Increased Pan-Type, A1-Type, and A2-Type Astrocyte Activation and Upstream Inflammatory Markers Are Induced by the P2X7 Receptor
Abstract
:1. Introduction
2. Results
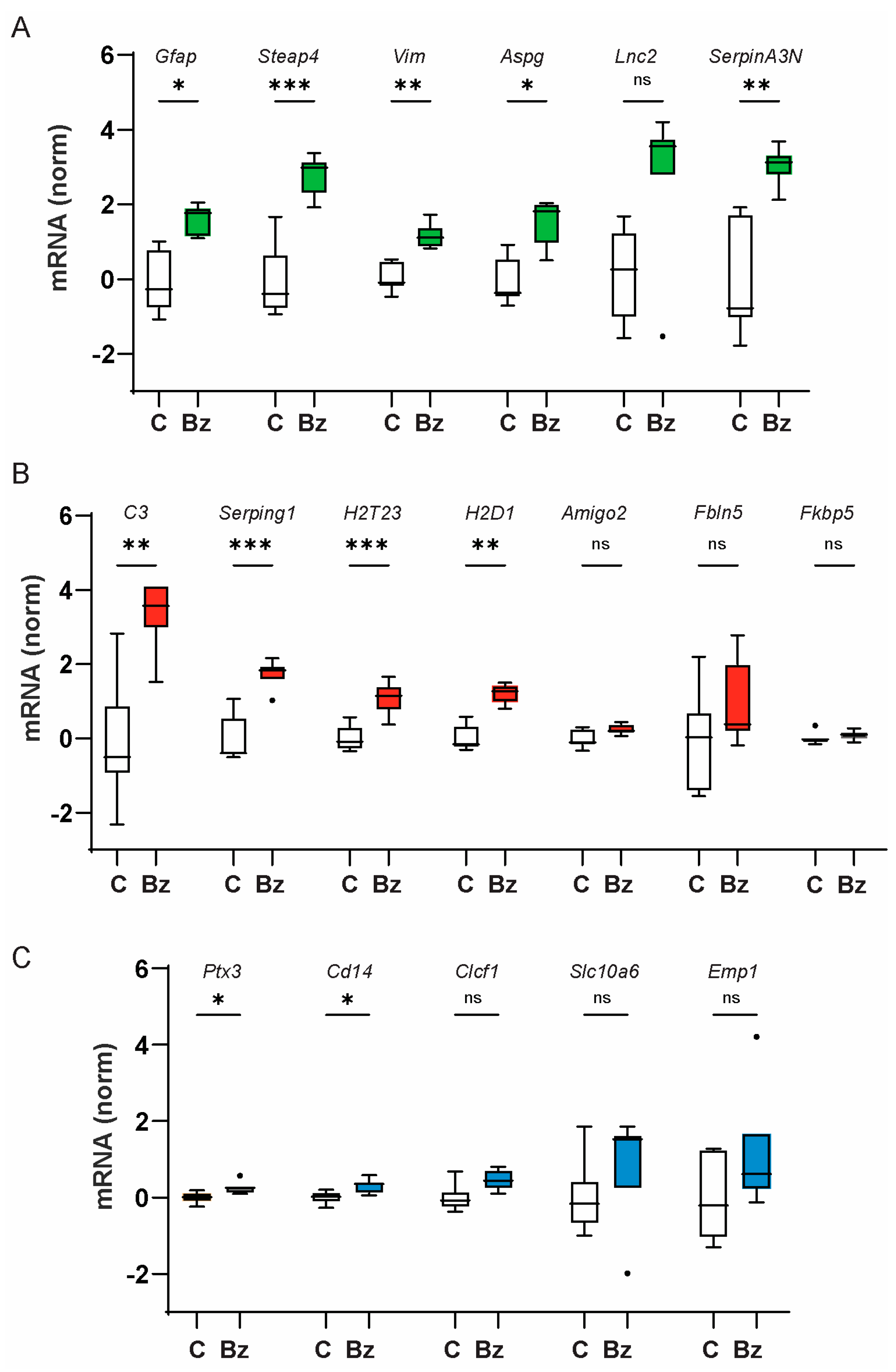
2.1. Injection of P2X7 Receptor Agonist BzATP Increases Expression of Genes Associated with A1-Type Astrocyte Inflammation
