BIO 300 Attenuates Whole Blood Transcriptome Changes in Mice Exposed to Total-Body Radiation
Abstract
:1. Introduction
2. Results
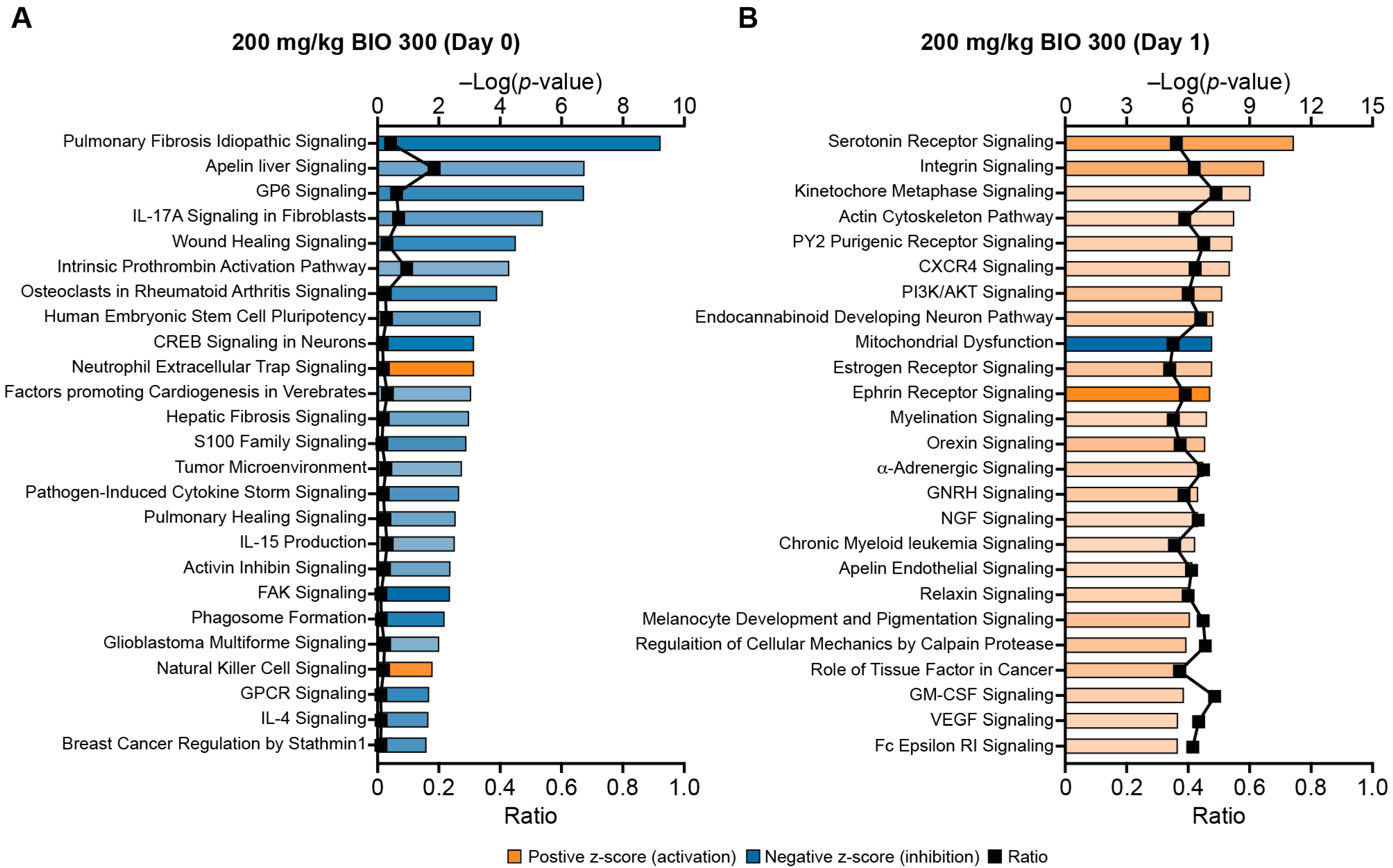
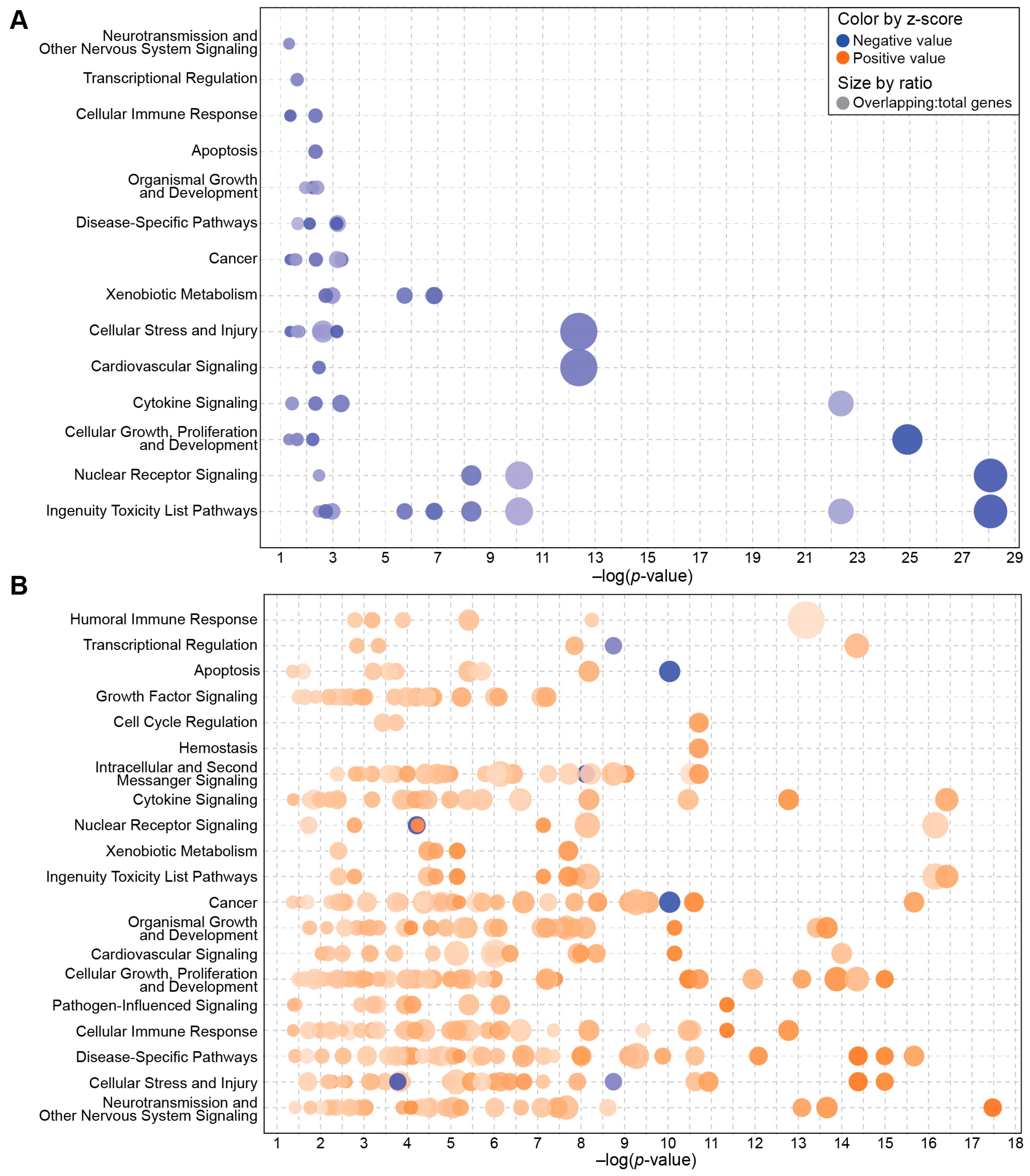
2.1. Profiling Whole-Blood Differential Gene Expression and Changes in Signaling Pathways in BIO 300-Treated Mice
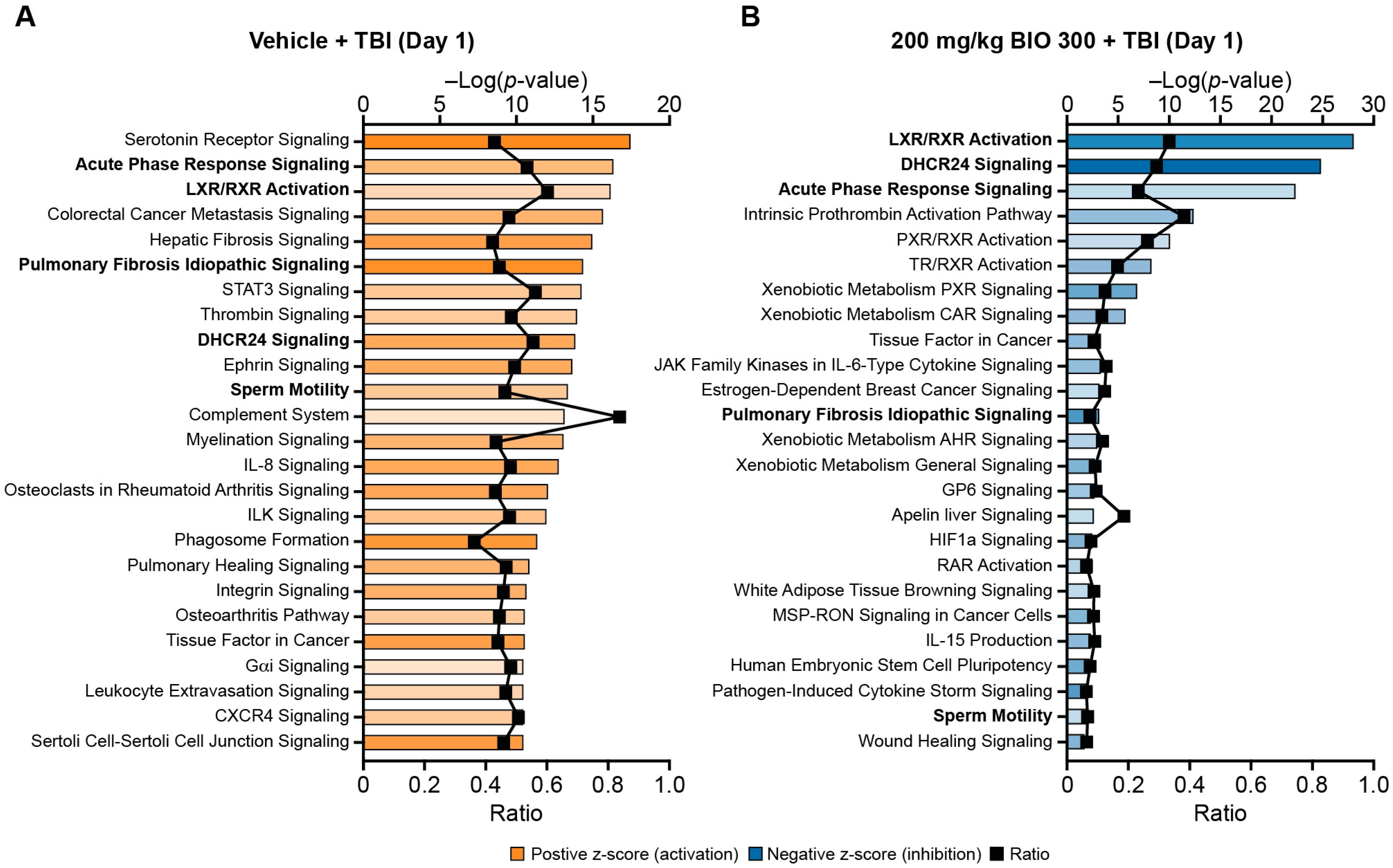
2.2. BIO 300 Attenuated Radiation-Induced Transcriptome Changes
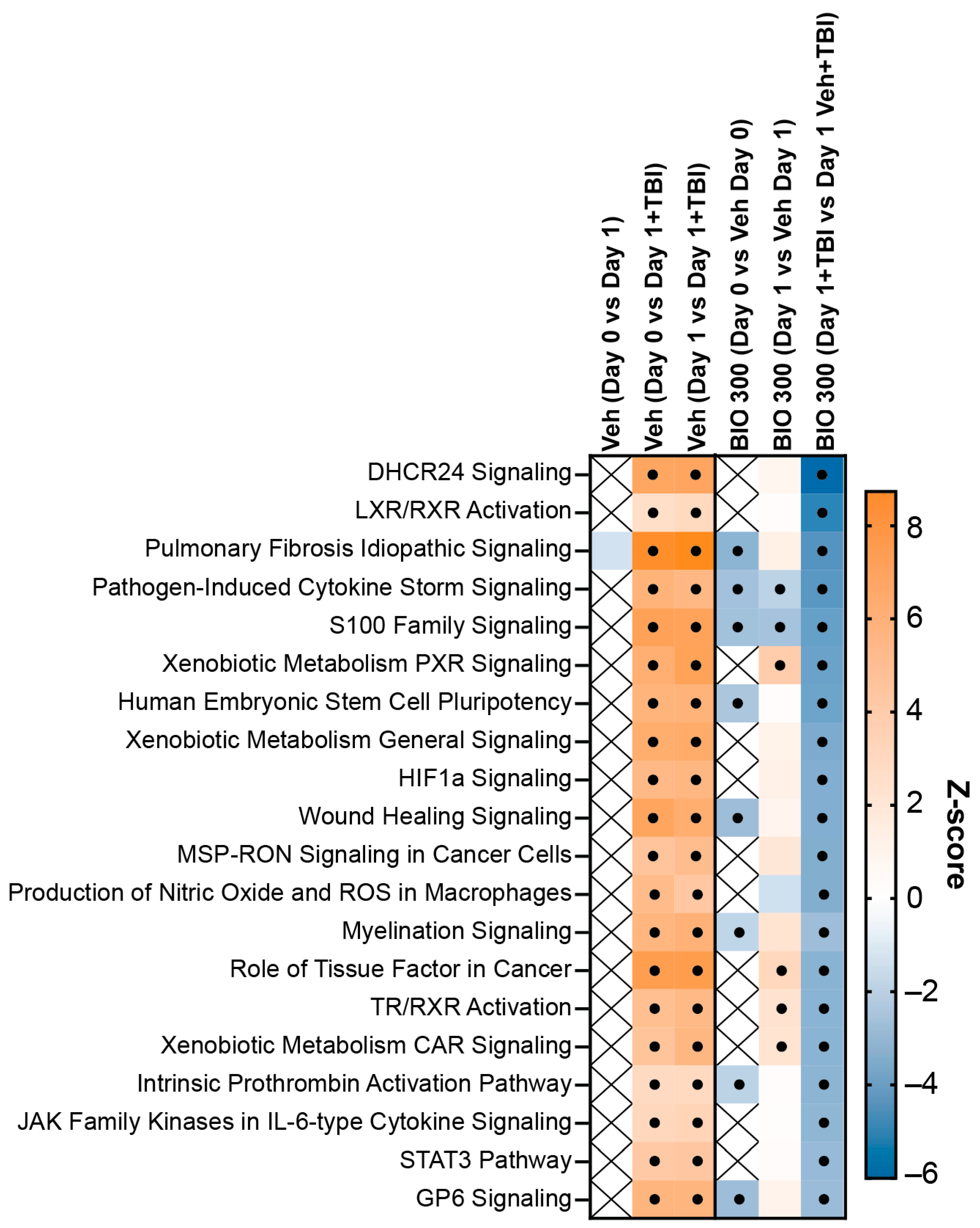
2.3. Identifying Putative Biomarkers of BIO 300 for Human Dose Selection
2.4. Comparing Transcriptome Changes between BIO 300-Treated Mice and Humans
3. Discussion
4. Materials and Methods
4.1. Study Design
4.2. Animal
4.3. Total-Body Irradiation
4.4. Drug Preparation and Dosing
4.5. Blood Sample Collection
4.6. RNA Extraction
4.7. RNA Sequencing and Analyses
