Contribution of Keratinocytes in Skin Cancer Initiation and Progression
Abstract
1. Introduction
2. Skin Architecture
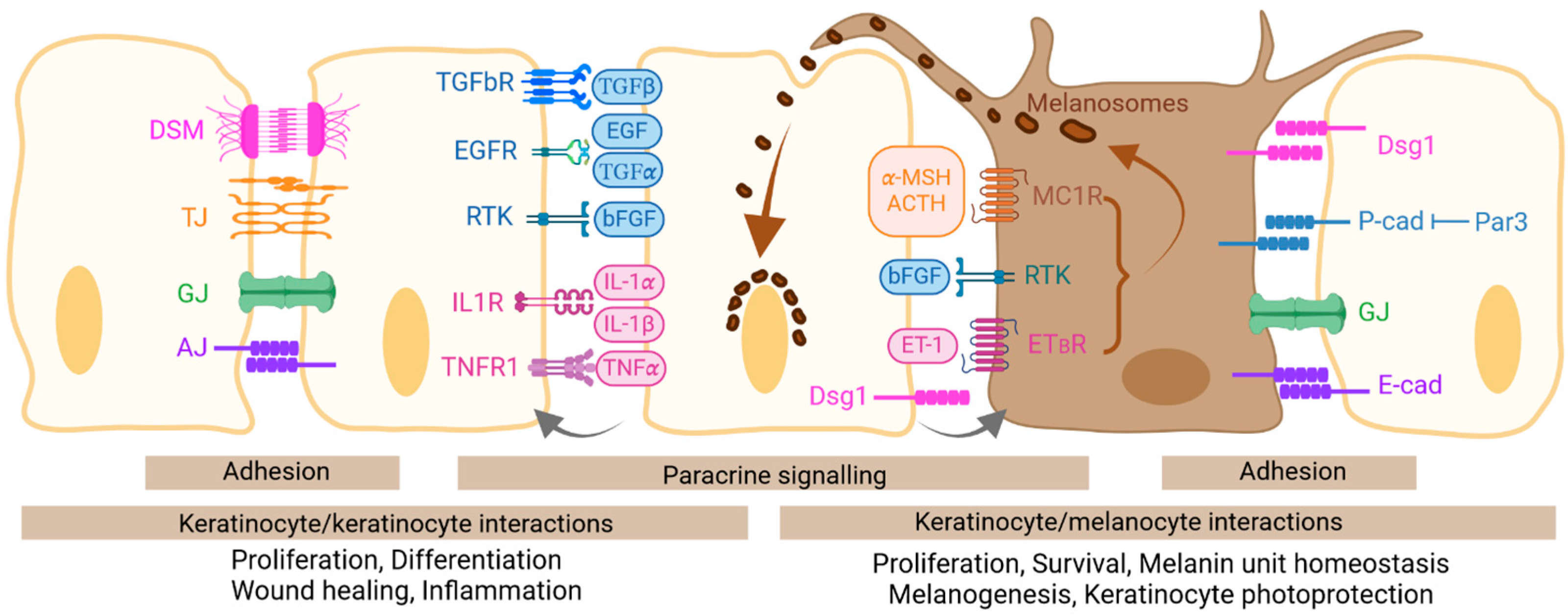
3. Keratinocyte Interactions with Their Neighboring Keratinocytes and Melanocytes
3.1. Interactions via Adhesion
3.2. Interactions via Notch Signaling
3.3. Interactions via Paracrine Signaling
4. Role of Keratinocytes in Non-Melanoma Skin Cancers
4.1. Basal Cell Carcinoma
4.2. Squamous Cell Carcinoma
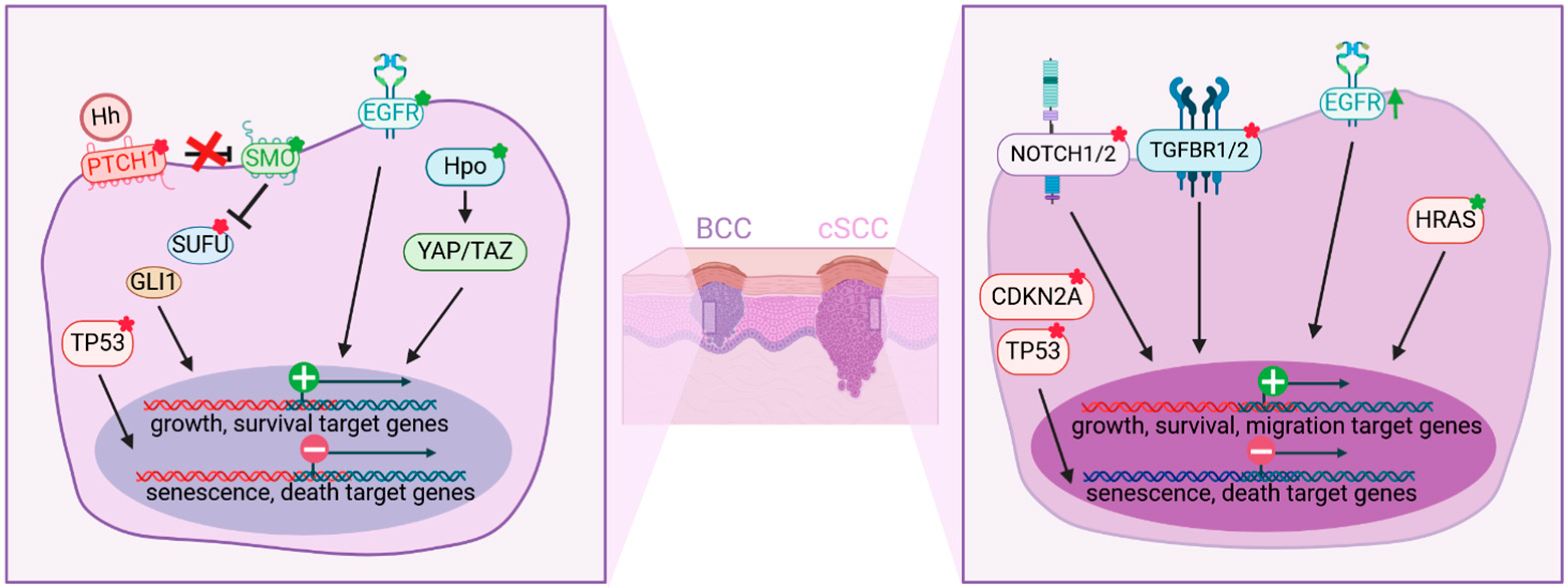
4.3. Epidermal Microenvironment of BCC and cSCC
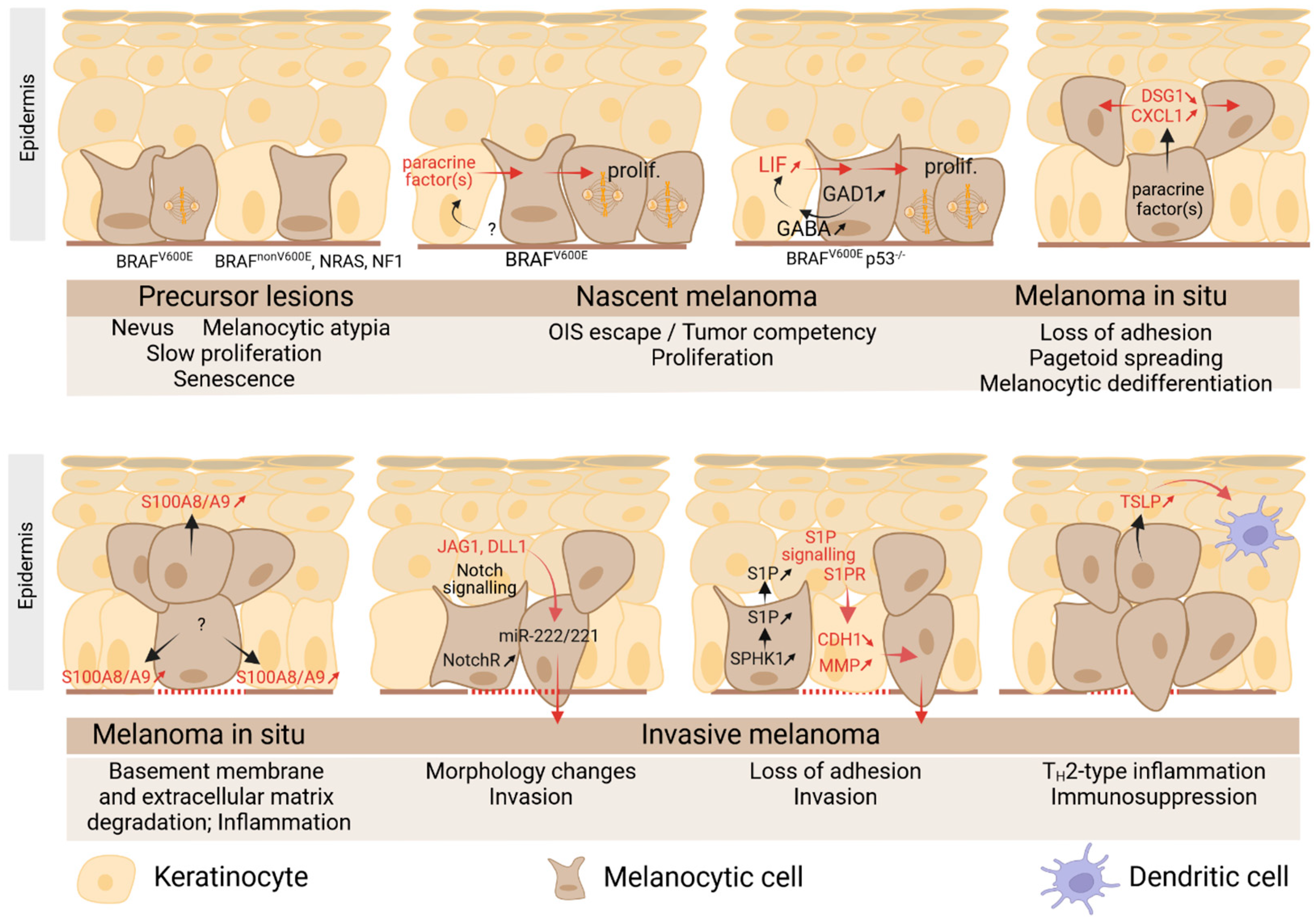
5. Role of Keratinocytes in Primary Cutaneous Melanoma
5.1. Primary Melanoma
5.2. Melanoma Induces Deep Changes in the Architectural Features of Epidermis
5.3. Role of Keratinocytes in Melanoma Initiation
5.4. Role of Keratinocytes in Melanoma Early Pagetoid Movement
5.5. Role of Keratinocytes in Melanoma Invasion/Progression
5.6. Role of Keratinocytes in Melanoma-Associated Inflammation
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Bouwstra, J.A.; Nădăban, A.; Bras, W.; McCabe, C.; Bunge, A.; Gooris, G.S. The Skin Barrier: An Extraordinary Interface with an Exceptional Lipid Organization. Prog. Lipid Res. 2023, 92, 101252. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.-Z.; Man, X.-Y. Biology of Melanocytes in Mammals. Front. Cell Dev. Biol. 2023, 11, 1309557. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.T.; Fisher, D.E. MITF and UV Responses in Skin: From Pigmentation to Addiction. Pigment. Cell Melanoma Res. 2019, 32, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Shain, A.H.; Bastian, B.C. From Melanocytes to Melanomas. Nat. Rev. Cancer 2016, 16, 345–358. [Google Scholar] [CrossRef] [PubMed]
- D’Arcy, C.; Kiel, C. Cell Adhesion Molecules in Normal Skin and Melanoma. Biomolecules 2021, 11, 1213. [Google Scholar] [CrossRef] [PubMed]
- Green, K.J.; Jaiganesh, A.; Broussard, J.A. Desmosomes: Essential Contributors to an Integrated Intercellular Junction Network. F1000Research 2019, 8, F1000 Faculty Rev-2150. [Google Scholar] [CrossRef] [PubMed]
- Simpson, C.L.; Patel, D.M.; Green, K.J. Deconstructing the Skin: Cytoarchitectural Determinants of Epidermal Morphogenesis. Nat. Rev. Mol. Cell Biol. 2011, 12, 565–580. [Google Scholar] [CrossRef]
- Zijl, S.; Salameti, V.; Louis, B.; Negri, V.A.; Watt, F.M. Dynamic Regulation of Human Epidermal Differentiation by Adhesive and Mechanical Forces. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2022; Volume 150, pp. 129–148. ISBN 978-0-12-820155-8. [Google Scholar]
- Green, K.J.; Niessen, C.M.; Rübsam, M.; Perez White, B.E.; Broussard, J.A. The Desmosome-Keratin Scaffold Integrates ErbB Family and Mechanical Signaling to Polarize Epidermal Structure and Function. Front. Cell Dev. Biol. 2022, 10, 903696. [Google Scholar] [CrossRef] [PubMed]
- Najor, N.A. Desmosomes in Human Disease. Annu. Rev. Pathol. 2018, 13, 51–70. [Google Scholar] [CrossRef]
- Hegazy, M.; Perl, A.L.; Svoboda, S.A.; Green, K.J. Desmosomal Cadherins in Health and Disease. Annu. Rev. Pathol. 2022, 17, 47–72. [Google Scholar] [CrossRef]
- Getsios, S.; Simpson, C.L.; Kojima, S.; Harmon, R.; Sheu, L.J.; Dusek, R.L.; Cornwell, M.; Green, K.J. Desmoglein 1-Dependent Suppression of EGFR Signaling Promotes Epidermal Differentiation and Morphogenesis. J. Cell Biol. 2009, 185, 1243–1258. [Google Scholar] [CrossRef] [PubMed]
- Rehman, A.; Cai, Y.; Hünefeld, C.; Jedličková, H.; Huang, Y.; Teck Teh, M.; Sharif Ahmad, U.; Uttagomol, J.; Wang, Y.; Kang, A.; et al. The Desmosomal Cadherin Desmoglein-3 Acts as a Keratinocyte Anti-Stress Protein via Suppression of P53. Cell Death Dis. 2019, 10, 750. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vega, L.; O’Shaughnessy, E.M.; Albuloushi, A.; Martin, P.E. Connexins and the Epithelial Tissue Barrier: A Focus on Connexin 26. Biology 2021, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Yasarbas, S.S.; Inal, E.; Yildirim, M.A.; Dubrac, S.; Lamartine, J.; Mese, G. Connexins in Epidermal Health and Diseases: Insights into Their Mutations, Implications, and Therapeutic Solutions. Front. Physiol. 2024, 15, 1346971. [Google Scholar] [CrossRef] [PubMed]
- Brissette, J.L.; Kumar, N.M.; Gilula, N.B.; Hall, J.E.; Dotto, G.P. Switch in Gap Junction Protein Expression Is Associated with Selective Changes in Junctional Permeability during Keratinocyte Differentiation. Proc. Natl. Acad. Sci. USA 1994, 91, 6453–6457. [Google Scholar] [CrossRef] [PubMed]
- Bäsler, K.; Bergmann, S.; Heisig, M.; Naegel, A.; Zorn-Kruppa, M.; Brandner, J.M. The Role of Tight Junctions in Skin Barrier Function and Dermal Absorption. J. Control. Release Off. J. Control. Release Soc. 2016, 242, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Brandner, J.M.; Zorn-Kruppa, M.; Yoshida, T.; Moll, I.; Beck, L.A.; De Benedetto, A. Epidermal Tight Junctions in Health and Disease. Tissue Barriers 2015, 3, e974451. [Google Scholar] [CrossRef] [PubMed]
- Rachow, S.; Zorn-Kruppa, M.; Ohnemus, U.; Kirschner, N.; Vidal-y-Sy, S.; von den Driesch, P.; Börnchen, C.; Eberle, J.; Mildner, M.; Vettorazzi, E.; et al. Occludin Is Involved in Adhesion, Apoptosis, Differentiation and Ca2+-Homeostasis of Human Keratinocytes: Implications for Tumorigenesis. PLoS ONE 2013, 8, e55116. [Google Scholar] [CrossRef] [PubMed]
- Haass, N.K.; Smalley, K.S.M.; Herlyn, M. The Role of Altered Cell-Cell Communication in Melanoma Progression. J. Mol. Histol. 2004, 35, 309–318. [Google Scholar] [CrossRef]
- Tang, A.; Eller, M.S.; Hara, M.; Yaar, M.; Hirohashi, S.; Gilchrest, B.A. E-Cadherin Is the Major Mediator of Human Melanocyte Adhesion to Keratinocytes in Vitro. J. Cell Sci. 1994, 107 Pt 4, 983–992. [Google Scholar] [CrossRef]
- Haass, N.K.; Herlyn, M. Normal Human Melanocyte Homeostasis as a Paradigm for Understanding Melanoma. J. Investig. Dermatol. Symp. Proc. 2005, 10, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Hsu, M.Y.; Meier, F.E.; Nesbit, M.; Hsu, J.Y.; Van Belle, P.; Elder, D.E.; Herlyn, M. E-Cadherin Expression in Melanoma Cells Restores Keratinocyte-Mediated Growth Control and down-Regulates Expression of Invasion-Related Adhesion Receptors. Am. J. Pathol. 2000, 156, 1515–1525. [Google Scholar] [CrossRef] [PubMed]
- Valyi-Nagy, I.T.; Hirka, G.; Jensen, P.J.; Shih, I.M.; Juhasz, I.; Herlyn, M. Undifferentiated Keratinocytes Control Growth, Morphology, and Antigen Expression of Normal Melanocytes through Cell-Cell Contact. Lab. Investig. J. Tech. Methods Pathol. 1993, 69, 152–159. [Google Scholar]
- Singh, S.K.; Baker, R.; Sikkink, S.K.; Nizard, C.; Schnebert, S.; Kurfurst, R.; Tobin, D.J. E-Cadherin Mediates Ultraviolet Radiation- and Calcium-Induced Melanin Transfer in Human Skin Cells. Exp. Dermatol. 2017, 26, 1125–1133. [Google Scholar] [CrossRef]
- Pauls, K.; Schön, M.; Kubitza, R.C.; Homey, B.; Wiesenborn, A.; Lehmann, P.; Ruzicka, T.; Parker, C.M.; Schön, M.P. Role of Integrin alphaE(CD103)Beta7 for Tissue-Specific Epidermal Localization of CD8+ T Lymphocytes. J. Investig. Dermatol. 2001, 117, 569–575. [Google Scholar] [CrossRef]
- Mescher, M.; Jeong, P.; Knapp, S.K.; Rübsam, M.; Saynisch, M.; Kranen, M.; Landsberg, J.; Schlaak, M.; Mauch, C.; Tüting, T.; et al. The Epidermal Polarity Protein Par3 Is a Non-Cell Autonomous Suppressor of Malignant Melanoma. J. Exp. Med. 2017, 214, 339–358. [Google Scholar] [CrossRef]
- Li, G.; Schaider, H.; Satyamoorthy, K.; Hanakawa, Y.; Hashimoto, K.; Herlyn, M. Downregulation of E-Cadherin and Desmoglein 1 by Autocrine Hepatocyte Growth Factor during Melanoma Development. Oncogene 2001, 20, 8125–8135. [Google Scholar] [CrossRef]
- Arnette, C.R.; Roth-Carter, Q.R.; Koetsier, J.L.; Broussard, J.A.; Burks, H.E.; Cheng, K.; Amadi, C.; Gerami, P.; Johnson, J.L.; Green, K.J. Keratinocyte Cadherin Desmoglein 1 Controls Melanocyte Behavior through Paracrine Signaling. Pigment Cell Melanoma Res. 2020, 33, 305–317. [Google Scholar] [CrossRef]
