Infestation of Rice Striped Stem Borer (Chilo suppressalis) Larvae Induces Emission of Volatile Organic Compounds in Rice and Repels Female Adult Oviposition
Abstract
1. Introduction
2. Results
2.1. Pre-Infestation of Rice by SSB Larvae Reduced Subsequent SSB Female Adults Oviposition
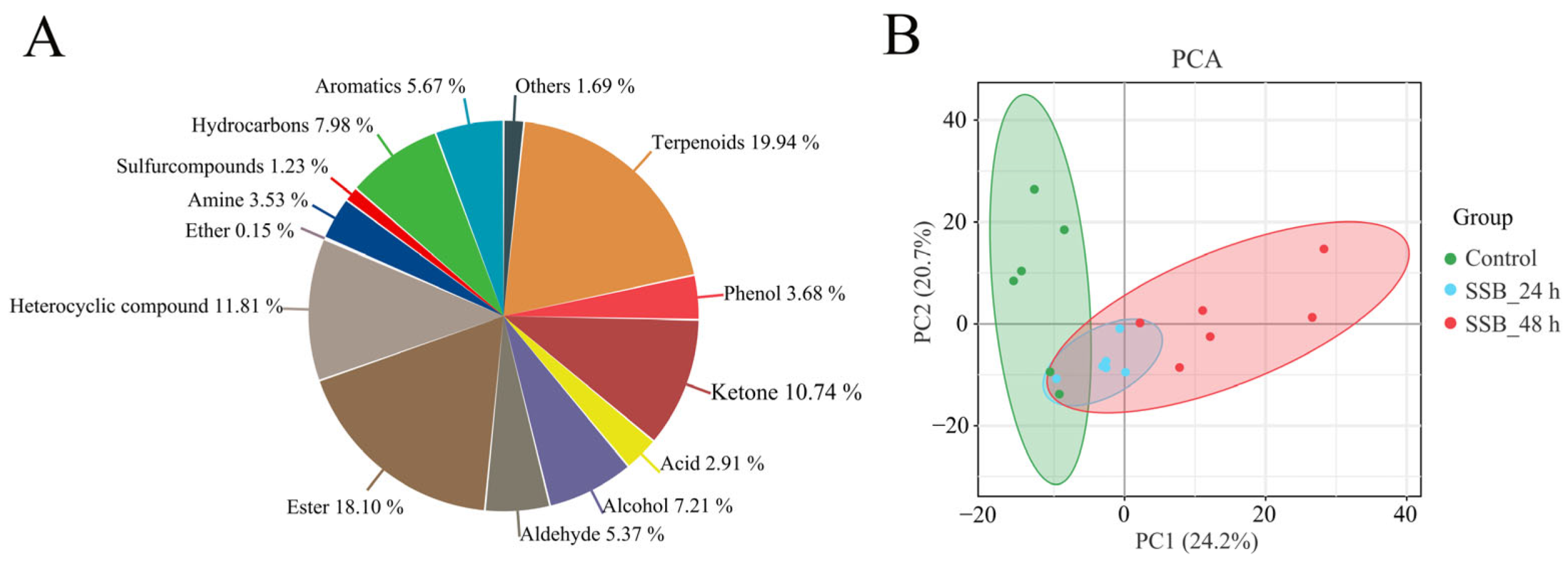
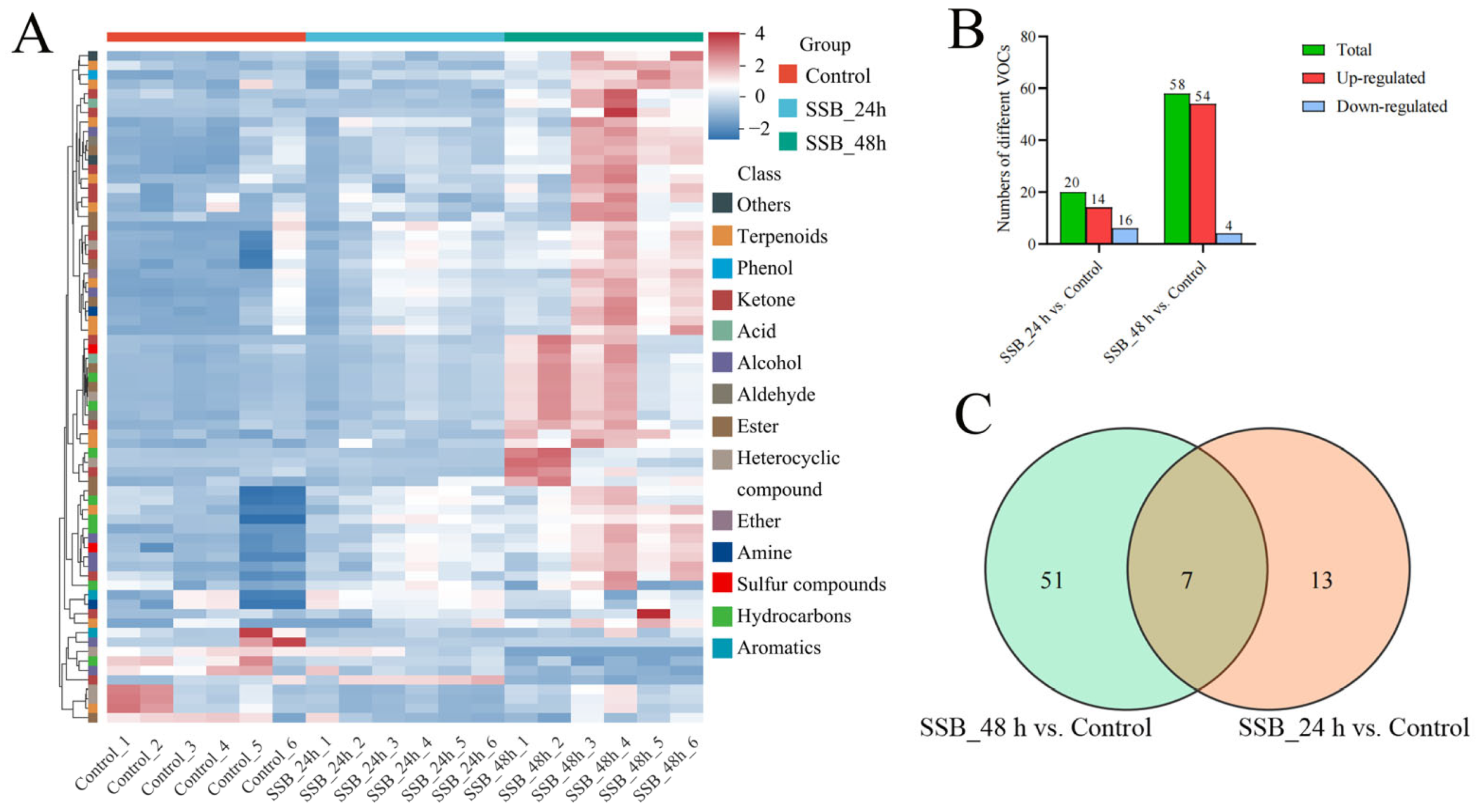
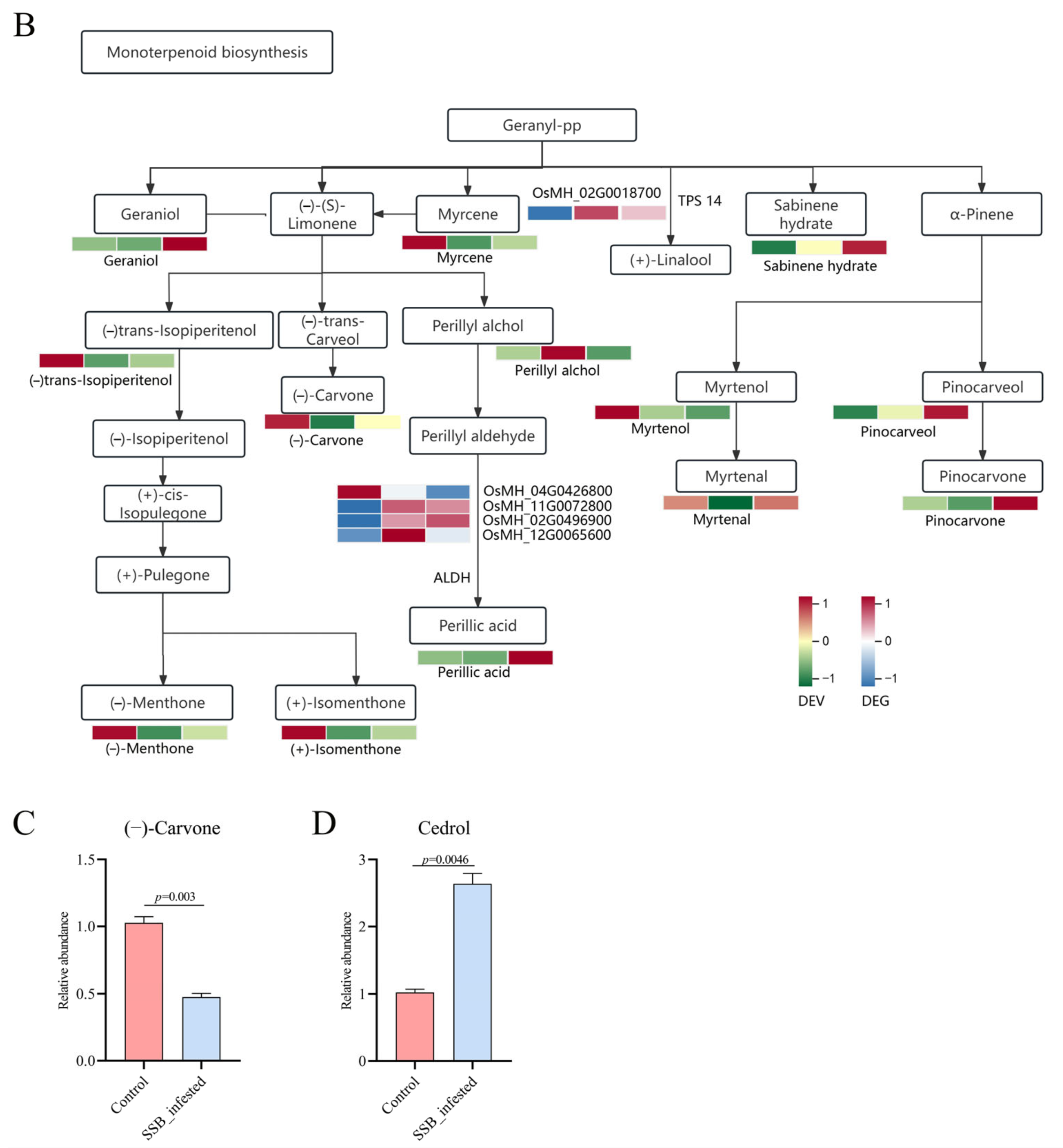
2.2. VOC Analysis of SSB-Infested Rice Plants
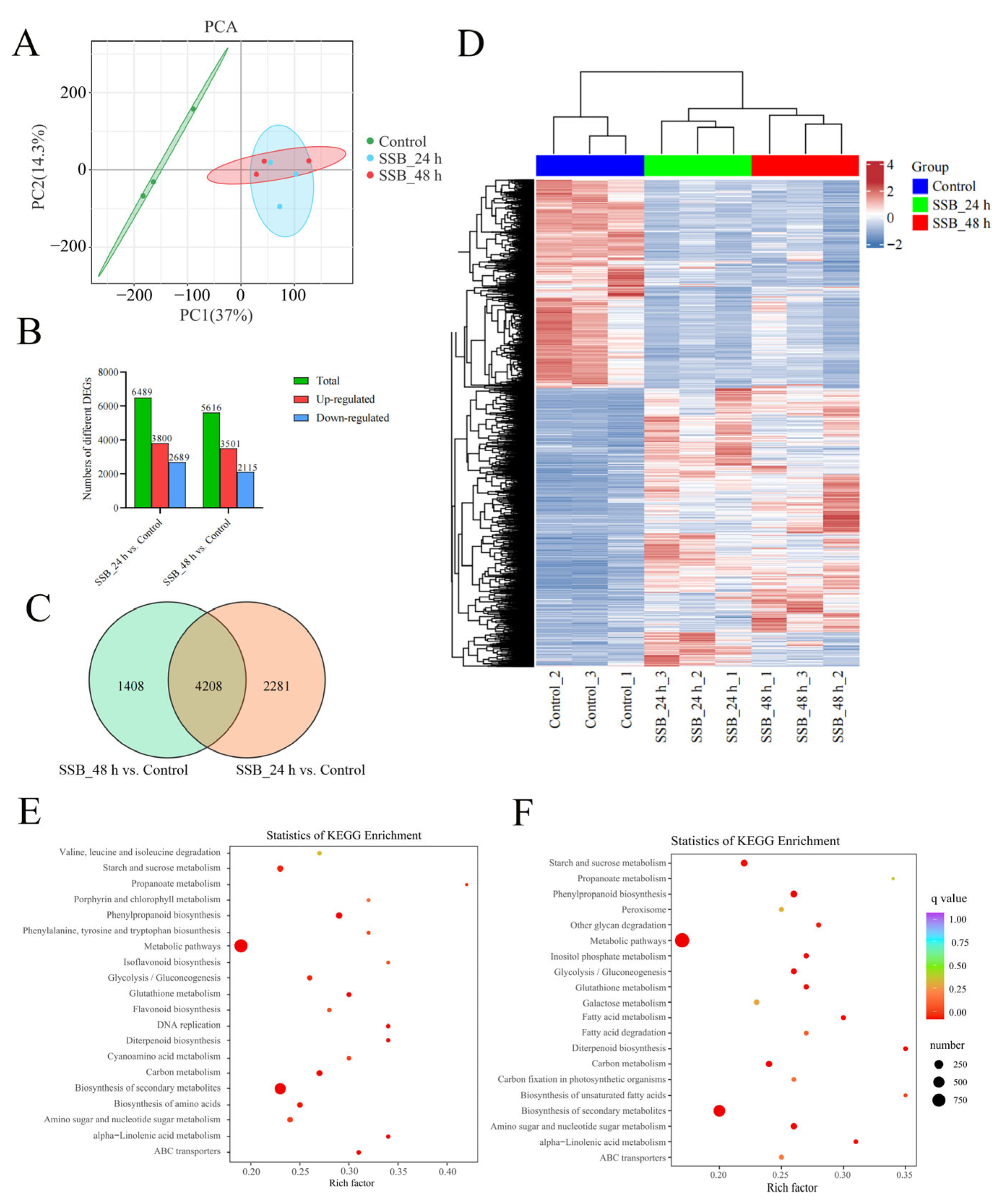
2.3. Responses of Rice Plants to SSB Infestation at the Transcriptional Level
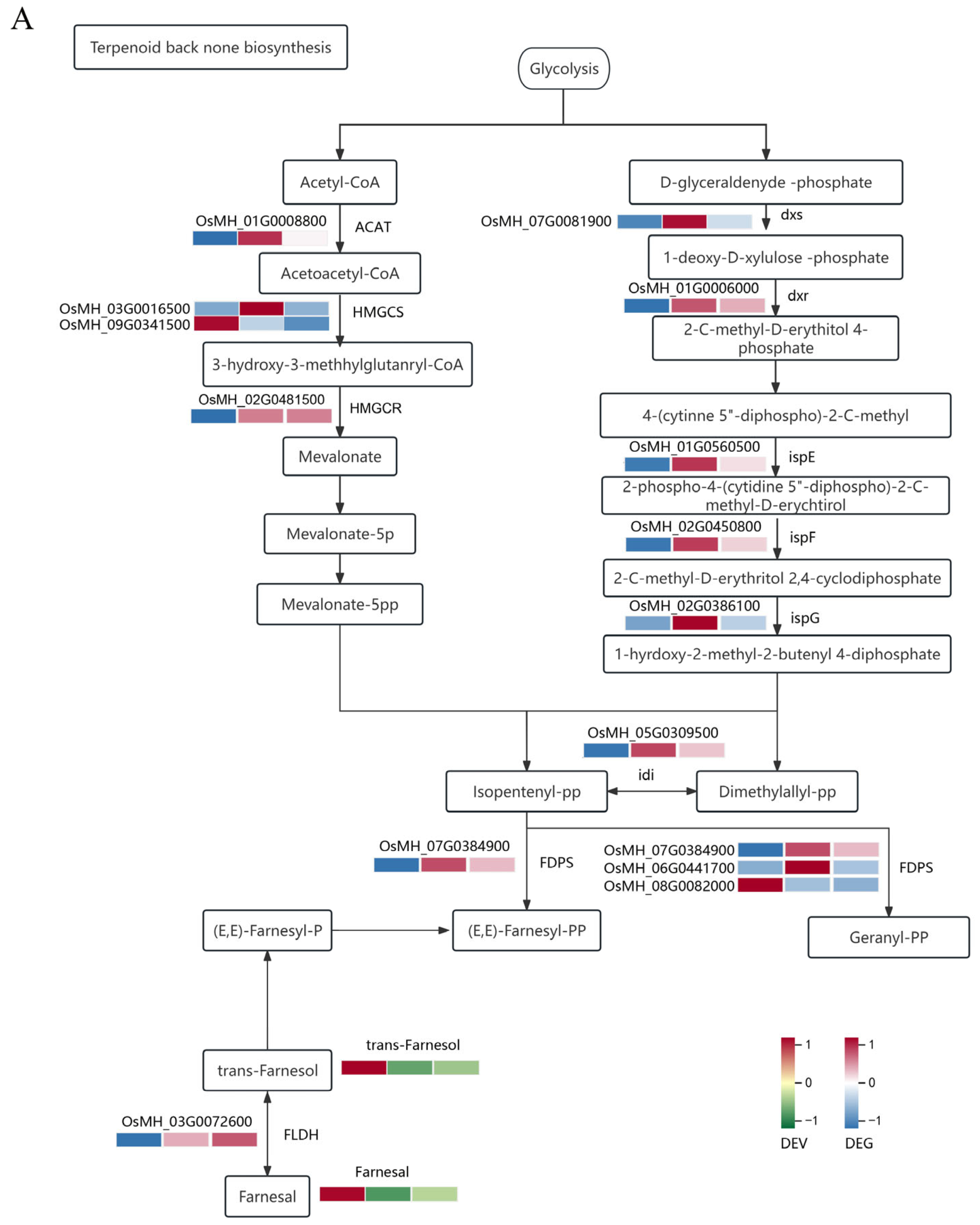
2.4. Correlation Analysis between the Transcriptome and Metabolome
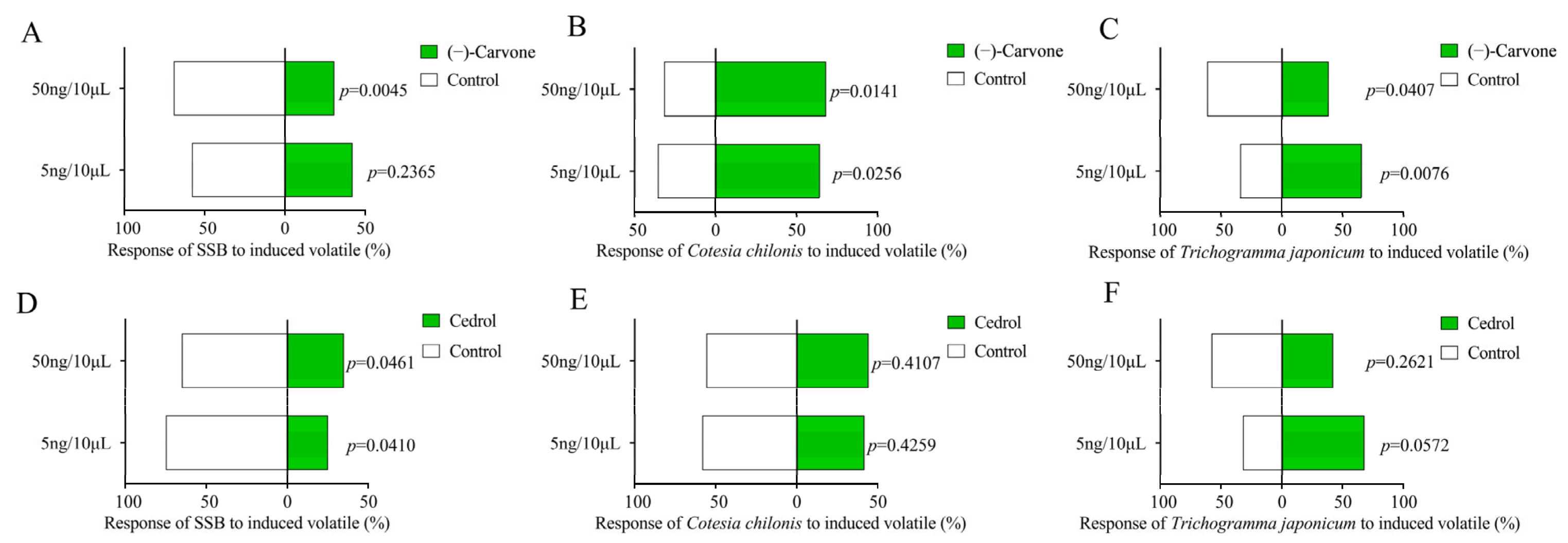
2.5. GC-MS Validation and Behaviour Response on SSB and Parasitic Wasps to Candidate Vocs
3. Discussion
4. Materials and Methods
4.1. Plant Growth and Insect Colony
4.2. Plant Treatment with SSB Caterpillars
4.3. SSB Larvae Bioassay
4.4. Widely Targeted Rice Volatilome by Headspace Solid-Phase Microextraction-Gas Chromatography-Mass Spectrometry (HS-SPME-GC-MS)
4.5. RNA Preparation and Construction of Sequencing Library
4.6. Vocs Detection and DEG Enrichment
4.7. Gas Chromatography-Mass Spectrometry (GC-MS)
4.8. Gene Expression Quantification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fürstenberg-Hägg, J.; Zagrobelny, M.; Bak, S. Plant defense against insect herbivores. Int. J. Mol. Sci. 2013, 14, 10242–10297. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Qi, L.X.; Tan, X.Y.; Li, S.F.; Fang, J.C.; Ji, R. Small brown planthopper nymph infestation regulates plant defenses by affecting secondary metabolite biosynthesis in rice. Int. J. Mol. Sci. 2023, 24, 4764. [Google Scholar] [CrossRef] [PubMed]