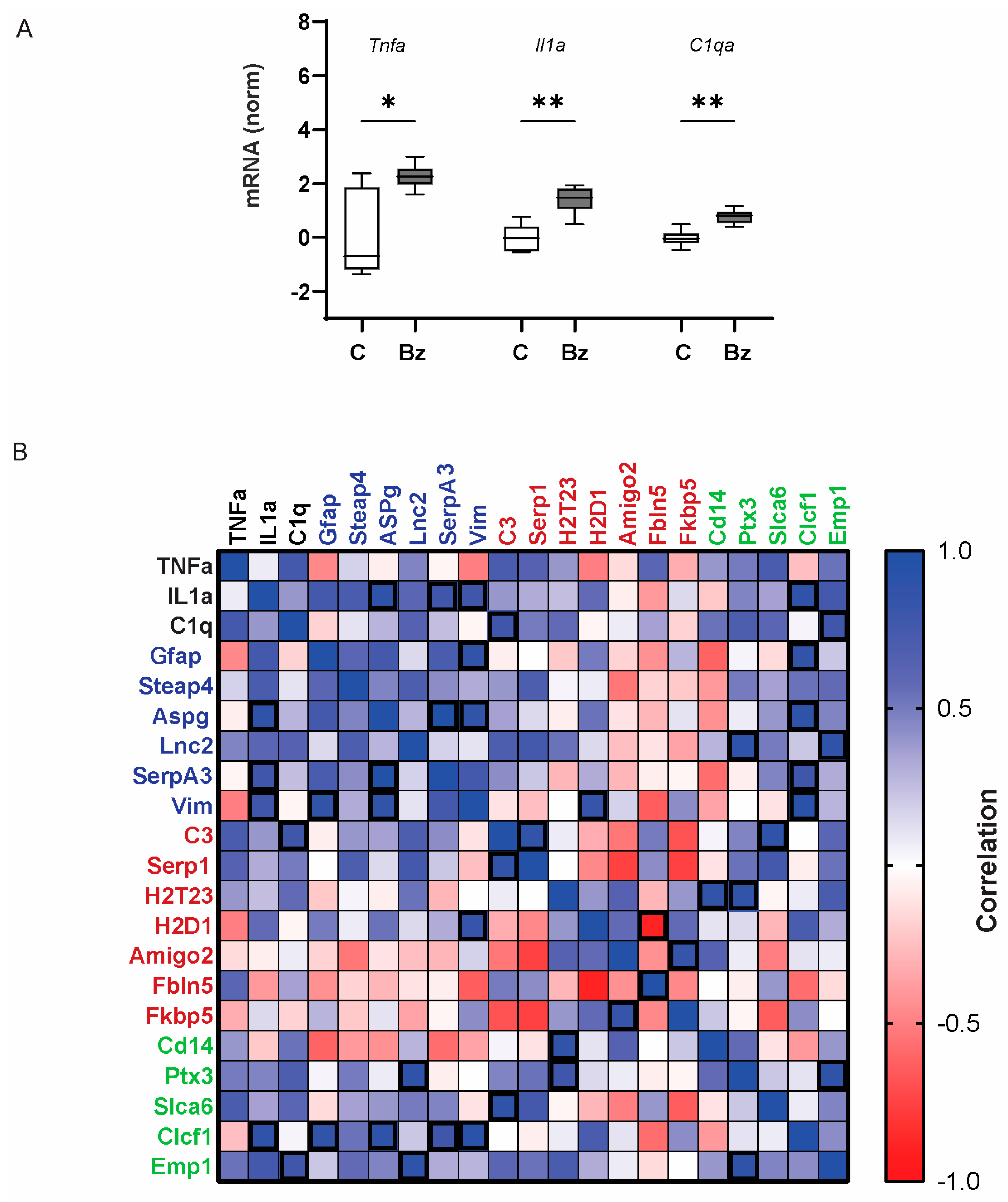
2.2. P2X7 Receptor Agonist BzATP Increases the Expression of Genes Upstream of Astrocyte Inflammation
2.3. P2X7 Receptor Contributes to Neurotoxic Astrocyte Activation after IOP Elevation
2.4. P2X7 Receptor Is Necessary for the Increased Expression of Tnfa and Il1a Following IOP Elevation
3. Discussion
3.1. The P2X7 Receptor Is Sufficient and Necessary for Glial Activation
3.2. Astrocyte Activation States: Mixed States or Mixed-Up Markers?
3.3. The P2X7 Receptor Is Sufficient and Necessary for Activation of Tnfa and Il1a
3.4. Physiological Implications
3.5. Summary
4. Materials and Methods
4.1. Animal Care and Use
4.2. Intravitreal Injections
4.3. Transient Elevation of IOP
4.4. Quantitative PCR
4.5. Data Analysis and Materials
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patani, R.; Hardingham, G.E.; Liddelow, S.A. Functional roles of reactive astrocytes in neuroinflammation and neurodegeneration. Nat. Rev. Neurol. 2023, 19, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Zamanian, J.L.; Xu, L.; Foo, L.C.; Nouri, N.; Zhou, L.; Giffard, R.G.; Barres, B.A. Genomic analysis of reactive astrogliosis. J. Neurosci. 2012, 32, 6391–6410. [Google Scholar] [CrossRef] [PubMed]
- Guttenplan, K.A.; Stafford, B.K.; El-Danaf, R.N.; Adler, D.I.; Munch, A.E.; Weigel, M.K.; Huberman, A.D.; Liddelow, S.A. Neurotoxic reactive astrocytes drive neuronal death after retinal injury. Cell Rep. 2020, 31, 107776. [Google Scholar] [CrossRef] [PubMed]
- Sterling, J.K.; Adetunji, M.O.; Guttha, S.; Bargoud, A.R.; Uyhazi, K.E.; Ross, A.G.; Dunaief, J.L.; Cui, Q.N. GLP-1 receptor agonist NLY01 reduces retinal inflammation and neuron death secondary to ocular hypertension. Cell Rep. 2020, 33, 108271. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yoo, I.D.; Lim, J.; Moon, J.-S. Pathological phenotypes of astrocytes in Alzheimer’s disease. Exp. Mol. Med. 2024, 56, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.B.; Song, L.J.; Wang, Q.; Kumar, G.; Yan, Y.Q.; Ma, C.G. Astrocytes: A double-edged sword in neurodegenerative diseases. Neural Regen. Res. 2021, 16, 1702–1710. [Google Scholar] [PubMed]
- Yao, Y.; Yuan, Y.; Sheng, S.; Li, Y.; Tang, X.; Gu, H. Observing astrocyte polarization in brains from mouse chronically infected with Toxoplasma gondii. Sci. Rep. 2024, 14, 10433. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.P.; Kam, T.I.; Panicker, N.; Kim, S.; Oh, Y.; Park, J.S.; Kwon, S.H.; Park, Y.J.; Karuppagounder, S.S.; Park, H.; et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat. Med. 2018, 24, 931–938. [Google Scholar] [CrossRef]
- Liu, Y.X.; Sun, H.; Guo, W.Y. Astrocyte polarization in glaucoma: A new opportunity. Neural Regen. Res. 2022, 17, 2582–2588. [Google Scholar]