- Hsu, M.; Andl, T.; Li, G.; Meinkoth, J.L.; Herlyn, M. Cadherin Repertoire Determines Partner-Specific Gap Junctional Communication during Melanoma Progression. J. Cell Sci. 2000, 113 Pt 9, 1535–1542. [Google Scholar] [CrossRef]
- Masuda, M.; Usami, S.; Yamazaki, K.; Takumi, Y.; Shinkawa, H.; Kurashima, K.; Kunihiro, T.; Kanzaki, J. Connexin 26 Distribution in Gap Junctions between Melanocytes in the Human Vestibular Dark Cell Area. Anat. Rec. 2001, 262, 137–146. [Google Scholar] [CrossRef]
- Ableser, M.J.; Penuela, S.; Lee, J.; Shao, Q.; Laird, D.W. Connexin43 Reduces Melanoma Growth within a Keratinocyte Microenvironment and during Tumorigenesis in Vivo. J. Biol. Chem. 2014, 289, 1592–1603. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch Signaling Pathway: Architecture, Disease, and Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 95. [Google Scholar] [CrossRef]
- Gratton, R.; Tricarico, P.M.; Moltrasio, C.; Lima Estevão de Oliveira, A.S.; Brandão, L.; Marzano, A.V.; Zupin, L.; Crovella, S. Pleiotropic Role of Notch Signaling in Human Skin Diseases. Int. J. Mol. Sci. 2020, 21, 4214. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, A.; Talora, C.; Okuyama, R.; Nicolas, M.; Mammucari, C.; Oh, H.; Aster, J.C.; Krishna, S.; Metzger, D.; Chambon, P.; et al. Notch Signaling Is a Direct Determinant of Keratinocyte Growth Arrest and Entry into Differentiation. EMBO J. 2001, 20, 3427–3436. [Google Scholar] [CrossRef] [PubMed]
- Devgan, V.; Mammucari, C.; Millar, S.E.; Brisken, C.; Dotto, G.P. p21WAF1/Cip1 Is a Negative Transcriptional Regulator of Wnt4 Expression Downstream of Notch1 Activation. Genes Dev. 2005, 19, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- El-Serafi, A.T.; El-Serafi, I.; Steinvall, I.; Sjöberg, F.; Elmasry, M. A Systematic Review of Keratinocyte Secretions: A Regenerative Perspective. Int. J. Mol. Sci. 2022, 23, 7934. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Tsoi, L.C.; Billi, A.C.; Ward, N.L.; Harms, P.W.; Zeng, C.; Maverakis, E.; Kahlenberg, J.M.; Gudjonsson, J.E. Cytokinocytes: The Diverse Contribution of Keratinocytes to Immune Responses in Skin. JCI Insight 2020, 5, e142067. [Google Scholar] [CrossRef] [PubMed]
- Liarte, S.; Bernabé-García, Á.; Nicolás, F.J. Role of TGF-β in Skin Chronic Wounds: A Keratinocyte Perspective. Cells 2020, 9, 306. [Google Scholar] [CrossRef] [PubMed]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. Growth Factors and Cytokines in Wound Healing. Wound Repair Regen. Off. Publ. Wound Health Soc. Eur. Tissue Repair Soc. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Pincelli, C.; Marconi, A. Autocrine Nerve Growth Factor in Human Keratinocytes. J. Dermatol. Sci. 2000, 22, 71–79. [Google Scholar] [CrossRef]
- Raja, S.K.; Garcia, M.S.; Isseroff, R.R. Wound Re-Epithelialization: Modulating Keratinocyte Migration in Wound Healing. Front. Biosci. J. Virtual Libr. 2007, 12, 2849–2868. [Google Scholar] [CrossRef]
- Hernández-Quintero, M.; Kuri-Harcuch, W.; González Robles, A.; Castro-Muñozledo, F. Interleukin-6 Promotes Human Epidermal Keratinocyte Proliferation and Keratin Cytoskeleton Reorganization in Culture. Cell Tissue Res. 2006, 325, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Haass, N.K.; Smalley, K.S.M.; Li, L.; Herlyn, M. Adhesion, Migration and Communication in Melanocytes and Melanoma. Pigment Cell Res. 2005, 18, 150–159. [Google Scholar] [CrossRef]
- Upadhyay, P.R.; Ho, T.; Abdel-Malek, Z.A. Participation of Keratinocyte- and Fibroblast-Derived Factors in Melanocyte Homeostasis, the Response to UV, and Pigmentary Disorders. Pigment Cell Melanoma Res. 2021, 34, 762–776. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Fukunaga-Kalabis, M.; Herlyn, M. Crosstalk in Skin: Melanocytes, Keratinocytes, Stem Cells, and Melanoma. J. Cell Commun. Signal. 2016, 10, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Halaban, R.; Langdon, R.; Birchall, N.; Cuono, C.; Baird, A.; Scott, G.; Moellmann, G.; McGuire, J. Basic Fibroblast Growth Factor from Human Keratinocytes Is a Natural Mitogen for Melanocytes. J. Cell Biol. 1988, 107, 1611–1619. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Yada, Y.; Miyagishi, M. Endothelins Secreted from Human Keratinocytes Are Intrinsic Mitogens for Human Melanocytes. J. Biol. Chem. 1992, 267, 24675–24680. [Google Scholar] [CrossRef] [PubMed]
- Jamal, S.; Schneider, R.J. UV-Induction of Keratinocyte Endothelin-1 Downregulates E-Cadherin in Melanocytes and Melanoma Cells. J. Clin. Investig. 2002, 110, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Mangahas, C.R.; dela Cruz, G.V.; Schneider, R.J.; Jamal, S. Endothelin-1 Upregulates MCAM in Melanocytes. J. Investig. Dermatol. 2004, 123, 1135–1139. [Google Scholar] [CrossRef]
- Abdel-Malek, Z.; Swope, V.B.; Suzuki, I.; Akcali, C.; Harriger, M.D.; Boyce, S.T.; Urabe, K.; Hearing, V.J. Mitogenic and Melanogenic Stimulation of Normal Human Melanocytes by Melanotropic Peptides. Proc. Natl. Acad. Sci. USA 1995, 92, 1789–1793. [Google Scholar] [CrossRef]
- Bigliardi-Qi, M.; Sumanovski, L.T.; Büchner, S.; Rufli, T.; Bigliardi, P.L. Mu-Opiate Receptor and Beta-Endorphin Expression in Nerve Endings and Keratinocytes in Human Skin. Dermatology 2004, 209, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Kauser, S.; Schallreuter, K.U.; Thody, A.J.; Gummer, C.; Tobin, D.J. Regulation of Human Epidermal Melanocyte Biology by Beta-Endorphin. J. Investig. Dermatol. 2003, 120, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Hunt, G.; Donatien, P.D.; Lunec, J.; Todd, C.; Kyne, S.; Thody, A.J. Cultured Human Melanocytes Respond to MSH Peptides and ACTH. Pigment Cell Res. 1994, 7, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Kadekaro, A.L.; Leachman, S.; Kavanagh, R.J.; Swope, V.; Cassidy, P.; Supp, D.; Sartor, M.; Schwemberger, S.; Babcock, G.; Wakamatsu, K.; et al. Melanocortin 1 Receptor Genotype: An Important Determinant of the Damage Response of Melanocytes to Ultraviolet Radiation. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2010, 24, 3850–3860. [Google Scholar] [CrossRef]
- Moreiras, H.; Seabra, M.C.; Barral, D.C. Melanin Transfer in the Epidermis: The Pursuit of Skin Pigmentation Control Mechanisms. Int. J. Mol. Sci. 2021, 22, 4466. [Google Scholar] [CrossRef] [PubMed]
- Nanz, L.; Keim, U.; Katalinic, A.; Meyer, T.; Garbe, C.; Leiter, U. Epidemiology of Keratinocyte Skin Cancer with a Focus on Cutaneous Squamous Cell Carcinoma. Cancers 2024, 16, 606. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, G.; Karagiannis, T.; Palmer, J.B.; Lotya, J.; O’Neill, C.; Kisa, R.; Herrera, V.; Siegel, D.M. Incidence and Prevalence of Basal Cell Carcinoma (BCC) and Locally Advanced BCC (LABCC) in a Large Commercially Insured Population in the United States: A Retrospective Cohort Study. J. Am. Acad. Dermatol. 2016, 75, 957–966.e2. [Google Scholar] [CrossRef] [PubMed]
- Boeckmann, L.; Martens, M.C.; Emmert, S. Molecular Biology of Basal and Squamous Cell Carcinomas. Adv. Exp. Med. Biol. 2020, 1268, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Bansaccal, N.; Vieugue, P.; Sarate, R.; Song, Y.; Minguijon, E.; Miroshnikova, Y.A.; Zeuschner, D.; Collin, A.; Allard, J.; Engelman, D.; et al. The Extracellular Matrix Dictates Regional Competence for Tumour Initiation. Nature 2023, 623, 828–835. [Google Scholar] [CrossRef]
- Dika, E.; Scarfì, F.; Ferracin, M.; Broseghini, E.; Marcelli, E.; Bortolani, B.; Campione, E.; Riefolo, M.; Ricci, C.; Lambertini, M. Basal Cell Carcinoma: A Comprehensive Review. Int. J. Mol. Sci. 2020, 21, 5572. [Google Scholar] [CrossRef]
- Abe, Y.; Tanaka, N. Roles of the Hedgehog Signaling Pathway in Epidermal and Hair Follicle Development, Homeostasis, and Cancer. J. Dev. Biol. 2017, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Skoda, A.M.; Simovic, D.; Karin, V.; Kardum, V.; Vranic, S.; Serman, L. The Role of the Hedgehog Signaling Pathway in Cancer: A Comprehensive Review. Bosn. J. Basic Med. Sci. 2018, 18, 8–20. [Google Scholar] [CrossRef]
