Chromosome-Level Assembly Reveals a Fifteen-Chromosome Aneuploid Genome and Environmental Adaptation Strategy of Chinese Traditional Medical Fungus Wolfiporia hoelen
Abstract
:1. Introduction
2. Results and Discussion
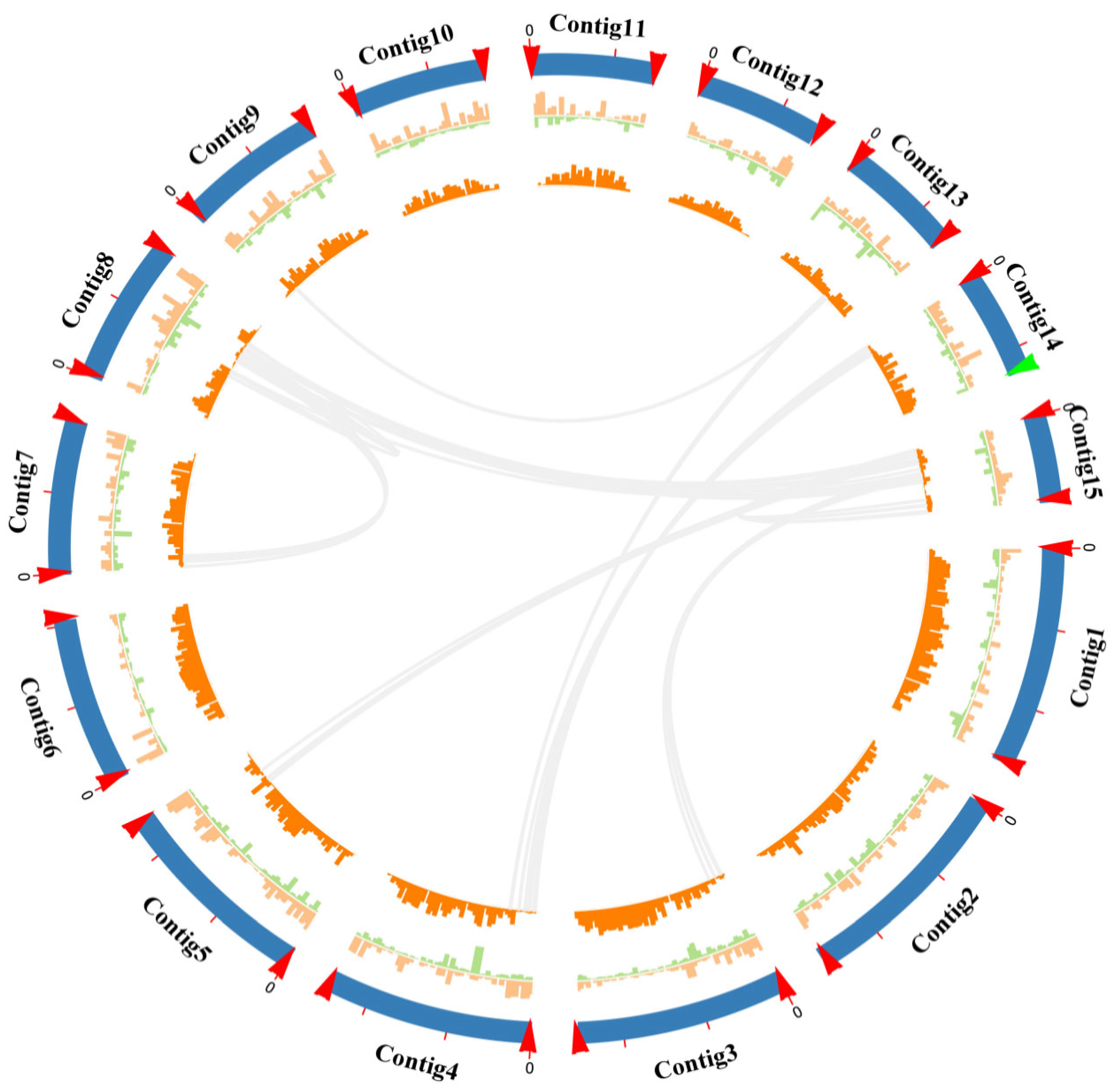
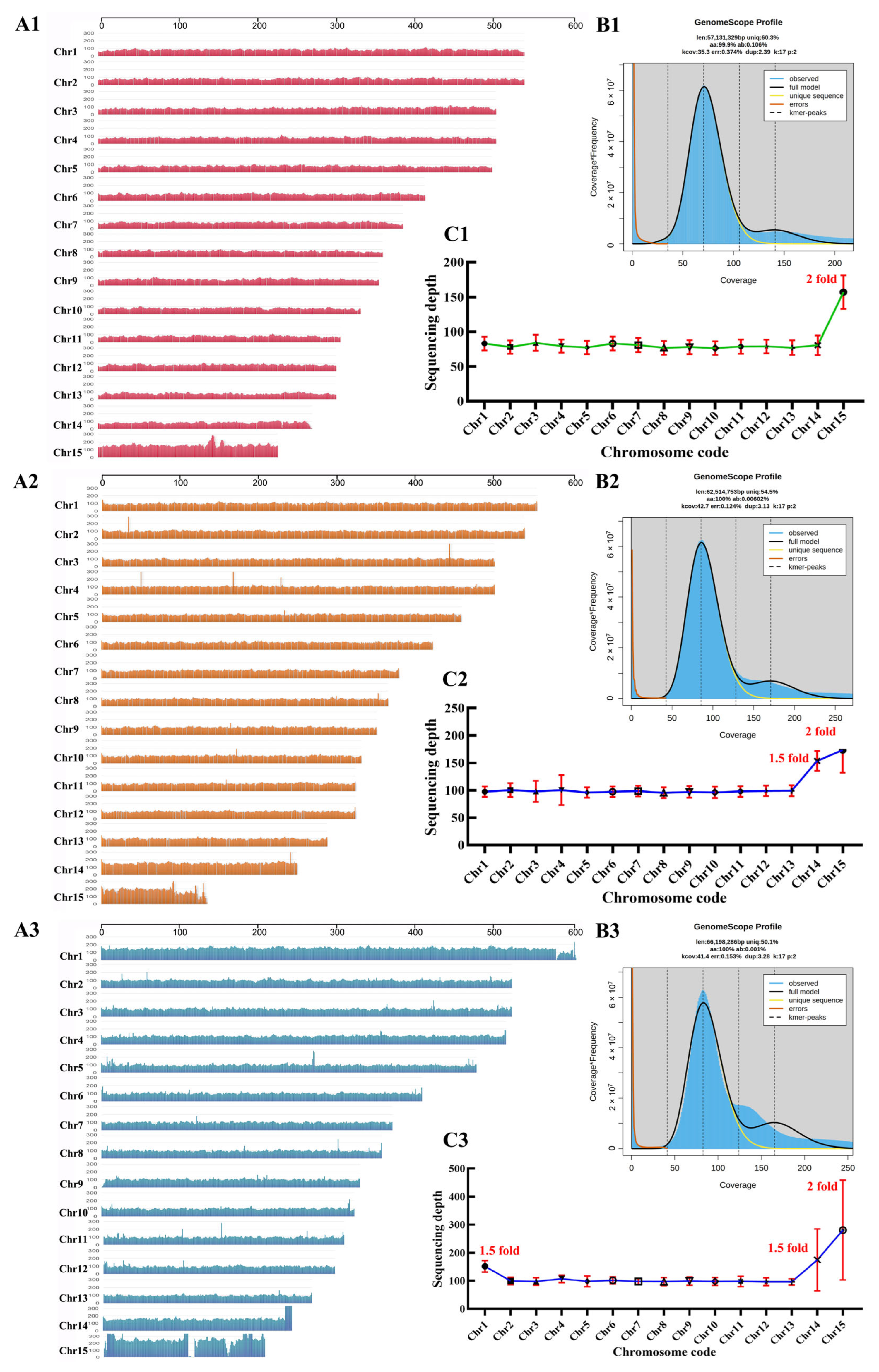
2.1. Chromosome-Level Genome Assembly of Wolfiporia hoelen Strain L7
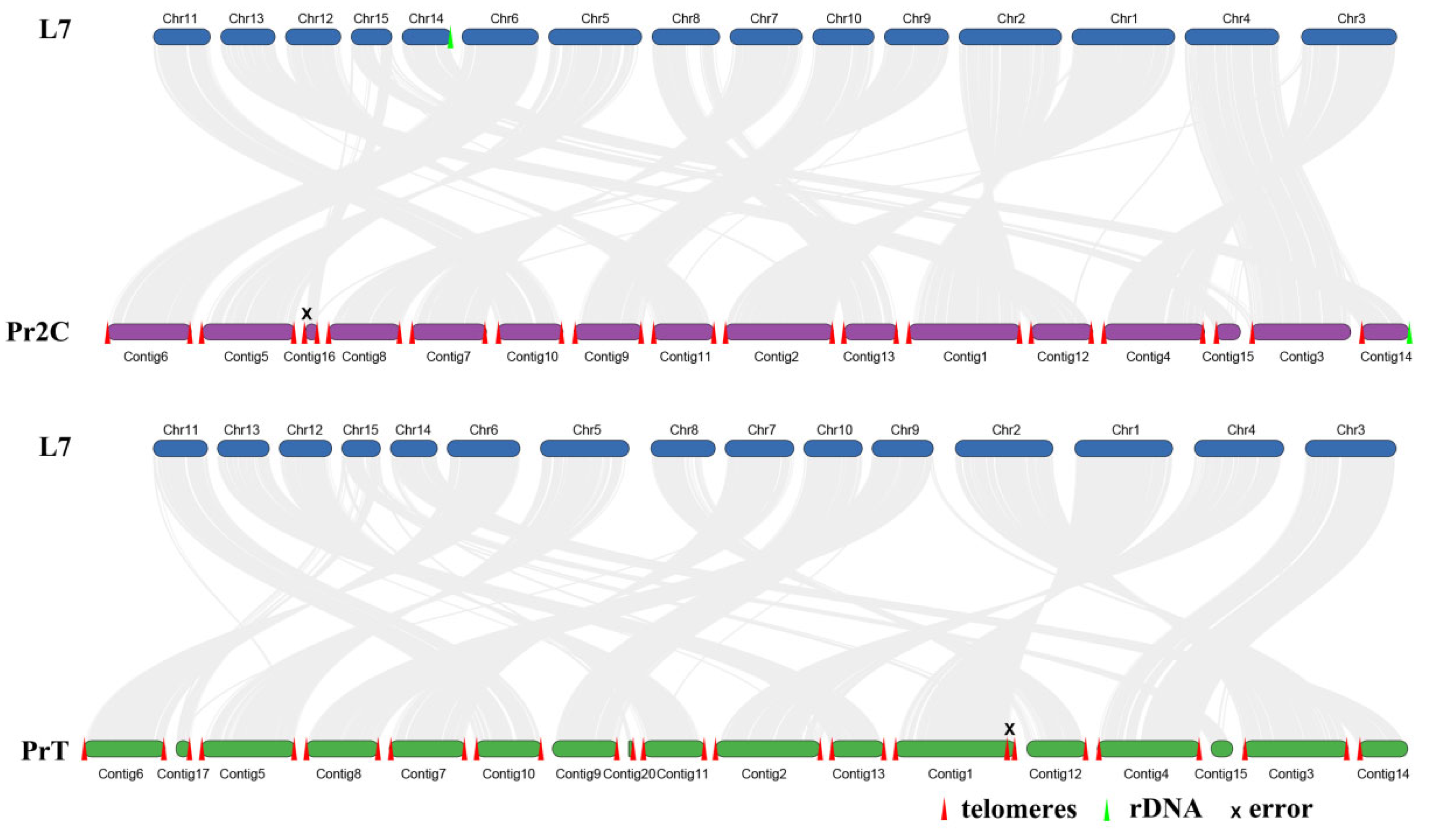
2.2. Genome Comparison and Collinearity Analysis of Different Wolfioria hoelen Strains
2.3. Aneuploidy Genome of Wolfiporia hoelen
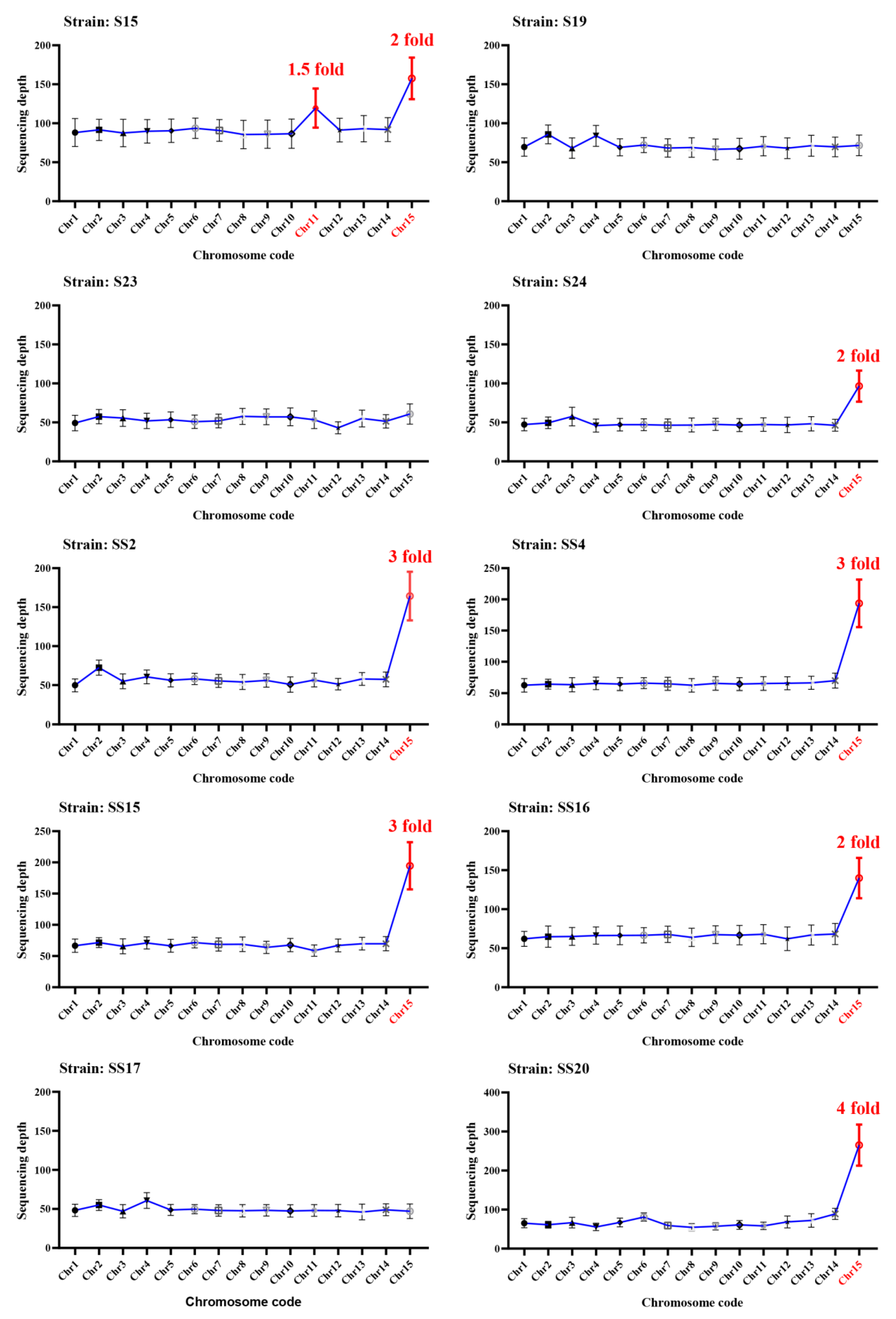
2.4. Aneuploidy Characters for Different-Type Heterokaryotic Strains
2.5. Aneuploidy Characters for Homokaryotic Offspring of L12 and L14
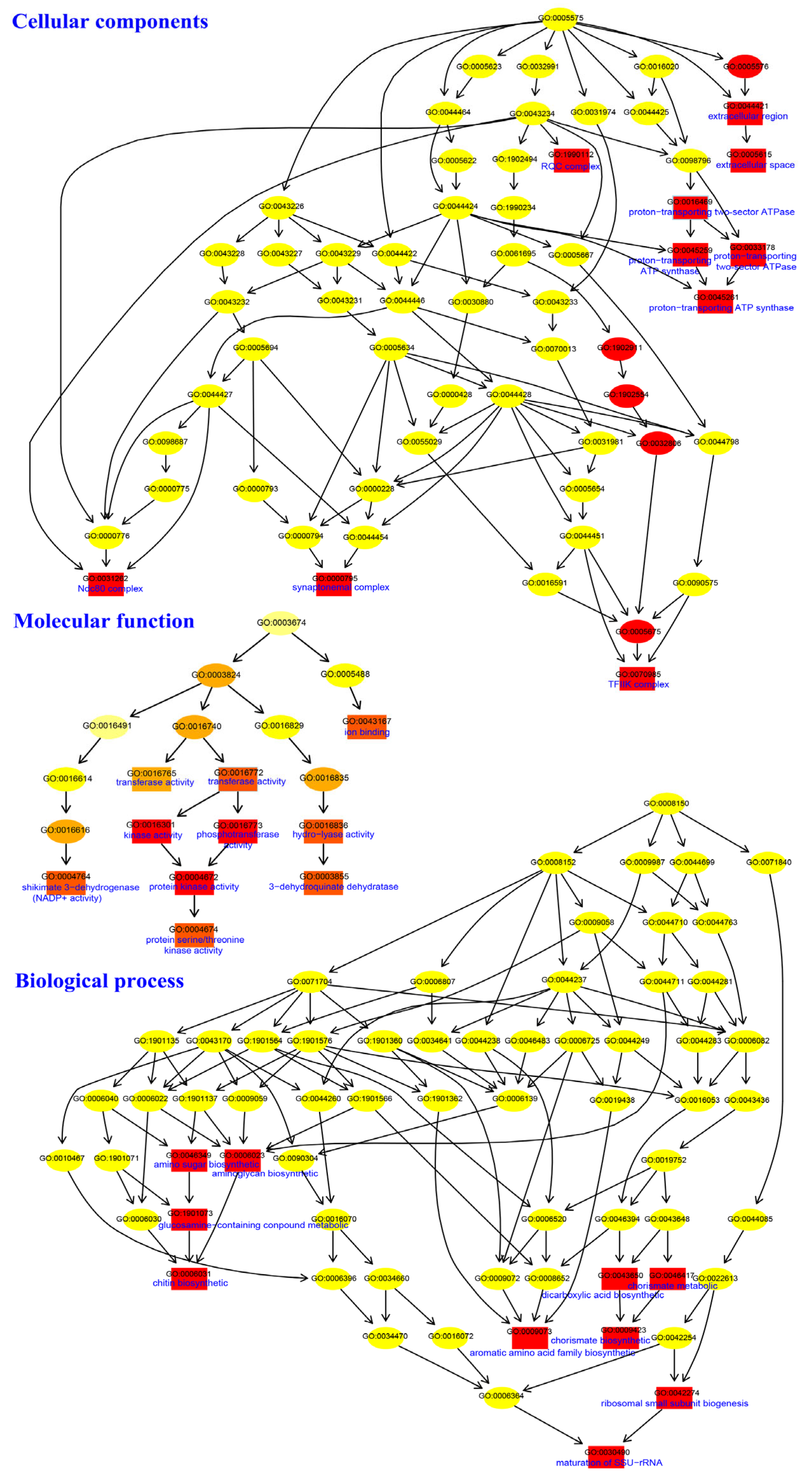
2.6. Gene Function Enrichment of Genes on Chromosome 15
3. Materials and Methods
3.1. Homokaryotic Strain Isolation
3.2. Genome Sequencing, Assembly, and Quality Assessment
3.3. Telomere and rDNA Sequences Location
3.4. Annotation of Gene Structure and Function
3.5. Whole-Genome Collinearity Analysis
3.6. Genome Resequencing
3.7. Sequencing Depth Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, H.L.; Zhang, L.J.; Shang, X.D.; Peng, B.; Li, Y.; Xiao, S.J.; Tan, Q.; Fu, Y.P. Chromosomal genome and population genetic analyses to reveal genetic architecture, breeding history and genes related to cadmium accumulation in Lentinula edodes. BMC Genom. 2022, 23, 120. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wu, F.; Si, J.; Zhao, Y.F.; Dai, Y.C. Whole genome sequence of Auricularia heimuer (Basidiomycota, Fungi), the third most important cultivated mushroom worldwide. Genomics 2019, 111, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Sonnenberg, A.S.M.; Gao, W.; Lavrijssen, B.; Hendrickx, P.; Sedaghat-Tellgerd, N.; Foulongne-Oriol, M.; Kong, W.; Schijlen, E.G.W.M.; Baars, J.J.P.; Visser, R.G.F. A detailed analysis of the recombination landscape of the button mushroom Agaricus bisporus var. bisporus. Fungal Genet. Biol. 2016, 93, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.B.; Zhao, M.R.; Hsiang, T.; Feng, X.X.; Zhang, J.X.; Huang, C.Y. Identification and characterization of small noncoding RNAs in genome sequences of the edible fungus Pleurotus ostreatus. BioMed Res. Int. 2016, 2016, 2503023. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, L.; Tang, W.Q.; Xia, W.W.; Zhong, Y.L.; Xu, X.Y.; Xie, B.G.; Tao, Y.X. Comprehensive genetic analysis of monokaryon and dikaryon populations provides insight into cross-breeding of Flammulina filiformis. Front. Microbiol. 