Uncovering the Expression Pattern of the Costimulatory Receptors ICOS, 4-1BB, and OX-40 in Exhausted Peripheral and Tumor-Infiltrating Natural Killer Cells from Patients with Cervical Cancer
Abstract
:1. Introduction
2. Results
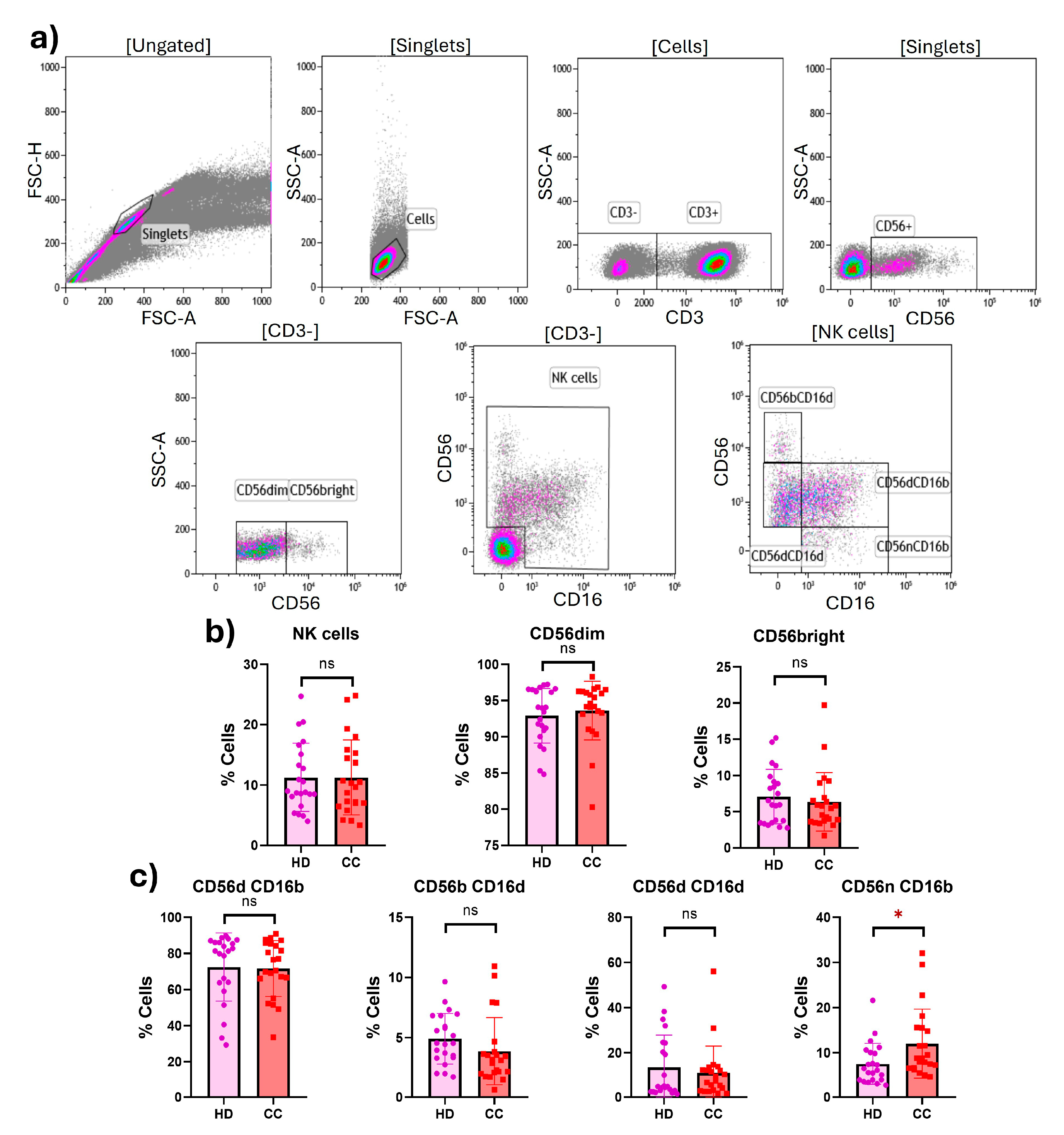
2.1. Expression of Costimulatory and Inhibitory Receptors in Peripheral NK Cells
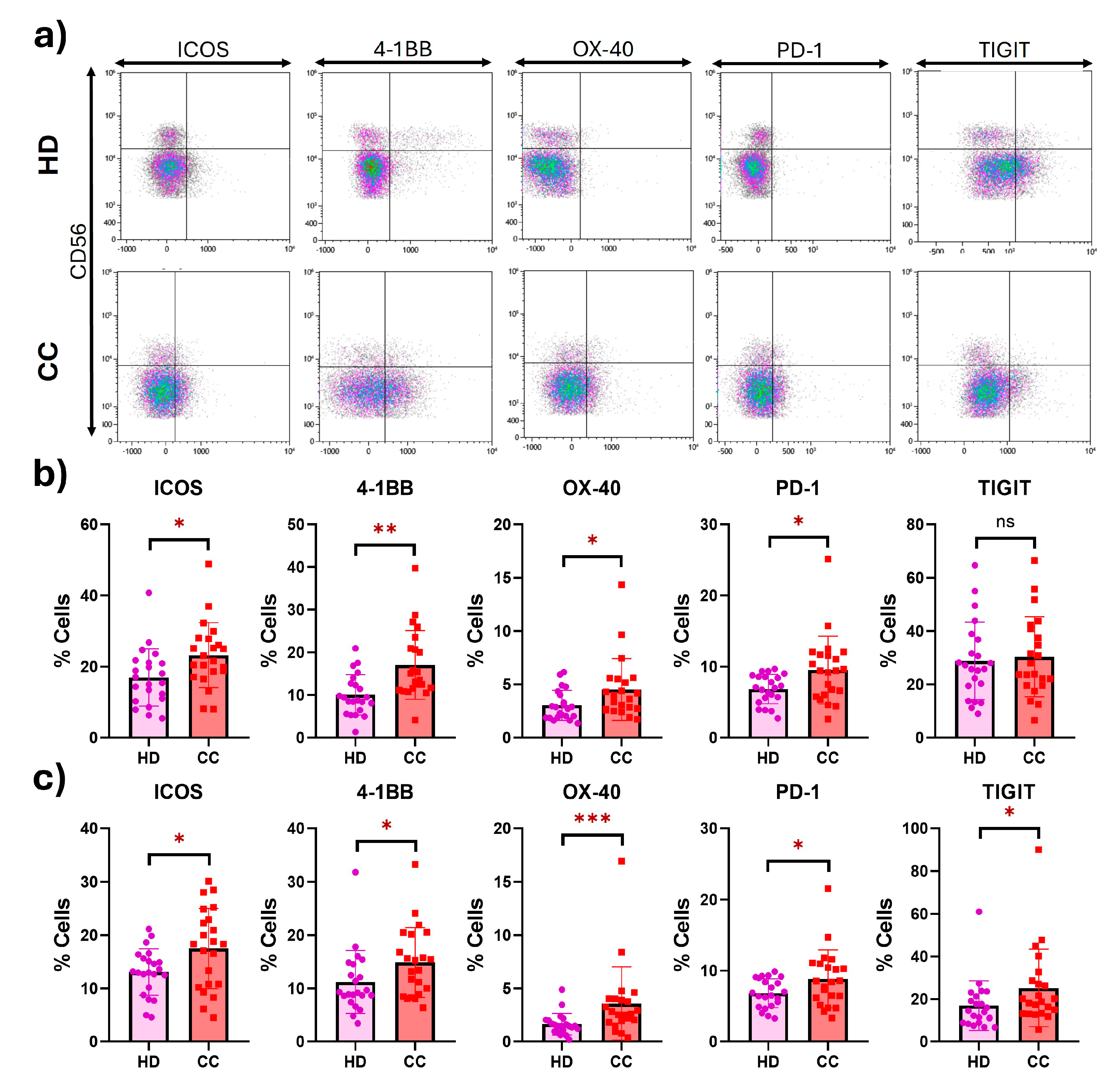
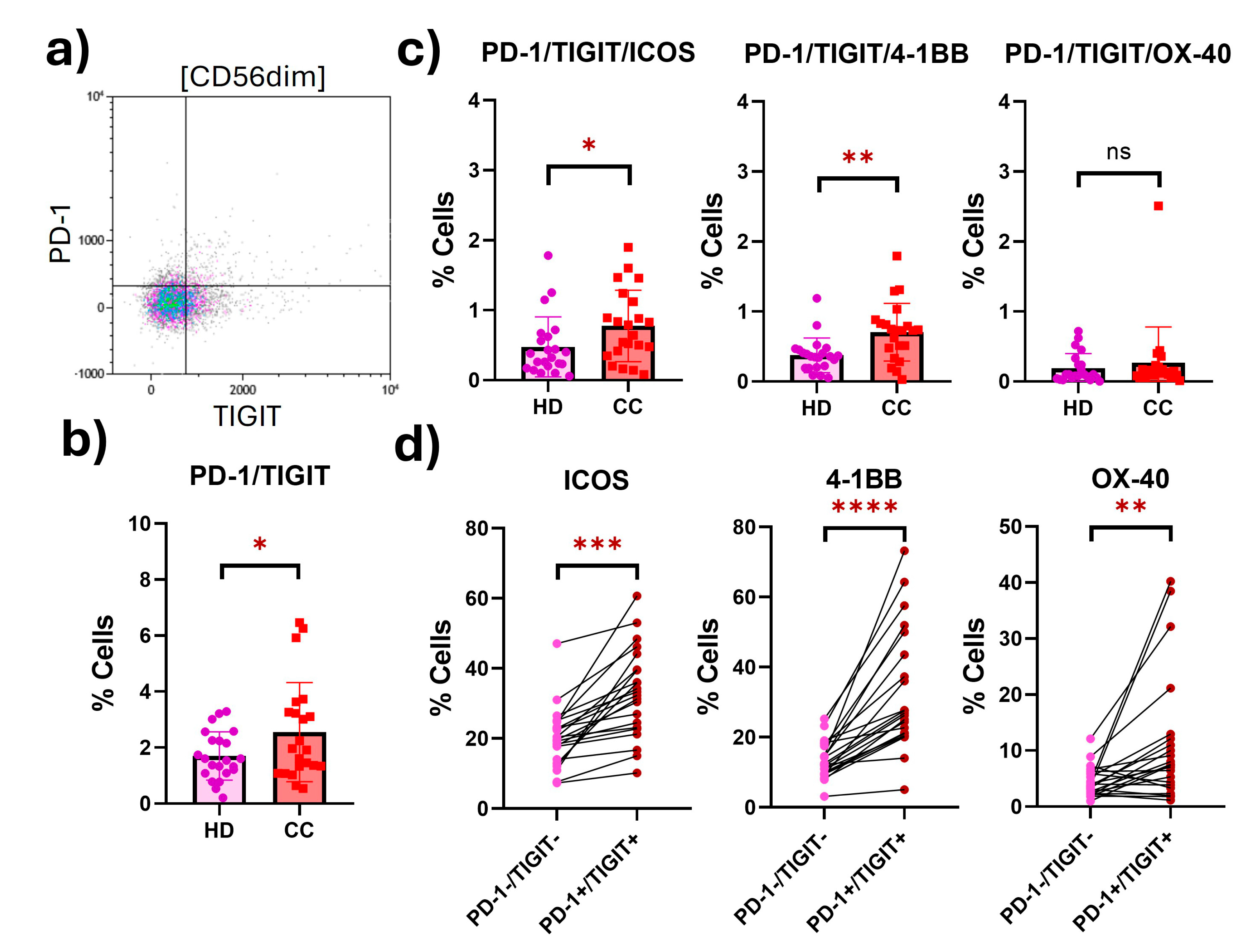
2.2. Peripheral NK Cells with an Exhausted Phenotype Overexpress Costimulatory Receptors
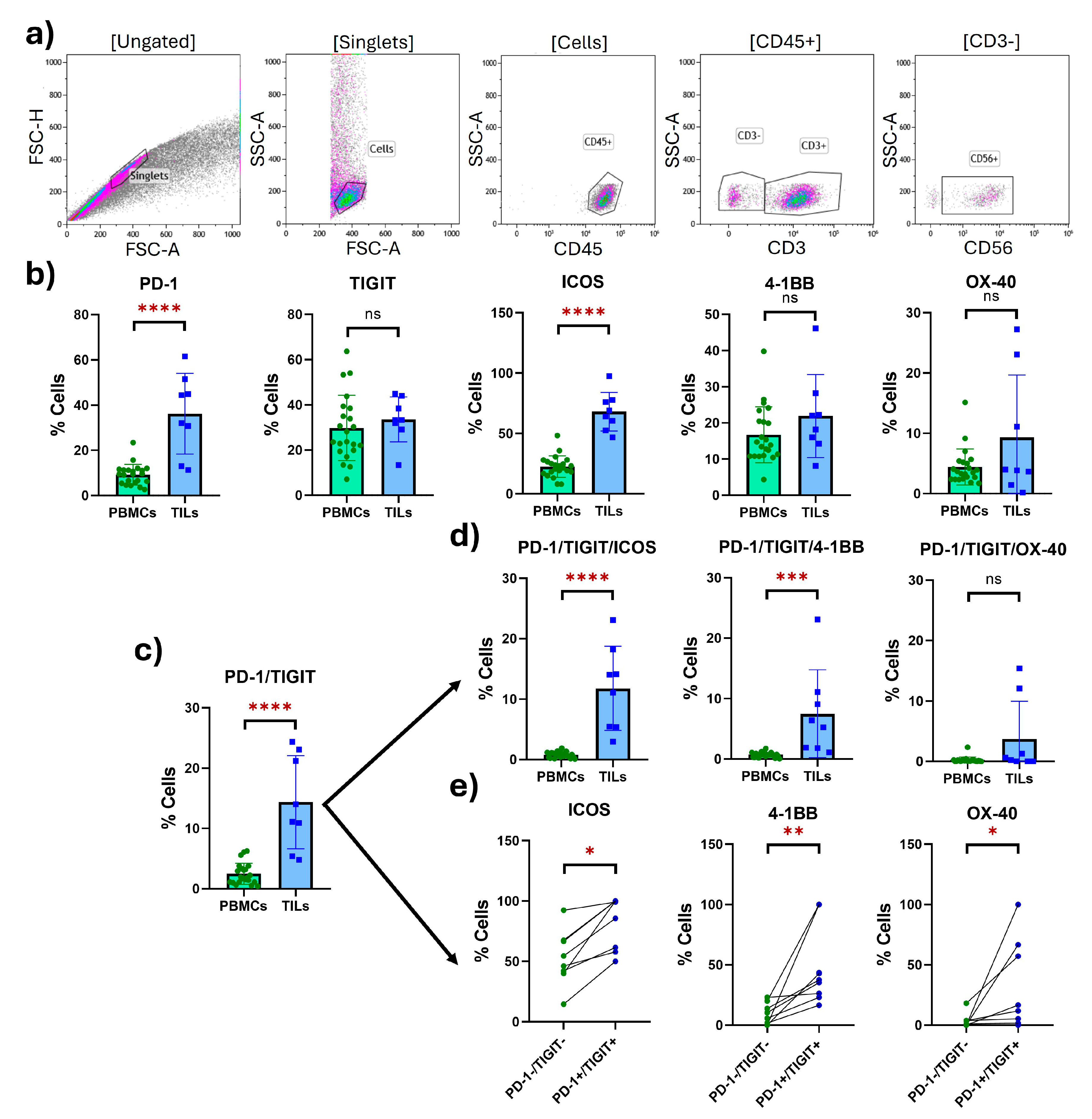
2.3. Tumor-Infiltrating NK Cells with an Exhausted Phenotype Overexpress Costimulatory Receptors
3. Discussion
Limitations and Strengths of the Study
4. Materials and Methods
4.1. Participants and Sample Collection
4.2. Flow Cytometry
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical Cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-Y.; Fu, T.; Jiang, Y.-Z.; Shao, Z.-M. Natural Killer Cells in Cancer Biology and Therapy. Mol. Cancer 2020, 19, 120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, M.; Yang, S.; Wang, J.; Feng, X.; Han, Z. Natural Killer Cells: Of-the-Shelf Cytotherapy for Cancer Immunosurveillance. Am. J. Cancer Res. 2021, 11, 1770–1791. [Google Scholar] [PubMed]
- Shimasaki, N.; Jain, A.; Campana, D. NK Cells for Cancer Immunotherapy. Nat. Rev. Drug Discov. 2020, 19, 200–218. [Google Scholar] [CrossRef] [PubMed]
- Montes-Casado, M.; Ojeda, G.; Aragoneses-Fenoll, L.; López, D.; de Andrés, B.; Gaspar, M.L.; Dianzani, U.; Rojo, J.M.; Portolés, P. ICOS Deficiency Hampers the Homeostasis, Development and Function of NK Cells. PLoS ONE 2019, 14, e0219449. [Google Scholar] [CrossRef] [PubMed]
