Activation of the TRPML1 Ion Channel Induces Proton Secretion in the Human Gastric Parietal Cell Line HGT-1
Abstract
1. Introduction
2. Results
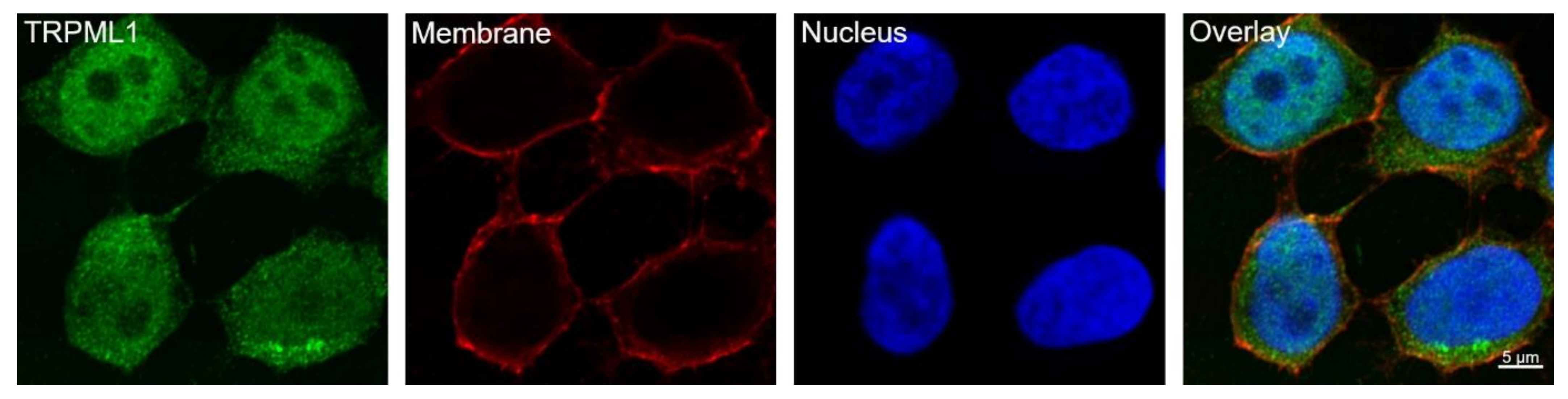
2.1. TRPML1 Is Expressed in the Parietal Cell Line HGT-1
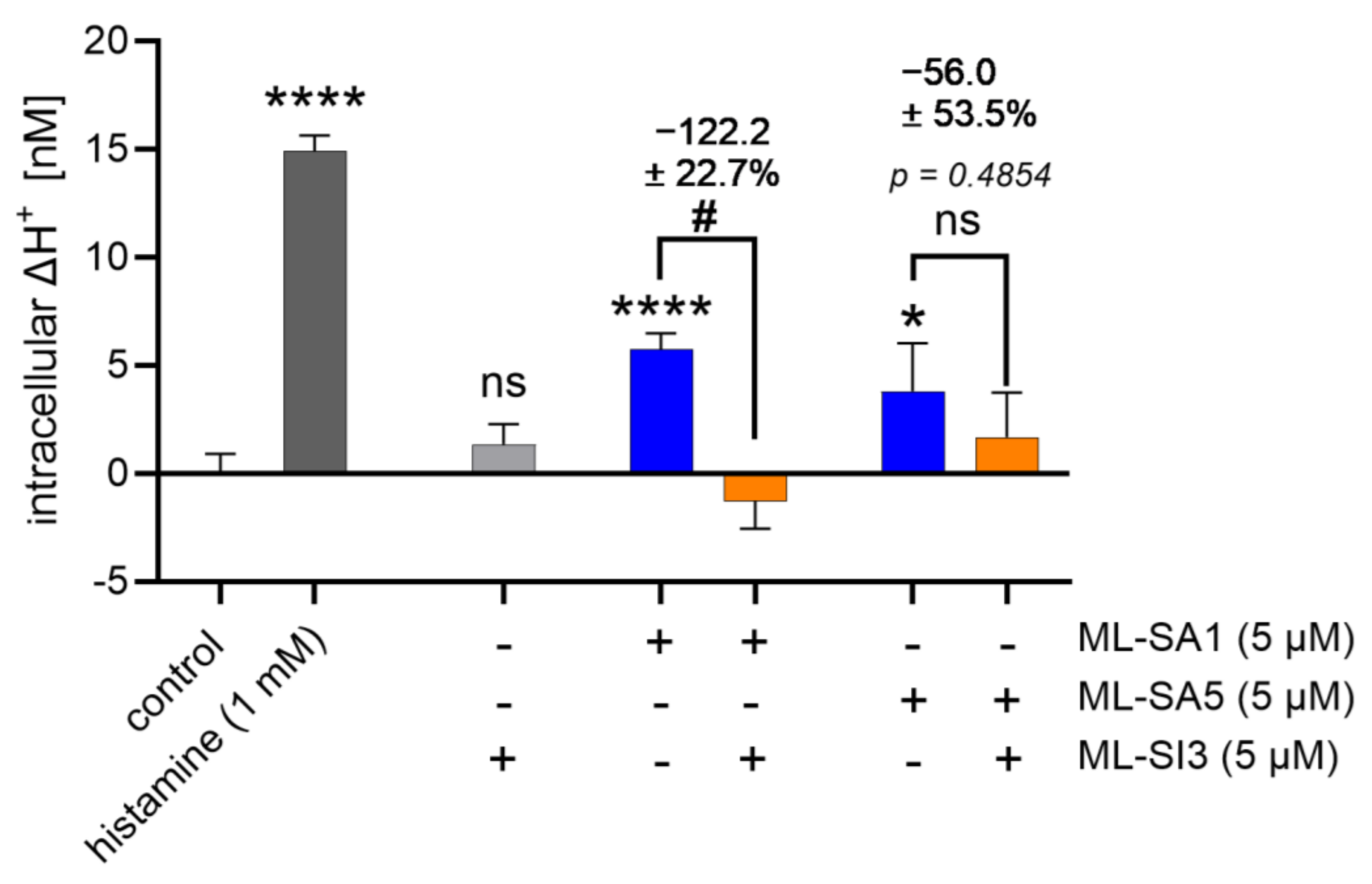
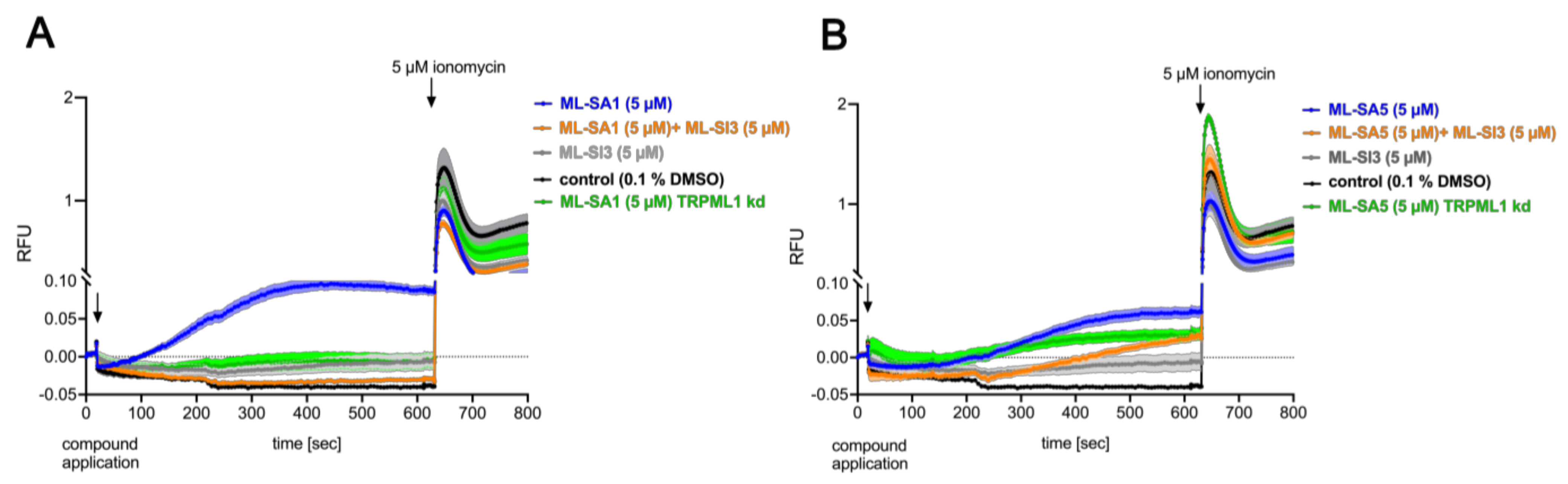
2.2. TRPML1 Activation Induces Proton Secretion in HGT-1 Cells
2.3. TRPML1 Activation Leads to Increased Cytosolic Ca2+ Concentrations
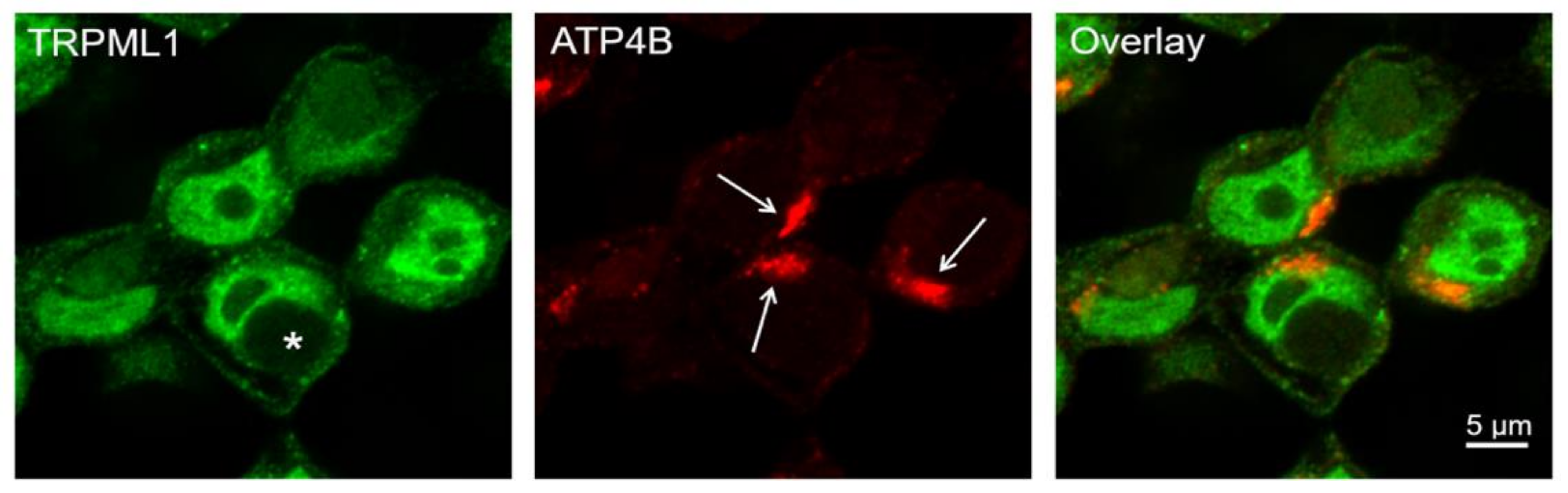
2.4. H+/K+-ATPase Accumulation and Vacuolar Apical Compartment Formation in HGT-1 Cells
2.5. Stimulation with Histamine and ML-SA1 Causes the Shifting of Lamp-1 to the Cell Membrane in Live-Cell Staining in HGT-1 Cells
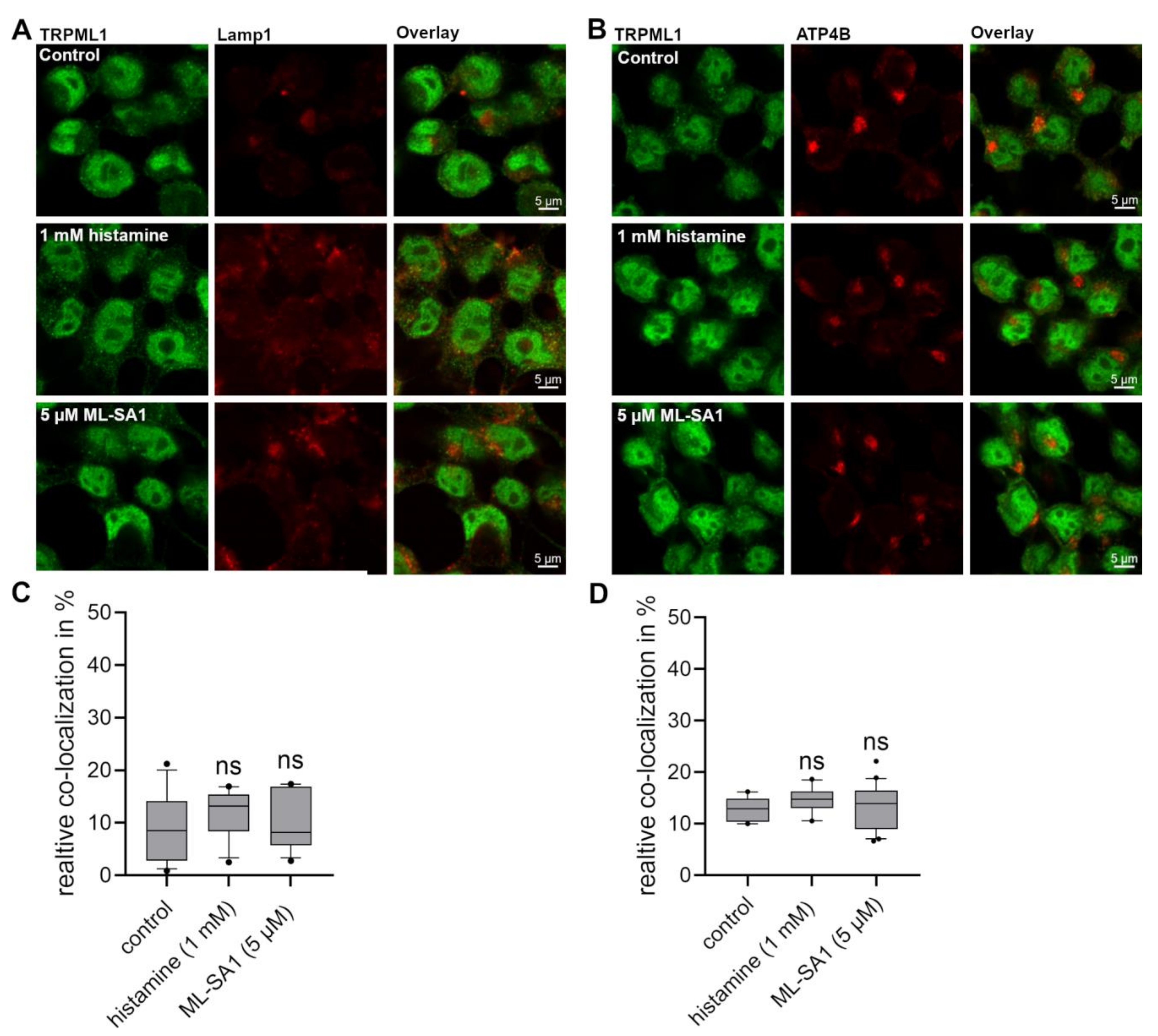
2.6. No Difference in the Co-Localization of TRPML1 with Lamp-1 and H+/K+-ATPase Could Be Detected in HGT-1 Cells after Exposure to Histamine and ML-SA1
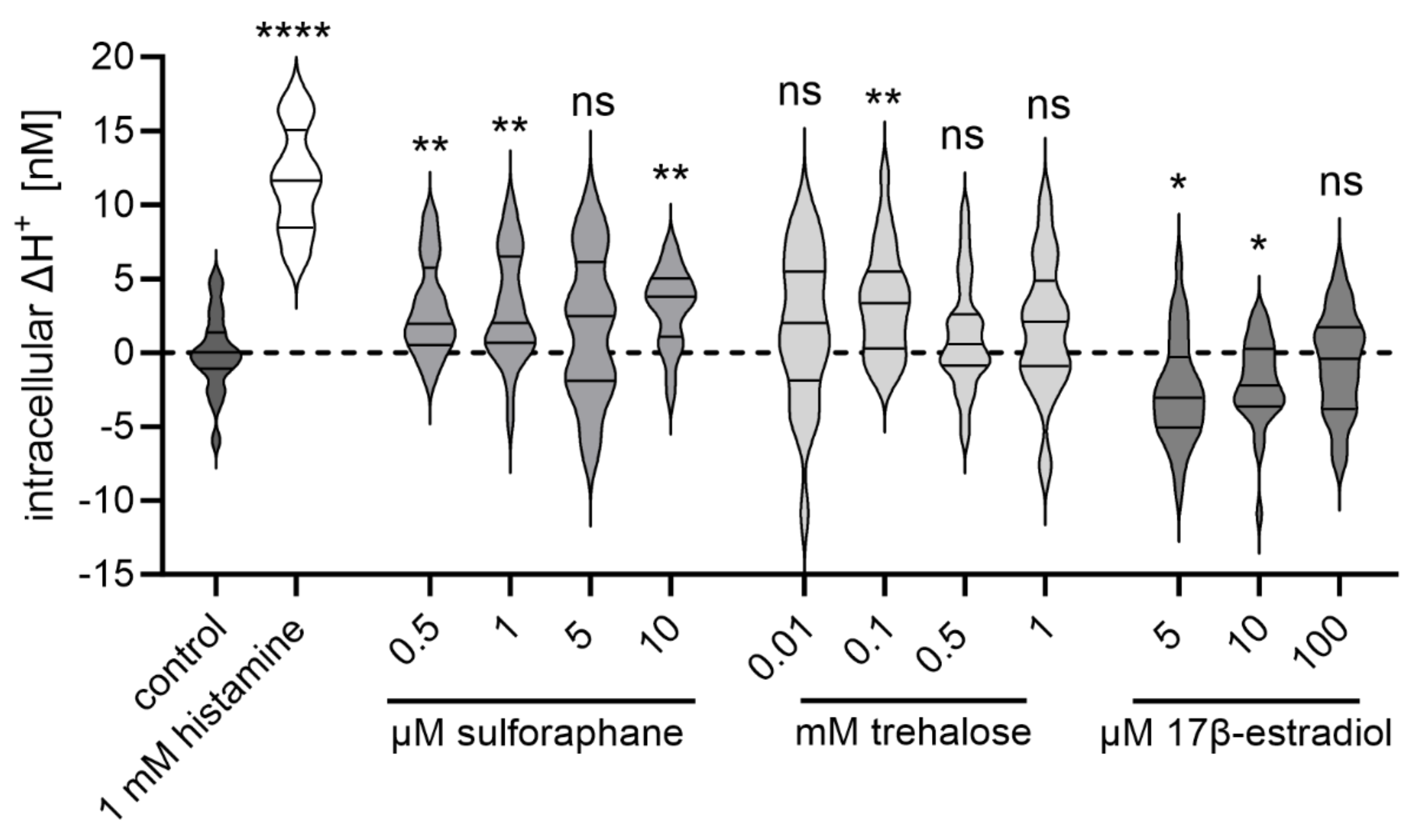
2.7. The Potential TRPML1 Activity Modulators Sulforaphane, Trehalose, and 17β-Estradiol Impact Proton Secretion in HGT-1 Cells
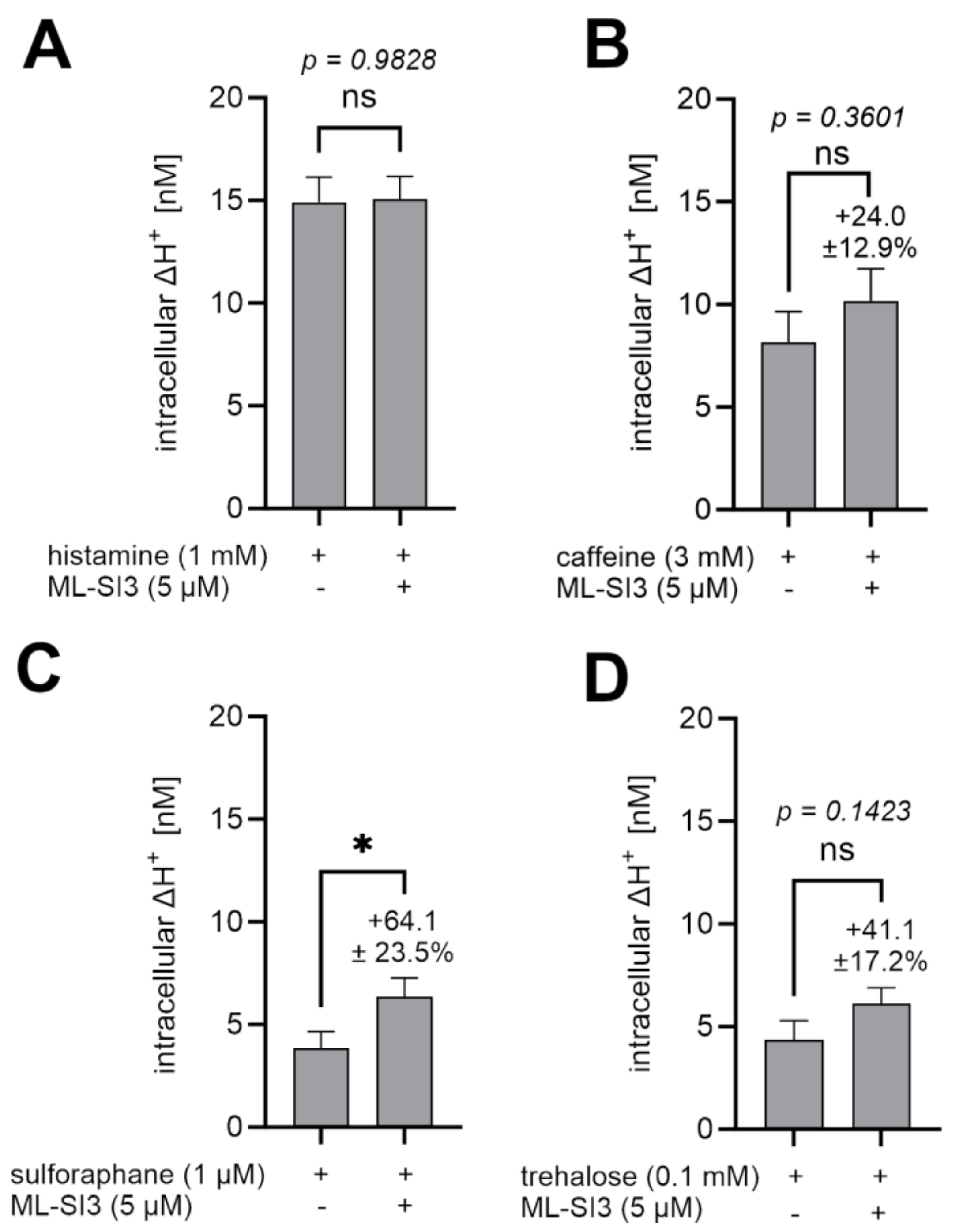
2.8. ML-SI3 Did Not Reduce the Proton Secretion Induced by Histamine, Caffeine, Trehalose, and Sulforaphane
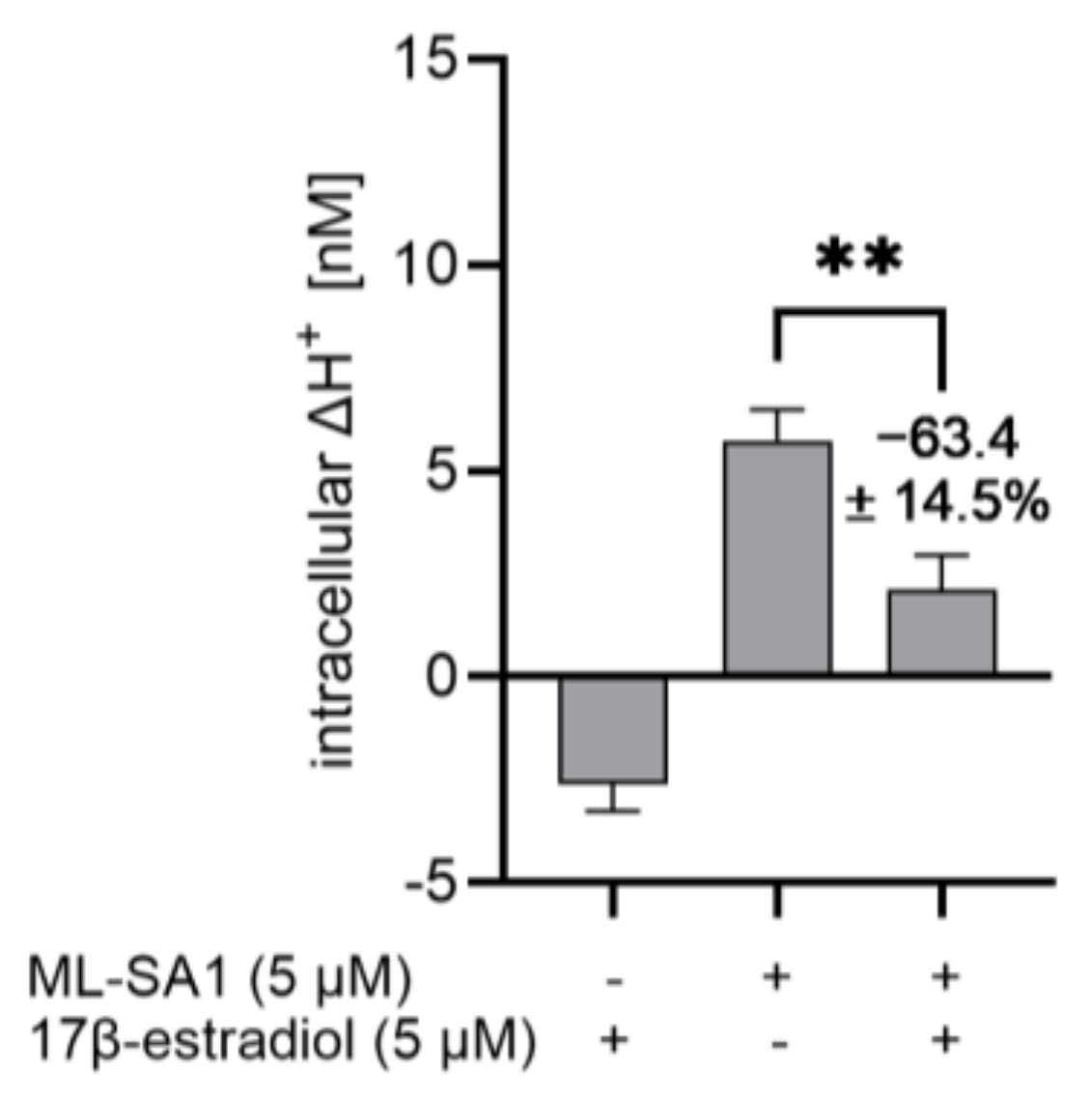
2.9. 17β-Estradiol Reduced the Proton Secretory Effect of ML-SA1 in HGT-1
2.10. Proton Secretion Induced by ML-SA1 and ML-SA5 Was Reduced in TRPML1 Knock-Down (kd) Cells
3. Discussion
3.1. HGT-1 Cells Localize TRPML1 in or around the Cell Nucleus