2022, 13, 887259. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.P.; Gao, X.; Zhang, M.H.; Hu, C.H.; Yang, W.J.; Guo, L.Z.; Yang, S.; Yu, H.L.; Yu, H. Whole genome sequence of an edible mushroom Oudemansiella raphanipes (Changgengu). J. Fungi 2023, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Y.X.; Jiang, H.; Yang, K.; Wang, S.Y.; Wang, R.; Li, S.; Lei, P.; Xu, H.; Qiu, Y.B.; et al. Whole genome sequencing and annotation of Naematelia aurantialba (Basidiomycota, edible-medicinal Fungi). J. Fungi 2021, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Meng, G.L.; Dong, C.H. Homokaryotic high-quality genome assembly of medicinal fungi Wolfiporia hoelen reveals auto-regulation and high-temperature adaption of probable two-speed genome. Int. J. Mol. Sci. 2022, 23, 10484. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.G.; Wang, Z.X.; Feng, X.L.; Zhang, R.Q.; Shen, Q.Y.; Du, S.T.; Gao, J.M.; Qi, J.Z. Chromosome-level genome sequences, comparative genomic analyses, and secondary-metabolite biosynthesis evaluation of the medicinal edible mushroom Laetiporus sulphureus. Microbiol. Spectr. 2022, 10, e0243922. [Google Scholar] [CrossRef]
- Sonnenberg, A.S.M.; Sedaghat-Telgerd, N.; Lavrijssen, B.; Ohm, R.A.; Hendrickx, P.M.; Scholtmeijer, K.; Baars, J.J.P.; Peer, A.V. Telomere-to-telomere assembled and centromere annotated genomes of the two main subspecies of the button mushroom Agaricus bisporus reveal especially polymorphic chromosome ends. Sci. Rep. 2020, 10, 14653. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, C.Y.; Gao, W. Research advances on molecular mushroom breeding. J. Fungal Res. 2019, 17, 229–239. [Google Scholar] [CrossRef]
- Wieloch, W. Chromosome visualization in filamentous fungi. J. Microbiol. Methods 2006, 67, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.C.; Cantor, C.R. Separation of yeast chromosome-sized DNAs by pulsed field gradient gel electrophoresis. Cell 1984, 37, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Millis, D.; McCluskey, K. Electrophoretic karyotypes of fungi: The new cytology. Mol. Plant-Microbe Interact. 1990, 3, 351–357. [Google Scholar] [CrossRef]
- Borbye, L.; Linde-Laursen, I.; Christiansen, S.; Giese, H. The chromosome complement of Erysiphe graminis f. sp. hordei analysed by light microscopy and field inversion gel electrophoresis. Mycol. Res. 1992, 96, 97–102. [Google Scholar] [CrossRef]