- Turlings, T.C.J.; Erb, M. Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Aratani, Y.; Uemura, T.; Hagihara, T.; Matsui, K.; Toyota, M. Green leaf volatile sensory calcium transduction in Arabidop. Nat. Commun. 2023, 14, 6236. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Wang, Y.; He, L.; Huang, F.; Zhang, D.; Wang, Y.; Wei, X.; Han, M.; Deng, H.; Luo, L.; et al. Molecular basis of methyl-salicylate-mediated plant airborne defence. Nature 2023, 622, 139–148. [Google Scholar] [CrossRef]
- Hu, X.Y.; Su, S.; Liu, Q.; Jiao, Y.; Peng, Y.; Li, Y.; Turlings, T.C.J. Caterpillar-induced rice volatiles provide enemy-free space for the offspring of the brown planthopper. Elife 2020, 9, e55421. [Google Scholar] [CrossRef]
- Li, S.; Tan, X.; He, Z.; Jiang, L.; Li, Y.; Yang, L.; Hoffmann, A.A.; Zhao, Q.; Fang, J.; Ji, R. Transcriptome-wide N6-methyladenosine profiling reveals growth-defense trade-offs in the response of rice to brown planthopper (Nilaparvata lugens) infestation. Pest Manag. Sci. 2024. [Google Scholar] [CrossRef]
- Stam, J.M.; Kroes, A.; Li, Y.; Gols, R.; van Loon, J.J.; Poelman, E.H.; Dicke, M. Plant interactions with multiple insect herbivores: From community to genes. Annu. Rev. Plant Biol. 2014, 65, 689–713. [Google Scholar] [CrossRef]
- Knolhoff, L.M.; Heckel, D.G. Behavioral assays for studies of host plant choice and adaptation in herbivorous insects. Annu. Rev. Entomol. 2014, 59, 263–278. [Google Scholar] [CrossRef]
- D’Alessandro, M.; Held, M.; Triponez, Y.; Turlings, T.C.J. The role of indole and other shikimic acid derived maize volatiles in the attraction of two parasitic wasps. J. Chem. Ecol. 2006, 32, 2733–2748. [Google Scholar] [CrossRef]
