, 1999; pp. 21–94. [Google Scholar]
- Rojkiewicz, M.; Kozik, W.; Czapka, M.; Jarzembek, K.; Zięba, G.; Kuś, P.; Kozik, V. Fotodynamiczna terapia nowotworów. Probl. Ekol. 2010, 14, 4. [Google Scholar]
- Kliber-Jasik, M.M.; Nackiewicz, J.; Broda, M. Ftalocyjaniny i ich rola w terapii fotodynamicznej. In Chemia; Nyćkowiak, J., Leśny, J., Eds.; Badania i Rozwój Młodych Naukowców w Polsce–Monografie 2017; Młodzi Naukowcy: Poznań, Poland, 2017; pp. 37–42. ISBN 978-83-65677-31-0. [Google Scholar]
- Bartusik-Aebisher, D.; Żołyniak, A.; Barnaś, E.; Machorowska Pieniążek, A.; Oleś, P.; Kawczyk-Krupka, A.; Aebisher, D. The Use of Photodynamic Therapy in the Treatment of Brain Tumors-A Review of the Literature. Molecules 2022, 27, 6847. [Google Scholar] [CrossRef]
- Żołyniak-Brzuchacz, A.; Barnaś, E.; Aebisher, D.; Szpunar, M.; Dynarowicz, K.; Ostańska, E.; Skręt-Magierło, J.; Kluz, T.; Bartusik-Aebisher, D. In vitro Photodynamic Therapy in the Treatment of Endometrial Cancer. ARCTIC J. 2024, 77, 27–48. [Google Scholar] [CrossRef]
- Corti, L.; Mazzarotto, R.; Belfontali, S.; De Luca, C.; Baiocchi, C.; Boso, C.; Calzavara, F. Gynecologic cancer recurrences and photodynamic therapy: Our experience. J. Clin. Laser. Med. Surg. 1995, 13, 325–328. [Google Scholar] [CrossRef]
- Koren, H.; Alth, G. Photodynamic therapy in gynaecologic cancer. J. Photochem. Photobiol. B. 1996, 36, 189–191. [Google Scholar] [CrossRef]
- Choi, Y.; Chang, J.E.; Jheon, S.; Han, S.J.; Kim, J.K. Enhanced production of reactive oxygen species in HeLa cells under concurrent low-dose carboplatin and Photofrin® photodynamic therapy. Oncol. Rep. 2018, 40, 339–345. [Google Scholar] [CrossRef]
- Ziółkowski, P.; Symonowicz, K.; Osiecka, B.J.; Rabczyński, J.; Gerber, J. Photodynamic treatment of epithelial tissue derived from patients with endometrial cancer: Acontribution to the role of laminin and epidermal growth factor receptor in photodynamic therapy. J. Biomed. Opt. 1999, 4, 272–275. [Google Scholar] [CrossRef][Green Version]
- Varriale, L.; Coppola, E.; Quarto, M.; Veneziani, B.M.; Palumbo, G. Molecular aspects of photodynamic therapy: Low energy pre-sensitization of hypericin-loaded human endometrial carcinoma cells enhances photo-tolerance, alters gene expression and affects the cell cycle. FEBS Lett. 2002, 512, 287–290. [Google Scholar] [CrossRef]