4.8. Signaling Pathway Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, V.K.; Seed, T.M. A review of radiation countermeasures focusing on injury-specific medicinals and regulatory approval status: Part I. Radiation sub-syndromes, animal models and FDA-approved countermeasures. Int. J. Radiat. Biol. 2017, 93, 851–869. [Google Scholar] [CrossRef] [PubMed]
- Van Dyk, J.; Keane, T.; Kan, S.; Rider, W.; Fryer, C. Radiation pneumonitis following large single dose irradiation: A re-evaluation based on absolute dose to lung. Int. J. Radiat. Oncol. Biol. Phys. 1981, 7, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Gross, N.J. Pulmonary Effects of Radiation Therapy. Ann. Intern. Med. 1977, 86, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.P.; Jackson, I.L.; Shah, J.R.; Czarniecki, C.W.; Maidment, B.W.; DiCarlo, A.L. Animal models and medical countermeasures development for radiation-induced lung damage: Report from an NIAID workshop. Radiat. Res. 2012, 177, e0025–e0039. [Google Scholar] [CrossRef]
- Elliott, T.B.; Deutz, N.E.; Gulani, J.; Koch, A.; Olsen, C.H.; Christensen, C.; Chappell, M.; Whitnall, M.H.; Moroni, M. Gastrointestinal acute radiation syndrome in Göttingen minipigs (Sus scrofa domestica). Comp. Med. 2014, 64, 456–463. [Google Scholar] [PubMed]
- Farese, A.; MacVittie, T. Filgrastim for the treatment of hematopoietic acute radiation syndrome. Drugs Today 2015, 51, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Seed, T. An update on sargramostim for treatment of acute radiation syndrome. Drugs Today 2018, 54, 679–693. [Google Scholar] [CrossRef] [PubMed]
- Hankey, K.G.; Farese, A.M.; Blaauw, E.C.; Gibbs, A.M.; Smith, C.P.; Katz, B.P.; Tong, Y.; Prado, K.L.; MacVittie, T.J. Pegfilgrastim Improves Survival of Lethally Irradiated Nonhuman Primates. Radiat. Res. 2015, 183, 643–655. [Google Scholar] [CrossRef]