- Allmann, S.; Baldwin, R.T. Insects Betray Themselves in nature to predators by rapid isomerization of green leaf volatiles. Science 2010, 329, 1075–1078. [Google Scholar] [CrossRef] [PubMed]
- Alborn, H.T.; Turlings, T.C.J.; Jones, T.H.; Stenhagen, G.S. An elicitor of plant volatiles from beet armyworm oral secretion. Science 1997, 276, 945–949. [Google Scholar] [CrossRef]
- Carroll, M.J.; Schmelz, E.A.; Meagher, R.L.; Teal, P.E. Attraction of spodoptera frugiperda larvae to volatiles from herbivore-damaged maize seedlings. J. Chem. Ecol. 2006, 32, 1911–1924. [Google Scholar] [CrossRef] [PubMed]
- Cajka, T.; Fiehn, O. Toward merging untargeted and targeted methods in mass spectrometry-based metabolomics and lipidomics. Anal. Chem. 2016, 88, 524–545. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Tsugawa, H.; Wohlgemuth, G.; Mehta, S.; Mueller, M.; Zheng, Y.; Ogiwara, A.; Meissen, J.; Showalter, M.; Takeuchi, K.; et al. Identifying metabolites by integrating metabolome databases with mass spectrometry cheminformatics. Nat. Methods 2017, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, A.; Qiu, Y.; Jia, W.; Walker, D.I.; Jones, D.P.; Du, X. ADAP-GC 4.0: Application of clustering-assisted multivariate curve resolution to spectral deconvolution of gas chromatographymass spectrometry metabolomics data. Anal. Chem. 2019, 91, 9069–9077. [Google Scholar] [CrossRef]
- Yuan, H.; Cao, G.; Hou, X.; Huang, M.; Du, P.; Tan, T.; Zhang, Y.; Zhou, H.; Liu, X.; Liu, L.; et al. Development of a widely targeted volatilomics method for profiling volatilomes in plants. Mol. Plant. 2022, 15, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xin, M.; Li, L.; He, X.; Yi, P.; Tang, Y.; Li, J.; Zheng, F.; Liu, G.; Sheng, J.; et al. Characterization of the aromatic profile of purple passion fruit (Passiflora edulis Sims) during ripening by HS-SPME-GC/MS and RNA sequencing. Food Chem. 2021, 355, 129685. [Google Scholar] [CrossRef]