- Rujas, E.; Cui, H.; Sicard, T.; Semesi, A.; Julien, J.-P. Structural Characterization of the ICOS/ICOS-L Immune Complex Reveals High Molecular Mimicry by Therapeutic Antibodies. Nat. Commun. 2020, 11, 5066. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.M.J.; Nemeth, M.R.; Lim, S.-O. 4-1BB: A Promising Target for Cancer Immunotherapy. Front. Oncol. 2022, 12, 968360. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhao, S.; Zhang, X.; Jia, K.; Wang, H.; Zhou, C.; He, Y. OX40 (CD134) and OX40 Ligand, Important Immune Checkpoints in Cancer. Onco Targets Ther 2019, 12, 7347–7353. [Google Scholar] [CrossRef] [PubMed]
- Bi, J.; Tian, Z. NK Cell Exhaustion. Front Immunol. 2017, 8, 760. [Google Scholar] [CrossRef] [PubMed]
- Roe, K. NK-Cell Exhaustion, B-Cell Exhaustion and T-Cell Exhaustion—The Differences and Similarities. Immunology 2022, 166, 155–168. [Google Scholar] [CrossRef]
- Jia, H.; Yang, H.; Xiong, H.; Luo, K.Q. NK Cell Exhaustion in the Tumor Microenvironment. Front. Immunol. 2023, 14, 1303605. [Google Scholar] [CrossRef] [PubMed]
- Merino, A.; Zhang, B.; Dougherty, P.; Luo, X.; Wang, J.; Blazar, B.R.; Miller, J.S.; Cichocki, F. Chronic Stimulation Drives Human NK Cell Dysfunction and Epigenetic Reprograming. J. Clin. Investig. 2019, 129, 3770–3785. [Google Scholar] [CrossRef]
- Solorzano-Ibarra, F.; Alejandre-Gonzalez, A.G.; Ortiz-Lazareno, P.C.; Bastidas-Ramirez, B.E.; Zepeda-Moreno, A.; Tellez-Bañuelos, M.C.; Banu, N.; Carrillo-Garibaldi, O.J.; Chavira-Alvarado, A.; Bueno-Topete, M.R.; et al. Immune Checkpoint Expression on Peripheral Cytotoxic Lymphocytes in Cervical Cancer Patients: Moving beyond the PD-1/PD-L1 Axis. Clin. Exp. Immunol. 2021, 204, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, Y.; Xu, Y.; Wang, Z.; Du, X.; Li, C.; Peng, J.; Gao, L.; Liang, X.; Ma, C. Increased Expression of Programmed Cell Death Protein 1 on NK Cells Inhibits NK-Cell-Mediated Anti-Tumor Function and Indicates Poor Prognosis in Digestive Cancers. Oncogene 2017, 36, 6143–6153. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, A.W., IV; Jillab, M.; Plimack, E.R.; Hudes, G.R.; Uzzo, R.G.; Litwin, S.; Dulaimi, E.; Al-Saleem, T.; Campbell, K.S. PD-1 Expression on Peripheral Blood Cells Increases with Stage in Renal Cell Carcinoma Patients and Is Rapidly Reduced after Surgical Tumor Resection. Cancer Immunol. Res. 2014, 2, 320–331. [Google Scholar] [CrossRef]
- Mariotti, F.R.; Petrini, S.; Ingegnere, T.; Tumino, N.; Besi, F.; Scordamaglia, F.; Munari, E.; Pesce, S.; Marcenaro, E.; Moretta, A.; et al. PD-1 in Human NK Cells: Evidence of Cytoplasmic mRNA and Protein Expression. OncoImmunology 2019, 8, 1557030. [Google Scholar] [CrossRef] [PubMed]
- Barshidi, A.; Ardeshiri, K.; Ebrahimi, F.; Alian, F.; Shekarchi, A.A.; Hojjat-Farsangi, M.; Jadidi-Niaragh, F. The Role of Exhausted Natural Killer Cells in the Immunopathogenesis and Treatment of Leukemia. Cell Commun. Signal. 2024, 22, 59. [Google Scholar] [CrossRef] [PubMed]
- Pesce, S.; Greppi, M.; Tabellini, G.; Rampinelli, F.; Parolini, S.; Olive, D.; Moretta, L.; Moretta, A.; Marcenaro, E. Identification of a Subset of Human Natural Killer Cells Expressing High Levels of Programmed Death 1: A Phenotypic and Functional Characterization. J. Allergy Clin. Immunol. 2017, 139, 335–346. [Google Scholar] [CrossRef]
- Beldi-Ferchiou, A.; Lambert, M.; Dogniaux, S.; Vély, F.; Vivier, E.; Olive, D.; Dupuy, S.; Levasseur, F.; Zucman, D.; Lebbé, C.; et al. PD-1 Mediates Functional Exhaustion of Activated NK Cells in Patients with Kaposi Sarcoma. Oncotarget 2016, 7, 72961–72977. [Google Scholar] [CrossRef]
- Zhang, Q.; Bi, J.; Zheng, X.; Chen, Y.; Wang, H.; Wu, W.; Wang, Z.; Wu, Q.; Peng, H.; Wei, H.; et al. Blockade of the Checkpoint Receptor TIGIT Prevents NK Cell Exhaustion and Elicits Potent Anti-Tumor Immunity. Nat. Immunol. 2018, 19, 723–732. [Google Scholar] [CrossRef]