- Poma, A.; Pacioni, G.; Ranalli, R.; Miranda, M. Ploidy and chromosomal number in Tuber aestivum. FEMS Microbiol. Lett. 1998, 167, 101–105. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, S.L.; Liu, B.; Wang, H.; Deng, J.; Liao, Y.C.; Wang, Q.; Cheng, F.; Wang, X.W.; Wu, J. A sequence-based genetic linkage map as a reference for Brassica rapa pseudochromosome assembly. BMC Genom. 2011, 12, 239. [Google Scholar] [CrossRef] [PubMed]
- Ethier, S.D.; Miura, H.; Dostie, J. Discovering genome regulation with 3C and 3C-related technologies. Biochim. Biophys. Acta 2012, 1819, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Tosh, J.; Tybulewicz, V.; Fisher, E.M.C. Mouse models of aneuploidy to understand chromosome disorders. Mamm. Genome 2022, 33, 157–168. [Google Scholar] [CrossRef]
- Zhou, J.N.; Tan, C.; Cui, C.; Ge, A.H.; Li, Z.Y. Distinct subgenome stabilities in synthesized Brassica allohexaploids. Theor. Appl. Genet. 2016, 129, 1257–1271. [Google Scholar] [CrossRef]
- Tsai, H.J.; Nelliat, A. A double-edged sword: Aneuploidy is a prevalent strategy in fungal adaptation. Genes 2019, 10, 787. [Google Scholar] [CrossRef]
- Rieseberg, L.H.; Willis, J.H. Plant speciation. Science 2007, 317, 910–914. [Google Scholar] [CrossRef] [PubMed]
- Knouse, K.A.; Wu, J.; Whittaker, C.A.; Amon, A. Single cell sequencing reveals low levels of aneuploidy across mammalian tissues. Proc. Natl. Acad. Sci. USA 2014, 111, 13409–13414. [Google Scholar] [CrossRef]
- Forche, A.; Solis, N.V.; Swidergall, M.; Thomas, R.; Guyer, A.; Beach, A.; Cromie, G.A.; Le, G.T.; Lowell, E.; Pavelka, N.; et al. Selection of Candida albicans trisomy during oropharyngeal infection results in a commensal-like phenotype. PLoS Genet. 2019, 15, e1008137. [Google Scholar] [CrossRef] [PubMed]
- Morard, M.; Macías, L.G.; Adam, A.C.; Lairón-Peris, M.; Pérez-Torrado, R.; Toft, C.; Barrio, E. Aneuploidy and ethanol tolerance in Saccharomyces cerevisiae. Front. Genet. 2019, 10, 82. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Teoh, F.; Tan, A.S.M.; Cao, Y.B.; Pavelka, N.; Berman, J. Aneuploidy enables cross-adaptation to unrelated drugs. Mol. Biol. Evol. 2019, 36, 1768–1782. [Google Scholar] [CrossRef]
- Sionov, E.; Chang, Y.C.; Kwon-Chung, K.J. Azole heteroresistance in Cryptococcus neoformans: Emergence of resistant clones with chromosomal disomy in the mouse brain during fluconazole treatment. Antimicrob. Agents Chemother. 2013, 57, 5127–5130. [Google Scholar] [CrossRef]
- Gresham, D.; Desai, M.M.; Tucker, C.M.; Jenq, H.T.; Pai, D.A.; Ward, A.; DeSevo, C.G.; Botstein, D.; Dunham, M.J. The repertoire and dynamics of evolutionary adaptations to controlled nutrient-limited environments in yeast. PLoS Genet. 2008, 4, e1000303. [Google Scholar] [CrossRef] [PubMed]