- Sha, H.; Li, S.; Li, J.; Zhao, J.; Su, D. Widely targeted metabolomics and network pharmacology reveal the nutritional potential of yellowhorn (Xanthoceras sorbifolium Bunge) leaves and flowers. Foods 2024, 13, 1274. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, X.; Tzin, V.; Romeis, J.; Peng, Y.; Li, Y. Combined transcriptome and metabolome analyses to understand the dynamic responses of rice plants to attack by the rice stem borer Chilo suppressalis (Lepidoptera: Crambidae). BMC Plant Biol. 2016, 16, 259. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.; Zhou, L.; You, S.; Deng, H.; Chen, Y.; Alseekh, S.; Yuan, Y.; Fu, R.; Zhang, Z.; et al. MicroTom metabolic network: Rewiring tomato metabolic regulatory network throughout the Growth Cycle. Mol. Plant. 2020, 13, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.A.; Purugganan, M.D.; Zhang, Q.F. The rice genome revolution: From an ancient grain to Green Super Rice. Nat. Rev. Genet. 2018, 19, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.X.; Zheng, X.S.; Yang, Y.J.; Tian, J.C.; Lu, Y.H.; Tan, K.H.; Heong, K.L.; Lu, Z.X. Methyl eugenol bioactivities as a new potential botanical insecticide against major insect pests and their natural enemies on rice (Oriza sativa). Crop Prot. 2015, 72, 144–149. [Google Scholar] [CrossRef]
- Ju, J.F.; Yang, L.; Shen, C.; Li, J.C.; Hoffmann, A.A.; Huang, Y.X.; Zhu, F.; Ji, R.; Luo, G.H.; Fang, J.C. Defence and nutrition synergistically contribute to the distinct tolerance of rice subspecies to the stem borer, Chilo suppressalis. Plant Cell Environ. 2024, 47, 2426–2442. [Google Scholar] [CrossRef]