- Fan, Y.-Y.; Huo, J. A1/A2 astrocytes in central nervous system injuries and diseases: Angels or devils? Neurochem. Int. 2021, 148, 105080. [Google Scholar] [CrossRef] [PubMed]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Paolicelli, R.C.; Sierra, A.; Stevens, B.; Tremblay, M.E.; Aguzzi, A.; Ajami, B.; Amit, I.; Audinat, E.; Bechmann, I.; Bennett, M.; et al. Microglia states and nomenclature: A field at its crossroads. Neuron 2022, 110, 3458–3483. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chen, Y.; Chen, D. The heterogeneity of astrocytes in glaucoma. Front. Neuroanat. 2022, 16, 995369. [Google Scholar] [CrossRef] [PubMed]
- Guttenplan, K.A.; Weigel, M.K.; Prakash, P.; Wijewardhane, P.R.; Hasel, P.; Rufen-Blanchette, U.; Münch, A.E.; Blum, J.A.; Fine, J.; Neal, M.C.; et al. Neurotoxic reactive astrocytes induce cell death via saturated lipids. Nature 2021, 599, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Corriden, R.; Insel, P.A. Basal release of ATP: An autocrine-paracrine mechanism for cell regulation. Sci. Signal 2010, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. The therapeutic potential of purinergic signalling. Biochem. Pharmacol. 2018, 151, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Linden, J.; Koch-Nolte, F.; Dahl, G. Purine release, metabolism, and signaling in the inflammatory response. Annu. Rev. Immunol. 2019, 37, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, E.; Giuliani, A.L.; De Marchi, E.; Pegoraro, A.; Orioli, E.; Di Virgilio, F. The P2X7 receptor: A main player in inflammation. Biochem. Pharmacol. 2018, 151, 234–244. [Google Scholar] [CrossRef]
- Savio, L.E.B.; de Andrade Mello, P.; da Silva, C.G.; Coutinho-Silva, R. The P2X7 receptor in inflammatory diseases: Angel or demon? Front. Pharmacol. 2018, 9, 52. [Google Scholar] [CrossRef]
- Giuliani, A.L.; Sarti, A.C.; Falzoni, S.; Di Virgilio, F. The P2X7 receptor-interleukin-1 liaison. Front. Pharmacol. 2017, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Beckel, J.M.; Gomez, N.M.; Lu, W.; Campagno, K.E.; Nabet, B.; Albalawi, F.; Lim, J.C.; Boesze-Battaglia, K.; Mitchell, C.H. Stimulation of TLR3 triggers release of lysosomal ATP in astrocytes and epithelial cells that requires TRPML1 channels. Sci. Rep. 2018, 8, 5726. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Guha, S.; Lu, W.; Campagno, K.E.; Beckel, J.M.; Mills, J.A.; Yang, W.; Mitchell, C.H. Polarized cytokine release triggered by P2X7 receptor from retinal pigmented epithelial cells dependent on calcium iInflux. Cells 2020, 9, 2537. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Hu, H.; Sevigny, J.; Gabelt, B.T.; Kaufman, P.L.; Johnson, E.C.; Morrison, J.C.; Zode, G.S.; Sheffield, V.C.; Zhang, X.; et al. Rat, mouse, and primate models of chronic glaucoma show sustained elevation of extracellular ATP and altered purinergic signaling in the posterior eye. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3075–3083. [Google Scholar] [CrossRef] [PubMed]