- Clayton, N.P.; Khan-Malek, R.C.; Dangler, C.A.; Zhang, D.; Ascah, A.; Gains, M.; Gardner, B.; Mockbee, C.; Keutzer, J.M.; McManus, J.; et al. Sargramostim (rhu GM-CSF) Improves Survival of Non-Human Primates with Severe Bone Marrow Suppression after Acute, High-Dose, Whole-Body Irradiation. Radiat. Res. 2021, 195, 191–199. [Google Scholar] [CrossRef]
- Zhong, Y.; Pouliot, M.; Downey, A.-M.; Mockbee, C.; Roychowdhury, D.; Wierzbicki, W.; Authier, S. Efficacy of delayed administration of sargramostim up to 120 hours post exposure in a nonhuman primate total body radiation model. Int. J. Radiat. Biol. 2021, 97, S100–S116. [Google Scholar] [CrossRef]
- Farese, A.M.; Cohen, M.V.; Katz, B.P.; Smith, C.P.; Gibbs, A.; Cohen, D.M.; MacVittie, T.J. Filgrastim Improves Survival in Lethally Irradiated Nonhuman Primates. Radiat. Res. 2013, 179, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, H.M.; McManus, J.; Gale, R.P. Sargramostim in acute radiation syndrome. Expert Opin. Biol. Ther. 2022, 22, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Chang, P.Y.; Fielden, M.; Downey, A.M.; Bunin, D.; Bakke, J.; Gahagen, J.; Iyer, L.; Doshi, S.; Wierzbicki, W.; et al. Pharmacodynamics of romiplostim alone and in combination with pegfilgrastim on acute radiation-induced thrombocytopenia and neutropenia in non-human primates. Int. J. Radiat. Biol. 2020, 96, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Bunin, D.I.; Javitz, H.S.; Gahagen, J.; Bakke, J.; Lane, J.H.; Andrews, D.A.; Chang, P.Y. Survival and Hematologic Benefits of Romiplostim after Acute Radiation Exposure Supported FDA Approval under the Animal Rule. Int. J. Radiat. Oncol. Biol. Phys. 2023, 117, 705–717. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. Radiological and Nuclear Emergency Preparedness Information from FDA. 2024. Available online: https://www.fda.gov/emergency-preparedness-and-response/mcm-issues/radiological-and-nuclear-emergency-preparedness-information-fda (accessed on 21 July 2024).