- Peris, K.; Fargnoli, M.C.; Kaufmann, R.; Arenberger, P.; Bastholt, L.; Seguin, N.B.; Bataille, V.; Brochez, L.; Del Marmol, V.; Dummer, R.; et al. European Consensus-Based Interdisciplinary Guideline for Diagnosis and Treatment of Basal Cell Carcinoma-Update 2023. Eur. J. Cancer 2023, 192, 113254. [Google Scholar] [CrossRef] [PubMed]
- Hahn, H.; Wicking, C.; Zaphiropoulous, P.G.; Gailani, M.R.; Shanley, S.; Chidambaram, A.; Vorechovsky, I.; Holmberg, E.; Unden, A.B.; Gillies, S.; et al. Mutations of the Human Homolog of Drosophila Patched in the Nevoid Basal Cell Carcinoma Syndrome. Cell 1996, 85, 841–851. [Google Scholar] [CrossRef]
- Gailani, M.R.; Ståhle-Bäckdahl, M.; Leffell, D.J.; Glynn, M.; Zaphiropoulos, P.G.; Pressman, C.; Undén, A.B.; Dean, M.; Brash, D.E.; Bale, A.E.; et al. The Role of the Human Homologue of Drosophila Patched in Sporadic Basal Cell Carcinomas. Nat. Genet. 1996, 14, 78–81. [Google Scholar] [CrossRef]
- Bonilla, X.; Parmentier, L.; King, B.; Bezrukov, F.; Kaya, G.; Zoete, V.; Seplyarskiy, V.B.; Sharpe, H.J.; McKee, T.; Letourneau, A.; et al. Genomic Analysis Identifies New Drivers and Progression Pathways in Skin Basal Cell Carcinoma. Nat. Genet. 2016, 48, 398–406. [Google Scholar] [CrossRef]
- Jayaraman, S.S.; Rayhan, D.J.; Hazany, S.; Kolodney, M.S. Mutational Landscape of Basal Cell Carcinomas by Whole-Exome Sequencing. J. Investig. Dermatol. 2014, 134, 213–220. [Google Scholar] [CrossRef]
- Pellegrini, C.; Maturo, M.G.; Di Nardo, L.; Ciciarelli, V.; Gutiérrez García-Rodrigo, C.; Fargnoli, M.C. Understanding the Molecular Genetics of Basal Cell Carcinoma. Int. J. Mol. Sci. 2017, 18, 2485. [Google Scholar] [CrossRef] [PubMed]
- Durante, G.; Comito, F.; Lambertini, M.; Broseghini, E.; Dika, E.; Ferracin, M. Non-Coding RNA Dysregulation in Skin Cancers. Essays Biochem. 2021, 65, 641–655. [Google Scholar] [CrossRef]
- Kashyap, M.P.; Sinha, R.; Mukhtar, M.S.; Athar, M. Epigenetic Regulation in the Pathogenesis of Non-Melanoma Skin Cancer. Semin. Cancer Biol. 2022, 83, 36–56. [Google Scholar] [CrossRef]
- Natarelli, N.; Boby, A.; Aflatooni, S.; Tran, J.T.; Diaz, M.J.; Taneja, K.; Forouzandeh, M. Regulatory miRNAs and lncRNAs in Skin Cancer: A Narrative Review. Life 2023, 13, 1696. [Google Scholar] [CrossRef]
- Sonkoly, E.; Lovén, J.; Xu, N.; Meisgen, F.; Wei, T.; Brodin, P.; Jaks, V.; Kasper, M.; Shimokawa, T.; Harada, M.; et al. MicroRNA-203 Functions as a Tumor Suppressor in Basal Cell Carcinoma. Oncogenesis 2012, 1, e3. [Google Scholar] [CrossRef]
- Heffelfinger, C.; Ouyang, Z.; Engberg, A.; Leffell, D.J.; Hanlon, A.M.; Gordon, P.B.; Zheng, W.; Zhao, H.; Snyder, M.P.; Bale, A.E. Correlation of Global MicroRNA Expression With Basal Cell Carcinoma Subtype. G3 Genes Genomes Genet. 2012, 2, 279–286. [Google Scholar] [CrossRef]
- Fastner, S.; Rahman, H.; Gutierrez, J.; Shen, N.; Florell, S.R.; Florell, A.; Stubben, C.J.; Boucher, K.M.; Deacon, D.C.; Judson-Torres, R.L.; et al. MicroRNA Signatures Associated with Basal Cell Carcinoma Subtypes. JID Innov. Skin Sci. Mol. Popul. Health 2024, 4, 100286. [Google Scholar] [CrossRef]
- Tamas, T.; Baciut, M.; Nutu, A.; Bran, S.; Armencea, G.; Stoia, S.; Manea, A.; Crisan, L.; Opris, H.; Onisor, F.; et al. Is miRNA Regulation the Key to Controlling Non-Melanoma Skin Cancer Evolution? Genes 2021, 12, 1929. [Google Scholar] [CrossRef]
- Asplund, A.; Gry Björklund, M.; Sundquist, C.; Strömberg, S.; Edlund, K.; Ostman, A.; Nilsson, P.; Pontén, F.; Lundeberg, J. Expression Profiling of Microdissected Cell Populations Selected from Basal Cells in Normal Epidermis and Basal Cell Carcinoma. Br. J. Dermatol. 2008, 158, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Litvinov, I.V.; Xie, P.; Gunn, S.; Sasseville, D.; Lefrançois, P. The Transcriptional Landscape Analysis of Basal Cell Carcinomas Reveals Novel Signalling Pathways and Actionable Targets. Life Sci. Alliance 2021, 4, e202000651. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Dai, H.; Zhang, X.; Liu, S.; Lin, Y.; Somani, A.-K.; Xie, J.; Han, J. Distinct Transcriptomic Landscapes of Cutaneous Basal Cell Carcinomas and Squamous Cell Carcinomas. Genes Dis. 2021, 8, 181–192. [Google Scholar] [CrossRef]
- Atwood, S.X.; Sarin, K.Y.; Whitson, R.J.; Li, J.R.; Kim, G.; Rezaee, M.; Ally, M.S.; Kim, J.; Yao, C.; Chang, A.L.S.; et al. Smoothened Variants Explain the Majority of Drug Resistance in Basal Cell Carcinoma. Cancer Cell 2015, 27, 342–353. [Google Scholar] [CrossRef]
- Sharpe, H.J.; Pau, G.; Dijkgraaf, G.J.; Basset-Seguin, N.; Modrusan, Z.; Januario, T.; Tsui, V.; Durham, A.B.; Dlugosz, A.A.; Haverty, P.M.; et al. Genomic Analysis of Smoothened Inhibitor Resistance in Basal Cell Carcinoma. Cancer Cell 2015, 27, 327–341. [Google Scholar] [CrossRef]
- Jiang, R.; Fritz, M.; Que, S.K.T. Cutaneous Squamous Cell Carcinoma: An Updated Review. Cancers 2024, 16, 1800. [Google Scholar] [CrossRef] [PubMed]
- Winge, M.C.G.; Kellman, L.N.; Guo, K.; Tang, J.Y.; Swetter, S.M.; Aasi, S.Z.; Sarin, K.Y.; Chang, A.L.S.; Khavari, P.A. Advances in Cutaneous Squamous Cell Carcinoma. Nat. Rev. Cancer 2023, 23, 430–449. [Google Scholar] [CrossRef] [PubMed]
- Czarnecki, D.; Meehan, C.J.; Bruce, F.; Culjak, G. The Majority of Cutaneous Squamous Cell Carcinomas Arise in Actinic Keratoses. J. Cutan. Med. Surg. 2002, 6, 207–209. [Google Scholar] [CrossRef] [PubMed]
- Marks, R.; Rennie, G.; Selwood, T.S. Malignant Transformation of Solar Keratoses to Squamous Cell Carcinoma. Lancet 1988, 1, 795–797. [Google Scholar] [CrossRef]
- Burton, K.A.; Ashack, K.A.; Khachemoune, A. Cutaneous Squamous Cell Carcinoma: A Review of High-Risk and Metastatic Disease. Am. J. Clin. Dermatol. 2016, 17, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Cassarino, D.S.; Derienzo, D.P.; Barr, R.J. Cutaneous Squamous Cell Carcinoma: A Comprehensive Clinicopathologic Classification. Part One. J. Cutan. Pathol. 2006, 33, 191–206. [Google Scholar] [CrossRef]
- Pickering, C.R.; Zhou, J.H.; Lee, J.J.; Drummond, J.A.; Peng, S.A.; Saade, R.E.; Tsai, K.Y.; Curry, J.L.; Tetzlaff, M.T.; Lai, S.Y.; et al. Mutational Landscape of Aggressive Cutaneous Squamous Cell Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 6582–6592. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.; Kim, J.-I. Genetic Studies of Actinic Keratosis Development: Where Are We Now? Ann. Dermatol. 2023, 35, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Corchado-Cobos, R.; García-Sancha, N.; González-Sarmiento, R.; Pérez-Losada, J.; Cañueto, J. Cutaneous Squamous Cell Carcinoma: From Biology to Therapy. Int. J. Mol. Sci. 2020, 21, 2956. [Google Scholar] [CrossRef]
- Brash, D.E.; Rudolph, J.A.; Simon, J.A.; Lin, A.; McKenna, G.J.; Baden, H.P.; Halperin, A.J.; Pontén, J. A Role for Sunlight in Skin Cancer: UV-Induced P53 Mutations in Squamous Cell Carcinoma. Proc. Natl. Acad. Sci. USA 1991, 88, 10124–10128. [Google Scholar] [CrossRef]
- Ziegler, A.; Jonason, A.S.; Leffell, D.J.; Simon, J.A.; Sharma, H.W.; Kimmelman, J.; Remington, L.; Jacks, T.; Brash, D.E. Sunburn and P53 in the Onset of Skin Cancer. Nature 1994, 372, 773–776. [Google Scholar] [CrossRef]
- Durinck, S.; Ho, C.; Wang, N.J.; Liao, W.; Jakkula, L.R.; Collisson, E.A.; Pons, J.; Chan, S.-W.; Lam, E.T.; Chu, C.; et al. Temporal Dissection of Tumorigenesis in Primary Cancers. Cancer Discov. 2011, 1, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Remenyik, E.; Zelterman, D.; Brash, D.E.; Wikonkal, N.M. Escaping the Stem Cell Compartment: Sustained UVB Exposure Allows P53-Mutant Keratinocytes to Colonize Adjacent Epidermal Proliferating Units without Incurring Additional Mutations. Proc. Natl. Acad. Sci. USA 2001, 98, 13948–13953. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.L.; Harwood, C.A.; Crook, T.; Cronin, J.G.; Kelsell, D.P.; Proby, C.M. p16INK4a and p14ARF Tumor Suppressor Genes Are Commonly Inactivated in Cutaneous Squamous Cell Carcinoma. J. Investig. Dermatol. 2004, 122, 1284–1292. [Google Scholar] [CrossRef] [PubMed]