- Meng, F.; Li, L.; Lu, F.; Yue, J.; Liu, Z.; Zhang, W.; Fu, R. Overexpression of TIGIT in NK and T Cells Contributes to Tumor Immune Escape in Myelodysplastic Syndromes. Front. Oncol. 2020, 10, 1595. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Chen, J.; Ji, T.; Cong, X. TIGIT, a Novel Immune Checkpoint Therapy for Melanoma. Cell Death Dis. 2023, 14, 466. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska, A.; Skiba, W.; Suszczyk, D.; Kuryło, W.; Jakubowicz-Gil, J.; Paduch, R.; Wertel, I. The Dual Blockade of the TIGIT and PD-1/PD-L1 Pathway as a New Hope for Ovarian Cancer Patients. Cancers 2022, 14, 5757. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ding, X.; Li, H.; Yang, C.; Ma, Z.; Xu, G.; Yang, S.; Zhang, D.; Xie, X.; Xin, L.; et al. Upregulation of TIGIT and PD-1 in Colorectal Cancer with Mismatch-Repair Deficiency. Immunol. Investig. 2021, 50, 338–355. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, Y.; Xu, J.; Chen, P.; Zhao, Z.; Cai, Q.; Li, L.; Tian, H.; Bai, G.; Liu, L.; et al. PVR/TIGIT and PD-L1/PD-1 Expression Predicts Survival and Enlightens Combined Immunotherapy in Lung Squamous Cell Carcinoma. Transl. Oncol. 2022, 24, 101501. [Google Scholar] [CrossRef] [PubMed]
- Chauvin, J.-M.; Pagliano, O.; Fourcade, J.; Sun, Z.; Wang, H.; Sander, C.; Kirkwood, J.M.; Chen, T.T.; Maurer, M.; Korman, A.J.; et al. TIGIT and PD-1 Impair Tumor Antigen–Specific CD8+ T Cells in Melanoma Patients. J. Clin. Investig. 2015, 125, 2046–2058. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Luo, J.; Chen, Y.; Cui, J.; Lei, Y.; Cui, Y.; Jiang, N.; Jiang, W.; Chen, L.; Chen, Y.; et al. Combined Evaluation of the Expression Status of CD155 and TIGIT Plays an Important Role in the Prognosis of LUAD (Lung Adenocarcinoma). Int. Immunopharmacol. 