- Rancati, G.; Pavelka, N.; Fleharty, B.; Noll, A.; Trimble, R.; Walton, K.; Perera, A.; Staehling-Hampton, K.; Seidel, C.W.; Li, R. Aneuploidy underlies rapid adaptive evolution of yeast cells deprived of a conserved cytokinesis motor. Cell 2008, 135, 879–893. [Google Scholar] [CrossRef]
- Selmecki, A.; Maruvka, Y.E.; Richmond, P.A.; Guillet, M.; Shoresh, N.; Sorenson, A.; De, S.; Kishony, R.; Michor, F.; Dowell, R.; et al. Polyploidy can drive rapid adaptation in yeast. Nature 2015, 519, 349–352. [Google Scholar] [CrossRef]
- Deng, Y.J.; Guo, L.; Lin, L.J.; Li, Y.F.; Zhang, J.X.; Zhang, Y.; Yuan, B.; Ke, L.N.; Xie, B.G.; Ming, R. Meiosis in an asymmetric dikaryotic genome of Tremella fuciformis Tr01 facilitates new chromosome formation. Genome Biol. 2023, 24, 280. [Google Scholar] [CrossRef] [PubMed]
- Stalpers, J.A.; Redhead, S.A.; May, T.W.; Rossman, A.Y.; Crouch, J.A.; Cubeta, M.A.; Dai, Y.C.; Kirschner, R.; Langer, G.J.; Larsson, K.H.; et al. Competing sexual-asexual generic names in Agaricomycotina (Basidiomycota) with recommendations for use. IMA Fungus 2021, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Papp, V.; Dai, Y.C. What is the correct scientific name for “Fuling” medicinal mushroom? Mycology 2022, 13, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Li, S.J.; Dong, C.H.; Dai, Y.C.; Papp, V. The genus Pachyma (syn. Wolfiporia) reinstated and species clarification of the cultivated medicinal mushroom “Fuling” in China. Front. Microbiol. 2020, 11, 590788. [Google Scholar] [CrossRef]
- Wang, K.Q.; Fu, J.; Su, W.; Fang, H.; Deng, F. Review of Chinese traditional medicinal fungus: Wolfiporia cocos. Res. Inf. Tradit. Chin. Med. 2002, 6, 16–17. [Google Scholar] [CrossRef]
- Jo, W.S.; Hong, I.P.; Yoo, Y.B.; Park, S.D. Improvement on artificial cultivation technique of Poria cocos in Korea. In Proceedings of the International Mycological Congress 10th IMC10, Bangkok, Thailand, 3–8 August 2014. [Google Scholar]
- Wu, F.; Zhou, L.W.; Yang, Z.L.; Bau, T.; Li, T.H.; Dai, Y.C. Resource diversity of Chinese macrofungi: Edible, medicinal and poisonous species. Fungal Divers. 2019, 98, 1–76. [Google Scholar] [CrossRef]