- Albalawi, F.; Lu, W.; Beckel, J.M.; Lim, J.C.; McCaughey, S.A.; Ch, M. The P2X7 receptor primes IL-1β and the NLRP3 inflammasome in astrocytes exposed to mechanical strain. Front. Cell Neurosci. 2017, 11, 227. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Barres, B.A. Reactive astrocytes: Production, function, and therapeutic potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Shestopalov, V.I.; Spurlock, M.; Gramlich, O.W.; Kuehn, M.H. Immune responses in the glaucomatous retina: Regulation and dynamics. Cells 2021, 10, 1973. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Zhang, X.; Zheng, D.; Ge, J.; Laties, A.M.; Mitchell, C.H. Sustained elevation of extracellular ATP in aqueous humor from humans with primary chronic angle-closure glaucoma. Exp. Eye Res. 2011, 93, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, A.; Ge, J.; Reigada, D.; Laties, A.M.; Mitchell, C.H. Acute increase of intraocular pressure releases ATP into the anterior chamber. Exp. Eye Res. 2007, 85, 637–643. [Google Scholar] [CrossRef]
- Jarvis, M.F.; Khakh, B.S. ATP-gated P2X cation-channels. Neuropharmacology 2009, 56, 208–215. [Google Scholar] [CrossRef]
- Romano, G.L.; Amato, R.; Lazzara, F.; Porciatti, V.; Chou, T.-H.; Drago, F.; Bucolo, C. P2X7 receptor antagonism preserves retinal ganglion cells in glaucomatous mice. Biochem. Pharmacol. 2020, 180, 114199. [Google Scholar] [CrossRef]
- Niyadurupola, N.; Sidaway, P.; Ma, N.; Rhodes, J.D.; Broadway, D.C.; Sanderson, J. P2X7 receptor activation mediates retinal ganglion cell death in a human retina model of ischemic neurodegeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2163–2170. [Google Scholar] [CrossRef]
- Lu, W.; Albalawi, F.; Beckel, J.M.; Lim, J.C.; Laties, A.M.; Mitchell, C.H. The P2X7 receptor links mechanical strain to cytokine IL-6 up-regulation and release in neurons and astrocytes. J. Neurochem. 2017, 141, 436–448. [Google Scholar] [CrossRef]
- Dong, L.; Hu, Y.; Zhou, L.; Cheng, X. P2X7 receptor antagonist protects retinal ganglion cells by inhibiting microglial activation in a rat chronic ocular hypertension model. Mol. Med. Rep. 2018, 17, 2289–2296. [Google Scholar] [CrossRef]
- Mac Nair, C.E.; Schlamp, C.L.; Montgomery, A.D.; Shestopalov, V.I.; Nickells, R.W. Retinal glial responses to optic nerve crush are attenuated in Bax-deficient mice and modulated by purinergic signaling pathways. J. Neuroinflamm. 2016, 13, 93. [Google Scholar] [CrossRef]
- Bennett, M.L.; Viaene, A.N. What are activated and reactive glia and what is their role in neurodegeneration? Neurobiol. Dis. 2021, 148, 105172. [Google Scholar] [CrossRef]
- Tezel, G. TNF-alpha signaling in glaucomatous neurodegeneration. Prog. Brain Res. 2008, 173, 409–421. [Google Scholar]