- Raab, G.H.; Schneider, A.F.; Eiermann, W.; Gottschalk-Deponte, H.; Baumgartner, R.; Beyer, W. Response of human endometrium and ovarian carcinoma cell-lines to photodynamic therapy. Arch. Gynecol. Obstet. 1990, 248, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Schneider-Yin, X.; Kurmanaviciene, A.; Roth, M.; Roos, M.; Fedier, A.; Minder, E.I.; Walt, H. Hypericin and 5-aminolevulinic acid-induced protoporphyrin IX induce enhanced phototoxicity in human endometrial cancer cells with non-coherent white light. Photodiagnosis Photodyn. Ther. 2009, 6, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-M.; Rhee, Y.-H.; Kim, J.-S. The Anticancer Effects of Radachlorin-mediated Photodynamic Therapy in the Human Endometrial Adenocarcinoma Cell Line HEC-1-A. Anticancer Res. 2017, 37, 6251–6258. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.C.; Jung, S.G.; Park, H.; Cho, Y.H.; Lee, C.; Kim, S.J. Fertility preservation via photodynamic therapy in young patients with early-stage uterine endometrial cancer: A long-term follow-up study. Int. J. Gynecol. Cancer 2013, 23, 698–704. [Google Scholar] [CrossRef]
- Choi, M.C.; Kim, G.; Hwang, Y.Y. Fertility-sparing management combined with photodynamic therapy for endometrial stromal sarcoma: A case report. Photodiagnosis Photodyn. Therapy 2014, 11, 533–536. [Google Scholar] [CrossRef]
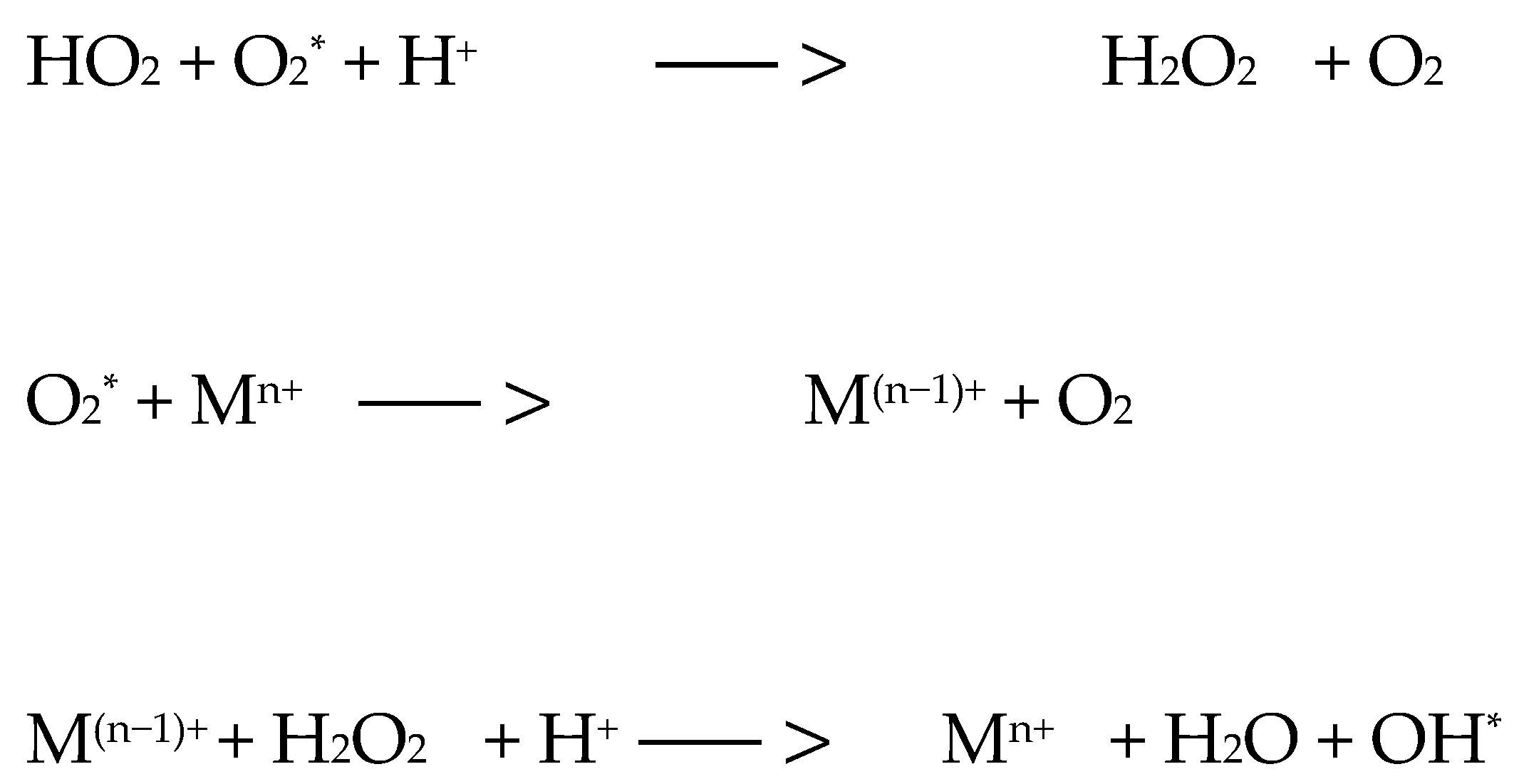
| PDT for Endometrial Cancer | |
|---|---|
| The most common cause of the development of the disease is | Excessive estrogen stimulation (both endogenous and exogenous), with simultaneous deficiency of progestogens |
| Excessive body weight | |
| A family history of endometrial cancer, colorectal cancer or breast cancer | |
| Hormonal disorders caused by ovarian tumors | |
| Postmenopausal age | |
| Early menarche | |
| Late age of last menstrual period no offspring | |
| Occurrence of anovulatory cycles | |
| Polycystic ovary syndrome | |
| Diabetes | |
| Presence of congenital predisposition syndromes (Lynch syndrome, Cowden syndrome) | |
| PDT Advantages | Fewer adverse effects |
| Low invasiveness | |
| Short treatment time | |
| Usable in outpatient settings | |
| Double selectivity | |
| Can be applied in the same time | |
| Little or no scar after healing | |
| Lower cost than other treatment | |
| PDT Disadvantages | Photosensitivity after treatment |
| Treatment efficacy depends on accurate light delivery to the tumor | |