- Fresenius Kabi. STIMUFEND (Pegfilgrastim-Fpgk) is Biosimilar* to NEULASTA (pegfilgrastim). 2023. Available online: https://www.fda.gov/media/172679/download?attachment (accessed on 28 December 2023).
- Coherus BioSciences Inc. UDENYCA (Pegfilgrastim-Cbqv) is Biosimilar* to NEULASTA (Pegfilgrastim). 2022. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/761039s014lbl.pdf (accessed on 28 December 2023).
- Singh, V.K.; Seed, T.M. Development of gamma-tocotrienol as a radiation medical countermeasure for the acute radiation syndrome: Current status and future perspectives. Expert Opin. Investig. Drugs 2023, 32, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Kiang, J.G.; Cannon, G.; Singh, V.K. An Overview of Radiation Countermeasure Development in Radiation Research from 1954 to 2024. Radiat. Res. 2024, 202, 420–431. [Google Scholar] [CrossRef]
- Singh, V.K.; Fatanmi, O.O.; Wise, S.Y.; Carpenter, A.; Nakamura-Peek, S.; Serebrenik, A.A.; Kaytor, M.D. A novel oral formulation of BIO 300 confers prophylactic radioprotection from acute radiation syndrome in mice. Int. J. Radiat. Biol. 2022, 98, 958–967. [Google Scholar] [CrossRef] [PubMed]
- Landauer, M.R.; Harvey, A.J.; Kaytor, M.D.; Day, R.M. Mechanism and therapeutic window of a genistein nanosuspension to protect against hematopoietic-acute radiation syndrome. J. Radiat. Res. 2019, 60, 308–317. [Google Scholar] [CrossRef]
- Singh, V.K.; Serebrenik, A.A.; Fatanmi, O.O.; Wise, S.Y.; Carpenter, A.D.; Janocha, B.L.; Kaytor, M.D. The Radioprotectant, BIO 300, Protects the Lungs from Total-Body Irradiation Injury in C57L/J Mice. Radiat. Res. 2023, 199, 294–300. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Guidance Document: Product Development under the Animal Rule. 2015. Available online: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm399217.pdf (accessed on 20 October 2022).