- He, K.; Xiao, H.; Sun, Y.; Ding, S.M.; Situ, G.M.; Li, F. Transgenic microRNA-14 rice shows high resistance to rice stem borer. Plant Biotechnol. J. 2019, 17, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Baldwin, I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 2021, 291, 2141–2144. [Google Scholar] [CrossRef]
- Yao, C.; Du, L.; Liu, Q.S.; Hu, X.Y.; Ye, W.F.; Turlings, T.C.J.; Li, Y.H. Stemborer-induced rice plant volatiles boost direct and indirect resistance in neighboring plants. New Phytol. 2023, 237, 2375–2387. [Google Scholar] [CrossRef]
- Kiryu, M.; Hamanaka, M.; Yoshitomi, K.; Mochizuki, S.; Akimitsu, K.; Gomi, K. Rice terpene synthase 18 (OsTPS18) encodes a sesquiterpene synthase that produces an antibacterial (E)-nerolidol against a bacterial pathogen of rice. J. Gen. Plant Pathol. 2018, 84, 221–229. [Google Scholar] [CrossRef]
- Wang, Y.D.; Qiu, L.; Wang, B.; Guan, Z.Y.; Dong, Z.; Zhang, J.; Cao, S.; Yang, L.L.; Wang, B.; Gong, Z.; et al. Structural basis for odorant recognition of the insect odorant receptor OR-Orco heterocomplex. Science 2024, 384, 1453–1460. [Google Scholar] [CrossRef]
- Yang, L.; Yao, X.M.; Liu, B.S.; Han, Y.C.; Ji, R.; Ju, J.F.; Zhang, X.N.; Wu, S.W.; Fang, J.C.; Sun, Y. Caterpillar-Induced rice volatile (E)-β-Farnesene Impairs the development and survival of Chilo suppressalis larvae by disrupting insect hormone balance. Front. Physiol. 2022, 13, 904482. [Google Scholar] [CrossRef]
- Ye, M.; Liu, M.M.; Erb, M.; Glauser, G.; Zhang, J.; Li, X.W.; Sun, X.L. Indole primes defence signalling and increases herbivore resistance in tea plants. Plant Cell Environ. 2021, 44, 1165–1177. [Google Scholar] [CrossRef]
- Hu, L.; Ye, M.; Erb, M. Integration of two herbivore-induced plant volatiles results in synergistic effects on plant defence and resistance. Plant Cell Environ. 2019, 42, 959–971. [Google Scholar] [CrossRef] [PubMed]