3.2. ML-SA5 and ML-SA1 Impact Proton Secretion and Intracellular Calcium Concentrations via Different Mechanisms
3.3. 17β-Estradiol Reduced the Proton Secretory Effect of ML-SA1 on HGT-1 Cells
3.4. Histamine and the Food-Derived Compounds Trehalose and Caffeine Do Not Act via TRPML1 in HGT-1 Cells
4. Material and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Immunostaining and Confocal Laser Scanning Microscopy (CLSM)
4.4. Cell Viability
4.5. Proton Secretion Assay
4.6. Transient Knock-Down of TRPML1
4.7. Relative Quantitation of RNA with qPCR and Real-Time PCR
4.8. Flow Cytometry
4.9. Intracellular Ca2+ Mobilization Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nilius, B.; Owsianik, G. The transient receptor potential family of ion channels. Genome Biol. 2011, 12, 218. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Gao, Q.; Xu, H. TRPML1: An ion channel in the lysosome. Handb. Exp. Pharmacol. 2014, 222, 631–645. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Xu, H. Phosphoinositide isoforms determine compartment-specific ion channel activity. Proc. Natl. Acad. Sci. USA 2012, 109, 11384–11389. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.-P.; Shen, D.; Wang, X.; Dawson, T.; Li, X.; Zhang, Q.; Cheng, X.; Zhang, Y.; Weisman, L.S.; Delling, M.; et al. PI(3,5)P2 controls membrane trafficking by direct activation of mucolipin Ca2+ release channels in the endolysosome. Nat. Commun. 2010, 1, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, W.; Gao, Q.; Yang, J.; Yan, X.; Zhao, H.; Su, L.; Yang, M.; Gao, C.; Yao, Y.; et al. Rapamycin directly activates lysosomal mucolipin TRP channels independent of mTOR. PLoS Biol. 2019, 17, e3000252. [Google Scholar] [CrossRef] [PubMed]