- Wang, N.N.; Zhang, Y.; Wang, X.P.; Huang, X.W.; Fei, Y.; Yu, Y.; Shou, D. Antioxidant property of water-soluble polysaccharides from Poria cocos wolf using different extraction methods. Int. J. Biol. Macromol. 2016, 83, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.Y.; Ma, Q.H.; Ren, M.Y.; Liang, D.D.; Yu, Q.T.; Luo, J.B. Antitumor pharmacological mechanism of the oral liquid of Poria cocos polysaccharide. J. Ethnopharmacol. 2017, 209, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.S.; Wang, S.X.; Liu, G.Q.; Bao, L.; Huang, Y.; Zhao, R.L.; Liu, H.W. Anti-inflammatory diterpenes and steroids from peels of the cultivated edible mushroom Wolfiporia cocos. Phytochem. Lett. 2020, 36, 11–16. [Google Scholar] [CrossRef]
- Pu, Y.W.; Liu, Z.J.; Tian, H.; Bao, Y.X. The immunomodulatory effect of Poria cocos polysaccharides is mediated by the Ca2+/PKC/p38/NF-κB signaling pathway in macrophages. Int. Immunopharmacol. 2019, 72, 252–257. [Google Scholar] [CrossRef]
- Luo, H.M.; Qian, J.; Xu, Z.C.; Liu, W.J.; Xu, L.; Li, Y.; Xu, J.; Zhang, J.H.; Xu, X.L.; Liu, C.; et al. The Wolfiporia cocos genome and transcriptome shed light on the formation of its edible and medicinal sclerotium. Genom. Proteom. Bioinform. 2020, 18, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Yang, Y.; Bi, G.Q.; Nelson, D.; Hu, S.; Makunga, N.P.; Yu, B.; Liu, X.; Li, X.H.; Hu, X.B. Genomic and transcriptomic insight of giant sclerotium formation of wood-decay fungi. Front. Microbiol. 2021, 12, 746121. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Min, B.; Han, J.G.; Park, H.; Baek, S.; Jeong, S.; Choi, I.G. Draft genome sequence of the reference strain of the Korean medicinal mushroom Wolfiporia cocos KMCC03342. Mycobiology 2022, 50, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, L.F.; Chen, M.T.; Xu, Z.Y. First report on the regulation and function of carbon metabolism during large sclerotia formation in medicinal fungus Wolfiporia cocos. Fungal Genet. Biol. 2023, 166, 103793. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Wang, Q.; Dong, C.H. Distinguishing homokaryons and heterokaryons in medicinal polypore mushroom Wolfiporia cocos (Agaricomycetes) based on cultural and genetic characteristics. Front. Microbiol. 2021, 11, 596715. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Wang, Q.; Dong, C.H. Bipolar system of sexual incompatibility and heterothallic life cycle in the basidiomycetes Pachyma hoelen Fr. (Fuling). Mycologia 2022, 114, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Dong, C.H. A new type of homokaryotic strain of Wolfiporia hoelen with indistinguishable phenotypes from the parent strains. Mycosystema 2022, 41, 1279–1292. [Google Scholar] [CrossRef]
- Li, S.J.; Dong, C.H. Mating and crossbreeding systems of Wolfiporia hoelen. J. Fungal Res. 2023, 21, 143–150. [Google Scholar] [CrossRef]