- South, A.P.; Purdie, K.J.; Watt, S.A.; Haldenby, S.; den Breems, N.; Dimon, M.; Arron, S.T.; Kluk, M.J.; Aster, J.C.; McHugh, A.; et al. NOTCH1 Mutations Occur Early during Cutaneous Squamous Cell Carcinogenesis. J. Investig. Dermatol. 2014, 134, 2630–2638. [Google Scholar] [CrossRef]
- Wang, N.J.; Sanborn, Z.; Arnett, K.L.; Bayston, L.J.; Liao, W.; Proby, C.M.; Leigh, I.M.; Collisson, E.A.; Gordon, P.B.; Jakkula, L.; et al. Loss-of-Function Mutations in Notch Receptors in Cutaneous and Lung Squamous Cell Carcinoma. Proc. Natl. Acad. Sci. USA 2011, 108, 17761–17766. [Google Scholar] [CrossRef]
- Lefort, K.; Dotto, G.P. Notch Signaling in the Integrated Control of Keratinocyte Growth/Differentiation and Tumor Suppression. Semin. Cancer Biol. 2004, 14, 374–386. [Google Scholar] [CrossRef]
- Demehri, S.; Turkoz, A.; Kopan, R. Epidermal Notch1 Loss Promotes Skin Tumorigenesis by Impacting the Stromal Microenvironment. Cancer Cell 2009, 16, 55–66. [Google Scholar] [CrossRef]
- Pierceall, W.E.; Goldberg, L.H.; Tainsky, M.A.; Mukhopadhyay, T.; Ananthaswamy, H.N. Ras Gene Mutation and Amplification in Human Nonmelanoma Skin Cancers. Mol. Carcinog. 1991, 4, 196–202. [Google Scholar] [CrossRef]
- Oberholzer, P.A.; Kee, D.; Dziunycz, P.; Sucker, A.; Kamsukom, N.; Jones, R.; Roden, C.; Chalk, C.J.; Ardlie, K.; Palescandolo, E.; et al. RAS Mutations Are Associated with the Development of Cutaneous Squamous Cell Tumors in Patients Treated with RAF Inhibitors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 316–321. [Google Scholar] [CrossRef]
- Su, F.; Viros, A.; Milagre, C.; Trunzer, K.; Bollag, G.; Spleiss, O.; Reis-Filho, J.S.; Kong, X.; Koya, R.C.; Flaherty, K.T.; et al. RAS Mutations in Cutaneous Squamous-Cell Carcinomas in Patients Treated with BRAF Inhibitors. N. Engl. J. Med. 2012, 366, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Cammareri, P.; Rose, A.M.; Vincent, D.F.; Wang, J.; Nagano, A.; Libertini, S.; Ridgway, R.A.; Athineos, D.; Coates, P.J.; McHugh, A.; et al. Inactivation of TGFβ Receptors in Stem Cells Drives Cutaneous Squamous Cell Carcinoma. Nat. Commun. 2016, 7, 12493. [Google Scholar] [CrossRef] [PubMed]
- Inman, G.J.; Wang, J.; Nagano, A.; Alexandrov, L.B.; Purdie, K.J.; Taylor, R.G.; Sherwood, V.; Thomson, J.; Hogan, S.; Spender, L.C.; et al. The Genomic Landscape of Cutaneous SCC Reveals Drivers and a Novel Azathioprine Associated Mutational Signature. Nat. Commun. 2018, 9, 3667. [Google Scholar] [CrossRef] [PubMed]
- Cañueto, J.; Cardeñoso, E.; García, J.L.; Santos-Briz, Á.; Castellanos-Martín, A.; Fernández-López, E.; Blanco Gómez, A.; Pérez-Losada, J.; Román-Curto, C. Epidermal Growth Factor Receptor Expression Is Associated with Poor Outcome in Cutaneous Squamous Cell Carcinoma. Br. J. Dermatol. 2017, 176, 1279–1287. [Google Scholar] [CrossRef]
- Oda, K.; Matsuoka, Y.; Funahashi, A.; Kitano, H. A Comprehensive Pathway Map of Epidermal Growth Factor Receptor Signaling. Mol. Syst. Biol. 2005, 1, 2005.0010. [Google Scholar] [CrossRef] [PubMed]
- Harwood, C.A.; Proby, C.M.; Inman, G.J.; Leigh, I.M. The Promise of Genomics and the Development of Targeted Therapies for Cutaneous Squamous Cell Carcinoma. Acta Derm. Venereol. 2016, 96, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Stratigos, A.J.; Garbe, C.; Dessinioti, C.; Lebbe, C.; Bataille, V.; Bastholt, L.; Dreno, B.; Concetta Fargnoli, M.; Forsea, A.M.; Frenard, C.; et al. European Interdisciplinary Guideline on Invasive Squamous Cell Carcinoma of the Skin: Part 2. Treatment. Eur. J. Cancer 2020, 128, 83–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, B.; Wen, X.; Hao, D.; Du, D.; He, G.; Jiang, X. The Roles of lncRNA in Cutaneous Squamous Cell Carcinoma. Front. Oncol. 2020, 10, 158. [Google Scholar] [CrossRef]
- Piipponen, M.; Nissinen, L.; Farshchian, M.; Riihilä, P.; Kivisaari, A.; Kallajoki, M.; Peltonen, J.; Peltonen, S.; Kähäri, V.-M. Long Noncoding RNA PICSAR Promotes Growth of Cutaneous Squamous Cell Carcinoma by Regulating ERK1/2 Activity. J. Investig. Dermatol. 2016, 136, 1701–1710. [Google Scholar] [CrossRef][Green Version]
- Piipponen, M.; Heino, J.; Kähäri, V.-M.; Nissinen, L. Long Non-Coding RNA PICSAR Decreases Adhesion and Promotes Migration of Squamous Carcinoma Cells by Downregulating A2β1 and A5β1 Integrin Expression. Biol. Open 2018, 7, bio037044. [Google Scholar] [CrossRef]
- Piipponen, M.; Nissinen, L.; Riihilä, P.; Farshchian, M.; Kallajoki, M.; Peltonen, J.; Peltonen, S.; Kähäri, V.-M. P53-Regulated Long Noncoding RNA PRECSIT Promotes Progression of Cutaneous Squamous Cell Carcinoma via STAT3 Signaling. Am. J. Pathol. 2020, 190, 503–517. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, R.; Chen, H.; Chen, L.; Zhou, X.; Liu, L.; Ju, M.; Chen, K.; Huang, D. Comprehensive Analysis of lncRNA-mRNAs Co-Expression Network Identifies Potential lncRNA Biomarkers in Cutaneous Squamous Cell Carcinoma. BMC Genom. 2022, 23, 274. [Google Scholar] [CrossRef]
- Lan, T.; Yan, Y.; Zheng, D.; Ding, L. Investigating Diagnostic Potential of Long Non-Coding RNAs in Head and Neck Squamous Cell Carcinoma Using TCGA Database and Clinical Specimens. Sci. Rep. 2024, 14, 7500. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberger, B.M.; Kasper, M. Cellular Heterogeneity and Microenvironmental Control of Skin Cancer. J. Intern. Med. 2021, 289, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Bystryn, J.C. Alterations in Antigenic Properties of Normal Epidermis Adjacent to Basal Cell Carcinomas. J. Investig. Dermatol. 1981, 76, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Said, J.W.; Sassoon, A.F.; Shintaku, I.P.; Banks-Schlegel, S. Involucrin in Squamous and Basal Cell Carcinomas of the Skin: An Immunohistochemical Study. J. Investig. Dermatol. 1984, 82, 449–452. [Google Scholar] [CrossRef]
- Haass, N.K.; Wladykowski, E.; Kief, S.; Moll, I.; Brandner, J.M. Differential Induction of Connexins 26 and 30 in Skin Tumors and Their Adjacent Epidermis. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2006, 54, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, A.; Gamallo, C.; Benito, N.; Palacios, J.; Quintanilla, M.; Cano, A.; Contreras, F. Differential Patterns of Placental and Epithelial Cadherin Expression in Basal Cell Carcinoma and in the Epidermis Overlying Tumours. Br. J. Cancer 1995, 72, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A.; Sakuraoka, K.; Shimizu, H.; Nishikawa, T. Immunohistochemical Evaluation of Epidermis Overlying Basal Cell Carcinomas. Br. J. Dermatol. 1993, 128, 644–649. [Google Scholar] [CrossRef]
- Lacina, L.; Smetana, K.; Dvoránková, B.; Pytlík, R.; Kideryová, L.; Kucerová, L.; Plzáková, Z.; Stork, J.; Gabius, H.-J.; André, S. Stromal Fibroblasts from Basal Cell Carcinoma Affect Phenotype of Normal Keratinocytes. Br. J. Dermatol. 2007, 156, 819–829. [Google Scholar] [CrossRef]
- Hoesl, C.; Zanuttigh, E.; Fröhlich, T.; Philippou-Massier, J.; Krebs, S.; Blum, H.; Dahlhoff, M. The Secretome of Skin Cancer Cells Activates the mTOR/MYC Pathway in Healthy Keratinocytes and Induces Tumorigenic Properties. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118717. [Google Scholar] [CrossRef] [PubMed]
- Yerly, L.; Pich-Bavastro, C.; Di Domizio, J.; Wyss, T.; Tissot-Renaud, S.; Cangkrama, M.; Gilliet, M.; Werner, S.; Kuonen, F. Integrated Multi-Omics Reveals Cellular and Molecular Interactions Governing the Invasive Niche of Basal Cell Carcinoma. Nat. Commun. 2022, 13, 4897. [Google Scholar] [CrossRef]