- Schmiege, P.; Fine, M.; Blobel, G.; Li, X. Human TRPML1 channel structures in open and closed conformations. Nature 2017, 550, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, N.; Gu, M.; Zhang, X.; Raval, N.; Yang, J.; Bekier, M.; Calvo, R.; Patnaik, S.; Wang, W.; King, G.; et al. Gastric Acid Secretion from Parietal Cells Is Mediated by a Ca2+ Efflux Channel in the Tubulovesicle. Dev. Cell 2017, 41, 262–273.e6. [Google Scholar] [CrossRef] [PubMed]
- Capurro, M.I.; Greenfield, L.K.; Prashar, A.; Xia, S.; Abdullah, M.; Wong, H.; Zhong, X.Z.; Bertaux-Skeirik, N.; Chakrabarti, J.; Siddiqui, I.; et al. VacA generates a protective intracellular reservoir for Helicobacter pylori that is eliminated by activation of the lysosomal calcium channel TRPML1. Nat. Microbiol. 2019, 4, 1411–1423. [Google Scholar] [CrossRef]
- Schmiege, P.; Fine, M.; Li, X. Atomic insights into ML-SI3 mediated human TRPML1 inhibition. Structure 2021, 29, 1295–1302.e3. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.-P.; Cheng, X.; Mills, E.; Delling, M.; Wang, F.; Kurz, T.; Xu, H. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature 2008, 455, 992–996. [Google Scholar] [CrossRef]
- Soyombo, A.A.; Tjon-Kon-Sang, S.; Rbaibi, Y.; Bashllari, E.; Bisceglia, J.; Muallem, S.; Kiselyov, K. TRP-ML1 regulates lysosomal pH and acidic lysosomal lipid hydrolytic activity. J. Biol. Chem. 2006, 281, 7294–7301. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Shao, R.; Wang, N.; Zhou, N.; Du, K.; Shi, J.; Wang, Y.; Zhao, Z.; Ye, X.; Zhang, X.; et al. Sulforaphane Activates a lysosome-dependent transcriptional program to mitigate oxidative stress. Autophagy 2021, 17, 872–887. [Google Scholar] [CrossRef] [PubMed]
- Cieslik, E.; Leszczynska, T.; Filipiakflorkiewicz, A.; Sikora, E.; Pisulewski, P. Effects of some technological processes on glucosinolate contents in cruciferous vegetables. Food Chem. 2007, 105, 976–981. [Google Scholar] [CrossRef]
- Rusmini, P.; Cortese, K.; Crippa, V.; Cristofani, R.; Cicardi, M.E.; Ferrari, V.; Vezzoli, G.; Tedesco, B.; Meroni, M.; Messi, E.; et al. Trehalose induces autophagy via lysosomal-mediated TFEB activation in models of motoneuron degeneration. Autophagy 2019, 15, 631–651. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Makhdoomi, M.; Singh, L.; Kumar, P.; Khan, N.; Singh, S.; Verma, H.N.; Luthra, K.; Sarkar, S.; Kumar, D. Trehalose limits opportunistic mycobacterial survival during HIV co-infection by reversing HIV-mediated autophagy block. Autophagy 2021, 17, 476–495. [Google Scholar] [CrossRef]
- Rühl, P.; Rosato, A.S.; Urban, N.; Gerndt, S.; Tang, R.; Abrahamian, C.; Leser, C.; Sheng, J.; Jha, A.; Vollmer, G.; et al. Estradiol analogs attenuate autophagy, cell migration and invasion by direct and selective inhibition of TRPML1, independent of estrogen receptors. Sci. Rep. 2021, 11, 8313. [Google Scholar] [CrossRef]