- Gramlich, O.W.; Godwin, C.R.; Wadkins, D.; Elwood, B.W.; Kuehn, M.H. Early functional impairment in experimental glaucoma is accompanied by disruption of the gabaergic system and inceptive neuroinflammation. Int. J. Mol. Sci. 2021, 22, 7581. [Google Scholar] [CrossRef]
- Williams, P.A.; Tribble, J.R.; Pepper, K.W.; Cross, S.D.; Morgan, B.P.; Morgan, J.E.; John, S.W.M.; Howell, G.R. Inhibition of the classical pathway of the complement cascade prevents early dendritic and synaptic degeneration in glaucoma. Mol. Neurodegener. 2016, 11, 26. [Google Scholar] [CrossRef]
- Shinozaki, Y.; Koizumi, S. Potential roles of astrocytes and Muller cells in the pathogenesis of glaucoma. J. Pharmacol. Sci. 2021, 145, 262–267. [Google Scholar] [CrossRef]
- Pitha, I.; Kambhampati, S.; Sharma, A.; Sharma, R.; McCrea, L.; Mozzer, A.; Kannan, R.M. Targeted microglial attenuation through dendrimer-drug conjugates improves glaucoma neuroprotection. Biomacromolecules 2023, 24, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Wurl, J.A.; Mac Nair, C.E.; Dietz, J.A.; Shestopalov, V.I.; Nickells, R.W. Contralateral astrocyte response to acute optic nerve damage is mitigated by PANX1 channel activity. Int. J. Mol. Sci. 2023, 24, 15641. [Google Scholar] [CrossRef] [PubMed]
- Boulos, M.E.; Bray, M.J.C. Complement C3 inhibition modulates neurodegeneration in chronic traumatic brain injury. J. Neurosci. 2018, 38, 7201–7203. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Dejanovic, B.; Gandham, V.D.; Gogineni, A.; Edmonds, R.; Schauer, S.; Srinivasan, K.; Huntley, M.A.; Wang, Y.; Wang, T.M.; et al. Complement C3 is activated in human ad brain and is required for neurodegeneration in mouse models of amyloidosis and tauopathy. Cell Rep. 2019, 28, 2111–2123.e2116. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhou, R.; Peng, H.; Peng, J.; Li, Q.; Mei, M. Microglia PKM2 mediates neuroinflammation and neuron loss in mice epilepsy through the astrocyte C3-neuron C3R signaling pathway. Brain Sci. 2023, 13, 262. [Google Scholar] [CrossRef] [PubMed]
- Gharagozloo, M.; Smith, M.D.; Jin, J.; Garton, T.; Taylor, M.; Chao, A.; Meyers, K.; Kornberg, M.D.; Zack, D.J.; Ohayon, J.; et al. Complement component 3 from astrocytes mediates retinal ganglion cell loss during neuroinflammation. Acta Neuropathol. 2021, 142, 899–915. [Google Scholar] [CrossRef]
- Hoppe, C.; Gregory-Ksander, M. The role of complement dysregulation in glaucoma. Int. J. Mol. Sci. 2024, 25, 2307. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Kocab, A.J.; Zacks, D.N.; Marshak-Rothstein, A.; Gregory-Ksander, M. A small peptide antagonist of the Fas receptor inhibits neuroinflammation and prevents axon degeneration and retinal ganglion cell death in an inducible mouse model of glaucoma. J. Neuroinflam. 2019, 16, 184. [Google Scholar] [CrossRef]