- Haensel, D.; Daniel, B.; Gaddam, S.; Pan, C.; Fabo, T.; Bjelajac, J.; Jussila, A.R.; Gonzalez, F.; Li, N.Y.; Chen, Y.; et al. Skin Basal Cell Carcinomas Assemble a Pro-Tumorigenic Spatially Organized and Self-Propagating Trem2+ Myeloid Niche. Nat. Commun. 2023, 14, 2685. [Google Scholar] [CrossRef] [PubMed]
- Ganier, C.; Mazin, P.; Herrera-Oropeza, G.; Du-Harpur, X.; Blakeley, M.; Gabriel, J.; Predeus, A.V.; Cakir, B.; Prete, M.; Harun, N.; et al. Multiscale Spatial Mapping of Cell Populations across Anatomical Sites in Healthy Human Skin and Basal Cell Carcinoma. Proc. Natl. Acad. Sci. USA 2024, 121, e2313326120. [Google Scholar] [CrossRef]
- Ji, A.L.; Rubin, A.J.; Thrane, K.; Jiang, S.; Reynolds, D.L.; Meyers, R.M.; Guo, M.G.; George, B.M.; Mollbrink, A.; Bergenstråhle, J.; et al. Multimodal Analysis of Composition and Spatial Architecture in Human Squamous Cell Carcinoma. Cell 2020, 182, 497–514.e22. [Google Scholar] [CrossRef]
- Guerrero-Juarez, C.F.; Lee, G.H.; Liu, Y.; Wang, S.; Karikomi, M.; Sha, Y.; Chow, R.Y.; Nguyen, T.T.L.; Iglesias, V.S.; Aasi, S.; et al. Single-Cell Analysis of Human Basal Cell Carcinoma Reveals Novel Regulators of Tumor Growth and the Tumor Microenvironment. Sci. Adv. 2022, 8, eabm7981. [Google Scholar] [CrossRef] [PubMed]
- Michaloglou, C.; Vredeveld, L.C.W.; Soengas, M.S.; Denoyelle, C.; Kuilman, T.; van der Horst, C.M.A.M.; Majoor, D.M.; Shay, J.W.; Mooi, W.J.; Peeper, D.S. BRAFE600-Associated Senescence-like Cell Cycle Arrest of Human Naevi. Nature 2005, 436, 720–724. [Google Scholar] [CrossRef]
- Pampena, R.; Kyrgidis, A.; Lallas, A.; Moscarella, E.; Argenziano, G.; Longo, C. A Meta-Analysis of Nevus-Associated Melanoma: Prevalence and Practical Implications. J. Am. Acad. Dermatol. 2017, 77, 938–945.e4. [Google Scholar] [CrossRef]
- Clark, W.H.; Elder, D.E.; Guerry, D.; Epstein, M.N.; Greene, M.H.; Van Horn, M. A Study of Tumor Progression: The Precursor Lesions of Superficial Spreading and Nodular Melanoma. Hum. Pathol. 1984, 15, 1147–1165. [Google Scholar] [CrossRef]
- Elder, D.E. Precursors to Melanoma and Their Mimics: Nevi of Special Sites. Mod. Pathol. 2006, 19 (Suppl. 2), S4–S20. [Google Scholar] [CrossRef]
- Clark, W.H.; Ainsworth, A.M.; Bernardino, E.A.; Yang, C.H.; Mihm, C.M.; Reed, R.J. The Developmental Biology of Primary Human Malignant Melanomas. Semin. Oncol. 1975, 2, 83–103. [Google Scholar] [PubMed]
- Guerry, D.; Synnestvedt, M.; Elder, D.E.; Schultz, D. Lessons from Tumor Progression: The Invasive Radial Growth Phase of Melanoma Is Common, Incapable of Metastasis, and Indolent. J. Investig. Dermatol. 1993, 100, 342S–345S. [Google Scholar] [CrossRef]
- Hossain, S.M.; Eccles, M.R. Phenotype Switching and the Melanoma Microenvironment; Impact on Immunotherapy and Drug Resistance. Int. J. Mol. Sci. 2023, 24, 1601. [Google Scholar] [CrossRef] [PubMed]
- Pedri, D.; Karras, P.; Landeloos, E.; Marine, J.-C.; Rambow, F. Epithelial-to-Mesenchymal-like Transition Events in Melanoma. FEBS J. 2022, 289, 1352–1368. [Google Scholar] [CrossRef]
- Rambow, F.; Marine, J.-C.; Goding, C.R. Melanoma Plasticity and Phenotypic Diversity: Therapeutic Barriers and Opportunities. Genes Dev. 2019, 33, 1295–1318. [Google Scholar] [CrossRef]
- Hanly, A.J.; Jorda, M.; Elgart, G.W. Cutaneous Malignant Melanoma Associated with Extensive Pseudoepitheliomatous Hyperplasia. Report of a Case and Discussion of the Origin of Pseudoepitheliomatous Hyperplasia. J. Cutan. Pathol. 2000, 27, 153–156. [Google Scholar] [CrossRef]
- Mott, R.T.; Rosenberg, A.; Livingston, S.; Morgan, M.B. Melanoma Associated with Pseudoepitheliomatous Hyperplasia: A Case Series and Investigation into the Role of Epidermal Growth Factor Receptor. J. Cutan. Pathol. 2002, 29, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Reis-Filho, J.S.; Gasparetto, E.L.; Schmitt, F.C.; Fillus-Neto, J. Pseudoepitheliomatous Hyperplasia in Cutaneous Malignant Melanoma: A Rare and Misleading Feature. J. Cutan. Pathol. 2001, 28, 496–497. [Google Scholar] [CrossRef]
- Drunkenmölle, E.; Marsch, W.C.; Lübbe, D.; Helmbold, P. Paratumoral Epidermal Hyperplasia: A Novel Prognostic Factor in Thick Primary Melanoma of the Skin? Am. J. Dermatopathol. 2005, 27, 482–488. [Google Scholar] [CrossRef]
- Kodet, O.; Lacina, L.; Krejčí, E.; Dvořánková, B.; Grim, M.; Štork, J.; Kodetová, D.; Vlček, Č.; Šáchová, J.; Kolář, M.; et al. Melanoma Cells Influence the Differentiation Pattern of Human Epidermal Keratinocytes. Mol. Cancer 2015, 14, 1. [Google Scholar] [CrossRef]
- McCarty, M.F.; Bielenberg, D.R.; Nilsson, M.B.; Gershenwald, J.E.; Barnhill, R.L.; Ahearne, P.; Bucana, C.D.; Fidler, I.J. Epidermal Hyperplasia Overlying Human Melanoma Correlates with Tumour Depth and Angiogenesis. Melanoma Res. 2003, 13, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Hantschke, M.; Bastian, B.C.; LeBoit, P.E. Consumption of the Epidermis: A Diagnostic Criterion for the Differential Diagnosis of Melanoma and Spitz Nevus. Am. J. Surg. Pathol. 2004, 28, 1621–1625. [Google Scholar] [CrossRef]
- Bandino, J.P.; Kazlouskaya, V.; Ergin, Ş.; Molés-Poveda, P.; Cleaver, N.J.; Kabigting, F.D.; Shackelton, J.B.; Thieu, K.; Elston, D.M. Epidermal Consumption in Benign and Malignant Melanocytic Neoplasms. J. Cutan. Pathol. 2015, 42, 937–943. [Google Scholar] [CrossRef]
- Corbalán-Vélez, R.; Oviedo-Ramírez, I.; Martínez-Barba, E.; Clemente-Ruiz De Almirón, A. Epidermal Effacement in Malignant Melanoma. Actas Dermo-Sifiliográficas Engl. Ed. 2011, 102, 634–635. [Google Scholar] [CrossRef]
- Gerger, A.; Koller, S.; Kern, T.; Massone, C.; Steiger, K.; Richtig, E.; Kerl, H.; Smolle, J. Diagnostic Applicability of in Vivo Confocal Laser Scanning Microscopy in Melanocytic Skin Tumors. J. Investig. Dermatol. 2005, 124, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Langley, R.G.; Rajadhyaksha, M.; Dwyer, P.J.; Sober, A.J.; Flotte, T.J.; Anderson, R.R. Confocal Scanning Laser Microscopy of Benign and Malignant Melanocytic Skin Lesions in Vivo. J. Am. Acad. Dermatol. 2001, 45, 365–376. [Google Scholar] [CrossRef]
- Pellacani, G.; Cesinaro, A.M.; Seidenari, S. Reflectance-Mode Confocal Microscopy for the in Vivo Characterization of Pagetoid Melanocytosis in Melanomas and Nevi. J. Investig. Dermatol. 2005, 125, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.Y.L.; Ellis, R.A.; Lovat, P.E. Prognostic Impact of Autophagy Biomarkers for Cutaneous Melanoma. Front. Oncol. 2016, 6, 236. [Google Scholar] [CrossRef]
- Cosgarea, I.; McConnell, A.T.; Ewen, T.; Tang, D.; Hill, D.S.; Anagnostou, M.; Elias, M.; Ellis, R.A.; Murray, A.; Spender, L.C.; et al. Melanoma Secretion of Transforming Growth Factor-Β2 Leads to Loss of Epidermal AMBRA1 Threatening Epidermal Integrity and Facilitating Tumour Ulceration. Br. J. Dermatol. 2022, 186, 694–704. [Google Scholar] [CrossRef]
- Ewen, T.; Husain, A.; Stefanos, N.; Barrett, P.; Jones, C.; Ness, T.; Long, A.; Horswell, S.; Bosomworth, H.; Lowenstein, J.; et al. Validation of Epidermal AMBRA1 and Loricrin (AMBLor) as a Prognostic Biomarker for Nonulcerated American Joint Committee on Cancer Stage I/II Cutaneous Melanoma. Br. J. Dermatol. 2024, 190, 549–558. [Google Scholar] [CrossRef]
- Blessing, K.; McLaren, K.M. Histological Regression in Primary Cutaneous Melanoma: Recognition, Prevalence and Significance. Histopathology 1992, 20, 315–322. [Google Scholar] [CrossRef]
- Aung, P.P.; Nagarajan, P.; Prieto, V.G. Regression in Primary Cutaneous Melanoma: Etiopathogenesis and Clinical Significance. Lab. Investig. J. Tech. Methods Pathol. 2017, 97, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Aivazian, K.; Ahmed, T.; El Sharouni, M.-A.; Stretch, J.R.; Saw, R.P.M.; Spillane, A.J.; Shannon, K.F.; Ch’ng, S.; Nieweg, O.E.; Thompson, J.F.; et al. Histological Regression in Melanoma: Impact on Sentinel Lymph Node Status and Survival. Mod. Pathol. 2021, 34, 1999–2008. [Google Scholar] [CrossRef] [PubMed]