- Peng, X.; Holler, C.J.; Alves, A.-M.F.; Oliviera, M.G.; Speake, M.; Pugliese, A.; Oskouei, M.R.; de Freitas, I.D.; Chen, A.Y.-P.; Gallegos, R.; et al. Discovery and characterization of novel TRPML1 agonists. Bioorg. Med. Chem. Lett. 2024, 98, 129595. [Google Scholar] [CrossRef]
- Sun, M.; Goldin, E.; Stahl, S.; Falardeau, J.L.; Kennedy, J.C.; Acierno, J.S., Jr.; Bove, C.; Kaneski, C.R.; Nagle, J.; Bromley, M.C.; et al. Mucolipidosis type IV is caused by mutations in a gene encoding a novel transient receptor potential channel. Hum. Mol. Genet. 2000, 9, 2471–2478. [Google Scholar] [CrossRef]
- Chandra, M.; Zhou, H.; Li, Q.; Muallem, S.; Hofmann, S.L.; Soyombo, A.A. A role for the Ca2+ channel TRPML1 in gastric acid secretion, based on analysis of knockout mice. Gastroenterology 2011, 140, 857–867.e1. [Google Scholar] [CrossRef]
- Richter, P.; Sebald, K.; Fischer, K.; Behrens, M.; Schnieke, A.; Somoza, V. Released during Gastric Digestion of Casein, Stimulate Mechanisms of Gastric Acid Secretion via Bitter Taste Receptors TAS2R16 and TAS2R38. J. Agric. Food Chem. 2022, 70, 11591–11602. [Google Scholar] [CrossRef]
- Richter, P.; Andersen, G.; Kahlenberg, K.; Mueller, A.U.; Pirkwieser, P.; Boger, V.; Somoza, V. Sodium-Permeable Ion Channels TRPM4 and TRPM5 are Functional in Human Gastric Parietal Cells in Culture and Modulate the Cellular Response to Bitter-Tasting Food Constituents. J. Agric. Food Chem. 2024, 72, 4906–4917. [Google Scholar] [CrossRef]
- Liszt, K.I.; Ley, J.P.; Lieder, B.; Behrens, M.; Stöger, V.; Reiner, A.; Hochkogler, C.M.; Köck, E.; Marchiori, A.; Hans, J.; et al. Caffeine induces gastric acid secretion via bitter taste signaling in gastric parietal cells. Proc. Natl. Acad. Sci. USA 2017, 114, E6260–E6269. [Google Scholar] [CrossRef] [PubMed]