| Tissue oxygenation is crucial to the photodynamic effect | |
| Impossible to treat metastatic cancers with current technology | |
| Contraindications to PDT treatment | A non-responsive tumor |
| Porphyria | |
| Systemic lupus erythematosus | |
| Other photosensitivity dermatoses | |
| Allergy to the photosensitiser (very rare) | |
| Evidence for endometrial cancer prevention strategies | Obesity is an established risk factor for endometrial cancer |
| Weight cycling and weight gain in middle age are risk factors for endometrial cancer. | |
| Bariatric surgery reduces the risk of endometrial cancer by up to 81% in obese women who attain and maintain a normal weight. | |
| Combined oral contraceptives provide durable protection against endometrial cancer for 30 years or more. | |
| Ever use of the levonorgestrel intrauterine system (LNG-IUS) and inert intrauterine devices reduce endometrial cancer risk. | |
| The first oestrogen-based non-progestin HRT for non-hysterectomised women that contains estradiol and bazedoxifene has an effective protective effect on endometrium. | |
| Weight cycling and weight gain in middle age are risk factors for endometrial cancer. | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żołyniak-Brzuchacz, A.; Barnaś, E.; Bartusik-Aebisher, D.; Aebisher, D. The Use of Photodynamic Therapy in the Treatment of Endometrial Cancer—A Review of the Literature. Int. J. Mol. Sci. 2024, 25, 8772. https://doi.org/10.3390/ijms25168772
Żołyniak-Brzuchacz A, Barnaś E, Bartusik-Aebisher D, Aebisher D. The Use of Photodynamic Therapy in the Treatment of Endometrial Cancer—A Review of the Literature. International Journal of Molecular Sciences. 2024; 25(16):8772. https://doi.org/10.3390/ijms25168772
Chicago/Turabian StyleŻołyniak-Brzuchacz, Aleksandra, Edyta Barnaś, Dorota Bartusik-Aebisher, and David Aebisher. 2024. "The Use of Photodynamic Therapy in the Treatment of Endometrial Cancer—A Review of the Literature" International Journal of Molecular Sciences 25, no. 16: 8772. https://doi.org/10.3390/ijms25168772
APA StyleŻołyniak-Brzuchacz, A., Barnaś, E., Bartusik-Aebisher, D., & Aebisher, D. (2024). The Use of Photodynamic Therapy in the Treatment of Endometrial Cancer—A Review of the Literature. International Journal of Molecular Sciences, 25(16), 8772. https://doi.org/10.3390/ijms25168772