- Marrapodi, R.; Bellei, B. The Keratinocyte in the Picture Cutaneous Melanoma Microenvironment. Cancers 2024, 16, 913. [Google Scholar] [CrossRef] [PubMed]
- Sadangi, S.; Milosavljevic, K.; Castro-Perez, E.; Lares, M.; Singh, M.; Altameemi, S.; Beebe, D.J.; Ayuso, J.M.; Setaluri, V. Role of the Skin Microenvironment in Melanomagenesis: Epidermal Keratinocytes and Dermal Fibroblasts Promote BRAF Oncogene-Induced Senescence Escape in Melanocytes. Cancers 2022, 14, 1233. [Google Scholar] [CrossRef] [PubMed]
- Tagore, M.; Hergenreder, E.; Perlee, S.C.; Cruz, N.M.; Menocal, L.; Suresh, S.; Chan, E.; Baron, M.; Melendez, S.; Dave, A.; et al. GABA Regulates Electrical Activity and Tumor Initiation in Melanoma. Cancer Discov. 2023, 13, 2270–2291. [Google Scholar] [CrossRef] [PubMed]
- Arnold, I.; Watt, F.M. C-Myc Activation in Transgenic Mouse Epidermis Results in Mobilization of Stem Cells and Differentiation of Their Progeny. Curr. Biol. CB 2001, 11, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Mill, P.; Mo, R.; Hu, M.C.; Dagnino, L.; Rosenblum, N.D.; Hui, C.-C. Shh Controls Epithelial Proliferation via Independent Pathways That Converge on N-Myc. Dev. Cell 2005, 9, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Burks, H.E.; Pokorny, J.L.; Koetsier, J.L.; Roth-Carter, Q.R.; Arnette, C.R.; Gerami, P.; Seykora, J.T.; Johnson, J.L.; Ren, Z.; Green, K.J. Melanoma Cells Repress Desmoglein 1 in Keratinocytes to Promote Tumor Cell Migration. J. Cell Biol. 2023, 222, e202212031. [Google Scholar] [CrossRef]
- Godsel, L.M.; Roth-Carter, Q.R.; Koetsier, J.L.; Tsoi, L.C.; Huffine, A.L.; Broussard, J.A.; Fitz, G.N.; Lloyd, S.M.; Kweon, J.; Burks, H.E.; et al. Translational Implications of Th17-Skewed Inflammation Due to Genetic Deficiency of a Cadherin Stress Sensor. J. Clin. Investig. 2022, 132, e144363. [Google Scholar] [CrossRef]
- Polivka, L.; Hadj-Rabia, S.; Bal, E.; Leclerc-Mercier, S.; Madrange, M.; Hamel, Y.; Bonnet, D.; Mallet, S.; Lepidi, H.; Ovaert, C.; et al. Epithelial Barrier Dysfunction in Desmoglein-1 Deficiency. J. Allergy Clin. Immunol. 2018, 142, 702–706.e7. [Google Scholar] [CrossRef] [PubMed]
- Eves, P.; Katerinaki, E.; Simpson, C.; Layton, C.; Dawson, R.; Evans, G.; Mac Neil, S. Melanoma Invasion in Reconstructed Human Skin Is Influenced by Skin Cells--Investigation of the Role of Proteolytic Enzymes. Clin. Exp. Metastasis 2003, 20, 685–700. [Google Scholar] [CrossRef]
- Eves, P.; Layton, C.; Hedley, S.; Dawson, R.A.; Wagner, M.; Morandini, R.; Ghanem, G.; Mac Neil, S. Characterization of an in Vitro Model of Human Melanoma Invasion Based on Reconstructed Human Skin. Br. J. Dermatol. 2000, 142, 210–222. [Google Scholar] [CrossRef]
- Van Kilsdonk, J.W.J.; Bergers, M.; Van Kempen, L.C.L.T.; Schalkwijk, J.; Swart, G.W.M. Keratinocytes Drive Melanoma Invasion in a Reconstructed Skin Model. Melanoma Res. 2010, 20, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Ayuso, J.M.; Sadangi, S.; Lares, M.; Rehman, S.; Humayun, M.; Denecke, K.M.; Skala, M.C.; Beebe, D.J.; Setaluri, V. Microfluidic Model with Air-Walls Reveals Fibroblasts and Keratinocytes Modulate Melanoma Cell Phenotype, Migration, and Metabolism. Lab. Chip 2021, 21, 1139–1149. [Google Scholar] [CrossRef]
- Golan, T.; Messer, A.R.; Amitai-Lange, A.; Melamed, Z.; Ohana, R.; Bell, R.E.; Kapitansky, O.; Lerman, G.; Greenberger, S.; Khaled, M.; et al. Interactions of Melanoma Cells with Distal Keratinocytes Trigger Metastasis via Notch Signaling Inhibition of MITF. Mol. Cell 2015, 59, 664–676. [Google Scholar] [CrossRef]
- Balint, K.; Xiao, M.; Pinnix, C.C.; Soma, A.; Veres, I.; Juhasz, I.; Brown, E.J.; Capobianco, A.J.; Herlyn, M.; Liu, Z.-J. Activation of Notch1 Signaling Is Required for Beta-Catenin-Mediated Human Primary Melanoma Progression. J. Clin. Investig. 2005, 115, 3166–3176. [Google Scholar] [CrossRef] [PubMed]
- Hoek, K.; Rimm, D.L.; Williams, K.R.; Zhao, H.; Ariyan, S.; Lin, A.; Kluger, H.M.; Berger, A.J.; Cheng, E.; Trombetta, E.S.; et al. Expression Profiling Reveals Novel Pathways in the Transformation of Melanocytes to Melanomas. Cancer Res. 2004, 64, 5270–5282. [Google Scholar] [CrossRef]
- Massi, D.; Tarantini, F.; Franchi, A.; Paglierani, M.; Di Serio, C.; Pellerito, S.; Leoncini, G.; Cirino, G.; Geppetti, P.; Santucci, M. Evidence for Differential Expression of Notch Receptors and Their Ligands in Melanocytic Nevi and Cutaneous Malignant Melanoma. Mod. Pathol. 2006, 19, 246–254. [Google Scholar] [CrossRef]
- Pinnix, C.C.; Lee, J.T.; Liu, Z.-J.; McDaid, R.; Balint, K.; Beverly, L.J.; Brafford, P.A.; Xiao, M.; Himes, B.; Zabierowski, S.E.; et al. Active Notch1 Confers a Transformed Phenotype to Primary Human Melanocytes. Cancer Res. 2009, 69, 5312–5320. [Google Scholar] [CrossRef]
- Bedogni, B. Notch Signaling in Melanoma: Interacting Pathways and Stromal Influences That Enhance Notch Targeting. Pigment Cell Melanoma Res. 2014, 27, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-J.; Xiao, M.; Balint, K.; Smalley, K.S.M.; Brafford, P.; Qiu, R.; Pinnix, C.C.; Li, X.; Herlyn, M. Notch1 Signaling Promotes Primary Melanoma Progression by Activating Mitogen-Activated Protein Kinase/Phosphatidylinositol 3-Kinase-Akt Pathways and up-Regulating N-Cadherin Expression. Cancer Res. 2006, 66, 4182–4190. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.-Z.; Stennett, L.; Bacon, P.; Bodner, B.; Hendrix, M.J.C.; Seftor, R.E.B.; Seftor, E.A.; Margaryan, N.V.; Pollock, P.M.; Curtis, A.; et al. P53-Independent NOXA Induction Overcomes Apoptotic Resistance of Malignant Melanomas. Mol. Cancer Ther. 2004, 3, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Mirandola, L.; Chiriva-Internati, M.; Basile, A.; Locati, M.; Lesma, E.; Chiaramonte, R.; Platonova, N. Cancer Cells Exploit Notch Signaling to Redefine a Supportive Cytokine Milieu. Front. Immunol. 2018, 9, 1823. [Google Scholar] [CrossRef] [PubMed]
- Tabach, Y.; Golan, T.; Hernández-Hernández, A.; Messer, A.R.; Fukuda, T.; Kouznetsova, A.; Liu, J.-G.; Lilienthal, I.; Levy, C.; Ruvkun, G. Human Disease Locus Discovery and Mapping to Molecular Pathways through Phylogenetic Profiling. Mol. Syst. Biol. 2013, 9, 692. [Google Scholar] [CrossRef]
- Noujarède, J.; Carrié, L.; Garcia, V.; Grimont, M.; Eberhardt, A.; Mucher, E.; Genais, M.; Schreuder, A.; Carpentier, S.; Ségui, B.; et al. Sphingolipid Paracrine Signaling Impairs Keratinocyte Adhesion to Promote Melanoma Invasion. Cell Rep. 2023, 42, 113586. [Google Scholar] [CrossRef] [PubMed]
- Hannun, Y.A.; Obeid, L.M. Principles of Bioactive Lipid Signalling: Lessons from Sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, S.; Milstien, S. Sphingosine-1-Phosphate: Signaling inside and Out. FEBS Lett. 2000, 476, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Chipuk, J.E.; McStay, G.P.; Bharti, A.; Kuwana, T.; Clarke, C.J.; Siskind, L.J.; Obeid, L.M.; Green, D.R. Sphingolipid Metabolism Cooperates with BAK and BAX to Promote the Mitochondrial Pathway of Apoptosis. Cell 2012, 148, 988–1000. [Google Scholar] [CrossRef]
- Alvarez, S.E.; Harikumar, K.B.; Hait, N.C.; Allegood, J.; Strub, G.M.; Kim, E.Y.; Maceyka, M.; Jiang, H.; Luo, C.; Kordula, T.; et al. Sphingosine-1-Phosphate Is a Missing Cofactor for the E3 Ubiquitin Ligase TRAF2. Nature 2010, 465, 1084–1088. [Google Scholar] [CrossRef]
- Park, K.; Ikushiro, H.; Seo, H.S.; Shin, K.-O.; Kim, Y.I.; Kim, J.Y.; Lee, Y.-M.; Yano, T.; Holleran, W.M.; Elias, P.; et al. ER Stress Stimulates Production of the Key Antimicrobial Peptide, Cathelicidin, by Forming a Previously Unidentified Intracellular S1P Signaling Complex. Proc. Natl. Acad. Sci. USA 2016, 113, E1334–E1342. [Google Scholar] [CrossRef]
- Hait, N.C.; Allegood, J.; Maceyka, M.; Strub, G.M.; Harikumar, K.B.; Singh, S.K.; Luo, C.; Marmorstein, R.; Kordula, T.; Milstien, S.; et al. Regulation of Histone Acetylation in the Nucleus by Sphingosine-1-Phosphate. Science 2009, 325, 1254–1257. [Google Scholar] [CrossRef]