- Liszt, K.I.; Walker, J.; Somoza, V. Identification of Organic Acids in Wine That Stimulate Mechanisms of Gastric Acid Secretion. J. Agric. Food Chem. 2012, 60, 7022–7030. [Google Scholar] [CrossRef]
- Liszt, K.I.; Hans, J.; Ley, J.P.; Köck, E.; Somoza, V. Characterization of Bitter Compounds via Modulation of Proton Secretion in Human Gastric Parietal Cells in Culture. J. Agric. Food Chem. 2018, 66, 2295–2300. [Google Scholar] [CrossRef]
- Schubert, M.L. Gastric secretion. Curr. Opin Gastroenterol 2011, 27, 536–542. [Google Scholar] [CrossRef] [PubMed]
- Laboisse, C.; Augeron, C.; Couturierturpin, M.; Gespach, C.; Cheret, A.; Potet, F. Characterization of a newly established human gastric cancer cell line HGT-1 bearing histamine H2-receptors. Cancer Res. 1982, 42, 1541–1548. [Google Scholar]
- Carmosino, M.; Procino, G.; Casavola, V.; Svelto, M.; Valenti, G. The cultured human gastric cells HGT-1 express the principal transporters involved in acid secretion. Pflugers Arch. 2000, 440, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Zopun, M.; Liszt, K.I.; Stoeger, V.; Behrens, M.; Redel, U.; Ley, J.P.; Hans, J.; Somoza, V. Human Sweet Receptor T1R3 is Functional in Human Gastric Parietal Tumor Cells (HGT-1) and Modulates Cyclamate and Acesulfame K-Induced Mechanisms of Gastric Acid Secretion. J. Agric. Food Chem. 2018, 66, 4842–4852. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Hell, J.; Liszt, K.I.; Dresel, M.; Pignitter, M.; Hofmann, T.; Somoza, V. Identification of Beer Bitter Acids Regulating Mechanisms of Gastric Acid Secretion. J. Agric. Food Chem. 2012, 60, 1405–1412. [Google Scholar] [CrossRef]