- Jiang, X.Y. Study on the Function of PC-5P-88292_34 and Its Target Gene CsMYB4 in Tea Tree Response to Pathogen of Water Chestnut Stem Blight. Master’s Thesis, Guizhou University, Guiyang, China, 2023. [Google Scholar] [CrossRef]
- Yan, S.P. Evaluation of Germplasm Resources and Physiological and Molecular Basis of Reaumuria soongorica in Response to Salt Stress. Ph.D. Thesis, Gansu Agricultural University, Lanzhou, China, 2022. [Google Scholar] [CrossRef]
- Wang, L. Molecular Mechanisms of Lead Tolerance and Accumulation in Tartary Buckwheat (Fagopyrum tataricum). Ph.D. Thesis, Northwest A&F University, Xi’an, China, 2020. [Google Scholar] [CrossRef]
- Wang, Q. Transcriptomics Analysis of Paeonia lactiflora in Response to Drought Stress and Functional Study of Related Transcription Factors. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2020. [Google Scholar] [CrossRef]
- Li, Y. Maize-Mediated Interaction between Fusarium graminearum and Antagonistic Bacteria. Master’s Thesis, Henan University of Technology, Zhengzhou, China, 2018. [Google Scholar]
- Lu, F.Z. Mechanism Characterization of the ZmPP2C26 Gene Alternative Splicing Variants Negatively Regulate Drought Tolerance in Maize. Ph.D. Thesis, Sichuan Agricultural University, Chengdu, China, 2023. [Google Scholar] [CrossRef]
- Yin, Y.Z. Study on the Function of ZmJMJ20 in Cadmium Tolerance of Maize. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2023. [Google Scholar] [CrossRef]
- Zhang, S.P. Comparative Transcriptomics Provide Insights into the Pathogenic Immune Response Mechanism of Leaf Brown Spot in Weeping Forsythia. Master’s Thesis, Henan University, Zhengzhou, China, 2023. [Google Scholar] [CrossRef]
- Han, Y.Q.; Wu, X.X.; Jiang, S.M.; Wei, A.Q.; Tian, N.N.; Wang, H. Exploring LRR-RLK genes involved in resistance of foxtail millet to Sclerospora graminicola based on the weighted gene co-expression network analysis. Acta Phytopathol. Sin. 2024. [Google Scholar] [CrossRef]
- Yu, Z.F. Study on Response Mechanism of ‘Wanfeng’ Almonds to Low Temperature Stress Based on Transcriptional and Metabolic Analysis. Ph.D. Thesis, Xinjiang Agricultural University, Urumqi, China, 2023. [Google Scholar] [CrossRef]
- Jiang, Y.L. Effects of Cold Stress on Adaptability of Oat. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2023. [Google Scholar] [CrossRef]
- Pan, N.H.; Guan, L.R.; Li, H.L.; Zhao, J.; Li, C.Y.; Wang, Y.Y.; Xie, Y. Screening of blast resistance-related candidate genes in rice landrace ‘Acuce’. Mol. Plant Breed. 2024. Available online: https://link.cnki.net/urlid/46.1068.S.20240313.1456.010 (accessed on 20 August 2023).