- Ogretmen, B. Sphingolipid Metabolism in Cancer Signalling and Therapy. Nat. Rev. Cancer 2018, 18, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Newton, J.; Lima, S.; Maceyka, M.; Spiegel, S. Revisiting the Sphingolipid Rheostat: Evolving Concepts in Cancer Therapy. Exp. Cell Res. 2015, 333, 195–200. [Google Scholar] [CrossRef]
- Vietri Rudan, M.; Watt, F.M. Mammalian Epidermis: A Compendium of Lipid Functionality. Front. Physiol. 2021, 12, 804824. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y. Ceramide Signaling in Mammalian Epidermis. Biochim. Biophys. Acta 2014, 1841, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-S.; Kim, S.-Y.; Kleuser, B.; Schäfer-Korting, M.; Kim, K.H.; Park, K.-C. Sphingosine-1-Phosphate Inhibits Human Keratinocyte Proliferation via Akt/Protein Kinase B Inactivation. Cell. Signal. 2004, 16, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Schüppel, M.; Kürschner, U.; Kleuser, U.; Schäfer-Korting, M.; Kleuser, B. Sphingosine 1-Phosphate Restrains Insulin-Mediated Keratinocyte Proliferation via Inhibition of Akt through the S1P2 Receptor Subtype. J. Investig. Dermatol. 2008, 128, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Vogler, R.; Sauer, B.; Kim, D.-S.; Schäfer-Korting, M.; Kleuser, B. Sphingosine-1-Phosphate and Its Potentially Paradoxical Effects on Critical Parameters of Cutaneous Wound Healing. J. Investig. Dermatol. 2003, 120, 693–700. [Google Scholar] [CrossRef]
- Manggau, M.; Kim, D.S.; Ruwisch, L.; Vogler, R.; Korting, H.C.; Schäfer-Korting, M.; Kleuser, B. 1Alpha,25-Dihydroxyvitamin D3 Protects Human Keratinocytes from Apoptosis by the Formation of Sphingosine-1-Phosphate. J. Investig. Dermatol. 2001, 117, 1241–1249. [Google Scholar] [CrossRef]
- Schmitz, E.I.; Potteck, H.; Schüppel, M.; Manggau, M.; Wahydin, E.; Kleuser, B. Sphingosine 1-Phosphate Protects Primary Human Keratinocytes from Apoptosis via Nitric Oxide Formation through the Receptor Subtype S1P3. Mol. Cell. Biochem. 2012, 371, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Lichte, K.; Rossi, R.; Danneberg, K.; ter Braak, M.; Kürschner, U.; Jakobs, K.H.; Kleuser, B.; Meyer zu Heringdorf, D. Lysophospholipid Receptor-Mediated Calcium Signaling in Human Keratinocytes. J. Investig. Dermatol. 2008, 128, 1487–1498. [Google Scholar] [CrossRef] [PubMed]
- Allende, M.L.; Sipe, L.M.; Tuymetova, G.; Wilson-Henjum, K.L.; Chen, W.; Proia, R.L. Sphingosine-1-Phosphate Phosphatase 1 Regulates Keratinocyte Differentiation and Epidermal Homeostasis. J. Biol. Chem. 2013, 288, 18381–18391. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.H.; Youm, J.-K.; Kwon, M.J.; Park, B.D.; Lee, Y.-M.; Lee, S.-I.; Shin, D.M.; Lee, S.H. K6PC-5, a Direct Activator of Sphingosine Kinase 1, Promotes Epidermal Differentiation through Intracellular Ca2+ Signaling. J. Investig. Dermatol. 2008, 128, 2166–2178. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Song, J.; Lee, D.; Kim, G.-T.; Park, S.-H.; Shin, D.-Y.; Shin, K.-O.; Park, K.; Shim, S.-M.; Park, T.-S. Inhibition of Sphingosine 1-Phosphate Lyase Activates Human Keratinocyte Differentiation and Attenuates Psoriasis in Mice. J. Lipid Res. 2020, 61, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Igawa, S.; Choi, J.E.; Wang, Z.; Chang, Y.-L.; Wu, C.-C.; Werbel, T.; Ishida-Yamamoto, A.; Di Nardo, A. Human Keratinocytes Use Sphingosine 1-Phosphate and Its Receptors to Communicate Staphylococcus Aureus Invasion and Activate Host Defense. J. Investig. Dermatol. 2019, 139, 1743–1752.e5. [Google Scholar] [CrossRef] [PubMed]
- Oizumi, A.; Nakayama, H.; Okino, N.; Iwahara, C.; Kina, K.; Matsumoto, R.; Ogawa, H.; Takamori, K.; Ito, M.; Suga, Y.; et al. Pseudomonas-Derived Ceramidase Induces Production of Inflammatory Mediators from Human Keratinocytes via Sphingosine-1-Phosphate. PLoS ONE 2014, 9, e89402. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Elias, P.M.; Shin, K.-O.; Lee, Y.-M.; Hupe, M.; Borkowski, A.W.; Gallo, R.L.; Saba, J.; Holleran, W.M.; Uchida, Y. A Novel Role of a Lipid Species, Sphingosine-1-Phosphate, in Epithelial Innate Immunity. Mol. Cell. Biol. 2013, 33, 752–762. [Google Scholar] [CrossRef]
- Uchida, Y.; Houben, E.; Park, K.; Douangpanya, S.; Lee, Y.-M.; Wu, B.X.; Hannun, Y.A.; Radin, N.S.; Elias, P.M.; Holleran, W.M. Hydrolytic Pathway Protects against Ceramide-Induced Apoptosis in Keratinocytes Exposed to UVB. J. Investig. Dermatol. 2010, 130, 2472–2480. [Google Scholar] [CrossRef]
- Lovric, S.; Goncalves, S.; Gee, H.Y.; Oskouian, B.; Srinivas, H.; Choi, W.-I.; Shril, S.; Ashraf, S.; Tan, W.; Rao, J.; et al. Mutations in Sphingosine-1-Phosphate Lyase Cause Nephrosis with Ichthyosis and Adrenal Insufficiency. J. Clin. Investig. 2017, 127, 912–928. [Google Scholar] [CrossRef]
- Smith, C.J.; Williams, J.L.; Hall, C.; Casas, J.; Caley, M.P.; O’Toole, E.A.; Prasad, R.; Metherell, L.A. Ichthyosis Linked to Sphingosine 1-Phosphate Lyase Insufficiency Is Due to Aberrant Sphingolipid and Calcium Regulation. J. Lipid Res. 2023, 64, 100351. [Google Scholar] [CrossRef] [PubMed]
- Kleuser, B.; Bäumer, W. Sphingosine 1-Phosphate as Essential Signaling Molecule in Inflammatory Skin Diseases. Int. J. Mol. Sci. 2023, 24, 1456. [Google Scholar] [CrossRef] [PubMed]
- Carrié, L.; Virazels, M.; Dufau, C.; Montfort, A.; Levade, T.; Ségui, B.; Andrieu-Abadie, N. New Insights into the Role of Sphingolipid Metabolism in Melanoma. Cells 2020, 9, 1967. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, L.; Carrié, L.; Dufau, C.; Nieto, L.; Ségui, B.; Levade, T.; Riond, J.; Andrieu-Abadie, N. Lipid Metabolic Reprogramming: Role in Melanoma Progression and Therapeutic Perspectives. Cancers 2020, 12, 3147. [Google Scholar] [CrossRef] [PubMed]
- Albinet, V.; Bats, M.-L.; Huwiler, A.; Rochaix, P.; Chevreau, C.; Ségui, B.; Levade, T.; Andrieu-Abadie, N. Dual Role of Sphingosine Kinase-1 in Promoting the Differentiation of Dermal Fibroblasts and the Dissemination of Melanoma Cells. Oncogene 2014, 33, 3364–3373. [Google Scholar] [CrossRef]
- Leclerc, J.; Garandeau, D.; Pandiani, C.; Gaudel, C.; Bille, K.; Nottet, N.; Garcia, V.; Colosetti, P.; Pagnotta, S.; Bahadoran, P.; et al. Lysosomal Acid Ceramidase ASAH1 Controls the Transition between Invasive and Proliferative Phenotype in Melanoma Cells. Oncogene 2019, 38, 1282–1295. [Google Scholar] [CrossRef]
- Montfort, A.; Bertrand, F.; Rochotte, J.; Gilhodes, J.; Filleron, T.; Milhès, J.; Dufau, C.; Imbert, C.; Riond, J.; Tosolini, M.; et al. Neutral Sphingomyelinase 2 Heightens Anti-Melanoma Immune Responses and Anti-PD-1 Therapy Efficacy. Cancer Immunol. Res. 2021, 9, 568–582. [Google Scholar] [CrossRef]
- Garandeau, D.; Noujarède, J.; Leclerc, J.; Imbert, C.; Garcia, V.; Bats, M.-L.; Rambow, F.; Gilhodes, J.; Filleron, T.; Meyer, N.; et al. Targeting the Sphingosine 1-Phosphate Axis Exerts Potent Antitumor Activity in BRAFi-Resistant Melanomas. Mol. Cancer Ther. 2019, 18, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Imbert, C.; Montfort, A.; Fraisse, M.; Marcheteau, E.; Gilhodes, J.; Martin, E.; Bertrand, F.; Marcellin, M.; Burlet-Schiltz, O.; de Peredo, A.G.; et al. Resistance of Melanoma to Immune Checkpoint Inhibitors Is Overcome by Targeting the Sphingosine Kinase-1. Nat. Commun. 2020, 11, 437. [Google Scholar] [CrossRef]
- Kuphal, S.; Bosserhoff, A.K. E-Cadherin Cell-Cell Communication in Melanogenesis and during Development of Malignant Melanoma. Arch. Biochem. Biophys. 2012, 524, 43–47. [Google Scholar] [CrossRef]
- Igawa, S.; Ohzono, A.; Pham, P.; Wang, Z.; Nakatsuji, T.; Dokoshi, T.; Di Nardo, A. Sphingosine 1-Phosphate Receptor 2 Is Central to Maintaining Epidermal Barrier Homeostasis. J. Investig. Dermatol. 2021, 141, 1188–1197.e5. [Google Scholar] [CrossRef] [PubMed]