- Simon, B.; Kather, H. Histamine-sensitive adenylate cyclase of human gastric mucosa. Gastroenterology 1977, 73, 429–431. [Google Scholar] [CrossRef]
- Nakada, S.L.; Crothers, J.M.; Machen, T.E.; Forte, J.G.; Natarajan, P.; Rosen, J.E.; Rakholia, M.; Okamoto, C.T. Apical vacuole formation by gastric parietal cells in primary culture: Effect of low extracellular Ca2+. Am. J. Physiol. Physiol. 2012, 303, C1301–C1311. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zavros, Y.; Orr, M.A.; Xiao, C.; Malinowska, D.H.; Donnelly, J.M.; Engevik, A.; Feng, R.; Boivin, G.P.; Li, J.; Houghton, J. Sonic hedgehog is associated with H+-K+-ATPase-containing membranes in gastric parietal cells and secreted with histamine stimulation. Am. J. Physiol. Gastrointest Liver Physiol. 2008, 295, G99–G111. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.-P.; Wang, X.; Shen, D.; Chen, S.; Liu, M.; Wang, Y.; Mills, E.; Cheng, X.; Delling, M.; Xu, H. Activating mutations of the TRPML1 channel revealed by proline-scanning mutagenesis. J. Biol. Chem. 2009, 284, 32040–32052. [Google Scholar] [CrossRef] [PubMed]
- Meyerhof, W.; Batram, C.; Kuhn, C.; Brockhoff, A.; Chudoba, E.; Bufe, B.; Appendino, G.; Behrens, M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem. Senses 2010, 35, 157–170. [Google Scholar] [CrossRef]
- Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 24 March 2024).