- James, D.G.; Price, T.S. Field-testing of methyl salicylate for recruitment and retention of beneficial insects in grapes and hops. J. Chem. Ecol. 2004, 30, 1613–1628. [Google Scholar] [CrossRef] [PubMed]
- Zakir1, A.M.; Sadek, M.; Bengtsson, M.; Hansson, B.; Witzgall, P. Herbivore-induced plant volatiles provide associational resistance against an ovipositing herbivore. J. Ecol. 2013, 101, 410–417. [Google Scholar] [CrossRef]
- Lou, Y.; Hua, X.; Turlings, T.C.J.; Cheng, J.; Chen, X.; Ye, G. Differences in induced volatile emissions among rice varieties result in differential attraction and parasitism of Nilaparvata lugens eggs by the parasitoid Anagrus nilaparvatae in the Field. J. Chem. Ecol. 2006, 32, 2375–2387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Su, R.; Yuan, H.; Zhou, H.; Jiangfang, Y.; Liu, X.; Luo, J. Widely targeted volatilomics and metabolomics analysis reveal the metabolic composition and diversity of zingiberaceae plants. Metabolites 2023, 13, 700. [Google Scholar] [CrossRef]
- Yuan, H.; Jiangfang, Y.; Liu, Z.; Su, R.; Li, Q.; Fang, C.; Huang, S.; Liu, X.; Fernie, A.R.; Luo, J. WTV2.0: A high-coverage plant volatilomics method with a comprehensive selective ion monitoring acquisition mode. Mol. Plant 2024, 17, 972–985. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, X.; Su, S.; Ning, Y.; Peng, Y.; Ye, G.; Lou, Y.; Turlings, T.C.J.; Li, Y. Cooperative herbivory between two important pests of rice. Nat. Commun. 2021, 12, 6772. [Google Scholar] [CrossRef]
- Han, L.Z.; Li, S.B.; Liu, P.L.; Peng, Y.F.; Hou, M.L. New artificial diet for continuous rearing of Chilo suppressalis (Lepidoptera: Crambidae). Ann. Entomol. Soc. Am. 2012, 105, 253–258. [Google Scholar] [CrossRef]