2020, 80, 106198. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Bu, J.; Zhou, M.; Sido, J.; Lin, Y.; Liu, G.; Lin, Q.; Xu, X.; Leavenworth, J.W.; Shen, E. CD8+T Cells Expressing Both PD-1 and TIGIT but Not CD226 Are Dysfunctional in Acute Myeloid Leukemia (AML) Patients. Clin. Immunol. 2018, 190, 64–73. [Google Scholar] [CrossRef]
- Farhat, M.; Croft, W.; Parry, H.M.; Verma, K.; Kinsella, F.A.M.; Xu, J.; Bone, D.; McSkeane, T.; Paneesha, S.; Pratt, G.; et al. PD-1 Expression Contributes to Functional Impairment of NK Cells in Patients with B-CLL. Leukemia 2024, 38, 1813–1817. [Google Scholar] [CrossRef]
- Pazina, T.; MacFarlane, A.W.; Bernabei, L.; Dulaimi, E.; Kotcher, R.; Yam, C.; Bezman, N.A.; Robbins, M.D.; Ross, E.A.; Campbell, K.S.; et al. Alterations of NK Cell Phenotype in the Disease Course of Multiple Myeloma. Cancers 2021, 13, 226. [Google Scholar] [CrossRef] [PubMed]
- Amatore, F.; Gorvel, L.; Olive, D. Role of Inducible Co-Stimulator (ICOS) in Cancer Immunotherapy. Expert Opin. Biol. Ther. 2020, 20, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yan, X.; Hong, B.; Xiao, Y.; Qian, Y. The Expression and Prognostic Significance of ICOS in NSCLC Integrated Pan-Cancer and Multi-Omics Analyses. Int. J. Med. Sci. 2024, 21, 795–808. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; He, M.; Ren, K.; Ma, H.; Xue, Q. Inducible Co-Stimulator ICOS Expression Correlates with Immune Cell Infiltration and Can Predict Prognosis in Lung Adenocarcinoma. Int. J. Gen. Med. 2022, 15, 3739–3751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, Y.; Qin, S.-L.; Mu, Y.-F.; Qi, Y.; Yu, M.-H.; Zhong, M. The Clinical Impact of ICOS Signal in Colorectal Cancer Patients. OncoImmunology 2016, 5, e1141857. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-R.; Chou, C.-H.; Liu, C.-J.; Lin, Y.-C.; Tu, H.-F.; Chang, K.-W.; Lin, S.-C. The Concordant Disruption of B7/CD28 Immune Regulators Predicts the Prognosis of Oral Carcinomas. Int. J. Mol. Sci. 2023, 24, 5931. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Tian, J.; Han, X.; Li, X. A Model of Seven Immune Checkpoint-Related Genes Predicting Overall Survival for Head and Neck Squamous Cell Carcinoma. Eur. Arch. Otorhinolaryngol. 2021, 278, 3467–3477. [Google Scholar] [CrossRef]
- Kaban, K.; Greiner, S.M.; Holzmayer, S.; Tandler, C.; Meyer, S.; Hinterleitner, C.; Salih, H.R.; Märklin, M.; Heitmann, J.S. Immunoprofiling of 4-1BB Expression Predicts Outcome in Chronic Lymphocytic Leukemia (CLL). Diagnostics 2021, 11, 2041. [Google Scholar] [CrossRef] [PubMed]
- Makkouk, A.; Sundaram, V.; Chester, C.; Chang, S.; Colevas, A.D.; Sunwoo, J.B.; Maecker, H.; Desai, M.; Kohrt, H.E. Characterizing CD137 Upregulation on NK Cells in Patients Receiving Monoclonal Antibody Therapy. 2017, 28, 415–420. [CrossRef]
- Zhu, X.; Feng, Y.; Fan, P.; Dong, D.; Yuan, J.; Chang, C.; Wang, R. Increased Co-Expression of 4-1BB with PD-1 on CD8+ Tumor-Infiltrating Lymphocytes Is Associated with Improved Prognosis and Immunotherapy Response in Cervical Cancer. Front. Oncol. 2024, 14, 1381381. [Google Scholar] [CrossRef]
- Hashimoto, K. CD137 as an Attractive T Cell Co-Stimulatory Target in the TNFRSF for Immuno-Oncology Drug Development. Cancers 2021, 13, 2288. [Google Scholar] [CrossRef]
- Vidard, L. 4-1BB and Cytokines Trigger Human NK, Γδ T, and CD8+ T Cell Proliferation and Activation, but Are Not Required for Their Effector Functions. Immun. Inflamm. Dis. 2023, 11, e749. [Google Scholar] [CrossRef] [PubMed]