- Vellichirammal, N.N.; Sethi, S.; Avuthu, N.; Wise, S.Y.; Carpenter, A.D.; Fatanmi, O.O.; Guda, C.; Singh, V.K. Transcriptome profile changes in the jejunum of nonhuman primates exposed to supralethal dose of total- or partial-body radiation. BMC Genom. 2023, 24, 274. [Google Scholar] [CrossRef]
- Vellichirammal, N.N.; Sethi, S.; Pandey, S.; Singh, J.; Wise, S.Y.; Carpenter, A.D.; Fatanmi, O.O.; Guda, C.; Singh, V.K. Lung transcriptome of nonhuman primates exposed to total- and partial-body irradiation. Mol. Ther.-Nucleic Acids 2022, 29, 584–598. [Google Scholar] [CrossRef]
- Li, Y.; Girgis, M.; Jayatilake, M.; Serebrenik, A.A.; Cheema, A.K.; Kaytor, M.D.; Singh, V.K. Pharmacokinetic and metabolomic studies with a BIO 300 Oral Powder formulation in nonhuman primates. Sci. Rep. 2022, 12, 13475. [Google Scholar] [CrossRef] [PubMed]
- Girgis, M.; Li, Y.; Ma, J.; Sanda, M.; Wise, S.Y.; Fatanmi, O.O.; Kaytor, M.D.; Cheema, A.K.; Singh, V.K. Comparative proteomic analysis of serum from nonhuman primates administered BIO 300: A promising radiation countermeasure. Sci. Rep. 2020, 10, 19343. [Google Scholar] [CrossRef] [PubMed]
- Serebrenik, A.A.; Verduyn, C.W.; Kaytor, M.D. Safety, Pharmacokinetics, and Biomarkers of an Amorphous Solid Dispersion of Genistein, a Radioprotectant, in Healthy Volunteers. Clin. Pharmacol. Drug Dev. 2023, 12, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Braunstein, Z.; Toomey, A.C.; Zhong, J.; Rao, X. S100 Proteins As an Important Regulator of Macrophage Inflammation. Front. Immunol. 2017, 8, 1908. [Google Scholar] [CrossRef]
- Carr, D.J. Increased levels of IFN-gamma in the trigeminal ganglion correlate with protection against HSV-1-induced encephalitis following subcutaneous administration with androstenediol. J. Neuroimmunol. 1998, 89, 160–167. [Google Scholar] [CrossRef]
- Haroun, R.; Naasri, S.; Oweida, A.J. Toll-Like Receptors and the Response to Radiotherapy in Solid Tumors: Challenges and Opportunities. Vaccines 2023, 11, 818. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. More about Biomarkers & Qualification. 2018. Available online: https://www.fda.gov/Drugs/DevelopmentApprovalProcess/DrugDevelopmentToolsQualificationProgram/BiomarkerQualificationProgram/ucm535408.htm (accessed on 18 May 2018).
- Ha, C.T.; Li, X.-H.; Fu, D.; Xiao, M.; Landauer, M.R. Genistein nanoparticles protect mouse hematopoietic system and prevent proinflammatory factors after gamma irradiation. Radiat. Res. 2013, 180, 316–325. [Google Scholar] [CrossRef]
- Jia, Z.; Babu, P.V.A.; Si, H.; Nallasamy, P.; Zhu, H.; Zhen, W.; Misra, H.P.; Li, Y.; Liu, D. Genistein inhibits TNF-α-induced endothelial inflammation through the protein kinase pathway A and improves vascular inflammation in C57BL/6 mice. Int. J. Cardiol. 2013, 168, 2637–2645. [Google Scholar] [CrossRef]
- Sutrisno, S.; Aprina, H.; Simanungkalit, H.M.; Andriyani, A.; Barlianto, W.; Sujuti, H.; Santoso, S.; Dwijayasa, P.M.; Wahyuni, E.S.; Mustofa, E. Genistein modulates the estrogen receptor and suppresses angiogenesis and inflammation in the murine model of peritoneal endometriosis. J. Tradit. Complement. Med. 2018, 8, 278–281. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. 2005. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/estimating-maximum-safe-starting-dose-initial-clinical-trials-therapeutics-adult-healthy-volunteers (accessed on 21 December 2023).