- Colazza, S.; Salerno, G.; Wajnberg, E. Volatile and contact chemicals released by Nezara viridula (Heteroptera: Pentatomidae) have a kairomonal effect on the egg parasitoid Trissolcus basalis (Hymenoptera: Scelionidae). Biol. Control 1999, 16, 310–317. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Varet, H.; Brillet-Guéguen, L.; Coppée, J.Y.; Dillies, M.A. SARTools: A DESeq2- and EdgeR-based R pipeline for comprehensive differential analysis of RNA-seq data. PLoS ONE 2016, 11, e0157022. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for linking genomes to life and the environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. the gene ontology consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the human adult urinary metabolome variations with age, body mass index, and gender by implementing a comprehensive workflow for univariate and OPLS Statistical Analyses. J. Proteome. Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Q.; Erb, M.; Turlings, T.C.J.; Ge, L.; Hu, L.; Li, J.; Han, X.; Zhang, T.; Lu, J.; et al. Specific herbivore-induced volatiles defend plants and determine insect community composition in the field. Ecol. Lett. 2012, 15, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- De Lange, E.S.; Laplanche, D.; Guo, H.; Xu, W.; Vlimant, M.; Erb, M.; Ton, J.; Turlings, T.C.J. Spodoptera frugiperda caterpillars suppress herbivore-induced volatile emissions in maize. J. Chem. Ecol. 2020, 46, 344–360. [Google Scholar] [CrossRef] [PubMed]
- Laveroni, S.L.; Parks, V.R. Quantitative PCR of Alu repeats using PowerUpTM SYBR® green master mix. Methods Mol. Biol. 2023, 2685, 149–174. [Google Scholar] [CrossRef]