- Cabo, M.; Santana-Hernández, S.; Costa-Garcia, M.; Rea, A.; Lozano-Rodríguez, R.; Ataya, M.; Balaguer, F.; Juan, M.; Ochoa, M.C.; Menéndez, S.; et al. CD137 Costimulation Counteracts TGFβ Inhibition of NK-Cell Antitumor Function. Cancer Immunol. Res. 2021, 9, 1476–1490. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.; Smits, E.; Lardon, F.; Pauwels, P.; Deschoolmeester, V. Immune Checkpoint Modulation in Colorectal Cancer: What’s New and What to Expect. J. Immunol. Res. 2015, 2015, 158038. [Google Scholar] [CrossRef]
- Massarelli, E.; Lam, V.K.; Parra, E.R.; Rodriguez-Canales, J.; Behrens, C.; Diao, L.; Wang, J.; Blando, J.; Byers, L.A.; Yanamandra, N.; et al. High OX-40 Expression in the Tumor Immune Infiltrate Is a Favorable Prognostic Factor of Overall Survival in Non-Small Cell Lung Cancer. J. Immunother. Cancer 2019, 7, 351. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Xu, L.; Wu, H.; Liao, H.; Luo, L.; Liao, M.; Gong, J.; Deng, Y.; Yuan, K.; Wu, H.; et al. OX40 Expression in Hepatocellular Carcinoma Is Associated with a Distinct Immune Microenvironment, Specific Mutation Signature, and Poor Prognosis. OncoImmunology 2018, 7, e1404214. [Google Scholar] [CrossRef] [PubMed]
- Pauken, K.E.; Wherry, E.J. Overcoming T cell exhaustion in infection and cancer. Trends Immunol. 2015, 36, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Judge, S.J.; Murphy, W.J.; Canter, R.J. Characterizing the Dysfunctional NK Cell: Assessing the Clinical Relevance of Exhaustion, Anergy, and Senescence. Front Cell Infect Microbiol. 2020, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- Okolo, O.; Yu, V.; Flashner, S.; Martin, C.; Nakagawa, H.; Lin, D.T.; Puram, S.V.; Parikh, A.S. Protocol for Tumor Dissociation and Fluorescence-Activated Cell Sorting of Human Head and Neck Cancers. STAR Protoc. 2023, 4, 102294. [Google Scholar] [CrossRef]