- Cameron, E.G.; Nahmou, M.; Toth, A.B.; Heo, L.; Tanasa, B.; Dalal, R.; Yan, W.; Nallagatla, P.; Xia, X.; Hay, S.; et al. A molecular switch for neuroprotective astrocyte reactivity. Nature 2024, 626, 574–582. [Google Scholar] [CrossRef]
- Liang, X.; Samways, D.S.; Wolf, K.; Bowles, E.A.; Richards, J.P.; Bruno, J.; Dutertre, S.; DiPaolo, R.J.; Egan, T.M. Quantifying Ca2+ current and permeability in ATP-gated P2X7 receptors. J. Biol. Chem. 2015, 290, 7930–7942. [Google Scholar] [CrossRef]
- Steegborn, C. Structure, mechanism, and regulation of soluble adenylyl cyclases—Similarities and differences to transmembrane adenylyl cyclases. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2014, 1842, 2535–2547. [Google Scholar] [CrossRef] [PubMed]
- Litvin, T.N.; Kamenetsky, M.; Zarifyan, A.; Buck, J.; Levin, L.R. Kinetic properties of “soluble” adenylyl cyclase: Synergism between calcium and bicarbonate*. J. Biol. Chem. 2003, 278, 15922–15926. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, E.; Pizzirani, C.; Idzko, M.; Panther, E.; Norgauer, J.; Di Virgilio, F.; Ferrari, D. P2X(7) receptor: Death or life? Purinergic Signal 2005, 1, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, E.L.; Wang, A.Y.; Jobling, A.I.; Rutar, M.V.; Greferath, U.; Gu, B.; Vessey, K.A. Targeting P2X7 receptors as a means for treating retinal disease. Drug Discov. Today 2019, 24, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Vessey, K.A.; Gu, B.J.; Jobling, A.I.; Phipps, J.A.; Greferath, U.; Tran, M.X.; Dixon, M.A.; Baird, P.N.; Guymer, R.H.; Wiley, J.S.; et al. Loss of function of P2X7 receptor scavenger activity in aging mice: A novel model for investigating the early pathogenesis of age-related macular degeneration. Am. J. Pathol. 2017, 187, 1670–1685. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.C.; Lu, W.; Beckel, J.M.; Mitchell, C.H. Neuronal release of cytokine IL-3 triggered by mechanosensitive autostimulation of the P2X7 receptor Is neuroprotective. Front. Cell Neurosci. 2016, 10, 270. [Google Scholar] [CrossRef]
- Hu, H.; Lu, W.; Zhang, M.; Zhang, X.; Argall, A.J.; Patel, S.; Lee, G.E.; Kim, Y.C.; Jacobson, K.A.; Laties, A.M.; et al. Stimulation of the P2X7 receptor kills rat retinal ganglion cells in vivo. Exp. Eye Res. 2010, 91, 425–432. [Google Scholar] [CrossRef]
- Morrison, J.C.; Cepurna, W.O.; Tehrani, S.; Choe, T.E.; Jayaram, H.; Lozano, D.C.; Fortune, B.; Johnson, E.C. A period of controlled elevation of IOP (CEI) produces the specific gene expression responses and focal injury pattern of experimental rat glaucoma. Investig. Ophthalmol. Vis. Sci. 2016, 57, 6700–6711. [Google Scholar] [CrossRef]


| Gene Name | Genbank Accession | Primer (F: 5′–3′; R: 3′–5′) | Size (bp) |
|---|---|---|---|