- Bevitori, R.; Oliveira, M.B.; Grossi-de-Sá, M.F.; Lanna, A.C.; Da, S.R.D.; Petrofeza, S. Selection of optimized candidate reference genes for qRT-PCR normalization in rice (Oryza sativa L.) during magnaporthe oryzae infection and drought. Genet. Mol. Res. 2014, 13, 9795–9805. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.; Yu, S.; Tan, X.; Luo, G.; Yu, Z.; Ju, J.; Yang, L.; Huang, Y.; Li, S.; Ji, R.; et al. Infestation of Rice Striped Stem Borer (Chilo suppressalis) Larvae Induces Emission of Volatile Organic Compounds in Rice and Repels Female Adult Oviposition. Int. J. Mol. Sci. 2024, 25, 8827. https://doi.org/10.3390/ijms25168827
Shen C, Yu S, Tan X, Luo G, Yu Z, Ju J, Yang L, Huang Y, Li S, Ji R, et al. Infestation of Rice Striped Stem Borer (Chilo suppressalis) Larvae Induces Emission of Volatile Organic Compounds in Rice and Repels Female Adult Oviposition. International Journal of Molecular Sciences. 2024; 25(16):8827. https://doi.org/10.3390/ijms25168827
Chicago/Turabian StyleShen, Chen, Shan Yu, Xinyang Tan, Guanghua Luo, Zhengping Yu, Jiafei Ju, Lei Yang, Yuxuan Huang, Shuai Li, Rui Ji, and et al. 2024. "Infestation of Rice Striped Stem Borer (Chilo suppressalis) Larvae Induces Emission of Volatile Organic Compounds in Rice and Repels Female Adult Oviposition" International Journal of Molecular Sciences 25, no. 16: 8827. https://doi.org/10.3390/ijms25168827
APA StyleShen, C., Yu, S., Tan, X., Luo, G., Yu, Z., Ju, J., Yang, L., Huang, Y., Li, S., Ji, R., Zhao, C., & Fang, J. (2024). Infestation of Rice Striped Stem Borer (Chilo suppressalis) Larvae Induces Emission of Volatile Organic Compounds in Rice and Repels Female Adult Oviposition. International Journal of Molecular Sciences, 25(16), 8827. https://doi.org/10.3390/ijms25168827