- Zuo, Y.Y. Evaluation of Mulberry Anthracnose Resistance and Mining of Resistance Related Genes. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2023. [Google Scholar] [CrossRef]
- Wang, S.C. Physiological and Molecular Mechanisms of Exogenous Thiamine Alleviating Drought Stress in Pterocarya stenoptera. Master’s Thesis, Henan Agricultural University, Zhengzhou, China, 2023. [Google Scholar] [CrossRef]
- Zhang, W. Cadmium Resistance and Regulatory Pathway of BpTT2-Overexpressed Broussonetia papyrifera. Ph.D. Thesis, Central South University of Forestry & Technology, Changsha, China, 2022. [Google Scholar] [CrossRef]
- Guo, X.L. Effects of TaGSK3 on Wheat Development and Stress Adaptation. Ph.D. Thesis, Northwest A&F University, Xi’an, China, 2023. [Google Scholar] [CrossRef]
- Zhao, H.C. Analysis of Genome and Mycoparasitic Mechanism of the Mycoparasite Coinithyrium minitans. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2020. [Google Scholar] [CrossRef]
- Wang, B. Drought Tolerance-Related Association Mapping and Natural Varitions of two MYB Genes in Cassava (Manihot esculenta Cranz). Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2018. [Google Scholar]
- Liu, Y. Functional Analysis of Apple bHLH137-like Gene in Drought and Salt Stress. Master’s Thesis, Northwest A&F University, Xi’an, China, 2022. [Google Scholar] [CrossRef]
- Qasim, M.U. Identification of Resistance Genes and Pathways Involved in Resistance for Sclerotinia Stem Rot in Brassica napus. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2020. [Google Scholar] [CrossRef]
- Jiang, S.Q. Meta analysis of Core Response Genes in Maize Induced by Abiotic and Biotic Stress. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2020. [Google Scholar] [CrossRef]
- Guo, Z.H. Identification of Cold Tolerance Associated Areas and Candidate Genes at the Booting Stage of Rice in Cold Region. Ph.D. Thesis, South China Agricultural University, Guangzhou, China, 2019. [Google Scholar] [CrossRef]
- Zhou, D. RNA-seq Analysis of Potato Leaves during the Early Stage of Phytophthora infestans Infection. Master’s Thesis, Northwest A&F University, Xi’an, China, 2018. [Google Scholar]
- Li, H.L. The Transcriptome Analysis of Apple Resistance to Diplocarpon mali. Master’s Thesis, Northwest A&F University, Xi’an, China, 2017. [Google Scholar] [CrossRef]
- Dong, H.H. Research of Brazilian Resistance Differences to Fusarium oxysporum f.sp. cubense Race 1 and Race 4. Ph.D. Thesis, South China Agricultural University, Guangzhou, China, 2019. [Google Scholar] [CrossRef]
- Wang, X. The Molecular Basis of Salicylic Acid Regulating Alfalfa Response to Low Temperature. Ph.D. Thesis, Gansu Agricultural University, Lanzhou, China, 2023. [Google Scholar] [CrossRef]
- Qiu, T. Study on Salt Tolerance and Molecular Regulation Mechanism of Allotriploid Populus cathayana. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2022. [Google Scholar] [CrossRef]
- Wei, C.M. Physiological Response and Transcriptome Analysis of Echeveria to Low Temperature Stress. Master’s Thesis, Guizhou University, Guiyang, China, 2023. [Google Scholar] [CrossRef]
- Chen, X.J. The Mechanism of Ascorbic Acid Regulating the Adaptation of Tomato Seedlings to Salt Stress. Ph.D. Thesis, Shihezi University, Shihezi, China, 2023. [Google Scholar] [CrossRef]
- Li, W.Y. Study on Resistance Response Mechanism of Sansevieria trifasciata var. laurentii to Indoor Benzene. Master’s Thesis, Shandong Jianzhu University, Jinan, China, 2023. [Google Scholar] [CrossRef]
- Zhu, Y.X. Alleviative Effects and Mechanisms of Silicon on Salt Stress-Induced Damage in Cucumber Seedlings. Ph.D. Thesis, Northwest A&F University, Xi’an, China, 2016. [Google Scholar]
- Jiang, L. Study on Panicle Proteomes of Temperature of Rice in Response to High Nighttime Temperature Stress at Early Milky Stage. Master’s Thesis, Jiangxi Agricultural University, Nanchang, China, 2019. [Google Scholar] [CrossRef]
- Fan, Y.L. Physiological and Molecular Response Mechanism of Potato Tubers to Fusarium sulphureum and the Functional Study of StPALs. Ph.D. Thesis, Gansu Agricultural University, Lanzhou, China, 2022. [Google Scholar] [CrossRef]
- Luo, W. Identification and Mechanism Analysis of Soda Tolerance of Sweet Sorghum Germplasm Resources. Master’s Thesis, Inner Mongolia Minzu University, Tongliao, China, 2023. [Google Scholar] [CrossRef]
- Xie, Y.J. Analysis of Differentially Expressed Genes and Construction of Gene Co-Expression Network in Rice under Abiotic Stresses. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2018. [Google Scholar]
- Li, S.J.; Dong, C.H. Protoplast monokaryogenesis and cross of the homokaryotic strains of Wolfiporia hoelen. Mycosystema 2023, 42, 1258–1272. [Google Scholar] [CrossRef]
- Walker, B.J.; Abeel, T.; Shea, T.; Priest, M.; Abouelliel, A.; Sakthikumar, S.; Cuomo, C.A.; Zeng, Q.D.; Wortman, J.; Young, S.K.; et al. Pilon: An integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS ONE 2014, 9, e112963. [Google Scholar] [CrossRef]
- Chen, C.J.; Wu, Y.; Li, J.W.; Wang, X.; Zeng, Z.H.; Xu, J.; Liu, Y.L.; Feng, J.T.; Chen, H.; He, Y.H.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Marcais, G.; Kingsford, C. A fast, lock-free approach for efficient parallel counting of occurrences of k-mers. Bioinformatics 2011, 27, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Ranallo-Benavidez, T.R.; Jaron, K.S.; Schatz, M.C. GenomeScope 2.0 and smudgeplot for reference-free profiling of polyploid genomes. Nat. Commun. 2020, 11, 1432. [Google Scholar] [CrossRef] [PubMed]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V.; Zdobnov, E.M. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 31, 3210–3212. [Google Scholar] [CrossRef]
- Meyne, J.; Ratliff, R.L.; Moyzis, R.K. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc. Natl. Acad. Sci. USA 1989, 86, 7046–7053. [Google Scholar] [CrossRef]
- Wang, M.; Meng, G.L.; Yang, Y.; Wang, X.F.; Xie, R.; Dong, C.H. Telomere-to-telomere genome assembly of Tibetan medicinal mushroom Ganoderma leucocontextum and the first Copia centromeric retrotransposon in macro-fungi genome. J. Fungi 2024, 10, 15. [Google Scholar] [CrossRef]