- Uhlén, M.; Zhang, C.; Lee, S.; Sjöstedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357, eaan2507. [Google Scholar] [CrossRef]
- Morelli, M.B.; Amantini, C.; Tomassoni, D.; Nabissi, M.; Arcella, A.; Santoni, G. Transient Receptor Potential Mucolipin-1 Channels in Glioblastoma: Role in Patient’s Survival. Cancers 2019, 11, 525. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Q.; Mårdh, S. Interactions between Ca2+- and cAMP-dependent stimulatory pathways in parietal cells. Biochim. Et Biophys. Acta (BBA)—Mol. Cell Res. 1996, 1311, 133–142. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ariyasu, T.; Matsumoto, S.; Kyono, F.; Hanaya, T.; Arai, S.; Ikeda, M.; Kurimoto, M. Taste receptor T1R3 is an essential molecule for the cellular recognition of the disaccharide trehalose. In Vitro Cell. Dev. Biol. Anim. 2003, 39, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Malekinejad, H.; Rezabakhsh, A. Hormones in Dairy Foods and Their Impact on Public Health—A Narrative Review Article. Iran. J. Public Health 2015, 44, 742–758. [Google Scholar]
- Aslam, M.A.; Hulst, M.; Hoving-Bolink, R.A.; Smits, M.A.; de Vries, B.; Weites, I.; Groothuis, T.G.; Woelders, H. Yolk concentrations of hormones and glucose and egg weight and egg dimensions in unincubated chicken eggs, in relation to egg sex and hen body weight. Gen. Comp. Endocrinol. 2013, 187, 15–22. [Google Scholar] [CrossRef]
- Yao, X.; Forte, J.G. Cell biology of acid secretion by the parietal cell. Annu. Rev. Physiol. 2003, 65, 103–131. [Google Scholar] [CrossRef] [PubMed]
- Forte, J.G. The gastric parietal cell: At home and abroad. Eur. Surg. 2010, 42, 134–148. [Google Scholar] [CrossRef]
- Forte, J.G.; Zhu, L. Apical recycling of the gastric parietal cell H,K-ATPase. Annu. Rev. Physiol. 2010, 72, 273–296. [Google Scholar] [CrossRef]
- Negulescu, P.A.; Reenstra, W.W.; Machen, T.E. Intracellular Ca requirements for stimulus-secretion coupling in parietal cell. Am. J. Physiol. 1989, 256, C241–C251. [Google Scholar] [CrossRef] [PubMed]
- Consortium, T.U. UniProt: The universal protein knowledgebase in 2021. Nucleic Acids Res. 2020, 49, D480–D489. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- UniPro. Available online: http://www.uniprot.org (accessed on 18 July 2024).
- Andersen, G.; Kahlenberg, K.; Krautwurst, D.; Somoza, V. [6]-Gingerol Facilitates CXCL8 Secretion and ROS Production in Primary Human Neutrophils by Targeting the TRPV1 Channel. Mol. Nutr. Food Res. 2023, 67, e2200434. [Google Scholar] [CrossRef]
- Tiroch, J.; Sterneder, S.; Di Pizio, A.; Lieder, B.; Hoelz, K.; Holik, A.-K.; Pignitter, M.; Behrens, M.; Somoza, M.; Ley, J.P.; et al. Bitter Sensing TAS2R50 Mediates the trans-Resveratrol-Induced Anti-inflammatory Effect on Interleukin 6 Release in HGF-1 Cells in Culture. J. Agric. Food Chem. 2021, 69, 13339–13349. [Google Scholar] [CrossRef]


| Staining Antibody/Chemical | Company | Working Solution |
|---|---|---|
| Hoechst-33342 | Fisher Scientific GmbH, Munich, Germany | 1:2000 |
| Concanavalin A biotin conjugate | Sigma-Aldrich, Munich, Germany | 1:2000 |
| Streptavidin, Alexa Fluor™ 633 conjugate (RRID:AB_2313500) | Fisher Scientific GmbH, Munich, Germany | 1 µg/mL |
| DyLight™ 554 Phalloidin | Cell Signaling Technology, Beverly, MA USA | 1:100 |
| Anti-TRPML1 antibody (RRID: AB_10915894) | Alomone labs, Jerusalem, Israel | 8 µg/mL |
| TRPML1 blocking peptide | Alomone labs, Jerusalem, Israel | 8 µg/mL |
| Alexa Fluor 488 anti-Rabbit IgG (RRID: AB_2536097) | Fisher Scientific GmbH, Munich, Germany | 1 µg/mL |
| Anti-human-CD107a (LAMP-1) (RRID:AB_467426) | Fisher Scientific GmbH, Munich, Germany | 10 µg/mL |
| Anti-ATP4B Monoclonal Antibody (2G11) (RRID:AB_2227726) | Fisher Scientific GmbH, Munich, Germany | 1:100 |
| eFluor™ 570 anti-Mouse IgG (RRID: AB_2573606) | Fisher Scientific GmbH, Munich, Germany | 1 µg/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mueller, A.U.; Andersen, G.; Richter, P.; Somoza, V. Activation of the TRPML1 Ion Channel Induces Proton Secretion in the Human Gastric Parietal Cell Line HGT-1. Int. J. Mol. Sci. 2024, 25, 8829. https://doi.org/10.3390/ijms25168829
Mueller AU, Andersen G, Richter P, Somoza V. Activation of the TRPML1 Ion Channel Induces Proton Secretion in the Human Gastric Parietal Cell Line HGT-1. International Journal of Molecular Sciences. 2024; 25(16):8829. https://doi.org/10.3390/ijms25168829
Chicago/Turabian StyleMueller, Alina Ulrike, Gaby Andersen, Phil Richter, and Veronika Somoza. 2024. "Activation of the TRPML1 Ion Channel Induces Proton Secretion in the Human Gastric Parietal Cell Line HGT-1" International Journal of Molecular Sciences 25, no. 16: 8829. https://doi.org/10.3390/ijms25168829
APA StyleMueller, A. U., Andersen, G., Richter, P., & Somoza, V. (2024). Activation of the TRPML1 Ion Channel Induces Proton Secretion in the Human Gastric Parietal Cell Line HGT-1. International Journal of Molecular Sciences, 25(16), 8829. https://doi.org/10.3390/ijms25168829