- Hölzel, M.; Tüting, T. Inflammation-Induced Plasticity in Melanoma Therapy and Metastasis. Trends Immunol. 2016, 37, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Richmond, A.; Yang, J.; Su, Y. The Good and the Bad of Chemokines/Chemokine Receptors in Melanoma. Pigment Cell Melanoma Res. 2009, 22, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.C.; Coussens, L.M. Soluble Mediators of Inflammation during Tumor Development. Adv. Cancer Res. 2005, 93, 159–187. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.; Moser, B.; Karagiannis, S.N.; Lacy, K.E. Chemokine Pathways in Cutaneous Melanoma: Their Modulation by Cancer and Exploitation by the Clinician. Cancers 2021, 13, 5625. [Google Scholar] [CrossRef] [PubMed]
- Attrill, G.H.; Ferguson, P.M.; Palendira, U.; Long, G.V.; Wilmott, J.S.; Scolyer, R.A. The Tumour Immune Landscape and Its Implications in Cutaneous Melanoma. Pigment Cell Melanoma Res. 2021, 34, 529–549. [Google Scholar] [CrossRef] [PubMed]
- Lázár-Molnár, E.; Hegyesi, H.; Tóth, S.; Falus, A. Autocrine and Paracrine Regulation by Cytokines and Growth Factors in Melanoma. Cytokine 2000, 12, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Nirmal, A.J.; Maliga, Z.; Vallius, T.; Quattrochi, B.; Chen, A.A.; Jacobson, C.A.; Pelletier, R.J.; Yapp, C.; Arias-Camison, R.; Chen, Y.-A.; et al. The Spatial Landscape of Progression and Immunoediting in Primary Melanoma at Single-Cell Resolution. Cancer Discov. 2022, 12, 1518–1541. [Google Scholar] [CrossRef]
- Kiuru, M.; Kriner, M.A.; Wong, S.; Zhu, G.; Terrell, J.R.; Li, Q.; Hoang, M.; Beechem, J.; McPherson, J.D. High-Plex Spatial RNA Profiling Reveals Cell Type–Specific Biomarker Expression during Melanoma Development. J. Investig. Dermatol. 2022, 142, 1401–1412.e20. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sumardika, I.W.; Tomonobu, N.; Winarsa Ruma, I.M.; Kinoshita, R.; Kondo, E.; Inoue, Y.; Sato, H.; Yamauchi, A.; Murata, H.; et al. Melanoma Cell Adhesion Molecule Is the Driving Force behind the Dissemination of Melanoma upon S100A8/A9 Binding in the Original Skin Lesion. Cancer Lett. 2019, 452, 178–190. [Google Scholar] [CrossRef]
- Hibino, T.; Sakaguchi, M.; Miyamoto, S.; Yamamoto, M.; Motoyama, A.; Hosoi, J.; Shimokata, T.; Ito, T.; Tsuboi, R.; Huh, N.-H. S100A9 Is a Novel Ligand of EMMPRIN That Promotes Melanoma Metastasis. Cancer Res. 2013, 73, 172–183. [Google Scholar] [CrossRef] [PubMed]
- Ribé, A.; McNutt, N.S. S100A Protein Expression in the Distinction between Lentigo Maligna and Pigmented Actinic Keratosis. Am. J. Dermatopathol. 2003, 25, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Srikrishna, G. S100A8 and S100A9: New Insights into Their Roles in Malignancy. J. Innate Immun. 2012, 4, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhao, C.; Shao, R.; Xu, Y.; Zhao, W. The Functions and Regulatory Pathways of S100A8/A9 and Its Receptors in Cancers. Front. Pharmacol. 2023, 14, 1187741. [Google Scholar] [CrossRef] [PubMed]
- Chessa, C.; Bodet, C.; Jousselin, C.; Wehbe, M.; Lévêque, N.; Garcia, M. Antiviral and Immunomodulatory Properties of Antimicrobial Peptides Produced by Human Keratinocytes. Front. Microbiol. 2020, 11, 1155. [Google Scholar] [CrossRef] [PubMed]
- Kerkhoff, C.; Voss, A.; Scholzen, T.E.; Averill, M.M.; Zänker, K.S.; Bornfeldt, K.E. Novel Insights into the Role of S100A8/A9 in Skin Biology. Exp. Dermatol. 2012, 21, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Nukui, T.; Ehama, R.; Sakaguchi, M.; Sonegawa, H.; Katagiri, C.; Hibino, T.; Huh, N.-H. S100A8/A9, a key mediator for positive feedback growth stimulation of normal human keratinocytes. J. Cell. Biochem. 2008, 104, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Hiratsuka, S.; Watanabe, A.; Sakurai, Y.; Akashi-Takamura, S.; Ishibashi, S.; Miyake, K.; Shibuya, M.; Akira, S.; Aburatani, H.; Maru, Y. The S100A8-Serum Amyloid A3-TLR4 Paracrine Cascade Establishes a Pre-Metastatic Phase. Nat. Cell Biol. 2008, 10, 1349–1355. [Google Scholar] [CrossRef]
- Ruma, I.M.W.; Putranto, E.W.; Kondo, E.; Murata, H.; Watanabe, M.; Huang, P.; Kinoshita, R.; Futami, J.; Inoue, Y.; Yamauchi, A.; et al. MCAM, as a Novel Receptor for S100A8/A9, Mediates Progression of Malignant Melanoma through Prominent Activation of NF-κB and ROS Formation upon Ligand Binding. Clin. Exp. Metastasis 2016, 33, 609–627. [Google Scholar] [CrossRef]
- Saha, A.; Lee, Y.-C.; Zhang, Z.; Chandra, G.; Su, S.-B.; Mukherjee, A.B. Lack of an Endogenous Anti-Inflammatory Protein in Mice Enhances Colonization of B16F10 Melanoma Cells in the Lungs. J. Biol. Chem. 2010, 285, 10822–10831. [Google Scholar] [CrossRef]
- Clarke, L.E.; Warf, M.B.; Flake, D.D.; Hartman, A.-R.; Tahan, S.; Shea, C.R.; Gerami, P.; Messina, J.; Florell, S.R.; Wenstrup, R.J.; et al. Clinical Validation of a Gene Expression Signature That Differentiates Benign Nevi from Malignant Melanoma. J. Cutan. Pathol. 2015, 42, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Wagner, N.B.; Weide, B.; Gries, M.; Reith, M.; Tarnanidis, K.; Schuermans, V.; Kemper, C.; Kehrel, C.; Funder, A.; Lichtenberger, R.; et al. Tumor Microenvironment-Derived S100A8/A9 Is a Novel Prognostic Biomarker for Advanced Melanoma Patients and during Immunotherapy with Anti-PD-1 Antibodies. J. Immunother. Cancer 2019, 7, 343. [Google Scholar] [CrossRef] [PubMed]
- Hai, J.; Meyer, S.N.; Wong, S.L.; Li, Y.; Simmons, E.; Miglioretti, D.; Fung, M.; Kiuru, M. Comparison of S100A8 and PRAME as Biomarkers for Distinguishing Melanoma from Melanocytic Nevus: A Case-Control Analysis. Clin. Exp. Dermatol. 2024, 49, llae005. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; German, B.; Chraa, D.; Braud, A.; Hugel, C.; Meyer, P.; Davidson, G.; Laurette, P.; Mengus, G.; Flatter, E.; et al. Keratinocyte-Derived Cytokine TSLP Promotes Growth and Metastasis of Melanoma by Regulating the Tumor-Associated Immune Microenvironment. JCI Insight 2022, 7, e161438. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, S.F.; Artis, D. Sensing the Outside World: TSLP Regulates Barrier Immunity. Nat. Immunol. 2010, 11, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Corren, J.; Ziegler, S.F. TSLP: From Allergy to Cancer. Nat. Immunol. 2019, 20, 1603–1609. [Google Scholar] [CrossRef] [PubMed]
- Protti, M.P.; De Monte, L. Thymic Stromal Lymphopoietin and Cancer: Th2-Dependent and -Independent Mechanisms. Front. Immunol. 2020, 11, 2088. [Google Scholar] [CrossRef]
- Di Piazza, M.; Nowell, C.S.; Koch, U.; Durham, A.-D.; Radtke, F. Loss of Cutaneous TSLP-Dependent Immune Responses Skews the Balance of Inflammation from Tumor Protective to Tumor Promoting. Cancer Cell 2012, 22, 479–493. [Google Scholar] [CrossRef]
- Demehri, S.; Turkoz, A.; Manivasagam, S.; Yockey, L.J.; Turkoz, M.; Kopan, R. Elevated Epidermal Thymic Stromal Lymphopoietin Levels Establish an Antitumor Environment in the Skin. Cancer Cell 2012, 22, 494–505. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dainese-Marque, O.; Garcia, V.; Andrieu-Abadie, N.; Riond, J. Contribution of Keratinocytes in Skin Cancer Initiation and Progression. Int. J. Mol. Sci. 2024, 25, 8813. https://doi.org/10.3390/ijms25168813
Dainese-Marque O, Garcia V, Andrieu-Abadie N, Riond J. Contribution of Keratinocytes in Skin Cancer Initiation and Progression. International Journal of Molecular Sciences. 2024; 25(16):8813. https://doi.org/10.3390/ijms25168813
Chicago/Turabian StyleDainese-Marque, Océane, Virginie Garcia, Nathalie Andrieu-Abadie, and Joëlle Riond. 2024. "Contribution of Keratinocytes in Skin Cancer Initiation and Progression" International Journal of Molecular Sciences 25, no. 16: 8813. https://doi.org/10.3390/ijms25168813
APA StyleDainese-Marque, O., Garcia, V., Andrieu-Abadie, N., & Riond, J. (2024). Contribution of Keratinocytes in Skin Cancer Initiation and Progression. International Journal of Molecular Sciences, 25(16), 8813. https://doi.org/10.3390/ijms25168813