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate long-read alignment with burrows-wheeler transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R.; 1000 Genome Project Data Processing Subgroup. The sequence alignment/map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef] [PubMed]
- Tarasov, A. Sambamba: Fast processing of NGS alignment formats. Bioinformatics 2015, 31, 2032–2034. [Google Scholar] [CrossRef] [PubMed]



| L7 | Pr2C | PrT | SS20 | |
|---|---|---|---|---|
| Sequencing strategy | Illumina NovaSeq 6000 and Oxford Nanopore | Illumina NovaSeq 6000 and Oxford Nanopore | Illumina NovaSeq 6000 and Oxford Nanopore | Illumina Novaseq 6000 and PacBio |
| Genome size (Mb) | 59.68 | 58.40 | 57.64 | 64.44 |
| Number of Contigs | 28 | 27 | 17 | 78 |
| Number of Chromosomes | 15 (OLC) | - | - | 14 (HI-C) |
| N50 of Contigs (kb) | 4114.4 | 4228.1 | 4105.74 | 3760 |
| Max Contig (kb) | 5459.1 | 5572.1 | 6050.4 | 5.29 Mb |
| Anchored to chromosome (Mb) | 57.88 | - | - | 58.26 |
| Number of protein-coding genes | 10,421 | 11,085 | 10,749 | 10,567 |
| Average gene length (bp) | 2907.02 | 2416.16 | 2605.85 | 2004 |
| Percentage of repeat sequences (%) | 44.2 | 43.47 | 43.68 | |
| Mapping rate of Illumina data (%) | 99.83 | 94.36 | 89.04 | |
| Genome coverage (%) | 99.94 | 99.90 | 99.93 | |
| GC content (%) | 52.07 | 52.03 | 52.04 | 50.15 |
| Complete BUSCO (%) | 97.9 | 97.9 | 97.9 | 97.36 |
| Reference | This study | This study | This study | [8] |
| GO Term and Related Response Biological Process | Reference |
|---|---|
| GO: 0003855 3-dehydroquinate dehydratase activity | |
| tea tree response to Didymella bellidis infection | [50] |
| Reaumuria soongorica response to low and high salt stress | [51] |
| Fagopyrum tataricum response to lead stress | [52] |
| Paeonia lactiflora response to drought stress | [53] |
| Pseudomonas fluorescens response to Fusarium graminearum infection | [54] |
| GO: 0004674 protein serinethreonine kinase activity | |
| maize response to drought stress | [55] |
| maize response to cadmium stress | [56] |
| Forsythia suspensa response to Alternaria alternata infection | [57] |
| foxtail millet response to Sclerospora graminicola infection | [58] |
| Prunus dulcis response to low-temperature stress | [59] |
| oat response to cold stress | [60] |
| rice response to Megnaporthe oryzae infection | [61] |
| mulberry response to Colletotrichum infection | [62] |
| Pterocarya stenoptera response to drought stress | [63] |
| GO: 0004764 shikimate 3-dehydrogenase(NADP+) activity | |
| tea tree response to Didymella bellidis infection | [50] |
| Reaumuria soongorica response to low and high salt stress | [51] |
| Fagopyrum tataricum response to lead stress | [52] |
| Paeonia lactiflora response to drought stress | [53] |
| Broussonetia paperifera response to cadmium stress | [64] |
| GO: 0009423 chorismate biosynthetic process | |
| Triticum aestivum response to drought and high-temperature stress | [65] |
| Coinithyrium minitans response to Scelrotinia infection | [66] |
| Manihot esculenta response to drought stress | [67] |
| Pseudomonas fluorescens response to Fusarium graminearum infection | [54] |
| GO: 0031262 Ndc80 complex | |
| Malus domestica response to drought and salt stress | [68] |
| GO: 0009073 aromatic amino acid family biosynthetic process | |
| maize response to cadmium stress | [56] |
| mulberry response to Colletotrichum infection | [62] |
| Broussonetia paperifera response to cadmium stress | [64] |
| Triticum aestivum response to drought and high-temperature stress | [65] |
| Coinithyrium minitans response to Scelrotinia infection | [66] |
| Brassica napus resist response to Sclerotinia sclerotiorum infection | [69] |
| maize response to biotic and abiotic stress | [70] |
| rice response to cold stress | [71] |
| potato response to Phytophthora infestans infection | [72] |
| apple response to Diplocarpon mali infection | [73] |
| Musa response to Fusarium oxysporum f.sp. cubense | [74] |
| GO: 0043167 ion binding | |
| maize response to drought stress | [55] |
| maize response to cadmium stress | [56] |
| foxtail millet response to Sclerospora graminicola infection | [58] |
| mulberry response to Colletotrichum infection | [62] |
| Medicago sativa response to low-temperature stress | [75] |
| Populus cathayana response to salt stress | [76] |
| Echeveria response to low-temperature stress | [77] |
| Solanum lycopersicum response to salt stress | [78] |
| Sansevieria trifasciata var. laurentii response to benzene stress | [79] |
| GO: 0043650 dicarboxylic acid biosynthetic process | |
| mulberry response to Colletotrichum infection | [62] |
| cucumber response to salt stress | [80] |
| rice response to high nighttime temperature stress | [81] |
| Solanum tuberosum response to Fusarium sulphureum infection | [82] |
| GO: 0045261 proton-transporting ATP synthase | |
| tea tree response to Didymella bellidis infection | [50] |
| Brassica napus resist response to Sclerotinia sclerotiorum infection | [69] |
| sweet sorghum response to soda stress | [83] |
| rice response to high-temperature stress | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Li, B.; Guo, S. Chromosome-Level Assembly Reveals a Fifteen-Chromosome Aneuploid Genome and Environmental Adaptation Strategy of Chinese Traditional Medical Fungus Wolfiporia hoelen. Int. J. Mol. Sci. 2024, 25, 8786. https://doi.org/10.3390/ijms25168786
Li S, Li B, Guo S. Chromosome-Level Assembly Reveals a Fifteen-Chromosome Aneuploid Genome and Environmental Adaptation Strategy of Chinese Traditional Medical Fungus Wolfiporia hoelen. International Journal of Molecular Sciences. 2024; 25(16):8786. https://doi.org/10.3390/ijms25168786
Chicago/Turabian StyleLi, Shoujian, Bing Li, and Shunxing Guo. 2024. "Chromosome-Level Assembly Reveals a Fifteen-Chromosome Aneuploid Genome and Environmental Adaptation Strategy of Chinese Traditional Medical Fungus Wolfiporia hoelen" International Journal of Molecular Sciences 25, no. 16: 8786. https://doi.org/10.3390/ijms25168786