- Del Zotto, G.; Antonini, F.; Pesce, S.; Moretta, F.; Moretta, L.; Marcenaro, E. Comprehensive Phenotyping of Human PB NK Cells by Flow Cytometry. Cytometry A 2020, 97, 891–899. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Diaz, J.M.; Solorzano-Ibarra, F.; Garcia-Barrientos, N.T.; Klimov-Kravtchenko, K.; Guitron-Aviña, M.S.; Cruz-Ramos, J.A.; Ortiz-Lazareno, P.C.; Urciaga-Gutierrez, P.I.; Bueno-Topete, M.R.; Garcia-Chagollan, M.; et al. Uncovering the Expression Pattern of the Costimulatory Receptors ICOS, 4-1BB, and OX-40 in Exhausted Peripheral and Tumor-Infiltrating Natural Killer Cells from Patients with Cervical Cancer. Int. J. Mol. Sci. 2024, 25, 8775. https://doi.org/10.3390/ijms25168775
Rojas-Diaz JM, Solorzano-Ibarra F, Garcia-Barrientos NT, Klimov-Kravtchenko K, Guitron-Aviña MS, Cruz-Ramos JA, Ortiz-Lazareno PC, Urciaga-Gutierrez PI, Bueno-Topete MR, Garcia-Chagollan M, et al. Uncovering the Expression Pattern of the Costimulatory Receptors ICOS, 4-1BB, and OX-40 in Exhausted Peripheral and Tumor-Infiltrating Natural Killer Cells from Patients with Cervical Cancer. International Journal of Molecular Sciences. 2024; 25(16):8775. https://doi.org/10.3390/ijms25168775
Chicago/Turabian StyleRojas-Diaz, Jose Manuel, Fabiola Solorzano-Ibarra, Nadia Tatiana Garcia-Barrientos, Ksenia Klimov-Kravtchenko, Marcela Sofia Guitron-Aviña, Jose Alfonso Cruz-Ramos, Pablo Cesar Ortiz-Lazareno, Pedro Ivan Urciaga-Gutierrez, Miriam Ruth Bueno-Topete, Mariel Garcia-Chagollan, and et al. 2024. "Uncovering the Expression Pattern of the Costimulatory Receptors ICOS, 4-1BB, and OX-40 in Exhausted Peripheral and Tumor-Infiltrating Natural Killer Cells from Patients with Cervical Cancer" International Journal of Molecular Sciences 25, no. 16: 8775. https://doi.org/10.3390/ijms25168775