| Tnfa | NM_013693.3 | F: AAATGGCCTCCCTCTCATCAG R: GTCACTCGAATTTTGAGAAGATGATC | 73 |
| Il1a | NM_010554.4 | F: CAACGTCAAGCAACGGGAAG R: AAGGTGCTGATCTGGGTTGG | 126 |
| C1qa | NM_007572.2 | F: GAAGGGCGTGAAAGGCAATC R: CAAGCGTCATTGGGTTCTGC | 86 |
| Gfap | NM_001131020.1 | F: CCTGCCAGCTCTCCCT R: AAAGGTGTGGCTGAAATGCG | 216 |
| Steap4 | NM_054098.3 | F: CCCGAATCGTGTCTTTCCTA R: GGCCTGAGTAATGGTTGCAT | 262 |
| Vim | NM_011701.4 | F: GATGGCCCTGGACATTGAGA R: TTGAGTGGGTGTCAACCAGAG | 146 |
| Lcn2 | NM_008491.1 | F: CCAGTTCGCCATGGTATTTT R: CACACTCACCACCCATTCAG | 206 |
| SerpinA3N | NM_009252.2 | F: GCTGGCTGGTTTCAGCTCT R: ATCCATTCCCAACGTGCCAT | 127 |
| Aspg | NM_001081169.1 | F: GCTGCTGGCCATTTACACTG R: GTGGGCCTGTGCATACTCTT | 133 |
| C3 | NM_009778.3 | F: TTCCTTCACTATGGGACCAGC R: CTCCAGCCGTAGGACATTGG | 127 |
| Serping1 | NM_009776.3 | F: ACAGCCCCCTCTGAATTCTT R: GGATGCTCTCCAAGTTGCTC | 299 |
| H2D1 | NM_010380.3 | F: TCCGAGATTGTAAAGCGTGAAGA R: ACAGGGCAGTGCAGGGATAG | 204 |
| H2T23 | NM_010398.3 | F: GGACCGCGAATGACATAGC R: GCACCTCAGGGTGACTTCAT | 212 |
| Amigo2 | NM_178114.4 | F: GAGGCGACCATAATGTCGTT R: GCATCCAACAGTCCGATTCT | 263 |
| Fkbp5 | NM_010220.4 | F: TATGCTTATGGCTCGGCTGG R: CAGCCTTCCAGGTGGACTTT | 194 |
| Fbln5 | NM_001361987.1 | F: CTTCAGATGCAAGCAACAA R: AGGCAGTGTCAGAGGCCTTA | 281 |
| CD14 | NM_009841.4 | F: GGACTGATCTCAGCCCTCTG R: GCTTCAGCCCAGTGAAAGAC | 232 |
| Ptx3 | NM_008987.3 | F: AACAAGCTCTGTTGCCCATT R: TCCCAAATGGAACATTGGAT | 147 |
| Clcf1 | NM_019952.5 | F: CTTCAATCCTCCTCGACTGG R: TACGTCGGAGTTCAGCTGTG | 176 |
| Emp1 | NM_010128.4 | F: GAGACACTGGCCAGAAAAGC R: TAAAAGGCAAGGGAATGCAC | 183 |
| Slc10a6 | NM_029415.2 | F: GCTTCGGTGGTATGATGCTT R: CCACAGGCTTTTCTGGTGAT | 217 |
| GAPDH | NM_017008 | F: TCACCACCATGGAGAAGGC R: GCTAAGCAGTTGGTGGTGCA | 169 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campagno, K.E.; Sripinun, P.; See, L.P.; Li, J.; Lu, W.; Jassim, A.H.; Más Gómez, N.; Mitchell, C.H. Increased Pan-Type, A1-Type, and A2-Type Astrocyte Activation and Upstream Inflammatory Markers Are Induced by the P2X7 Receptor. Int. J. Mol. Sci. 2024, 25, 8784. https://doi.org/10.3390/ijms25168784
Campagno KE, Sripinun P, See LP, Li J, Lu W, Jassim AH, Más Gómez N, Mitchell CH. Increased Pan-Type, A1-Type, and A2-Type Astrocyte Activation and Upstream Inflammatory Markers Are Induced by the P2X7 Receptor. International Journal of Molecular Sciences. 2024; 25(16):8784. https://doi.org/10.3390/ijms25168784
Chicago/Turabian StyleCampagno, Keith E., Puttipong Sripinun, Lily P. See, Jiaqi Li, Wennan Lu, Assraa Hassan Jassim, Néstor Más Gómez, and Claire H. Mitchell. 2024. "Increased Pan-Type, A1-Type, and A2-Type Astrocyte Activation and Upstream Inflammatory Markers Are Induced by the P2X7 Receptor" International Journal of Molecular Sciences 25, no. 16: 8784. https://doi.org/10.3390/ijms25168784