- Simone, C.B.; Serebrenik, A.A.; Gore, E.M.; Mohindra, P.; Brown, S.L.; Wang, D.; Chetty, I.J.; Vujaskovic, Z.; Menon, S.; Thompson, J.; et al. Multicenter phase 1b/2a clinical trial of radioprotectant BIO 300 oral suspension for patients with non-small cell lung cancer receiving concurrent chemoradiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2023, 118, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.J.; Deoliveira, D.; Spasojevic, I.; Sempowski, G.D.; Jiang, C.; Owzar, K.; Wang, X.; Gesty-Palmer, D.; Cline, J.M.; Bourland, J.D.; et al. Growth hormone mitigates against lethal irradiation and enhances hematologic and immune recovery in mice and nonhuman primates. PLoS ONE 2010, 5, e11056. [Google Scholar] [CrossRef] [PubMed]
- Jackson, I.L.; Zodda, A.; Gurung, G.; Pavlovic, R.; Kaytor, M.D.; Kuskowski, M.A.; Vujaskovic, Z. BIO 300, a nanosuspension of genistein, mitigates pneumonitis/fibrosis following high-dose radiation exposure in the C57L/J murine model. Br. J. Pharmacol. 2017, 174, 4738–4750. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yu, J.; Jones, J.W.; Carter, C.L.; Pierzchalski, K.; Tudor, G.; Booth, C.; MacVittie, T.J.; Kane, M.A. Proteomic evaluation of the acute radiation syndrome of the gastrointestinal tract in a murine total-body irradiation model. Health Phys. 2019, 116, 516–528. [Google Scholar] [CrossRef]
- Patterson, A.M.; Vemula, S.; Plett, P.A.; Sampson, C.H.; Chua, H.L.; Fisher, A.; Wu, T.; Sellamuthu, R.; Feng, H.; Katz, B.P.; et al. Age and Sex Divergence in hematopoietic radiosensitivity in aged mouse models of the hematopoietic acute radiation syndrome. Radiat. Res. 2022, 198, 221–242. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Singh, J.; Varghese, R.; Zhang, Y.; Fatanmi, O.O.; Cheema, A.K.; Singh, V.K. Transcriptome of rhesus macaque (Macaca mulatta) exposed to total-body irradiation. Sci. Rep. 2021, 11, 6295. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. The Subread aligner: Fast, accurate and scalable read mapping by seed-and-vote. Nucleic Acids Res. 2013, 41, e108. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]


| Control | Treatment | Up | Down | Total DEGs |
|---|---|---|---|---|
| Vehicle–Day 1 | BIO 300 (200 mg/kg)–Day 1 | 3555 | 4125 | 7680 |
| Vehicle–Day 1 | BIO 300 (100 mg/kg)–Day 1 | 3612 | 4036 | 7648 |
| Vehicle–Day 1 | Vehicle + TBI–Day 1 | 4506 | 2676 | 7182 |
| Vehicle–Day 0 | Vehicle + TBI–Day 1 | 3244 | 2146 | 5390 |
| Vehicle–Day 0 | BIO 300 (50 mg/kg)–Day 0 | 8 | 1131 | 1139 |
| Vehicle–Day 1 | BIO 300 (50 mg/kg)–Day 1 | 35 | 20 | 55 |
| Vehicle–Day 0 | Vehicle–Day 1 | 1 | 37 | 38 |
| Vehicle–Day 0 | BIO 300 (200 mg/kg)–Day 0 | 3 | 0 | 3 |
| Vehicle–Day 0 | BIO 300 (100 mg/kg)–Day 0 | 0 | 2 | 2 |
| Vehicle + TBI–Day 1 | BIO 300 (200 mg/kg) + TBI–Day 1 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serebrenik, A.A.; Fatanmi, O.O.; Wise, S.Y.; Petrus, S.A.; Kaytor, M.D.; Singh, V.K. BIO 300 Attenuates Whole Blood Transcriptome Changes in Mice Exposed to Total-Body Radiation. Int. J. Mol. Sci. 2024, 25, 8818. https://doi.org/10.3390/ijms25168818
Serebrenik AA, Fatanmi OO, Wise SY, Petrus SA, Kaytor MD, Singh VK. BIO 300 Attenuates Whole Blood Transcriptome Changes in Mice Exposed to Total-Body Radiation. International Journal of Molecular Sciences. 2024; 25(16):8818. https://doi.org/10.3390/ijms25168818
Chicago/Turabian StyleSerebrenik, Artur A., Oluseyi O. Fatanmi, Stephen Y. Wise, Sarah A. Petrus, Michael D. Kaytor, and Vijay K. Singh. 2024. "BIO 300 Attenuates Whole Blood Transcriptome Changes in Mice Exposed to Total-Body Radiation" International Journal of Molecular Sciences 25, no. 16: 8818. https://doi.org/10.3390/ijms25168818






